Abstract
DYT1 dystonia is a movement disorder caused by a trinucleotide deletion (ΔGAG) in DYT1 (TOR1A), corresponding to a glutamic acid loss in the C-terminal region of torsinA. Functional alterations in the basal ganglia circuits have been reported in both DYT1 dystonia patients and rodent models. Dyt1 ΔGAG heterozygous knock-in (KI) mice exhibit motor deficits and decreased striatal dopamine receptor 2 (D2R) binding activity, suggesting a malfunction of the indirect pathway. However, the role of the direct pathway in pathogenesis of dystonia is not yet clear. Here, we report that Dyt1 KI mice exhibit significantly decreased striatal dopamine receptor 1 (D1R) binding activity and D1R protein levels, suggesting the alteration of the direct pathway. The decreased D1R may be caused by translational or post-translational processes since Dyt1 KI mice had normal levels of striatal D1R mRNA and a normal number of striatal neurons expressing D1R. Levels of striatal ionotropic glutamate receptor subunits, dopamine transporter, acetylcholine muscarinic M4 receptor and adenosine A2A receptor were not altered suggesting a specificity of affected polytopic membrane-associated proteins. Contribution of the direct pathway to motor-skill learning has been suggested in another pharmacological rat model injected with a D1R antagonist. In the present study, we developed a novel motor skill transfer test for mice and found deficits in Dyt1 KI mice. Further characterization of both the direct and the indirect pathways in Dyt1 KI mice will aid the development of novel therapeutic drugs.
Keywords: Direct pathway; Dopamine receptor; DYT1, Dystonia; Motor-skill transfer; TorsinA
1. Introduction
Dystonia is defined as a movement disorder characterized by muscle contractions causing twisting, repetitive movements or abnormal postures [1]. Dystonia is often initiated or worsened by voluntary action and associated with an overflow of muscle activation. DYT1 early-onset generalized torsion dystonia (DYT1 dystonia; OMIM 128100, dystonia 1) is caused by mutations in one allele of DYT1 (TOR1A), coding for torsinA. Most DYT1 dystonia patients have a heterozygous mutation of a trinucleotide deletion (ΔGAG), corresponding to a glutamic acid deletion in the C-terminal region [2]. An 18 bp-deletion [3], an Arg288Gln missense mutation [4], and a frame-shift mutation caused by a 4 bp-deletion [5] have also been reported in isolated dystonia patient families.
TorsinA is an ATP-binding protein with a distant relationship to the HSP100/Clp family of proteins [2]. ATPase activities have been reported in recombinant torsinA prepared from Escherichia coli [6] and baculovirus expression system [7]. Molecular chaperon-like activities of torsinA have also been reported in multiple experimental models. Examples include, overexpression of torsinA prevents aggregations of luciferase in vitro [8], accumulations of α-synuclein in cultured mammalian cells [9] and polyglutamine-repeat proteins in Caenorhabditis elegans [10]. The expression of genes associated with glutamate receptor-mediated synaptic plasticity is altered in cultured cell lines overexpressing human mutant torsinA [11, 12]. TorsinA contributes to the stability of snapin, which functions in exocytosis [13] and other synaptic proteins [14]. Moreover, torsinA contributes to trafficking of polytopic membrane-associated proteins [15] and protein processing in the secretory pathway [16] in vitro.
