Abstract
Exposure to arsenic in drinking water increases incidence of cardiovascular diseases. However, the basic mechanisms and genetic changes that promote these diseases are unknown. This study investigated the effects of chronic arsenic exposure on vessel growth and expression of angiogenic and tissue remodeling genes in cardiac tissues. Male mice were exposed to low to moderately high levels of arsenite (AsIII) for 5, 10, or 20 wk in their drinking water. Vessel growth in Matrigel implants was tested during the last 2 wk of each exposure period. Implant vascularization increased in mice exposed to 5–500 ppb AsIII for 5 wk. Similar increases were seen following exposure to 50–250 ppb of AsIII over 20 wk, but the response to 500 ppb decreased with time. RT-PCR analysis of cardiac mRNA revealed differential expression of angiogenic or tissue remodeling genes, such as vascular endothelial cell growth factor (VEGF), VEGF receptors, plasminogen activator inhibitor-1, endothelin-1, and matrix metalloproteinase-9, which varied with time or amount of exposure. VEGF receptor mRNA and cardiac microvessel density were reduced by exposure to 500 ppb AsIII for 20 wk. These data demonstrate differential concentration and time-dependent effects of chronic arsenic exposure on cardiovascular phenotype and vascular remodeling that may explain the etiology for AsIII-induced disease.
Keywords: Arsenic, angiogenesis, neovascularization, Matrigel, vascular endothelial cell growth factor, matrix metalloproteinase-9, endothelin-1, plasminogen activator inhibitor-1
Introduction
The understanding of environmental contributions to human cardiovascular diseases remains poor. Arsenic is an example of a significant contaminant in drinking water worldwide that has been repeatedly associated with a variety of cardiovascular diseases and cancers in humans (1–6). It is estimated that >50 million people worldwide are exposed to concentrations of arsenic in drinking water that are above the WHO maximum containment level (MCL) of 10 ppb (mg/L) (7). Despite the strong epidemiological associations, the mechanisms and dose dependence for arsenic-induced vascular diseases remain unresolved.
Arsenic contamination of drinking water is not uncommon, because it is highly abundant in the Earth's crust and easily leaches from rocks and soils into groundwater. The most common forms of arsenic in the environment are inorganic pentavalent arsenate and trivalent arsenite (AsIII). AsIII is the more toxic species because it freely enters cells, possibly through water channels, at neutral pH (8,9). Arsenate can be reduced to AsIII by environmental bacteria, as well as by reductases found in most mammalian organs (10,11). The cellular effects of AsIII and its more toxic methylated trivalent metabolites activate cell signaling and cause toxicity by reacting with cellular thiols or by causing the formation of reactive oxidants (12–16).
The most well characterized cardiovascular effects of arsenic are peripheral vascular disease, hypertension, ischemic disease, cardiomyopathies, and cardiac arrhythmias. These effects have been observed in rats and rabbits drinking large amounts of arsenic (17), in mice injected with clinically relevant arsenite therapies (18), and in humans receiving trivalent arsenic to treat leukemia (19,20). The worst cardiovascular manifestation of environmental arsenic exposure in humans is blackfoot disease: arteriosclerosis obliterans that results in severe peripheral vascular disease and loss of extremities (1,21–23). Blackfoot disease is rarely seen outside of the Taiwanese population that is exposed to arsenic drinking water levels of up to 1.5 ppm (23). However, there is substantial evidence of arsenic-induced occlusive disease worldwide (for reviews, see refs. 12 and 22). Ischemic heart disease and hypertension have been observed in several studies in Asia and the United States (6,21,24). Even chronic exposures at the current United States MCL of 50 ppb arsenic may double the risk of hyper-tension in susceptible populations (22,25). Finally, there has been a resurgence of therapeutic use of AsIII to treat leukemias and other cancers. However, a major dose-limiting side effect in up to 40% of patients receiving these therapies is cardiac arrhythmias that can progress to torsades de pointes in susceptible patients (19,20).
Mechanistic studies of arsenic toxicities have been confounded by the nonlinear effects of arsenic on cell signaling and the lack of adequate in vivo models that respond to environmentally relevant levels of arsenic (1,25). However, this lack of animal models may relate more to defining mechanisms for the carcinogenic effects of arsenic than to its cardiovascular effects. In animal models, high-dose or injected arsenic has been shown to decrease vasodilator responses (17,26), to decrease cardiac output (17), to cause cardiomyopathies (18), and, most recently, to promote angiogenesis (27). However, few studies have investigated the chronic effects of environmentally relevant arsenic exposures on the vascular remodeling that may underlie arsenic-induced vascular diseases.
Angiogenesis, the common term for the formation of new blood vessels from existing vessels in adult tissue, is dysregulated in many diseases, including cancer, atherosclerosis, diabetes, and ischemic diseases (29–31). However, there is a wealth of recent evidence that new vessel formation, called neovascularization, is a complex process that involves both the recruitment of progenitor cells and angiogenic remodeling of vessels (for a review, see ref. 29). AsIII has been shown to stimulate the angiogenic process in cell culture and neovascularization in vivo (27,31). However, this stimulation occurs only at lower concentrations of AsIII and endothelial cell function is generally compromised at AsIII concentrations >5 μM (15,33) in confluent cell cultures or >1 μM in proliferating cells or newly forming vessels (15,33). Arsenic-stimulated neovascularization may result from induction of angiogenic factors, such as vascular endothelial cell growth factor (VEGF), in vascular tisues (31,33); however, this has not been examined in vivo.
The current in vivo study was designed to address the hypothesis that chronic exposure to environmentally relevant concentrations of AsIII in drinking water enhances neovascularization and cardiovascular expression of genes that promote neovascularization and tissue remodeling. This was accomplished by feeding mice normal drinking water or the same water containing 1–500 ppb of AsIII for 5, 10, or 20 wk. Implanted Matrigel was used to test the effects of AsIII on fibroblast growth factor (FGF)-2-induced neovascularization. Cardiac tissue was collected for mRNA analysis and histological measurement of blood vessel density. Blood was collected to test changes in circulating protein levels. These are the first studies to examine the chronic profile of arsenic effects on vessel growth and remodeling following oral dosing. The data demonstrate that low to moderate levels of AsIII promote neovascularization and time-dependent systemic effects on cardiovascular gene expression. The significance of the results is enhanced by their potential relevance to the etiology of cardiovascular disease and possibly tumorigenesis.
