Summary
Background
A 61-year-old woman received standard immunizations, including Haemophilus influenzae type B, diphtheria, tetanus and Pneumovax, one year after undergoing non-myeloablative hematopoietic stem cell transplantation (HSCT) for acute myelogenous leukemia. Five days later she developed fatigue with progressive weight gain and edema. 14 days after immunization she presented with anasarca and was found to have acute renal failure and nephrotic proteinuria.
Investigations
Physical examination, serum chemistries, examination of urine sediment, renal ultrasound with Doppler, 24-hour urine collection and renal biopsy.
Diagnosis
Minimal change nephrotic syndrome with acute tubular injury.
Management
Aggressive diuresis and oral corticosteroid therapy.
Keywords: Acute renal failure, bone marrow transplant, immunization, minimal change disease, nephrotic syndrome
The Case
Two years prior to presentation a 59-year-old woman developed fatigue and pancytopenia, and bone marrow biopsy revealed acute myelogenous leukemia (AML). She had normal cytogenetics and entered remission after induction chemotherapy with anthracycline and cytarabine. She subsequently received consolidation therapy with high dose cytarabine, and did well until one year later, when pancytopenia recurred and marrow showed relapsed AML. Soon thereafter the patient underwent NON-MYELOABLATIVE HEMATOPOIETIC STEM CELL TRANSPLANTATION (HSCT) from an unrelated donor. She had no pre-transplantation proteinuria. CONDITIONING included fludarabine phosphate and busulfan (1), and GRAFT-VERSUS-HOST DISEASE (GVHD) prophylaxis consisted of methotrexate, sirolimus and tacrolimus (2).
The patient's post-transplantation course was uneventful. Immunosuppression was tapered and then discontinued after six months. One year after HSCT she received routine recommended immunizations, including hemophilus influenzae type B, diphtheria, tetanus toxoid and unconjugated 23-valent pneumococcal vaccine (also known as Pneumovax). Her serum creatinine at this visit was 71 μmol/L (0.8 mg/dl). Five days after immunization she began to experience fatigue and progressive weight gain. Two weeks after immunization she had gained 10 kg and had generalized edema. She had a serum creatinine of 301 μmol/L (3.4 mg/dl), hypoalbuminemia of 0.28 mg/L, proteinuria of 4+ and no hematuria. Urinary sediment showed fine granular casts (Figure 1 A). The patient was admitted to hospital for kidney biopsy and further evaluation. A Doppler renal ultrasound showed normal sized kidneys, normal vascular flow and no hydronephrosis. A 24-hr urine collection disclosed 14 g of urine protein.
Figure 1.
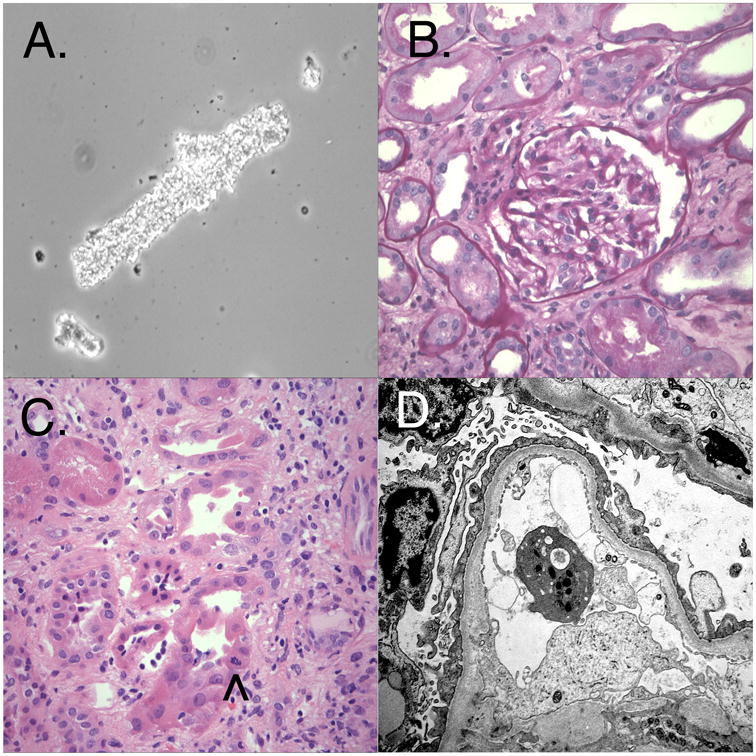
The patient's urinary sediment and kidney biopsy findings. (A) Fine granular cast on urinary sediment at presentation, 14 days after vaccination. (B) Unremarkable glomerulus by light microscopy (Periodic acid Schiff reaction). (C) Tubules show evidence of injury, with mild vacuolization, epithelial atypia, and scattered mitotic activity (arrowhead). The interstitium appears expanded with a mild increase in mononuclear inflammatory cells. (D) Ultrastructural evidence of glomerular visceral epithelial cell injury, including effacement of foot processes. The capillary endothelium does not appear injured, as the fenestrations are well preserved and there is no evidence of glomerular basement membrane remodeling or subendothelial expansion. No dense deposits are present.