Functional alterations in the basal ganglia circuits have been reported in both DYT1 dystonia patients and rodent models [17, 18]. According to a classical hypothesis of the basal ganglia circuits, the striatal medium spiny neurons expressing D1R contribute to the direct pathway and those expressing D2R contribute to the indirect pathway. Reduced striatal D2R binding activity was reported in focal dystonic patients by PET scans [19]. Reductions of D2R binding activity were also seen in both manifesting and non-manifesting DYT1 mutant carriers with the degree of reduction higher in symptomatic than asymptomatic patients, suggesting that reduction of D2R binding activity may affect the disease penetrance of the mutation [20]. Dyt1 ΔGAG heterozygous KI mice exhibit reduced striatal torsinA and D2R binding activity [21] and motor deficits [22]. Transgenic mice expressing human mutant torsinA using the CMV promoter also showed reduced striatal D2R [23]. Moreover, striatum-specific Dyt1 conditional knockout mice exhibit reduced striatal D2R binding activity and motor deficits, suggesting that the reduction of D2R is caused by loss of striatal torsinA function by a cell-autonomous mechanism and contributes to the motor deficits [24]. Taken together, these results suggest the malfunction of the indirect pathway in DYT1 dystonia.
Deep brain stimulation in the globus pallidus internus, which is a component of both the direct and indirect pathways in the basal ganglia circuits, is an effective surgical treatment for DYT1 dystonia [25–27]. Dyt1 ΔGAG heterozygous KI mice exhibit impaired corticostriatal LTD and motor deficits, which are restored by trihexyphenidyl, an anticholinergic which is commonly used for DYT1 dystonia patients to release their dystonic symptoms [21]. Therefore, the motor deficits in the genetic mouse models appear to be relevant to dystonic symptoms in humans. Dopamine plays complementary roles in both D1R- and D2R-expressing medium spiny neurons [28]. Cholinergic interneurons mediate dopaminergic control of corticostriatal LTD in medium spiny neurons [29]. Since muscarinic receptors are expressed in both D1R- and D2R-expressing medium spiny neurons, trihexyphenidyl may affect both direct and indirect pathways and improve the symptoms. However, contribution of the direct pathway to the pathogenesis of DYT1 dystonia has not been clear, due to a lack of proper radioligand for D1R for in vivo study [30]. A postmortem study showed only trends of decreased striatal binding activities of [3H]YM-09151-2 radio-ligand to D2R and [3H]SCH-23390 radio-ligand to D1R because of the limited sample size [31]. Here, D1R binding activities in the striatal membrane fractions and brain slices from Dyt1 KI mice were measured to determine the contribution of the direct pathway in DYT1 dystonia pathogenesis.
2. Materials and methods
2.1. Mice
All experimental procedures were carried out in compliance with the USPHS Guide for Care and Use of Laboratory Animals and approved by the University of Illinois Institutional Animal Care and Use Committee. The mice had 129/SvJ, BALB/c and C57BL/6 mixed genetic background. Dyt1 ΔGAG heterozygous KI mice and WT mice were cross-bred and generated approximately equal proportions of Dyt1 ΔGAG heterozygous KI and WT littermate mice as described earlier [22]. Adult male mice of each genotype were used in this study. Mice were kept in a 12-hour light/12-hour dark cycle. Food and water were given ad libitum.
2.2. D1R binding assay
The striata were prepared from three Dyt1ΔGAG heterozygous KI and three WT littermates at least six months of age and homogenized in nine volumes of ice-cold 50 mM Tris∙Cl (pH 7.1), 8 mM MgCl2, 5 mM EDTA∙2Na. The homogenate was centrifuged at 18,000 × g for 20 min at 4 °C and the pellet was suspended in the same buffer and stored at −80 °C. The protein concentration of the membrane preparation was determined by Bradford assay using bovine serum albumin as a standard [32]. [3H]SCH-23390 binding was determined based on a method reported previously [33]. An aliquot of the homogenate was suspended in 200 µl of binding buffer [50 mM Tris∙Cl (pH 7.4), 120 mM NaCl, 5mM KCl, 5 mM MgCl2, 1.5 mM CaCl2, 1mM EDTA∙2Na, 10 µM pargyline hydrochloride (Sigma-Aldrich) and 0.1% ascorbic acid] with 0.03 - 1.92 nM [3H]SCH-23390 (Perkin Elmer). The reaction mixture was incubated at 30°C for 60 min, and then rapidly filtered under vacuum through glass microfibre GF/B filter (Whatman). The filter was then washed four times with 4 ml of the ice-cold binding buffer without the isotope and then dried. The radioactivity of the filters was measured in 6 ml of ScintiSafe Econo1 (Fisher Scientific) by a Beckman liquid scintillation counter. Non-specific binding of [3H]SCH-23390 was measured in presence of 30 µM (+)-butaclamol (Sigma-Aldrich). The experiments were done in duplicates.