Materials and Methods
Mouse Exposure to Arsenic in Drinking Water
All animal exposures and procedures were performed in accordance with institutional guidelines for animal safety and welfare at Dartmouth College. Normal male C57BL/6NCr mice, 6–8 wk of age and weighing 20 g were obtained from the National Cancer Institute (Frederick, MD) and allowed to acclimate for 3–4 d prior to the start of treatments. Mice were housed in boxes of five and fed prepared drinking water solutions and standard mouse chow ad libitum. Drinking water solutions were prepared fresh twice weekly by dissolving 1–500 μg of sodium arsenite (Fisher Scientific, Pittsburgh, PA) in 1 L of commercially bottled drinking water (Vermont Pure, Randolf, VT), which contained <1 ppb of arsenic. There was no significant conversion of AsIII to AsV in these preparations (data not shown). The amount of water consumed by each mouse in a group and individual arsenic levels were not measured.
Matrigel Implant Assay
To test neovascularization potential, we implanted Matrigel for a 2-wk period prior to euthanizing the animals. Briefly, the mice were anesthetized with 125–250 mg/kg avertin. Matrigel (0.3–0.4 mL; BD Bioscience, Bedford, MA) or Matrigel supplemented with 50 ng/mL recombinant FGF-2 (PeproTech, Rocky Hill, NJ) was injected between the skin and abdominal muscle of each mouse. To end exposure, mice were euthanized with carbon monoxide. The Matrigel implants were then removed with a portion of adjacent skin and muscle for orientation and were then fixed in 10% neutral buffered formalin prior to paraffin embedding. One hundred-micron thick cross-sections were made and stained with H&E. Vessels were identified as cell-lined luminal structures containing red blood cells and scored according to a ranking algorithm to account for differences in vessel size and length in the cross-sections (see arrows in Fig. 2). Blood vessels were counted in 10 nonoverlapping fields of each section magnified ×400. The number of the vessels in each of 10 nonoverlapping fields from a single cross-section was summed and the data were reported as the mean sum ±SD for each treatment group.
Fig. 2.
Morphological features of Matrigel implants after chronic arsenic exposure. Mice were exposed to the indicated concentrations of AsIII in their drinking water for 5, 10, or 20 wk. Matrigel implants were inserted 2 wk before termination of exposures by euthanasia. The implants were removed and processed as in Fig. 1. The representative images are ×200 magnifications of H&E-stained cross-sections (bar = 10 μm). Arrows point to blood vessels, which are luminal structures containing red cells and M denotes the abdominal muscle layer. Note the multiple layers of cells in the vessel walls (e.g., 50 ppb at 10 wk) and additional cell infiltrates.
Cardiac Histopathology
Midsections of cardiac ventricular tissue were formalin fixed, paraffin embedded, and then sectioned. The sections were stained with Masson's trichrome and vessels were measured and counted using digital image analysis (ImagePro Plus, Media Cybernetics, Inc., Silver Spring, MD). The interior and exterior of approx 100 resistance vessels were traced and the average thickness of the vessel wall and the area of the lumen were then measured. Lumen diameter was automatically calculated from the lumen areas and the calculated ratio of 2(wall thickness)/lumen diameter was used as a measure of vascular hyperplasia. A digital imaging subroutine was used to determine microvessel density in a separate set of images captured at ×400 magnification. Vessels with cross-sectional diameters of <12 mm were detected and the number of microvessels was normalized to tissue area in the same image.
RT-PCR
Total cellular RNA was harvested with Trizol® from mouse hearts that had been snap frozen immediately after organ retrieval. The frozen hearts were pulverized in liquid nitrogen and then the powder was mixed with Trizol to extract total RNA. Reverse transcription (RT) was performed on 0.5 to 1.0 μg of RNA, followed by conventional or quantitative real-time PCR using gene specific primers and primers for 18S RNA (Table 1). Conventional PCR products were separated on a 2% agarose gel and stained with ethidium bromide. Intensity of the bands was determined using NIH Scion Image. Real-time PCR was performed only on gene products that produced a single band on gels and uniform melting curves in real-time reactions. Reactions were performed with an MJ Research Opticon II (Waltham, MA), and the number of PCR rounds required to reach a determined threshold abundance value was compared with a standard curve of known amounts of cDNA specific for a given gene. All specific products were normalized to the amount of 18S product, which did not vary as a result of AsIII treatments in mouse hearts.
Table 1.
PCR Primer Sequences
| cDNA | Sequences |
|---|---|
| VEGF | Forward: AAG CCA GCA CAT AGG AGA GAT |
| Reverse: CAT CTG CAA GTA CGT TCG TTT | |
| VEGFR1 | Forward: GAA GGA GAG GAC CTG AAA CTG |
| Reverse: GGC ACC TAT AGA CAC CCT CAT | |
| VEGFR2 | Forward: GTC GGG TTA CAG GCG AGT TC |
| Reverse: GTC TCC ACC CAG CAG AAA CC | |
| PAI-1 | Forward: TCA CTT TAC CCC TCC GAG AAT |
| Reverse: TCC CAT AGC ATC TTG GAT CTG | |
| Endothelin-1 | Forward: TCT GCT GTT CGT GAC TTT CCA |
| Reverse: TTG GCA GAA ATT CCA GCA CTT | |
| MMP-9 | Forward: CAC TGG GCT TAG ATC ATT CCA |
| Reverse: CGT CCT TGA AGA AAT GCA GAG | |
| 18S RNA | Forward: AAA TCA GTT ATG GTT CCT TTG G |
| Reverse: ATG GAT CCT CGT TAA AGG ATT T |
VEGF, vascular endothelial cell growth factor; PAI-1, plasminogen activator inhibitor-1; MMP-9, matrix metalloproteinase-9.
Protein Levels
Total plasminogen activator inhibitor-1 (PAI-1) protein levels in citrated (platelet poor) plasma from mice were measured by enzyme-linked immunosor-bent assay (ELISA) according to manufacturers’ instructions (Innovative Technologies, Southfield, MI). Whole blood was collected into sodium citrate specimen vials and spun at 2500g for 15 min, plasma was removed to a fresh vial, and 100-μL aliquots were assayed for PAI-1 content.
Statistics
There were five mice in each treatment group. One-way analysis of variance (ANOVA) was used to test for differences when the mice were exposed for a single time period. Two-way ANOVA was used to test for differences related to AsIII concentration and time of exposure. Differences between treatments were compared using Bonferroni post hoc analysis.