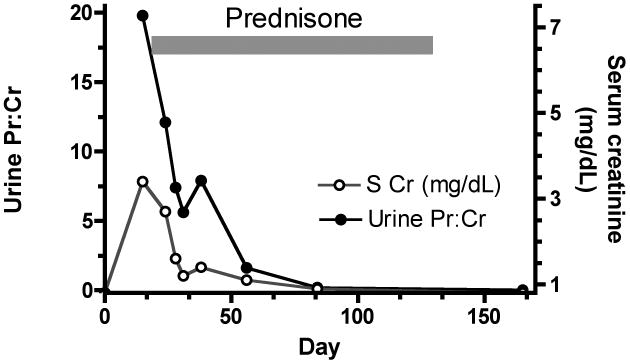
Light microscopy of the kidney biopsy (Figure 1 B & C) showed evidence of tubular injury and a mild but diffuse interstitial inflammatory infiltrate consisting of mononuclear cells and scattered eosinophils. It also showed fifteen glomeruli without evidence of segmental or global sclerosis, active inflammation, basement membrane changes, or thrombosis. No immune deposits or fibrin thrombi were identified by immunofluorescence and electron microscopy. Ultrastructural evaluation (Figure 1 D) revealed severe injury of the glomerular visceral epithelial cells, including microvillous degeneration and diffuse foot process effacement. The fenestrations of the glomerular capillary endothelium were well preserved and there was no significant basement membrane remodeling. Minimal change disease (MCD), acute tubular injury, and mild active interstitial nephritis were diagnosed. The patient was diuresed and started on 60 mg per day of oral prednisone. The proteinuria and acute renal failure (ARF) resolved rapidly. The urine protein:creatinine ratio decreased from 19.8 to 0.2 within two months after starting therapy (Figure 2). Within three weeks of the renal biopsy the patient developed mildly elevated TRANSAMINASES, dry eyes and oral ulcerations. Mild chronic GVHD was diagnosed. Steroids were tapered and ultimately discontinued after 10 weeks. After six months, the patient remains in clinical remission from AML and nephrotic syndrome. Her oral chronic GVHD responded to topical steroids, and her elevated transaminases have normalized.
Figure 2.
Time course for development and resolution of proteinuria after vaccination. Spot urine protein:creatinine ratio is plotted against time from vaccination (day 0). Prednisone therapy was started on day 16 and tapered after 8 weeks. Serum creatinine concentration is also indicated. S Cr, serum creatinine concentration; Urine Pr:Cr, urine protein:creatinine ratio.
Discussion of Diagnosis
HSCT is the most common non-renal transplant, with 18,000 performed annually in the US alone. The renal complications of this procedure can be divided into early and late events. Late events usually begin about 3 months after transplantation. The main causes of late renal disease in this population are summarized in Box 1 (3). At least 20% of survivors will develop chronic kidney disease (CKD), and this is most commonly caused by either bone marrow transplant nephropathy or chronic calcineurin inhibitor toxicity. Independent risk factors for development of CKD in non-myeloablative HSCT recipients include peri-transplantation ARF, previous autologous HSCT, long-term use of calcineurin inhibitors and chronic GVHD (4).
Box 1. Late renal complications after hematopoietic stem cell transplantation.
Chronic kidney disease
Bone marrow transplant nephropathy/thrombotic microangiopathy syndrome
Chronic calcineurin inhibitor toxicity
Glomerular disease
Membranous nephropathy
Minimal change disease
Glomerular disease is a less common late complication of HSCT than chronic kidney disease. Membranous nephropathy predominates, usually in the setting of chronic GVHD. Proteinuria may be in the nephrotic range and hematuria is often present. In the present case, the abrupt onset of massive proteinuria argued against membranous nephropathy and renal biopsy subsequently showed minimal change disease. MCD is less common than membranous nephropathy in HSCT recipients, and has never been described after non-myeloablative HSCT (5,6). Although the precise incidence of glomerular disease after HSCT has not been rigorously examined, a growing number of reports indicate that both membranous nephropathy and MCD may be more common in HSCT recipients compared to the general population. Other glomerulopathies also occasionally occur in HSCT recipients including focal and segmental glomerulosclersosis, IgA nephropathy and immune complex glomerulonephritis, but probably not more commonly than among non-transplanted patients.
