Abstract
A prospective randomized controlled trial comparing two groups of ICSI (intra-cytoplasmatic sperm injection) patients with a different form of triggering the final oocyte maturation has been performed. All patients received an ovarian stimulation for in vitro fertilisation (IVF) using an antagonist protocol using recombinant-FSH (rec-FSH) and Ganirelix. 120 Patients were randomized into two groups with similar clinical parameters. The first group had triggering with hCG, whereas the second group received a combination of hCG + GnRH agonist (Gonadotropin Releasing Hormone). As the primary endpoint, the number of metaphase II oocytes were analysed, the secondary endpoints were the number of cumulus oocyte complexes (COC), the number of fertilized oocytes, embryo morphology, pregnancy rate and the number of cryopreserved embryos. The mean number of MII oocytes in the hCG triggered group was 9.2 compared with 10.3 in the hCG-GnRH agonist group. There was no statistically significant difference in the number of COCs or pregnancy rates. However, the number of patients who received at least one embryo of excellent quality was significantly higher (p = 0.001) in the group with the combined triggering (45 out of 61 patients or 73.8%) versus the group with hCG triggering alone (28 out of 59 patients or 47.5%). The number of cryopreserved embryos was also higher in this group.
Keywords: Dual triggering, final oocyte maturation, FSH surge, GnRH agonist triggering, hCG triggering, GnRH antagonist, IVF, ICSI
Introduction
Ovarian stimulation with FSH, combined with GnRH agonist and triggered with hCG is a standard procedure in in vitro fertilization (IVF) and intra cytoplasmic sperm injection (ICSI) (Albano et al., 1998; Devroey et al., 2009; Kolibianakis et al., 2004). In the last decade the use of GnRH antagonists for down regulation has become more popular. It has been demonstrated before that the triggering for final oocyte maturation by hCG can be replaced by GnRH agonist (Gonen et al., 1990; Griesinger et al., 2007; Humaidan et al., 2009; Humaidan, 2011; Shapiro et al., 2011; Imbar et al., 2012). In several randomized controlled trials it was demonstrated that the number of cumulus oocyte complexes (COCs) was certainly not lower and even higher in patients triggered by either GnRH agonist or hCG (Humaidan et al., 2005).
In spontaneous cycles FSH and LH show a midcycle surge. However, if hCG is used for triggering final oocyte maturation, FSH and LH concentrations do not rise and oocyte maturation relies entirely on the LH activity of hCG (Kol and Humaidan, 2010; Kyrou et al., 2011).
On the other hand, GnRH agonist triggering of final oocyte maturation mimics the naturally occurring surge of gonadotropin hormones (Gonen et al., 1990). This strategy will initiate a flare-up of both FSH and LH (Kol and Humaidan, 2013). However the mechanism of the additional FSH effect on the periovulatory hormonal changes requires further investigation (Griffin et al., 2012; Humaidan, 2012; Humaidan et al., 2012; Kummer et al., 2013; Segal and Casper, 1992).
In this study we aim to investigate the role of the GnRH agonist induced FSH surge on the number of metaphase II (MII) oocytes. It could be expected that improved embryo quality would be obtained because of the mimicking of the natural mid cycle endocrinology. This phenomenon was investigated by comparing hCG triggering versus GnRH agonist combined with hCG triggering of final oocyte maturation in patients undergoing ICSI in a prospective randomized controlled trial (Palermo et al., 1992). The choice for dual triggering in the second group was initiated by the negative effect on implantation by the induction of final oocyte maturation by GnRH agonist alone (Kol and Humaidan, 2013).
Materials and Methods
The current randomized controlled trial was performed at the IVF centre of the hospital Jan Palfijn, Gent between November 2011 and September 2013. The inclusion criteria were tubal or male infertility, BMI < 32, age ≤ 38 years, absence of major endocrinologal pathology, first, second and third IVF cycle. Exclusion was made for couples with azospermia and patients with female uterine abnormalities and major endocrinological disorders including PCOS. Patients with ovarian endome triotic cysts were also excluded from the study. Recruitment took place at the first visit. Patients willing to participate, and meeting the inclusion and exclusion criteria were, after signing the informed consent form, randomized in two groups. Group A was assigned to the hCG triggering alone, whereas group B received the dual triggering. The randomization was performed by the study coordinator through a computer generated random number list. A statistician who was not involved in the recruitment or follow up of the patients performed the statistical analysis.