2.3. D1R autoradiography
Brains from three pairs of Dyt1ΔGAG heterozygous KI and WT littermates of six months old were frozen in isopentane (Sigma-Aldrich) cooled by dry ice. Coronal sections were cut in a cryostat and thaw-mounted onto gelatin-coated glass slides. Sections that corresponded to plates 20 to 25 [34] were used which included the cerebral cortex, caudate-putamen, and nucleus accumbens. Binding was performed according to a published protocol [35]. The sections were incubated in 1.65 nM [3H] SCH-23390 in incubation buffer [25 mM Tris∙Cl (pH 7.5), 100 mM NaCl, 1 mM MgCl2, 1 µM pargyline, 100 mg/l ascorbate] for 2.5 h at room temperature. After incubation, sections were washed for 10 min in cold incubation buffer followed by a dip in cold H2O. When sections were dry, they were exposed to BAS-TR 2025 imaging plate for 72 hr and scanned on an FLA-7000 bioimager analyzer (Fuji Film).
2.4. Western blot
The striata were dissected from eight Dyt1ΔGAG heterozygous KI and six their WT littermate brains and homogenized in 200 µl of ice-cold RIPA buffer (Santa Cruz) containing protease inhibitor cocktail (Roche). The homogenate was incubated for 30 minutes on ice and centrifuged at 10,000 × g for 10 minutes at 4°C. The supernatant was used as protein samples for Western blot. The protein concentration of the supernatant was measured by protein assay reagent (Bio-Rad). Aliquot of the supernatant corresponding to 30 µg of protein was mixed with 2 × loading buffer containing 2-mercaptoethanol and boiled for 5 minutes, chilled on ice for 1 minute and spun down. The proteins were separated on 10% SDS-PAGE gel and transferred to Millipore Immobilon –FL transfer membranes (PVDF). The PVDF membranes were washed in 0.1M PBS for 5 min and blocked with LICOR Odyssey blocking buffer for 1 hour. The membranes were cut into two at about 40 kDa and the membranes for higher molecular weight were incubated overnight at 4°C with goat polyclonal D1R antibody (Santa cruz, sc-31478) at 1:200 dilution and the other membranes for lower molecular weight were incubated with rabbit Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (Santa cruz, sc-25778) at 1:1000 dilution in the blocking buffer. The membranes were washed with 0.1M PBS containing 0.1% Tween 20, for 5 min four times, then treated for 1 hour with LI-COR IRDye 800CW donkey anti-goat IgG (H+L) or LI-COR IRDye 680RD donkey anti-rabbit IgG (H+L) at 1:15,556 dilution, respectively. After washing with 0.1M PBS containing 0.1% Tween 20 for 5 min four times and 0.1M PBS for 5 min three times, the membranes were dried and the signals were detected by a LI-COR Odyssey imaging system.