Most cases of MCD are idiopathic but a variety of secondary causes are recognized and a partial list is presented in Box 2. The case presented here was associated with an allergic reaction to vaccination. Various vaccines have been linked to MCD, including influenza, pneumococcal and hepatitis B vaccines (7-9). A circulating factor produced by activated T lymphocytes might be involved in the pathogenesis of MCD. Following injection in rats, T cell HYBRIDOMAS or the supernatants of CONCANAVALIN A-stimulated mononuclear cells from MCD patients induce proteinuria and podocyte foot process fusion (10). MCD is also associated with atopy and lymphopoliferative diseases such as Hodgkin's disease, and usually responds rapidly to corticosteroids.
Box 2. Some secondary causes of minimal change nephropathy.
Drugs
Nonsteroidal anti-inflammatory drugs
Ampicillin
Gold
Allergens
Hymenoptera stings
Food
Pollen
Poison ivy and poison oak
Immunization
Infections
Viral
Cancer
Hodgkin's disease
Acute renal failure often coexists with nephrotic syndrome. Many patients with acute renal failure plus nephrotic syndrome have active tubulointerstitial nephritis in the setting of a hypersensitivity reaction, with signs of tubular injury typical of ischemic or toxic insults. History and temporal associations with the onset of symptoms reveal that non-steroidal anti-inflammatory drugs, angiotensin converting enzyme inhibitors and diuretics are the most frequent causative agents. The patient in the case presented here had neither fever nor eosinophilia at diagnosis and was on no medications around the time of vaccination. A drug-associated type-I hypersensitivity reaction is, therefore, unlikely to account for her tubulo-interstitial nephritis or tubular injury. We speculate that vaccination itself triggered the hypersensitivity reaction. Two other causes of ARF in the setting of massive proteinuria should be considered. The prothrombotic state of nephrotic syndrome may cause bilateral renal vein thrombosis, presenting with flank pain and hematuria. This was excluded by renal ultrasound with Doppler. Patients with massive proteinuria may additionally develop ARF as a consequence of intrarenal edema with impairment in glomerular hemodynamics (11). A hallmark of this syndrome of “nephrosarca” is improvement in ARF with diuresis. The renal biopsy lacked significant interstitial edema, excluding this etiology of ARF.
Several factors support vaccination as the cause of both MCD and acute renal failure in the case presented: symptoms began five days after vaccination; MCD has previously been associated with both vaccination and HSCT; and recent data indicate nephrotic syndrome might be more common than previously thought in non-myeloablative HSCT recipients. In a report of one HSCT recipient, MCD developed after withdrawal of cyclosporine immunosuppression but resolved after cyclosporine was reintroduced (12). In the case presented here we propose that simultaneous immunization with three different vaccines might have resulted in hyperstimulation of the recovering immune system, leading to dysregulated T lymphocyte activation and, subsequently, MCD. The fact that the patient developed chronic GVHD soon after MCD was diagnosed suggests that MCD might be an unusual manifestation of GVHD in HSCT recipients. Nevertheless, we cannot rule out the possibility that the association between vaccination and MCD is coincidental, since almost all HSCT recipients receive immunizations after successful engraftment and, to our knowledge, no other case of MCD has been reported in this context.
It is not known whether non-myeloablative recipients are at greater risk of nephrotic syndrome than myeloablative transplant recipients. Patients in both groups might develop chronic GVHD, which is itself associated with heightened risk of nephrotic syndrome. In a consecutive series of 163 non-myeloablative HSCT recipients, Srinivisan et al. reported an unexpectedly high incidence of nephrotic syndrome (13). They found seven cases of nephrotic syndrome and biopsy findings revealed that four of these patients had membranous nephropathy. Although minimal change was not diagnosed, the remaining three cases were not biopsied. A majority also had limited chronic GVHD. The reasons for a possibly higher risk of nephrotic syndrome in non-myeloablative HSCT recipients than myeloablative transplant recipients are speculative. Earlier withdrawal of immunosuppression in non-myeloablative protocols could heighten susceptibility to immune-mediated glomerular disease. Host/donor marrow chimerism is another important difference between the two types of HSCT. Whether persistence of host lymphocytes that survive conditioning alters susceptibility to either membranous nephropathy or MCD, is not yet known and certainly warrants further study.