Since the primary endpoint was the number of metaphase II (MII) oocytes, sample size calculation was performed in order to detect a difference in the mean number of MII oocytes between the two groups, one (oocyte) with a power of 80%, tested by means of a t-test, and the two-sided at 5% significance level and standard deviation estimated at two. According to this power calculation 120 patients were needed to be randomized in two groups: 59 patients were assigned to group A (hCG triggering alone) and 61 patients were assigned to group B (dual triggering group).
The study was registered at EudraCT in November 2011, and also at clinicalstudies.gov in November 2011. This second registration was reinstored in 2012 because of the replacement of the study coordinator. Institutional Review Board approval was obtained. Patient recruitment started in November 2011 and the last patient was randomized in September 2013. The cycle results were available in November 2013.
Ovarian stimulation was performed using a starting dose of 200 IU of recombinant FSH (Puregon®, MSD, Brussels, Belgium) for all patients. Suppression of endogenous LH was performed by using 0.25 mg GnRH antagonist (Orgalutran®, MSD, Brussels, Belgium) from day six of FSH stimulation onwards. On day 7 of the stimulation an ultrasonic evaluation of the number and size of ovarian effect follicles was performed and the dose of recombinant FSH was adapted according to the individual reaction of the patient. Further ultrasonic and hormonal analysis followed every two days. Triggering of final oocyte maturation was performed as soon as three follicles of > = 17 mm diameter were present by either injecting 5000IU of hCG (Pregnyl®, MSD, Brussels, Belgium) or a combination of 0.2 mg GnRH agonist triptorelin-acetate (Gonapeptyl®, Ferring, Aalst, Belgium) plus 5000IU of hCG (Pregnyl®, MSD, Brussels, Belgium) concomitantly (Kolibianakis et al., 2004; Kyrou et al., 2011; Polyzos et al., 2012).
Serum oestradiol, progesterone, LH and FSH were measured at day two of the menstrual cycle before initiation of stimulation, on the day of triggering final oocyte maturation, on day one after triggering, on day two after triggering (day of oocyte retrieval), on day five after triggering (day of embryo replacement) and on day eight after triggering.
Thirty-six hours after triggering oocyte retrieval was performed by ultrasound guided vaginal follicle aspiration (Hoff et al., 1983; Morley et al., 2012). The luteal phase support was started after egg retrieval by vaginal administration of 200 mg micronized progesterone three times daily (Utrogestan®, Besins, Brussels, Belgium) (Germond et al., 2002). The number COCs was registered before being decumulated and the number of MII oocytes was analysed. ICSI was applied to all MII oocytes (Palermo et al., 1992). In accordance with the Belgian legislation, one or two embryos were replaced on day three after egg retrieval (Gordts et al., 2005; Ombelet et al., 2005). Supernumerary embryos of excellent quality were cryopreserved (slow freezing protocol) immediately after the fresh transfer (day three after oocyte collection). Embryos of excellent quality which met the following criteria: 7 or 8-cell stage at day three after fertilization, less than 10% fragmentation and homogenous blastomeres. According to the study protocol all embryo transfers were performed on day three after oocyte retrieval. Thirteen days after embryo transfer hCG and progesterone were measured. In case of positive hCG the vaginal administration of 600 mg micronized progesterone was continued until 12 weeks of pregnancy. If hCG serum test was positive, an ultrasound confirmation of the pregnancy was performed 23 days after embryo transfer.
At the end of the treatment cycle we recorded in each patient the number of mature oocytes (MII), cumulus oocyte complexes, fertilized oocytes (2PN), excellent quality embryos and cryopreserved embryos. Hormonal evolution over the treatment cycle was analysed.
Comparison of continuous variables was performed using t-test and for comparing distribution of categorical variables, a Fisher Exact was used. For the analysis of the cycle ranking Pearson Chi2 and Mantel-HaenszelChi2 were used. All tests were performed two-sided at 5% significance level. For statistical analysis of hormone profiles over time a mixed modelling approach was used, assessing the correlation between measurements within the same subject.