The striatal proteins were also extracted from other six to twelve of Dyt1ΔGAG heterozygous KI and their WT littermate mice in the RIPA buffer or 1% Triton X100 as previously described [36]. The proteins were separated on SDS-PAGE gels and transferred to PROTRAN nitrocellulose transfer membranes (Whatman). After blocking with 5% Non-fat milk (Bio-rad) in TBS-T buffer of 20mM Tris-Cl (pH7.6), 137mM NaCl, 0.1% Tween 20, the protein levels of glutamate receptor subunits GluR1, GluR2, GluR2/3, GluR4, dopamine transporter, muscarinic acetylcholine receptor M4, and adenosine A2A-R were also analyzed using rabbit anti-glutamate receptor 1 affinity purified polyclonal antibody (Chemicon, AB1504), affinity purified, polyclonal rabbit anti-glutamate receptor (GluR2) antibody (BD biosciences, 556342), rabbit anti-glutamate receptor 2 & 3 affinity purified polyclonal antibody (Chemicon, AB1506), and rabbit anti-glutamate receptor 4 affinity purified polyclonal antibody (Chemicon, AB1508), rabbit polyclonal dopamine transporter (DAT) antibody (ProSci Inc., 50–204), mouse monoclonal anti-Muscarinic acetylcholine receptor M4 (abcam, ab77956) antibody and mouse monoclonal adenosine A2A-R (sc-32261) antibody, respectively. Peroxidase AffiniPure donkey anti-rabbit IgG (H+L) antibody (Jackson ImmunoResearch, 711-035-152) and bovine anti-mouse IgG-HRP (Santa Cruz, sc-2370) were used as the secondary antibodies for the rabbit and mouse primary antibodies, respectively. The β-actin was detected with mouse anti β-actin antibody (Sigma, A5441) at 10,000 dilution and peroxidase AffiniPure donkey anti-mouse IgG (H+L) antibody (Jackson ImmunoResearch, 715-035-151) as a loading control for GluRs. The GAPDH was detected with HRP-conjugated anti-GAPDH antibody (Santa Cruz, sc-25778 HRP) at 1,000 dilution as a loading control for DAT, M4 and A2A-R. The signals were detected with SuperSignal substrate (Pierce, #34096) and X-ray film (Hawkins X-ray supply, HXR film) or an Alpha Innotech FluorChem FC2 camera after washing the membranes. The density of the bands was quantified by UN-SCAN-IT gel (Silk Scientific).
2.5. Quantitative real-time RT-PCR
The striata were dissected from five Dyt1 ΔGAG heterozygous KI and five WT littermate male mice of 4 months old and put in RNAlater RNA stabilization reagent (Qiagen). The RNA was isolated by RNeasy mini kit (Qiagen). The RNA concentration was measured by NanoDrop 2000c (Thermo Scientific) and the cDNA was synthesized by SuperScript III First-strand Synthesis SupeMix for qRT-PCR kit (Invitrogen). Quantitative PCR was performed using CFX real-time PCR detection system (Bio-Rad) with SYBR Select Master Mix for CFX (life technologies) and the following primer sets: GAPDH-F (5’-ACAGTCCATGCCATCACTGCC-3’) and GAPDH-R (5’-GCCTGCTTCACCACCTTCTTG-3’) for GAPDH cDNA as previously described [37], D1E1E2 (5’-GGTCTCCCAGATCGGGCATTTGG-3’) and D1E2 (5’-GGCATCCGCTGGTCCCTAGATTC-3’) for D1R cDNA, and E1 (5’-CACCCGCGAGCACAGCTTCTTTG-3’) and E2 (5’-AATACAGCCCGGGGAGCATCGTC- 3’) for β-actin cDNA. The CFX real-time system was programmed in the following way: 50°C for 2 min, 95°C for 2 min, 50 cycles of 95°C for 15 sec, 55°C for 15 sec and 72°C for 1 min + plate read, followed by the melting curve analysis: 95°C for 1min, 55°C for 1 min and melt curve 55°C to 95°C, increment 0.5°C (80 cycles) for 10 sec + plate read. The relative quantity of cDNA for D1R to that for GAPDH or β-actin was calculated by ΔCT method [38].