Discussion of Management
The cornerstone for therapy in MCD is corticosteroid treatment, usually at a dose of 1 mg/kg of body weight to 80 mg per kg of body weight prednisone per day. We recommend pneumocystis carinii pneumonia prophylaxis for patients receiving 60 mg or greater of prednisone per day.
Although 90% of children respond within weeks, approximately 75% of adults with MCD are steroid-sensitive and achieving remission takes longer, usually 1-3 months (14). In adult patients with corticosteroid-resistant MCD or frequently relapsing MCD, alternative immunosuppressive agents, such as cyclophosphamide, cyclosporine or mycophenalate mofetil are often required. The precise role of these agents has not yet been defined by trial data, however.
Control of edema in MCD is important and diuretics, combined with a low salt diet, are the mainstays of therapy. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers can be used to reduce proteinuria, particularly in cases with heavy proteinuria or slow response to corticosteroid therapy. In the case presented here, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers were not prescribed because the patient had acute renal failure and a rapid response to corticosteroid therapy. The thrombotic tendency in nephrotic syndrome can be treated with aspirin (325 mg daily).
Conclusion
This report describes a case of minimal change nephrotic syndrome associated with vaccination in a non-myeloablative HSCT recipient. The benefits of vaccination in HSCT recipients are clear (15), and this report should not alter current practice. Because of the expanding indications and growing numbers of HSCTs performed worldwide combined with improved survival, nephrologists need to be familiar with the renal complications of this life-saving procedure. Further study is needed to determine the incidence of both MCD and membranous nephropathy after HSCT, and to explore their association with GVHD and non-myeloblative transplantation protocols.
Acknowledgments
B.D.H. is supported by NIH Career Development Award DK073628-01.
Glossary
- NON-MYELOABLATIVE HEMATOPOIETIC STEM CELL TRANSPLANTATION (HSCT)
A procedure employing reduced intensity conditioning to allow engraftment of healthy bone marrow without myeloablation; cancer eradication in this type of transplant is accomplished by the graft-versus-tumor effect
- CONDITIONING
High dose chemo-radiotherapy given prior to hematopoietic stem cell transplantation; preparative regimen that eradicates diseased immune cells and provides a niche for donor stem cells to engraft
- GRAFT-VERSUS-HOST DISEASE (GVHD)
A complication of hematopoietic stem cell transplantation; donor immune cells react against host tissues, most commonly the skin, liver and gastrointestinal tract
- TRANSAMINASES
The enzymes alanine aminotransferase (ALT) and aspartate aminotransferase (AST); located in liver cells and released into the bloodstream following damage to the liver
- HYBRIDOMA
A cell that results from fusion of a normal cell with a tumor cell; when an antibody producing lymphocyte is fused to a tumor cell, the resulting immortalized cell line will generate a continuous supply of monoclonal antibodies
- CONCANAVALIN A
A glycoprotein that potently stimulates lymphocytes to proliferate
Footnotes
Competing Interests: The authors declare they have no competing interests.
Contributor Information
Benjamin D Humphreys, Harvard Medical School and Staff Nephrologist in the Renal Division, Brigham and Women's Hospital, Boston, MA, USA.
Vijay K Vanguri, Department of Pathology, Brigham and Women's Hospital, Boston, MA, USA.
Joel Henderson, Department of Pathology, Brigham and Women's Hospital, Boston, MA, USA.
Joseph H Antin, Harvard Medical School, and Chief, Stem Cell Transplant Program, Department of Medical Oncology, Dana Farber Cancer Institute, Boston, MA, USA.