Results
General Health of Chronic AsIII Exposures
The range of As(III) concentrations used in these studies caused few overt signs of toxicity or beha vioral changes in the animals. There were no significant differences in individual body weights or average group body weights over the course of the 20-wk study. This indicates that all animals consumed similar volumes of water and food. At approx 15 wk, animals in the 250- and 500-ppb-dose groups displayed hair loss without signs of dermatitis. This confirmed the observations of a previous study that reported hair loss in Sprague-Dawley rats exposed to 50 ppm arsenate for 1 yr (34). Necropsy of the mice demonstrated no signs of infection at the Matrigel injection site. There were no obvious changes in any of the internal organs.
Low Levels of AsIII-Induced Matrigel Vascularization
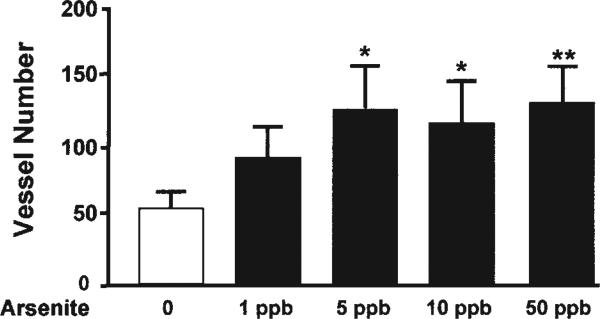
Daily or periodic injections of AsIII synergize with FGF-2 to induce vascularization in the mouse Matrigel model and increase vascularization of injected tumors (27). To demonstrate whether environmental exposures to AsIII cause a similar enhancement of neovascularization, we implanted Matrigel implants in control and AsIII-exposed mice for 2 wk prior to the end of the exposure period. The first 5-wk exposure study was designed to determine the threshold for AsIII enhancement of Matrigel vascularization. The range of AsIII exposures was chosen to include 1 ppb, which is below the current international MCL of 10 ppb, ranging up to the current MCL in the United States of 50 ppb. The data in Fig. 1 demonstrate that vessel density was significantly increased at all exposures tested that were >5 ppb.
Fig. 1.
Low-level arsenic exposure enhanced neovascularization in Matrigel. Male C57BL/6NCr mice were fed drinking water ad libitum supplemented with the indicated concentrations of AsIII for 5 wk. During the final 2 wk, Matrigel implants containing 50 ng/mL FGF-2 were inserted between the abdominal wall and the skin. Euthanasia with carbon monoxide terminated the exposures. The implants were removed, formalin fixed, and embedded in paraffin. Functional vessels in H&E-stained cross-sections were identified as cell-lined luminal structures containing red blood cells and scored for size and length. The vessels in 10 nonoverlapping fields at ×400 were counted and summed. Each bar represents the mean ± SD of summed vessels in sections from five mice. Significant differences are designated by *p < 0.05, **p < 0.01.
Neovascularization in Response to High AsIII Exposures Varies With Time
AsIII-induced vascular diseases in humans develop over many years, even though evidence of vascular remodeling is evident even in young children (1). Therefore, the second study was designed to examine whether AsIII enhancement of neovascularization varied with time and amount of exposure. Different groups of five mice were exposed for 5, 10, or 20 wk to 0, 50, 250, or 500 ppb of AsIII in drinking water. Again, 2 wk before terminating the exposures, Matrigel implants containing threshold amounts of FGF-2 were injected to test neovascularization potential. Figure 2 presents cross-sectional morphological features of representative Matrigel implants from the different exposure groups, and the graphs in Fig. 3 present the average vessel counts in 10 microscopic fields from the five animals in each group. At 5 wk of exposure, AsIII caused a concentration-dependent increase in vascularity of the Matrigel implants, with 500 ppb enhancing vascularity by five- to sixfold (Fig. 3). The Matrigel assay is a model of inflammatory neovascularization and it is important to note the concentration-dependent increase in total cellularity of the implants. This may reflect potentiation of the general inflammatory response by AsIII.
Fig. 3.
Chronic arsenic exposure via drinking water increased neovascularization. The vessels in 10 nonoverlapping fields in sections of Matrigel implants from the treatment groups in Fig. 2 were scored and summed. Each bar in the graph represents the mean sum ± SD of vessels from five mice. Significance was determined by two-way ANOVA followed by Bonferroni post hoc analysis. Significant difference from time-matched control is designated by *p < 0.05, **p < 0.01, ***p < 0.001.
The baseline vascularity in the 2-wk Matrigel assays increased slightly, but not significantly, over the 20-wk period (Fig. 3). The amount of neovascularization produced by 50 ppb AsIII relative to the baseline at each time-point was highly significant and this response did not change over the 20-wk study. In contrast, the magnitude of the response to the higher AsIII concentrations relative to the baseline diminished with time. The response in the 250-ppb-exposed mice remained significant. However, by 20 wk, the response in the mice exposed to 500 ppb became more variable and lost significance.
An Additional Angiogenic Factor Is Required for AsIII-Enhanced Neovascularization
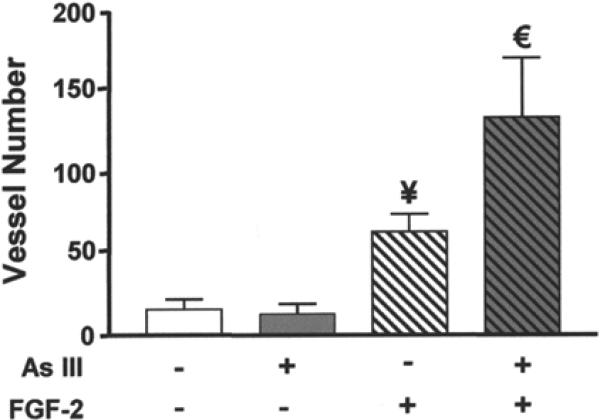
In keeping with our previous studies (27), the data in Fig. 4 demonstrate that exposure to 50 ppb arsenic did not increase the vascularity of Matrigel implants lacking FGF-2. Supplementing the Matrigel with 50 ng/mL FGF-2 increased vascularity of the implants in control animals. However, the placement of FGF-2-containing implants in mice exposed to 50 ppb for 5 wk resulted in a further twofold increase in vessel number. The data suggest that AsIII potentiates, but does not directly cause, neovascularization in this model. They are consistent with the failure of the Matrigel to vascularize in response to many known angiogenic agents unless FGF-2 is added.
Fig. 4.
FGF-2 was required for AsIII-enhanced neovascularization. Mice were exposed to normal drinking water or drinking water containing 50 ppb AsIII for 5 wk. Matrigel with or without 50 ng/mL of FGF-2 was implanted for the final 2 wk of exposure. Implants were collected and processed as in Fig. 1. Each bar represents the mean ± SD of summed vessel counts from five mice. Significant differences at p < 0.01 from non-FGF control or FGF control are designated by ¥ or €, respectively.