2.6. Immunohistochemistry
Immunohistochemistry was performed using the frozen brain sections as described earlier [39]. Briefly, three Dyt1 ΔGAG KI and three WT littermates of five to six months old were anesthetized and perfused with 0.1 M phosphate-buffered saline (pH 7.4; PBS) followed by 4% paraformaldehyde in 0.1 M PBS. The brains were soaked in the paraformaldehyde-PBS at 4°C overnight and soaked in 30% sucrose in 0.1 M PBS until they sank. The brains were frozen by dry-ice powder and cut into 20 µm sections with a Histoslide 2000 sliding microtome (Reichert-Jung). Sections were blocked and then incubated overnight at 4°C with rabbit polyclonal anti-dopamine receptor D1R antibody (Sigma, D6692, 1:300 dilution) and rabbit polyclonal anti-dopamine D2 receptor antibody (Chemicon, AB5084P; 1:300 dilution). Sections were stained with Vectastain ABC kit for peroxidase rabbit IgG and DAB peroxidase substrate kit with Nickel solution (Vector Lab). This staining produces gray-black reaction product in the immunopositive cells. The positive cell numbers in the striatum were counted by using BH2 light microscope (Olympus) with StereoInvestigator software (MicroBrightFields Bioscience). At least eight computerselected fields of 100 × 100 µm on five sections per animal were counted.
2.7. Motor-skill transfer test
Two groups consisting of eight to ten pairs of male Dyt1 ΔGAG heterozygous KI and WT littermate mice were used in this experiment. The treadmill apparatus used was a treadmill (Weslo) for humans converted to one for mice with an eight-lane (7 × 20 cm/lane) rectangular wood box suspended 1 cm above the running belt. Acrylic beads strung from a thin wire were placed along the back wall of each lane to serve as a non-noxious stimulus for running. Mice in treated group were trained for two weeks on the treadmill with a starting running speed of approximately 13 m/min (0.5 mph) for 5 minutes each day. When more than 90% of the mice could run comfortably at that speed, it was increased by approximately 2.5 m/min (0.1 mph) to a maximum speed of approximately 18.5 m/min (0.7 mph). Mice in the control (untrained) group were each placed on the treadmill for 5 minutes each day, but with the treadmill belt off. Two days after the last day of training, all mice were tested on an Economex accelerating rotarod (Columbus Instruments) for three trials in one day as previously described [22]. Latency to fall off the rotarod was measured for each mouse. Comparisons were then made between the four groups of mice, WT with and without treadmill training and KI with and without treadmill training.
2.8. Statistics
Bmax and Kd of striatal D1R binding activity in the membrane fractions were individually calculated for each mouse by Scatchard’s plot and those of each genotype were analyzed by Student’s t-test. D1R binding activities in the bran slices, Western blot data, relative quantities of cDNA for D1R to that for GAPDH or β-actin in qPCR analysis, and the number of stained neurons using D1R and D2R antibodies per the area in the immunohistochemistry, were analyzed by Student’s t-test. The latency to fall in the accelerated rotarod test was analyzed by two-way ANOVA with repeated measurement using SAS 9.1 software after natural log transformation to obtain a normal distribution. Significance was assigned at p < 0.05.