Results
Analysis of the covariates age, body mass index (BMI), basal FSH level, basal progesterone level, total dose of recombinant FSH administered during the stimulation, the number of days of stimulation as well as the cycle ranking did not demonstrate any differences between the groups compared, nor did the mean number of transferred embryos show any difference as shown in Table I. The mean number of embryos transferred in both groups was similar. There was no statistical difference in the number of patients that received a single or double embryo transfer (SET or DET).
Table I. Basal patient characteristics and ovarian stimulation parameters between patients triggered with hCG and those triggered by GnRH agonist + hCG.
| Group A hCG triggering |
Group B Dual triggering |
P-value | |
| Age (y) (mean + SD) | 30.5 ± 4.1 | 30.0 ± 3.6 | NS |
| BMI (kg/m2) (mean + SD) | 23.5 ± 5.1 | 23.8 ± 4.6 | NS |
| Basal FSH level (IU/L)(mean + SD) | 7.5 ± 2.3 | 6.9 ± 3.4 | NS |
| Basal progesterone level (ng/mL) (mean + SD) | 0.7 ± 0.3 | 0.8 ± 0.6 | NS |
| Total recFSH dose (IU)(mean + SD) | 2 006 ± 457 | 2 083 ± 590 | NS |
| Duration of stimulation (days) (mean + SD) | 11.4 ± 1.7 | 11.7 ± 2.1 | NS |
| Cycle ranking (mean + SD) | 1.47 ± 0.65 | 1.67 ± 0.76 | NS |
| Max E2 value (mean + SD) | 2207.78 ± 173.71 | 2164.57 ± 262.13 | NS |
| Single embryo transfer / dual embryo transfer | 34/25 | 29/28 | NS |
As shown in Table II, no significant differences were observed between the groups compared regarding the number of MII oocytes, the number of COCs, the number of fertilized oocytes (2PN) and the number of cryopreserved embryos. The number of patients with surplus embryos for cryopreservation was 35.6% (21/59) in group A and 54.1% (33/61) in the group with dual triggering (P = 0.04). In the group with hCG triggering alone (group A) 47.5% (28/59) of patients did have at least one embryo of excellent embryological morphology, as compared with 73.8% (45/61) in the group with dual triggering (group B) (P = 0.001) (Table II).
Table II. Embryological parameters between patients triggered with hCG and those triggered with GnRH agonist + hCG.
| Group A hCG triggering |
Group B Dual triggering |
P-value | |
| MII oocytes (mean + SD) | 9.2 ± 6.7 | 10.3 ± 6.8 | NS |
| Cumulus Oocyte Complexes (mean + SD) | 12.0 ± 7.2 | 13.9 ± 8.7 | NS |
| 2PN oocytes (mean + SD) | 6.0 ± 4.5 | 7.4 ± 5.2 | NS |
| Cryopreserved embryos (mean + SD) | 1.5 ± 2.9 | 2.2 ± 2.9 | NS |
| Patients with at least one top quality embryo | 28/59 ( 47.5%) | 45/61 (73.8%) | 0.001 |
| Patients with embryos for cryopreservation | 21/59 (35.6%) | 33/61 (54.1%) | 0.04 |
No significant difference was observed in the implantation rate between the two groups (Table III). In group A (hCG) the implantation rate was 34% (28/82) whereas in group B (hCG + agonist) the implantation rate was 22% (19/86). Finally 26 out of 59 (44.1%) patients became pregnant in the group with hCG triggering alone and 19 out of 61 (33.1%) in the group with the hCG combined with GnRH agonist triggering.
Table III. Comparison of clinical outcome.
| Endpoint | Group A hCG triggering |
Group B Dual triggering |
P-value |
| Implantation rate (mean number of foetal sacs per embryo transferred) | 28/82 (34%) | 19/86 (22%) | NS |
| On-going Pregnancy rate | 26/59 (44.1%) | 19/61 (31.1%) | NS |
To date 36 patients had frozen embryos transferred after thawing, 12 in group A and 24 in group B, leading to one (8.3%) and five (20.8%) pregnancies respectively. The combination of fresh embryo transfer with frozen/thawed embryo replacement brought the total pregnancy rate in group A to 27/59 (45.8%) whereas group B (dual triggering) achieved 24/61 pregnancies (39.3%).