Chronic AsIII Exposures Differentially Affect Cardiac Gene Induction
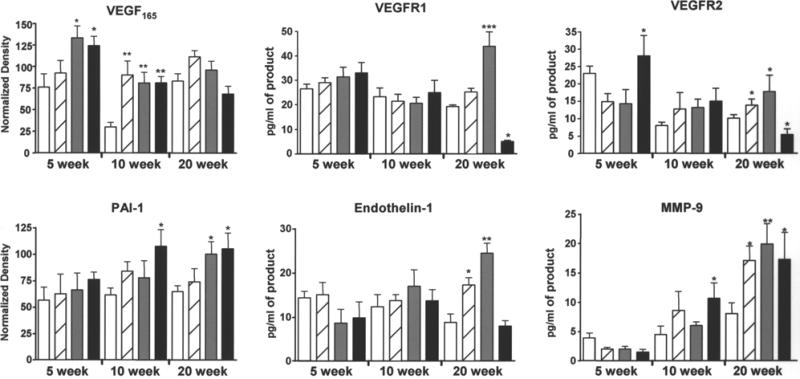
Total cardiac RNA was isolated from the animals presented in Fig. 3 to examine the effects of AsIII on expression of endogenous genes relevant to angio-genesis, vascular remodeling, and cardiovascular injury. Expression of VEGF and its receptors, VEGFR1 (flt-1) and VEGFR2 (Flk-1, KDR), was chosen for the major role of these proteins in both endothelial cell progenitor recruitment and endothelial cell proliferation in vasculogenesis and angiogenesis (28–30). Endothelin-1 was chosen for its role in promoting hypertension, angiogenesis, and cardiac tissue remodeling (35). PAI-1 was chosen for its role in supporting vessel stabilization and remodeling (29) and the demonstration of increased circulating PAI-1 levels in humans exposed to arsenic in drinking water (36,37). Finally, matrix metalloproteinase-9 (MMP-9) was chosen for its known role in promoting vessel remodeling through promotion of smooth muscle cell migration and proliferation (29,38,39). To reduce assay variability and to provide direct comparison between different mRNAs, we performed PCR reactions using a single RT reaction from each heart extract. The mRNA levels for these different genes are shown in Fig. 5 and illustrate several important points. First, despite variations in basal expression, differential effects of AsIII were observed as the exposures progressed. Further, these differential effects were selective in that they were gene specific. At 5 wk of exposure, when the neovascularization response is the highest (Fig. 3), there was a significant increase in VEGF expression and a modest increase in VEGF receptor expression (Fig. 5). In keeping with the Matrigel results, there is no time dependence of the response to 50 ppb over the 20-wk period. The exception is at 10 wk, when the significant response was because of a drop in basal VEGF expression, rather than an increase in the response to 50 ppb of AsIII. In contrast, VEGF expression was significantly increased above control by 250 and 500 ppb AsIII at 5 wk, but this response was lost at 10 and 20 wk. An initial increase of VEGFR2 expression in response to 500 ppb was lost by 10 wk and progressed to significant inhibition by 20 wk. VEGFR1 expression was relatively unchanged, except for a significant increase following 250 ppb and a significant decrease following 500 ppb for 20 wk. Endothelin-1 mRNA levels were relatively unaffected at 5 wk, but demonstrated progressive dose-dependent increases in response to 50 and 250 ppb of AsIII. This significance was enhancing by a downward trend in basal expression over the 20-wk period. In contrast, MMP-9 expression displayed a progressive increase in basal expression and highly significant increased expression at all doses. Basal PAI-1 mRNA levels remained unchanged and AsIII stimulation progressed to significance for 500 ppb by 10 wk and for both 250 and 500 ppb by 20 wk. Thus, AsIII clearly has different effects on cardiovascular genes and these effects occur with different patterns depending on the amount or duration of exposure.
Fig. 5.
Chronic AsIII exposure differentially affected cardiac angiogenic and remodeling gene expression. At the termination of exposure, hearts were collected from the mice in Fig. 3. Apical and atrial sections were snap frozen and RNA was isolated and analyzed as describe in Materials and Methods. Conventional (vascular endothelial cell growth factor [VEGF165] and plasminogen activator inhibitor-1 [PAI-1]) or real-time RT-PCR was used to measure specific mRNA levels relative to 18S RNA. Each bar represents the mean ± SD of corrected mRNA levels from five mice. Treatments were as follows: no added AsIII (open bar), 50 ppb AsIII (striped bar), 250 ppb AsIII (gray bar), and 500 ppb AsIII (black bar). The data were analyzed for differences by two-way ANOVA followed by Bonferroni post hoc test for significance. Significant difference from time-matched control is designated by *p < 0.05, **p < 0.01, ***p < 0.001. MMP-9, matrix metalloproteinase-9.
Chronic Arsenic Exposure Results in Increased Plasma PAI-1 Levels
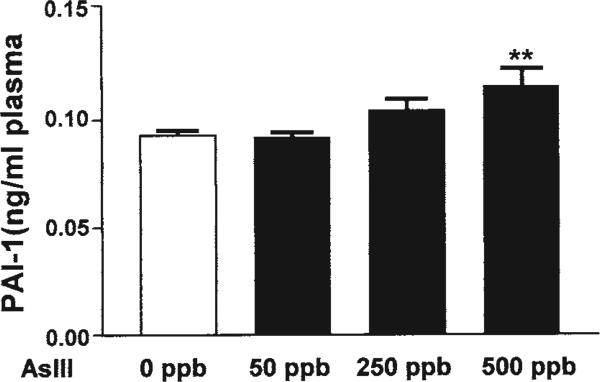
PAI-1 protein levels are elevated in human populations and human endothelial cells exposed to AsIII (36,37). To demonstrate that the dose-dependent increases in PAI-1 mRNA at 20 wk reflect changes in protein, we measured total plasma levels of PAI-1 by ELISA. As shown in Fig. 6, there was a dose-dependent increase in circulating PAI-1 that was significant only in response to 500 ppb AsIII.
Fig. 6.