3. Results
3.1. Reduction of striatal D1R in Dyt1 KI mice
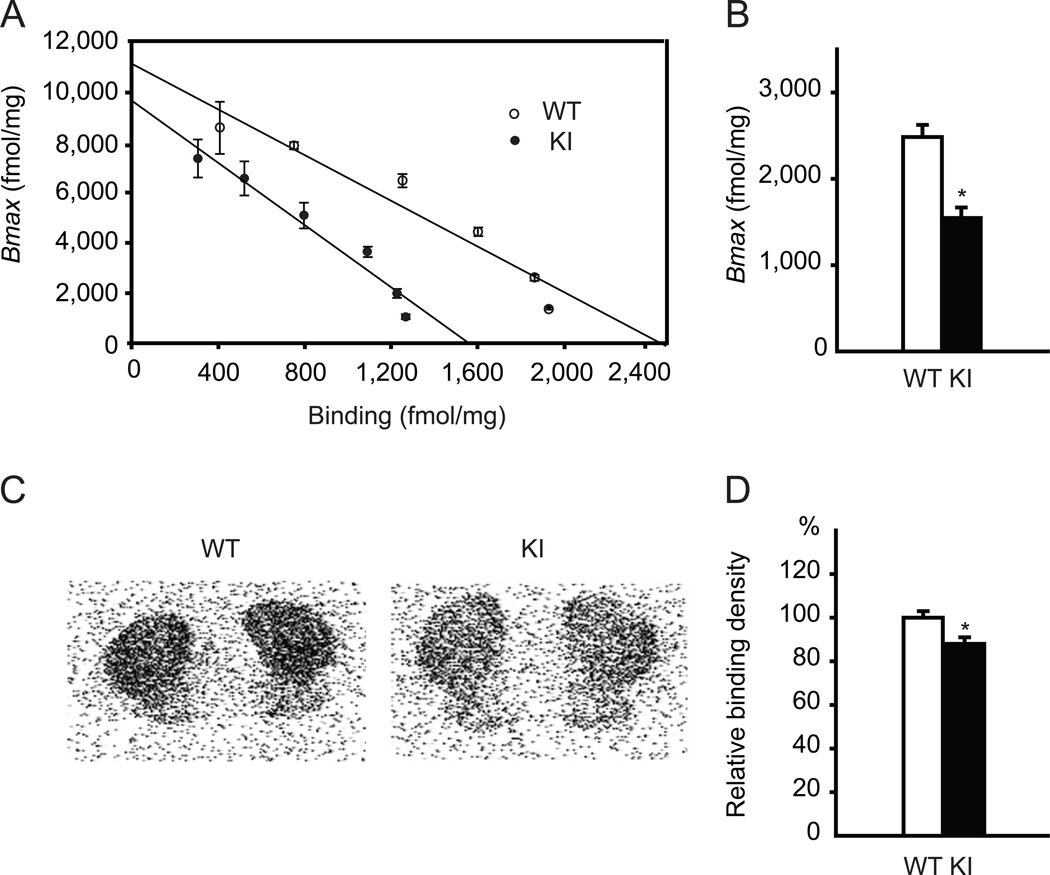
To examine the functional alteration of direct pathway in Dyt1 KI mice, a receptor binding assay was performed with a radioligand [3H] SCH-23390 for D1R in striatal membrane fractions of Dyt1 KI and WT littermate mice (Fig. 1A). D1R binding activity was significantly reduced by approximately 37% in Dyt1 KI mice compared to WT mice (means ± standard errors; WT: 2,484 ± 141 fmol/mg, n = 3; KI: 1,546 ± 122 fmol/mg, n = 3; p = 0.004; Fig. 1B), while receptor affinities for the ligand were not significantly different between the two groups (WT: 231 ± 26 pM, n = 3; KI: 168 ± 22 pM, n = 3; p = 0.12). To further analyze the functional D1R binding activity in Dyt1 KI mice, autoradiographic binding of [3H] SCH-23390 was assayed on coronal brain sections (Fig. 1C). Dyt1 KI mice showed significantly reduced D1R binding activity in comparison to WT (WT: 100 ± 3 %, n = 3; KI: 88 ± 3 %, n = 3; p = 0.040; Fig. 1D). The results suggest reduction of functional striatal D1R in Dyt1 KI mice.
Fig. 1.
Reduction of D1R binding activities in the striatal membrane fractions and brain slices of Dyt1 ΔGAG heterozygous KI mice. (A) Scatchard plot of D1R binding activity in the striatal membrane fractions shows that Dyt1 ΔGAG heterozygous KI mice had reduced binding of D1R antagonist [3H] SCH-23390. (B) Dyt1 ΔGAG heterozygous KI mice had an over 30% reduction in D1R binding. (C) Representative autoradiographs with D1R radioligand [3H] SCH-23390 in WT and Dyt1 ΔGAG heterozygous KI mice. (D) Radioligand autoradiographs confirmed the reduction of D1R in Dyt1 ΔGAG heterozygous KI mice. Vertical bars represent means ± standard errors. *p < 0.05.