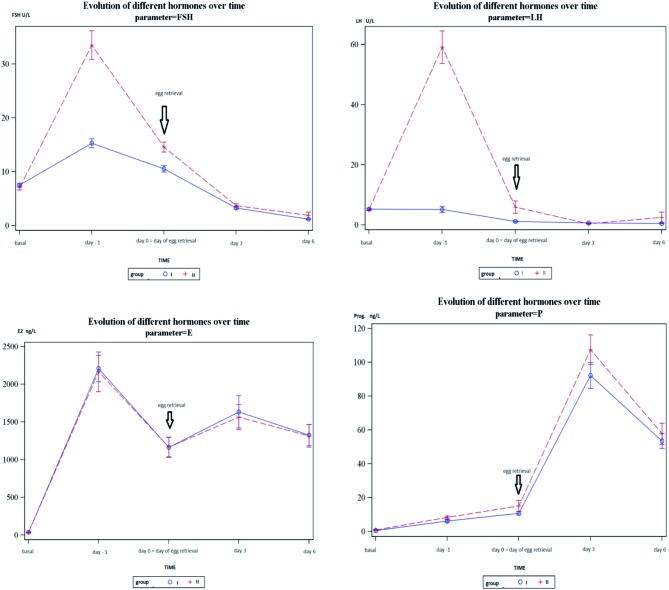
In Figure 1a graphical display of the evolution of the hormones following triggering of final oocyte maturation was made. There were no differences between the hCG and the GnRH agonist-hCG groups in terms of oestradiol (E2) and progesterone following triggering of final oocyte maturation. However, the FSH curve showed a more distinct surge after administration of GnRH agonist in the group with combined triggering. The FSH levels remain at a higher level for a longer period after combined triggering. There is no LH surge in the group of hCG triggering whereas a clear and very explicit LH-peak can be observed in the group with combined triggering.
Fig. 1. Graphic display of the evolution of FSH, LH, Estradiol and progesterone levels following triggering of final oocyte maturation (blue line: hCG group, red line: dual triggering group).
No OHSS was diagnosed in the study population, either in the group with hCG triggering, or in the dual triggering group.
Discussion
Both groups in the current study were comparable regarding female age, BMI, basal FSH, basal progesterone, total dose of recombinant FSH needed and the number of days required for stimulation.
So far it has been demonstrated that the administration of GnRH analogues for final maturation of oocytes after stimulation with recombinant FSH in combination with suppression of the LH surge by GnRH antagonists is a valuable alternative for classical hCG-triggering (Griesinger et al., 2006; Humaidan et al., 2005, 2009; Humaidan, 2010; Itskovitz et al., 1991; Kol et al., 2011; Kol and Humaidan, 2013). However, simultaneous administration of 5000 IUhCG (normal triggering dose) and GnRH agonists for triggering the final maturation of the oocytes has only been studied in a limited number of studies (Schachter et al., 2008; Lin et al., 2013). The present randomized trial is a prospective study dealing with the specific research question as to whether there is a difference between triggering with hCG alone and triggering with the combination of GnRH analogues in collaboration with hCG in terms of number and quality of oocytes, embryological and clinical outcome and hormonal profiles. Concerning the hormonal curves it has been noted that there was no difference in the comparison of hormone evolution over time between both groups for both the oestradiol and progesterone levels in the luteal phase. However there was a significantly higher level of LH immediately after triggering in the combination group. Also the induction of a distinct and significant additional FSH surge has been observed in all patients of the group in which dual triggering took place. As far as the primary endpoint is concerned, there was no significant difference in the number of MII oocytes, neither was there a difference in the number of COCs or in the number of 2PN oocytes. However, as could be expected from the literature (Lin et al., 2013; Schachter et al., 2008) there was a significantly higher number of patients with at least one embryo of excellent quality in patients who were triggered by a combination of GnRH agonist and hCG.
In the same group, there was also a significantly higher number of patients with frozen embryos and more embryos frozen per patient. In group II, 132 embryos were frozen versus 91 in group I (2.2 versus 1.5 per patient).
The pregnancy rate in the dual triggering group was lower than that obtained in the group triggered by hCG alone, despite the fact that the number of good quality embryos was significantly lower in the hCG-only triggered group. The reason for this discrepancy – better embryo-quality yet lower pregnancy rate – is still unclear. A possible explanation could be a negative effect on the endometrial receptivity, induced by the higher LH levels and the additional FSH surge. Further research is required to analyse these effects.