AsIII exposure in drinking water leads to increased plasminogen activator inhibitor-1 (PAI-1) in plasma. Circulating PAI-1 protein levels were determined in the plasma of mice after 20 wk of exposure to normal or AsIII-containing drinking water. During necropsy, blood was collected by cardiac puncture into sodium citrate and spun at 2500g for 10 min at 4°C and platelet-poor plasma collected and stored at −80°C until assay for total PAI-1 by enzyme-linked immunosorbent assay. Each bar represents the mean ± SD PAI-1 level from five mice. Significance is designated by **p < 0.01.
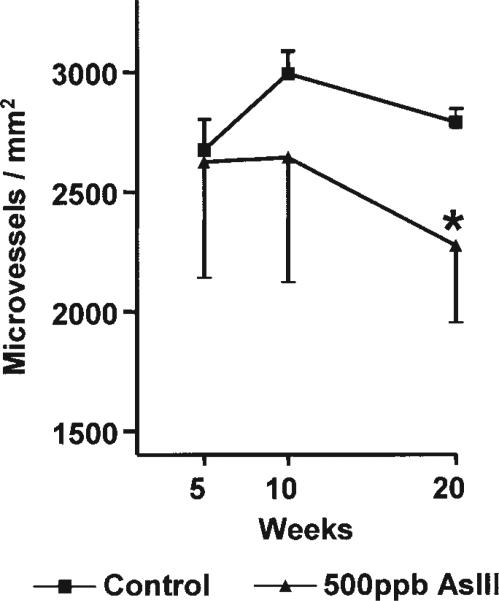
Chronic High-Level AsIII Exposures Decrease Microvessel Density in the Heart
The inhibitory effects of 500 ppb AsIII on expression of VEGF receptors suggested that chronic exposure at this level was toxic to the cardiac vasculature. Morphological analysis revealed no difference in wall thickness or luminal diameter for cardiac arterioles measuring between 12 and 100 μm (data not shown). However, as shown in Fig. 7, prolonged exposure to 500 ppb AsIII reduced the tissue density of microvessels of <12 μm. These data are consistent with the decreased magnitude of the neovascularization response in Matrigel implants from the same animals (Fig. 3).
Fig. 7.
Chronic AsIII exposure reduced cardiac micro-vessel density. Middle ventricular sections of the hearts collected in Fig. 5 were formalin fixed, paraffin embedded, and cross-sectioned. The tissue was stained with Masson's trichrome and imaged at ×400. Microvessels were automatically detected using a digital imaging subroutine and vessels with cross-sectional diameters of <12 μm were enumerated and sums were normalized to tissue area in the same image. Each point represents the mean ± SD microvessel density from the hearts of five mice. Significant differences from time-matched control are designated by *p < 0.05.
Discussion
The data presented are the first to characterize dose- and time-dependent effects of AsIII on neovascularization following environmentally relevant exposures in adult animals. In addition, these data have implications toward identifying mechanisms for the ischemic diseases that are associated with exposure to AsIII in drinking water and for AsIII-en-hanced tumorigenesis. Neovascularization and angio-genesis are enhanced by levels of AsIII that are near the current and future US regulatory limits for arsenic in drinking water (Fig.1). This potentiation in response to low-level exposure does not vary with time, which has significant implications for human health risk. In contrast, higher levels of exposure provide robust neovascularization early in exposure, but lead to vessel rarefaction after prolonged exposure. This toxicity may have added health significance, because these arsenic levels have been associated with ischemic heart disease. Gene expression changes in the hearts of the exposed mice may explain the time-dependent changes in dose–response relationships of the cardiovascular system to prolonged AsIII exposures.
Enhancement of pathologic neovascularization by AsIII in adult tissues could partially explain the etiology of AsIII-associated cancers and cardiovascular diseases. The finding in Fig. 4 that AsIII required additional inflammatory factors to increase neovascularization implied that AsIII contributes to longitudinal risks for disease, but does not directly cause disease. This was consistent with the previous demonstration that injected AsIII enhanced angiogenesis and tumor growth in vivo (27), even in animals that are poor models for arsenic-induced carcinogenesis (25). It was also consistent with the current theories proposing that arsenic is a cocarcinogen, not a primary carcinogen (25,40). The correlate in the cardiovascular system would be that AsIII-induced neovascularization could contribute to the enhanced blood vessel wall growth, smooth muscle cell proliferation, and malignancy of atherosclerosis. AsIII has been shown to enhance all of these processes in humans and animal models (1,41,42), as well as in cell culture models (33).
The observed AsIII-enhanced microvessel density presents an apparent paradox with reports of AsIII-related ischemic disease. This paradox may be explained by enhancement of the blood vessel wall microcirculation (i.e., vasa vasorum) and potential contributions of the vasa vasorum to both arteriosclerosis and atherosclerosis (29,43). Stimulation of smooth muscle cell growth and the vasa vasorum without an atherosclerotic or arteriogenic signal could result in the arteriosclerosis and the loss of perfusion seen in arsenic-induced peripheral vascular diseases (1). In contrast, AsIII-enhanced growth of the vasa vasorum would account for the vessel wall thickening and enhanced malignancy attributed to arsenic in the atherosclerotic lesions of exposed patients (41). Further investigation in a more appropriate animal model or in humans is needed to establish the role of AsIII in enhancing wall thickening or stiffening by promoting neovascularization of the vasa vasorum.
The data in Figs. 2 and 3 present several important implications of the time- and dose-dependent effects of AsIII-enhanced neovascularization. First, enhancement of small vessel density was much greater after a shorter period of exposure to higher AsIII concentrations relative to prolonged exposures. There was nearly a sixfold enhancement of FGF-2-stimulated vessel density following 5-wk exposure to 500 ppb AsIII (Fig. 3). By 10 wk of exposure, this response was reduced to a twofold increase relative to the time-matched control. A further reduction in the response, as well as an increase in variability, was seen by 20 wk. The assay truly measured neovascularization potential, because the Matrigel was implanted for 2-wk intervals at 3, 8, or 18 wk in the exposure period. Angiogenic potential has been reported to decrease with prolonged aging when 12-wk and 2-yr-old mice were compared (44). However, this would not explain the diminished neovascularization response in the mice exposed to 500 ppb AsIII (Fig. 3). If anything, there was an upward trend in baseline neovascularization of the Matrigel implants in these animals with only a 15-wk age difference. Also, there was no decrease in the magnitude of the response to either 50 or 250 ppb over the 20-wk period. The implication of the data is that individuals in high-exposure areas would have more severe disease that is supported by AsIII-enhanced neovascularization if they receive the disease signal (e.g., tumor development, hypertension, arteriosclerosis) earlier in exposure. In contrast, the risk of enhanced disease would remain constant for those individuals drinking water containing 250 ppb or less. This is important because, even at 50 ppb, arsenic in drinking water may raise the risk of hypertension by twofold in selected human populations (24). The second main implication is that there are different thresholds for the mechanisms of the vascular effects of AsIII. Inappropriate enhancement of neovascularization in the adult results from positive growth signals that contribute to proliferative diseases (e.g., arteriosclerosis, tumori-genesis). The threshold for AsIII enhancement of neovascularization appears to be between 1 and 5 ppb (Fig. 1). However, loss of microvessels or vessel rarefaction results from negative signals or apoptosis and contributes to fibrotic processes and tissue damage (e.g., fibrosis, cardiomyopathies). Prolonged exposure at a threshold between 250 and 500 ppb appeared to be required for these negative stimuli.