References
- 1.Alyea EP, Kim HT, Ho V, Cutler C, Gribben J, DeAngelo DJ, Lee SJ, Windawi S, Ritz J, Stone RM, et al. Comparative outcome of nonmyeloablative and myeloablative allogeneic hematopoietic cell transplantation for patients older than 50 years of age. Blood. 2005;105(4):1810–1814. doi: 10.1182/blood-2004-05-1947. [DOI] [PubMed] [Google Scholar]
- 2.Antin JH, Kim HT, Cutler C, Ho VT, Lee SJ, Miklos DB, Hochberg EP, Wu CJ, Alyea EP, Soiffer RJ. Sirolimus, tacrolimus, and low-dose methotrexate for graft-versus-host disease prophylaxis in mismatched related donor or unrelated donor transplantation. Blood. 2003;102(5):1601–1605. doi: 10.1182/blood-2003-02-0489. [DOI] [PubMed] [Google Scholar]
- 3.Humphreys BD, Soiffer RJ, Magee CC. Renal failure associated with cancer and its treatment: an update. J Am Soc Nephrol. 2005;16(1):151–161. doi: 10.1681/ASN.2004100843. [DOI] [PubMed] [Google Scholar]
- 4.Weiss AS, Sandmaier BM, Storer B, Storb R, McSweeney PA, Parikh CR. Chronic kidney disease following non-myeloablative hematopoietic cell transplantation. Am J Transplant. 2006;6(1):89–94. doi: 10.1111/j.1600-6143.2005.01131.x. [DOI] [PubMed] [Google Scholar]
- 5.Stevenson WS, Nankivell BJ, Hertzberg MS. Nephrotic syndrome after stem cell transplantation. Clin Transplant. 2005;19(1):141–144. doi: 10.1111/j.1399-0012.2004.00294.x. [DOI] [PubMed] [Google Scholar]
- 6.Romagnani P, Lazzeri E, Mazzinghi B, Lasagni L, Guidi S, Bosi A, Cirami C, Salvadori M. Nephrotic syndrome and renal failure after allogeneic stem cell transplantation: novel molecular diagnostic tools for a challenging differential diagnosis. Am J Kidney Dis. 2005;46(3):550–556. doi: 10.1053/j.ajkd.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 7.Kielstein JT, Termuhlen L, Sohn J, Kliem V. Minimal change nephrotic syndrome in a 65-year-old patient following influenza vaccination. Clin Nephrol. 2000;54(3):246–248. [PubMed] [Google Scholar]
- 8.Kikuchi Y, Imakiire T, Hyodo T, Higashi K, Henmi N, Suzuki S, Miura S. Minimal change nephrotic syndrome, lymphadenopathy and hyperimmunoglobulinemia after immunization with a pneumococcal vaccine. Clin Nephrol. 2002;58(1):68–72. doi: 10.5414/cnp58068. [DOI] [PubMed] [Google Scholar]
- 9.Islek I, Cengiz K, Cakir M, Kucukoduk S. Nephrotic syndrome following hepatitis B vaccination. Pediatr Nephrol. 2000;14(1):89–90. [PubMed] [Google Scholar]
- 10.Koyama A, Fujisaki M, Kobayashi M, Igarashi M, Narita M. A glomerular permeability factor produced by human T cell hybridomas. Kidney Int. 1991;40(3):453–460. doi: 10.1038/ki.1991.232. [DOI] [PubMed] [Google Scholar]
- 11.Lowenstein J, Schacht RG, Baldwin DS. Renal failure in minimal change nephrotic syndrome. Am J Med. 1981;70(2):227–233. doi: 10.1016/0002-9343(81)90754-3. [DOI] [PubMed] [Google Scholar]
- 12.Walker JV, Morich D, Anasetti C. Minimal-change nephrotic syndrome after cyclosporine withdrawal in a marrow transplant recipient. Am J Kidney Dis. 1995;26(3):532–534. doi: 10.1016/0272-6386(95)90503-0. [DOI] [PubMed] [Google Scholar]
- 13.Srinivasan R, Balow JE, Sabnis S, Lundqvist A, Igarashi T, Takahashi Y, Austin H, Tisdale J, Barrett J, Geller N, et al. Nephrotic syndrome: an under-recognised immune-mediated complication of non-myeloablative allogeneic haematopoietic cell transplantation. Br J Haematol. 2005;131(1):74–79. doi: 10.1111/j.1365-2141.2005.05728.x. [DOI] [PubMed] [Google Scholar]
- 14.Nolasco F, Cameron JS, Heywood EF, Hicks J, Ogg C, Williams DG. Adult-onset minimal change nephrotic syndrome: a long-term follow-up. Kidney Int. 1986;29(6):1215–1223. doi: 10.1038/ki.1986.130. [DOI] [PubMed] [Google Scholar]
- 15.Avigan D, Pirofski LA, Lazarus HM. Vaccination against infectious disease following hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2001;7(3):171–183. doi: 10.1053/bbmt.2001.v7.pm11302551. [DOI] [PubMed] [Google Scholar]