Since the combination of hCG and GnRH agonist for triggering appears to have a beneficial effect on embryo morphology, there might be an opportunity for this strategy for final oocyte maturation i.e. especially in patients with repeated poor embryo morphology although a potentially negative effect of this dual triggering on implantation cannot be excluded (Fanchin et al., 2001). Therefore vitrification of all oocytes and embryos obtained and replacement of the frozen/thawed embryos in a natural or artificial cycle might be considered (Galindo et al., 2009; Griesinger et al., 2007, 2011; Groenewoud et al., 2012). In case of an egg donation program in which all oocytes are vitrified for subsequent use, the combined triggering as used in the current study might be advantageous considering the increase in number of good quality embryos (Griesinger et al., 2006). However, it should be noted that such a strategy is only acceptable if there is no risk for OHSS (Engmann et al., 2008; Devroey et al., 2011; DiLuigi et al., 2010).
Notwithstanding the higher incidence of good quality embryos after dual triggering, a similar on-going pregnancy rate was observed; future randomized trials are still needed to assess the effect of the observed increase in embryo quality on pregnancy rate.
Our data corroborates the findings of Fatemi et al. (2010) concerning spontaneous LH surge and hCG triggering in natural cycles for the replacement of frozen/thawed embryos. In that study, the on-going pregnancy rates after spontaneous LH surge and after hCG triggering were analysed. Surprisingly, when hCG was added to a spontaneous LH surge, the pregnancy rate was significantly decreased (Fatemi et al., 2010). Although this study is dealing with a completely different patient population, it shows that hCG in combination with natural LH may have a negative effect on implantation.
Further research is needed to elaborate whether pregnancy rates are indeed lower after the combined triggering despite the improvement of embryo quality. This research question might be answered very soon when the outcome of the cryopreserved embryos is assessed, since the frozen/thawed cycles are not affected by the potential negative effect of the dual triggering on the endometrium.
Neither in the group with hCG triggering nor in the dual triggering group one case of OHSS was diagnosed. This is remarkable, especially for the dual triggering group, since in most studies concerning GnRH triggering, only low dose hCG (1500 IU) is added after oocyte retrieval in order to avoid ovarian hyperstimulation syndrome. (Humaidan et al., 2010). In our population 5000 IU hCG was used, but only at the time of triggering. No hCG was administered later in the cycle. This might explain the low incidence of OHSS in our study population.
Conclusion
In a randomized population of 120 patients there was no difference in the number of mature oocytes obtained between those patients that had induction of final oocyte maturation by the simultaneous administration of GnRH analogues and hCG, in comparison to the patients in the group that was triggered by the administration of hCG alone.
In the group with dual triggering of final maturation of oocytes a significant increase of morphologically excellent embryos accompanied by a significant increase in the number of patients with cryopreserved supernumerary embryos was observed. However, this did not result a higher pregnancy rate which requires further investigation.
Acknowledgments
>A special contribution was made by Lieve Declercq and Caroline Van de Steene, study coordinators, who performed the randomization and the collection of all hormonal and embryological data presented in this study. We thank Katrien Verschueren, Ir., M.Sc., of Living Statistics, for the statistical analysis. We would also like to thank Prof. John Hobbs for reviewing this paper.