The data in Fig. 2 also present the fact that, in addition to affecting the degree of vascularization, AsIII may alter different phases of vascularization depending on the exposure duration. In general, in animals euthanized at 5 wk, AsIII increased the Matrigel density of vessels that contained only endothelial cells or had at most one extra cell layer surrounding the luminal endothelial cells. In contrast, large vessels, such as those shown for 50 ppb at 10 wk (Fig. 2), were common in the implants from 50- and 250-ppb mice. These vessels were of note, because there were at least two cell layers in the wall surrounding the lumen based on the number of stained nuclei. This implied that lower concentrations of AsIII contributed to vessel stabilization in addition to promoting vessel invasion or angiogenesis. However, further morphometric analysis will be needed to determine how AsIII contributes to vessel remodeling or which parts of the remodeling program are stimulated or inhibited by AsIII. For example, if AsIII contributes to formation of larger vessels with limited branching, then tissue perfusion would be limited relative to stimulation of a more highly branched microvascular bed.
The mechanisms underlying differential dose- and time-dependent changes in vascularity or vessel remodeling following AsIII exposure may relate to the differential effects on gene expression shown in Fig. 5. VEGF is an autocrine and paracrine factor. Despite the difficulty of measuring localized changes in whole tissue, 250-and 500-ppb AsIII exposures were shown to increase total cardiac VEGF mRNA by nearly twofold after 5 wk of exposure. This increase and the trend for higher VEGFR2 mRNA levels would be expected to provide the greater angiogenic potential seen at this time period (Fig. 3). However, these responses were lost by 10 wk and progressed to inhibition of receptor mRNA expression in response to 500 ppb AsIII at 20 wk. The decline in both VEGFR1 and VEGFR2 mRNA following 500 ppb AsIII for 20 wk was dramatic. Because both of these proteins are survival factors for microvessels, this inhibition could explain the rarefaction of cardiac microvessels illustrated in Fig. 7. A similar systemic loss of VEGF receptor expression could also explain the diminished neovascularization response in Matrigel assays following 10 and 20 wk of exposure to 500 ppb. There is no change or a slightly enhanced expression of VEGF and its receptors in response to 50 and 250 ppb AsIII over the 20-wk exposure period. This would explain the sustained neovascularization responses to these two exposure levels (Fig. 3). The progressive increased induction of endothelin-1 mRNA by these two concentrations of AsIII that reaches significance at 20 wk (Fig. 5) may also contribute to enhanced angiogenesis. The role of endothelin-1 in the vascular effects of AsIII is unknown, but the protein has been implicated in receptor-mediated stimulation of tumor angiogenesis (35). However, chronic increases in endothelin-1 can be deleterious, because it is a vasoconstrictor and contributes to both hypertension and cardiac hypertrophy (35,45). A caveat to the data presented is that, with the exception of PAI-1, only mRNA levels were examined. Functional protein expression is not always matched to changes in mRNA and more detailed studies are needed to demonstrate the full mechanistic roles of these proteins in AsIII-enhanced neovascularization.
The time-dependent increase in the concentration response for AsIII-induced PAI-1 and MMP-9 (Fig. 5) expression would compound the negative impacts of AsIII on the vasculature. Both genes may enhance angiogenesis and vessel remodeling by facilitating smooth muscle cell migration, vessel stabilization, and wall thickening (29,38,46,47). However, over-expression of either gene contributes to vascular disease and thrombosis (29,39,47). It is interesting to note that both MMP-9 and PAI-1 have been implicated in AsIII-promoted disease. MMP-9 expression has been suggested to be a biomarker of arsenite-induced malignant transformation of prostate epithelial cells (48). PAI-1 was increased in the circulation of individuals from areas where AsIII exposures are endemic (36,37). Subtle changes in circulating PAI-1, such as those seen in Fig. 6, can be highly significant physiologically, because only a portion of the expressed protein is released to the circulation, its actions are much greater at the level of the tissue, and its inhibitory activity is exponentially related to its abundance (49).
In summary, this is the first study demonstrating long-term changes in cardiovascular gene expression and vascular remodeling that may underlie AsIII-induced vascular disease. The data demonstrate that vascular changes can be modeled in mice drinking environmentally relevant levels of AsIII. The threshold for AsIII-induced changes in neovascularization appears to be below the proposed MCL for arsenic in drinking water. The findings begin to explain epidemiological findings of a greater than twofold risk of cardiovascular disease in humans even at 50 ppb of arsenic (4,24). Although chronic changes in gene expression may explain some of the etiology of this disease risk, much more work will be needed to establish the clear links between individual exposure to arsenic in drinking water and disease outcomes.
Acknowledgments
This work was supported by the Superfund Basic Research Program (ES 07373) and the services of the Norris Cotton Cancer Center at Dartmouth Hitchcock Medical Center.