References
- Albano C, Grimbizis G, Smitz J, et al. The luteal phase of nonsupplemented cycles after ovarian superovulation with human menopausal gonadotropin and the gonadotropin-releasing hormone antagonist Cetrorelix. Fertil Steril. 1998;70:357–359. doi: 10.1016/s0015-0282(98)00135-6. [DOI] [PubMed] [Google Scholar]
- Devroey P, Aboulghar M, Garcia-Velasco J, et al. Improving the patient’s experience of IVF/ICSI: a proposal for an ovarian stimulation protocol with GnRH antagonist co-treatment. Hum Reprod. 2009;24:764–774. doi: 10.1093/humrep/den468. [DOI] [PubMed] [Google Scholar]
- Devroey P, Polyzos N, Blockeel C. An OHSS-Free Clinic by segmentation of IVF treatment. Hum Reprod. 2011;26:2593–2597. doi: 10.1093/humrep/der251. [DOI] [PubMed] [Google Scholar]
- DiLuigi A, Engmann L, Schmidt D, et al. Gonadotropin-releasing hormone agonist to induce final oocyte maturation prevents the development of ovarian hyperstimulation syndrome in high-risk patients and leads to improved clinical outcomes compared with coasting. Fertil Steril. 2010;94:1111–1114. doi: 10.1016/j.fertnstert.2009.10.034. [DOI] [PubMed] [Google Scholar]
- Engmann L, Di Luigi A, Schmidt D, et al. The use of gonadotropin-releasing hormone (GnRH) agonist to induce oocyte maturation after cotreatment with GnRH antagonist in high-risk patients undergoing in vitro fertilization prevents the risk of ovarian hyperstimulation syndrome: A prospective randomized controlled study. Fertil Steril. 2008:84–91. doi: 10.1016/j.fertnstert.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Fanchin R, Peltier E, Frydman R, et al. Human Chorionic Gonadotropin: Does it Affect Human Endometrial Morphology In Vivo? Semin Reprod Med. 2001;19:31–35. doi: 10.1055/s-2001-13908. [DOI] [PubMed] [Google Scholar]
- Fatemi H, Kyrou D, Bourgain C, et al. Cryopreserved-thawed human embryo transfer: spontaneous natural cycle is superior to human chorionicgonadotropin-induced natural cycle. Fertil Steril. 2010;94:2054–2058. doi: 10.1016/j.fertnstert.2009.11.036. [DOI] [PubMed] [Google Scholar]
- Galindo A, Bodri D, Guillén J, et al. Triggering with HCG or GnRH agonist in GnRH antagonist treated oocyte donation cycles: a randomizedclinical trial. Gynecol Endocrinol. 2009;25:60–66. doi: 10.1080/09513590802404013. [DOI] [PubMed] [Google Scholar]
- Germond M, Capelli P, Bruno G, et al. Comparison of the efficacy and safety of two formulations of micronized progesterone (Ellios and Utrogestan) used as luteal phase support after in vitro fertilization. Fertil Steril. 2002;77:313–317. doi: 10.1016/s0015-0282(01)02979-x. [DOI] [PubMed] [Google Scholar]
- Gonen Y, Balakier H, Powell W, et al. Use of Gonadotropin-Releasing Hormone Agonist to trigger Follicular Maturation for in Vitro Fertilization. J Clin Endocrinol Metab. 1990;71:918–922. doi: 10.1210/jcem-71-4-918. [DOI] [PubMed] [Google Scholar]
- Gordts S, Campo R, Puttemans P, et al. Belgian legislation and the effect of elective single embryo transfer on IVF outcome. 10. 2005:436–441. doi: 10.1016/s1472-6483(10)60818-8. [DOI] [PubMed] [Google Scholar]
- Griesinger G, Diedrich K, Devroey P, et al. GnRH agonist for triggering final oocyte maturation in the GnRH antagonist ovarian hyperstimulation protocol: a systematic review and meta-analysis. Hum Reprod Update. 2006;12:159–168. doi: 10.1093/humupd/dmi045. [DOI] [PubMed] [Google Scholar]
- Griesinger G, Kolibianakis E, Papanikolaou E, et al. Triggering of final oocyte maturation with gonadotropin-releasing hormone agonist or human chorionic gonadotropin. Live birth after frozen-thawed embryo replacement cycles. Fertil Steril. 2007;88:616–621. doi: 10.1016/j.fertnstert.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Griesinger G, Schultz L, Bauer T, et al. Ovarian hyperstimulation syndrome prevention by gonadotropin-releasing hormone agonist triggering of final oocyte maturation in a gonadotropin-releasing hormone antagonist protocol in combination with a “freeze-all” strategy: a prospective multicentric study. Fertil Steril. 2011;95:2029–2033. doi: 10.1016/j.fertnstert.2011.01.163. [DOI] [PubMed] [Google Scholar]
- Griesinger G, von Otte S, Schroer A, et al. Elective cryopreservation of all pronuclear oocytes after GnRH agonist triggering of final oocyte maturation in patients at risk of developing OHSS: a prospective, observational proof-of-concept study. Hum Reprod. 2007;22:1348–1352. doi: 10.1093/humrep/dem006. [DOI] [PubMed] [Google Scholar]
- Griffin D, Benadiva C, Kummer N, et al. Dual trigger of oocyte maturation with gonadotropin-releasing hormone agonist and low-dose human chorionic gonadotropin to optimize live birth rates in high responders. Fertil Steril. 2012;97:1316–1320. doi: 10.1016/j.fertnstert.2012.03.015. [DOI] [PubMed] [Google Scholar]
- Groenewoud ER, Macklon NS, Cohlen BJ. Cryo-thawed embryo transfer: natural versus artificial cycle. A non-inferiority trial. BMC Womens Health. 2012;12(doi: 10.1186/1472-6874-12-27):27. doi: 10.1186/1472-6874-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff J, Quigley M, Yen S. Hormonal dynamics at midcycle: A reevaluation. J Clin Endocrinol Metab. 1983;57:792–796. doi: 10.1210/jcem-57-4-792. [DOI] [PubMed] [Google Scholar]
- Humaidan P. Agonist trigger: What is the best approach? Agonist trigger and low dose hCG. Fertil Steril. 2012;97:529–530. doi: 10.1016/j.fertnstert.2011.11.016. [DOI] [PubMed] [Google Scholar]
- Humaidan P. Luteal phase rescue in high-risk OHSS patients by GnRH triggering in combination with low-dose HCG: a pilot study. Reprod Biomed Online. 2009;18:630–634. doi: 10.1016/s1472-6483(10)60006-5. [DOI] [PubMed] [Google Scholar]
- Humaidan P, Bredkjaer HE, Bungum L, et al. GnRH agonist (buserelin) or hCG for ovulation induction in GnRH antagonist IVF/ICSI cycles: a prospective randomized study. Hum Reprod. 2005;20:1213–1220. doi: 10.1093/humrep/deh765. [DOI] [PubMed] [Google Scholar]
- Humaidan P, Bredkjaer HE, Westergaard L, et al. 1,500 IU human chorionic gonadotropin administered at oocyte retrieval rescues the luteal phase when gonadotropin-releasing hormone agonist is used for ovulation induction: a prospective, randomized, controlled study. Fertil Steril. 2010;93:847–854. doi: 10.1016/j.fertnstert.2008.12.042. [DOI] [PubMed] [Google Scholar]
- Humaidan P, Kol S, Papanikolaou EG. GnRH agonist for triggering of final oocyte maturation: time for a change of practice? Hum Reprod Update. 2011;17:510–524. doi: 10.1093/humupd/dmr008. [DOI] [PubMed] [Google Scholar]
- Humaidan P, Papanikolaou EG, Tarlatzis BC. GnRH to trigger final oocyte maturation: a time to reconsider. Hum Reprod. 2009;24:2389–2394. doi: 10.1093/humrep/dep246. [DOI] [PubMed] [Google Scholar]
- Humaidan P, Quartarolo J, Papanikolaou E. Preventing ovarian hyperstimulation syndrome: guidance for the clinician. Fertil Steril. 2010;94:1–-9. doi: 10.1016/j.fertnstert.2010.03.028. [DOI] [PubMed] [Google Scholar]
- Imbar T, Kol S, Lossos F, et al. Reproductive outcome of fresh or frozen-thawed embryo transfer is similar in high-risk patients for ovarian hyperstimulation syndrome using GnRH agonist for final oocyte maturation and intensive luteal support. Hum Reprod. 2012;27:753–759. doi: 10.1093/humrep/der463. [DOI] [PubMed] [Google Scholar]
- Itskovitz J, Boldes R, Levron J, et al. Induction of preovulatory luteinizing hormone surge and prevention of ovarian hyperstimulation syndrome by gonadotropin-releasing hormone agonist. Fertil Steril. 1991;56:213–220. [PubMed] [Google Scholar]
- Kol S, Humaidan P. LH (as hCG) and FSH surges for final oocyte maturation: sometimes it takes two to tango? Reprod Biomed Online. 2010;21:590–592. doi: 10.1016/j.rbmo.2010.06.031. [DOI] [PubMed] [Google Scholar]