References
- 1.Engel RR, Hopenhayn-Rich C, Receveur O, Smith AH. Vascular effects of chronic arsenic exposure: a review. Epidemiol. Rev. 1994;16:184–208. doi: 10.1093/oxfordjournals.epirev.a036150. [DOI] [PubMed] [Google Scholar]
- 2.Tseng CH, Chong CK, Tseng CP, Hsueh YM, Chiou HY, Tseng CC, et al. Long-term arsenic exposure and ischemic heart disease in arseniasis-hyper-endemic villages in Taiwan. Toxicol. Lett. 2003;137:15–21. doi: 10.1016/s0378-4274(02)00377-6. [DOI] [PubMed] [Google Scholar]
- 3.Wang SL, Chiou JM, Chen CJ, Tseng CH, Chou WL, Wang CC, et al. Prevalence of non-insulin-dependent diabetes mellitus and related vascular diseases in southwestern arseniasis-endemic and nonendemic areas in taiwan. Environ. Health Perspect. 2003;111:155–160. doi: 10.1289/ehp.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahman M, Tondel M, Ahmad SA, Chowdhury IA, Faruquee MH, Axelson O. Hypertension and arsenic exposure in Bangladesh. Hypertension. 1999;33:74–78. doi: 10.1161/01.hyp.33.1.74. [DOI] [PubMed] [Google Scholar]
- 5.Calderon RL. The epidemiology of chemical contaminants of drinking water. Food Chem. Toxicol. 2000;38:S13–S20. doi: 10.1016/s0278-6915(99)00133-7. [DOI] [PubMed] [Google Scholar]
- 6.Lewis DR, Southwick JW, Ouellet-Hellstrom R, Rench J, Calderon RL. Drinking water arsenic in Utah: a cohort mortality study. Environ. Health Perspect. 1999;107:359–365. doi: 10.1289/ehp.99107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaofen J, Wai CM. Arsenic in drinking water—a global environmental problem. J. Chem. Educ. 2004;81:207–213. [Google Scholar]
- 8.Adachi M, Iwaki H, Shindoh M, Akao Y, Hachiya T, Ikeda M, et al. Predominant expression of the src homology 2-containing tyrosine phosphatase protein SHP2 in vascular smooth muscle cells. Virchows Arch. 1997;430:321–325. doi: 10.1007/BF01092755. [DOI] [PubMed] [Google Scholar]
- 9.Liu Z, Shen J, Carbrey JM, Mukhopadhyay R, Agre P, Rosen BP. Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9. Proc. Natl. Acad. Sci. USA. 2002;99:6053–6058. doi: 10.1073/pnas.092131899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oremland RS, Stolz JF. The ecology of arsenic. Science. 2003;300:939–944. doi: 10.1126/science.1081903. [DOI] [PubMed] [Google Scholar]
- 11.Aposhian HV, Zakharyan RA, Avram MD, Kopplin MJ, Wollenberg ML. Oxidation and detoxification of trivalent arsenic species. Toxicol. Appl. Pharmacol. 2003;193:1–8. doi: 10.1016/s0041-008x(03)00324-7. [DOI] [PubMed] [Google Scholar]
- 12.Carter DE, Aposhian HV, Gandolfi AJ. The metabolism of inorganic arsenic oxides, gallium arsenide, and arsine: a toxicochemical review. Toxicol. Appl. Pharmacol. 2003;193:309–334. doi: 10.1016/j.taap.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Barchowsky A, Roussel RR, Klei LR, James PE, Ganju N, Smith KR, et al. Low levels of arsenic trioxide stimulate proliferative signals in primary vascular cells without activating stress effector pathways. Toxicol. Appl. Pharmacol. 1999;159:65–75. doi: 10.1006/taap.1999.8723. [DOI] [PubMed] [Google Scholar]
- 14.Drobna Z, Jaspers I, Thomas DJ, Styblo M. Differential activation of AP-1 in human bladder epithelial cells by inorganic and methylated arsenicals. FASEB J. 2003;17:67–69. doi: 10.1096/fj.02-0287fje. [DOI] [PubMed] [Google Scholar]
- 15.Barchowsky A, Dudek EJ, Treadwell MD, Wetterhahn KE. Arsenic induces oxidant stress and NF-KappaB activation in cultured aortic endothelial cells. Free Radic. Biol. Med. 1996;21:783–790. doi: 10.1016/0891-5849(96)00174-8. [DOI] [PubMed] [Google Scholar]
- 16.Barchowsky A, Klei LR, Dudek EJ, Swartz HM, James PE. Stimulation of reactive oxygen, but not reactive nitrogen species, in vascular endothelial cells exposed to low levels of arsenite. Free Radic. Biol. Med. 1999;27:1405–1412. doi: 10.1016/s0891-5849(99)00186-0. [DOI] [PubMed] [Google Scholar]
- 17.Carmignani M, Boscolo P, Castellino N. Metabolic fate and cardiovascular effects of arsenic in rats and rabbits chronically exposed to trivalent and pentavalent arsenic. Arch. Toxicol. 1985;8(Suppl.):452–455. doi: 10.1007/978-3-642-69928-3_103. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Sun X, Wang L, Zhou Z, Kang YJ. Myocardial toxicity of arsenic trioxide in a mouse model. Cardiovasc. Toxicol. 2002;2:63–73. doi: 10.1385/ct:2:1:63. [DOI] [PubMed] [Google Scholar]
- 19.Unnikrishnan D, Dutcher JP, Varshneya N, Lucariello R, Api M, Garl S, et al. Torsades de pointes in 3 patients with leukemia treated with arsenic trioxide. Blood. 2001;97:1514–1516. doi: 10.1182/blood.v97.5.1514. [DOI] [PubMed] [Google Scholar]
- 20.Drolet B, Simard C, Roden DM. Unusual effects of a QT-prolonging drug, arsenic trioxide, on cardiac potassium currents. Circulation. 2004;109:26–29. doi: 10.1161/01.CIR.0000109484.00668.CE. [DOI] [PubMed] [Google Scholar]
- 21.Chen CJ, Chiou HY, Chiang MH, Lin LJ, Tai TY. Dose-response relationship between ischemic heart disease mortality and long-term arsenic exposure. Arterioscler. Thromb. Vasc. Biol. 1996;16:504–510. doi: 10.1161/01.atv.16.4.504. [DOI] [PubMed] [Google Scholar]
- 22.National Research Council . Arsenic in Drinking Water: Update 2001. National Academy Press; 2001. pp. 1–333. [Google Scholar]
- 23.Yu HS, Lee CH, Chen GS. Peripheral vascular diseases resulting from chronic arsenical poisoning. J. Dermatol. 2002;29:123–130. doi: 10.1111/j.1346-8138.2002.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 24.Rahman M. Arsenic and hypertension in Bangladesh. Bull. World Health Organ. 2002;80:173. [PMC free article] [PubMed] [Google Scholar]
- 25.Abernathy CO, Liu YP, Longfellow D, Aposhian HV, Beck B, Fowler B, et al. Arsenic: health effects, mechanisms of actions, and research issues. Environ. Health Perspect. 1999;107:593–597. doi: 10.1289/ehp.99107593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee MY, Jung BI, Chung SM, Bae ON, Lee JY, Park JD, et al. Arsenic-induced dysfunction in relaxation of blood vessels. Environ. Health Perspect. 2003;111:513–517. doi: 10.1289/ehp.5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soucy NV, Ihnat MA, Kamat CD, Hess L, Post MJ, Klei LR, et al. Arsenic stimulates angiogenesis and tumorigenesis in vivo. Toxicol. Sci. 2003;76:271–279. doi: 10.1093/toxsci/kfg231. [DOI] [PubMed] [Google Scholar]
- 28.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 29.Hayden MR, Tyagi SC. Vasa vasorum in plaque angiogenesis, metabolic syndrome, type 2 diabetes mellitus, and atheroscleropathy: a malignant transformation. Cardiovasc. Diabetol. 2004;3:1–16. doi: 10.1186/1475-2840-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loomans CJ, Dao HH, van Zonneveld AJ, Rabe-link TJ. Is endothelial progenitor cell dysfunction involved in altered angiogenic processes in patients with hypertension? Curr. Hypertens. Rep. 2004;6:51–54. doi: 10.1007/s11906-004-0011-y. [DOI] [PubMed] [Google Scholar]
- 31.Kao YH, Yu CL, Chang LW, Yu HS. Low concentrations of arsenic induce vascular endothelial growth factor and nitric oxide release and stimulate angio-genesis in vitro. Chem. Res. Toxicol. 2003;16:460–468. doi: 10.1021/tx025652a. [DOI] [PubMed] [Google Scholar]
- 32.Roboz GJ, Dias S, Lam G, Lane WJ, Soignet SL, Warrell RP, Jr., et al. Arsenic trioxide induces dose- and time-dependent apoptosis of endothelium and may exert an antileukemic effect via inhibition of angio-genesis. Blood. 2000;96:1525–1530. [PubMed] [Google Scholar]
- 33.Soucy NV, Klei LR, Mayka DD, Barchowsky A. Signaling pathways for arsenic-stimulated vascular endothelial growth factor-a expression in primary vascular smooth muscle cells. Chem. Res. Toxicol. 2004;17:555–563. doi: 10.1021/tx034193q. [DOI] [PubMed] [Google Scholar]
- 34.Carmignani M, Boscolo P, Iannaccone A. Effects of chronic exposure to arsenate on the cardiovascular function of rats. Br. J. Ind. Med. 1983;40:280–284. doi: 10.1136/oem.40.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bagnato A, Spinella F. Emerging role of endothelin-1 in tumor angiogenesis. Trends Endocrinol. Metab. 2003;14:44–50. doi: 10.1016/s1043-2760(02)00010-3. [DOI] [PubMed] [Google Scholar]
- 36.Jiang SJ, Lin TM, Wu HL, Han HS, Shi GY. Decrease of fibrinolytic activity in human endothelial cells by arsenite. Thromb. Res. 2002;105:55–62. doi: 10.1016/s0049-3848(01)00397-8. [DOI] [PubMed] [Google Scholar]
- 37.Wu HL, Yang WH, Wang MY, Shi GY. Impaired fibrinolysis in patients with Blackfoot disease. Thromb. Res. 1993;72:211–218. doi: 10.1016/0049-3848(93)90188-t. [DOI] [PubMed] [Google Scholar]
- 38.Cho A, Reidy MA. Matrix metalloproteinase-9 is necessary for the regulation of smooth muscle cell replication and migration after arterial injury. Circ. Res. 2002;91:845–851. doi: 10.1161/01.res.0000040420.17366.2e. [DOI] [PubMed] [Google Scholar]
- 39.Morishige K, Shimokawa H, Matsumoto Y, Eto Y, Uwatoku T, Abe K, et al. Overexpression of matrix metalloproteinase-9 promotes intravascular thrombus formation in porcine coronary arteries in vivo. Cardiovasc. Res. 2003;57:572–585. doi: 10.1016/s0008-6363(02)00710-1. [DOI] [PubMed] [Google Scholar]
- 40.Rossman TG, Uddin AN, Burns FJ, Bosland MC. Arsenite cocarcinogenesis: an animal model derived from genetic toxicology studies. Environ. Health Perspect. 2002;110(Suppl. 5):749–752. doi: 10.1289/ehp.02110s5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang CH, Jeng JS, Yip PK, Chen CL, Hsu LI, Hsueh YM, et al. Biological gradient between long-term arsenic exposure and carotid atherosclerosis. Circulation. 2002;105:1804–1809. doi: 10.1161/01.cir.0000015862.64816.b2. [DOI] [PubMed] [Google Scholar]
- 42.Simeonova PP, Hulderman T, Harki D, Luster MI. Arsenic exposure accelerates atherogenesis in apolipoprotein E(−/−) mice. Environ. Health Perspect. 2003;111:1744–1748. doi: 10.1289/ehp.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang DG, Conti CJ. Endothelial cell development, vasculogenesis, angiogenesis, and tumor neovascularization: an update. Semin. Thromb. Hemost. 2004;30:109–117. doi: 10.1055/s-2004-822975. [DOI] [PubMed] [Google Scholar]
- 44.Rivard A, Fabre JE, Silver M, Chen D, Murohara T, Kearney M, et al. Age-dependent impairment of angiogenesis. Circulation. 1999;99:111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 45.Scotland R, Vallance P, Ahluwalia A. Endothelin alters the reactivity of vasa vasorum: mecha nisms and implications for conduit vessel physiology and pathophysiology. Br. J. Pharmacol. 1999;128:1229–1234. doi: 10.1038/sj.bjp.0702930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galis ZS, Johnson C, Godin D, Magid R, Shipley JM, Senior RM, et al. Targeted disruption of the matrix metalloproteinase-9 gene impairs smooth muscle cell migration and geometrical arterial remodeling. Circ. Res. 2002;91:852–859. doi: 10.1161/01.res.0000041036.86977.14. [DOI] [PubMed] [Google Scholar]
- 47.Schneider DJ, Ricci MA, Taatjes DJ, Baumann PQ, Reese JC, Leavitt BJ, et al. Changes in arterial expression of fibrinolytic system proteins in atherogenesis. Arterioscler. Thromb. Vasc. Biol. 1997;17:3294–3301. doi: 10.1161/01.atv.17.11.3294. [DOI] [PubMed] [Google Scholar]
- 48.Achanzar WE, Brambila EM, Diwan BA, Webber MM, Waalkes MP. Inorganic arsenite-induced malignant transformation of human prostate epithelial cells. J. Natl. Cancer Inst. 2002;94:1888–1891. doi: 10.1093/jnci/94.24.1888. [DOI] [PubMed] [Google Scholar]
- 49.Koenig W. Haemostatic risk factors for cardiovascular diseases. Eur. Heart J. 1998;19(Suppl. C):C39–C43. [PubMed] [Google Scholar]