- Kol S, Humaidan P. GnRH agonist triggering: recent developments. Reprod Biomed Online. 2013;26:226–230. doi: 10.1016/j.rbmo.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Kol S, Humaidan P, Itskovitz-Eldor J. GnRH agonist ovulation trigger and hCG-based, progesterone-free luteal support: a proof of concept study. Hum Reprod. 2011;26:2874–2877. doi: 10.1093/humrep/der220. [DOI] [PubMed] [Google Scholar]
- Kolibianakis EM, Albano C, Camus M, et al. Prolongation of the follicular phase in in vitro fertilization results in a lower ongoing pregnancy rate in cycles stimulated with recombinant follicle-stimulating hormone and gonadotropin-releasing hormone antagonists. Fertil Steril. 2004;82:102–107. doi: 10.1016/j.fertnstert.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Kummer NE, Feinn RS, Griffin DW, et al. Predicting successful induction of oocyte maturation after gonadotropin-releasing hormone agonist (GnRH) trigger. Hum Reprod. 2013;28:152–159. doi: 10.1093/humrep/des361. [DOI] [PubMed] [Google Scholar]
- Kyrou D, Kolibianakis EM, Fatemi HM, et al. Is earlier administration of human chorionic gonadotropin (hCG) associated with the probability of pregnancy in cycles stimulated with recombinant follicle-stimulating hormone and gonadotropin-releasing hormone (GnRH) antagonists? A prospective randomized trial. Fertil Steril. 2011;96:1112–1115. doi: 10.1016/j.fertnstert.2011.08.029. [DOI] [PubMed] [Google Scholar]
- Lin MH, Ying-Wu F, Kuo Kuang Lee R. Dual trigger with combination of gonadotropin-releasing hormone agonist and human chorionic gonadotropin significantly improves the live-birth rate for normal responders in GnRH-antagonist cycles. Fertil Steril. 2013;100:1296–1302. doi: 10.1016/j.fertnstert.2013.07.1976. [DOI] [PubMed] [Google Scholar]
- Morley L, Tang T, Yasmin E, et al. Timing of human chorionic gonadotropin (hCG) hormone administration in IVF protocols using GnRH antagonists: a randomized controlled trial. Hum Fertil (Camb) 2012;15:134–139. doi: 10.3109/14647273.2012.712739. [DOI] [PubMed] [Google Scholar]
- Ombelet W, De Sutter P, Van der Elst J, et al. Multiple gestation and infertility treatment: registration, reflection and reaction--the Belgian project. Hum Reprod Update. 2005;11:3–14. doi: 10.1093/humupd/dmh048. [DOI] [PubMed] [Google Scholar]
- Palermo G, Joris H, Devroey P, et al. Pregnancies after intra cytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;4:17–18. doi: 10.1016/0140-6736(92)92425-f. [DOI] [PubMed] [Google Scholar]
- Polyzos NP, Blockeel C, Verpoest W, et al. Live birth rates following natural cycle IVF in women with poor ovarian response according to the Bologna criteria. Hum Reprod. 2012;27:3481–3486. doi: 10.1093/humrep/des318. [DOI] [PubMed] [Google Scholar]
- Schachter M, Friedler S, Ron-El R, et al. Can pregnancy rate be improved in gonadotropin-releasing hormone (GnRH) antagonist cycles by administering GnRH agonist before oocyte retrieval? A prospective, randomized study. Fertil Steril. 2008;90:1087–1093. doi: 10.1016/j.fertnstert.2007.07.1316. [DOI] [PubMed] [Google Scholar]
- Segal S, Casper RF. Gonadotropin-releasing hormone agonist versus chorionic gonadotropin for triggering follicular maturation in in vitro fertilization. Fertil Steril. 1992;57:1254–1258. [PubMed] [Google Scholar]
- Shapiro BS, Daneshmand ST, Garner FC. Comparison of “triggers” using leuprolide acetate alone or in combination with low-dose human chorionic gonadotropin. Fertil Steril. 2011;95:2715–2717. doi: 10.1016/j.fertnstert.2011.03.109. [DOI] [PubMed] [Google Scholar]
- Shapiro BS, Daneshmand ST, Restrepo H, et al. Efficacy of induced luteinizing hormone surge after “trigger” with gonadotropin-releasing hormone agonist. Fertil Steril. 2011;95:826. doi: 10.1016/j.fertnstert.2010.09.009. [DOI] [PubMed] [Google Scholar]