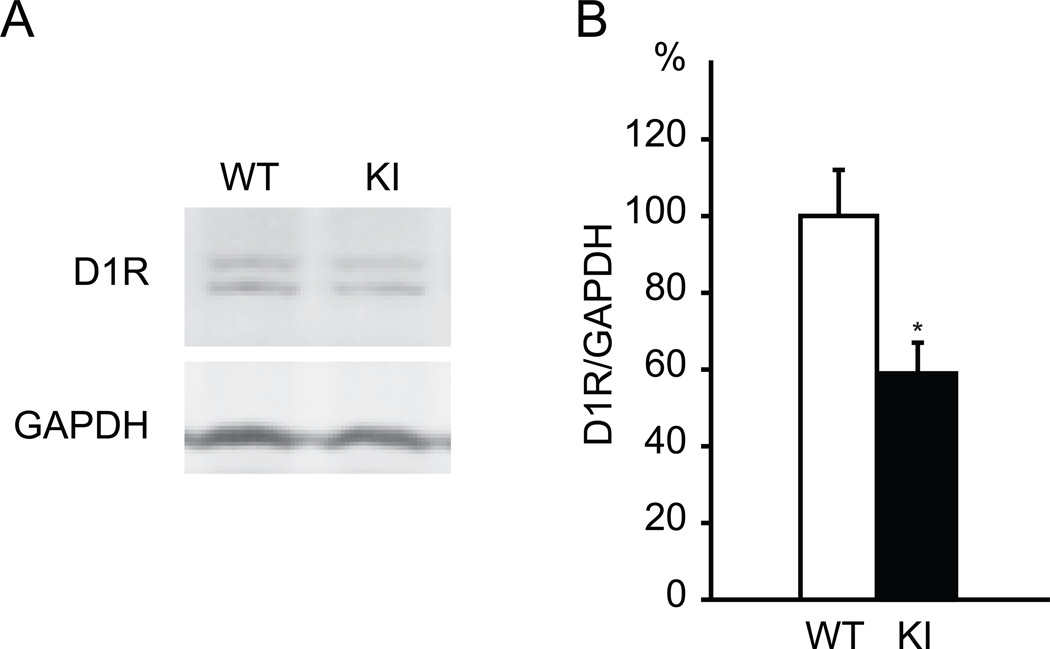
To determine whether the decreased D1R binding activity is the result of a decrease in D1R protein levels, Western blot analysis was performed using a D1R antibody (Fig. 2A). Relative striatal D1R level normalized to GAPDH was significantly reduced in Dyt1 KI mice (WT: 100 ± 12 %, n = 6; KI: 59 ± 8 %, n = 8; p = 0.013; Fig. 2B). Although SCH-23390 has binding activity to both D1R and dopamine receptor 5 [40], the Western blot data clearly showed the reduction of striatal D1R at protein level. The reduction levels in Western blot and ligand binding assay using the membrane fraction were similar. On the other hand, the ligand binding assay using the brain slice showed less reduction. It should be noted that Western blot and ligand binding assay of the membrane faction used samples derived from brain homogenates, while the ligand binding assay of the brain slices measures mostly surface receptors using brain tissues without homogenization. Regardless of the reduction levels, both ligand binding and Western blot results suggested statistically significant reduction of D1R.
Fig. 2.
Reduction of striatal D1R in Dyt1 ΔGAG heterozygous KI mice. (A) The representative bands of D1R and GAPDH in Western blot experiments. (B) The quantified D1R standardized to GAPDH. The striatal D1R level in Dyt1 ΔGAG heterozygous KI mice was significantly decreased in comparison to that in WT littermates. Vertical bars represent means ± standard errors. *p < 0.05.
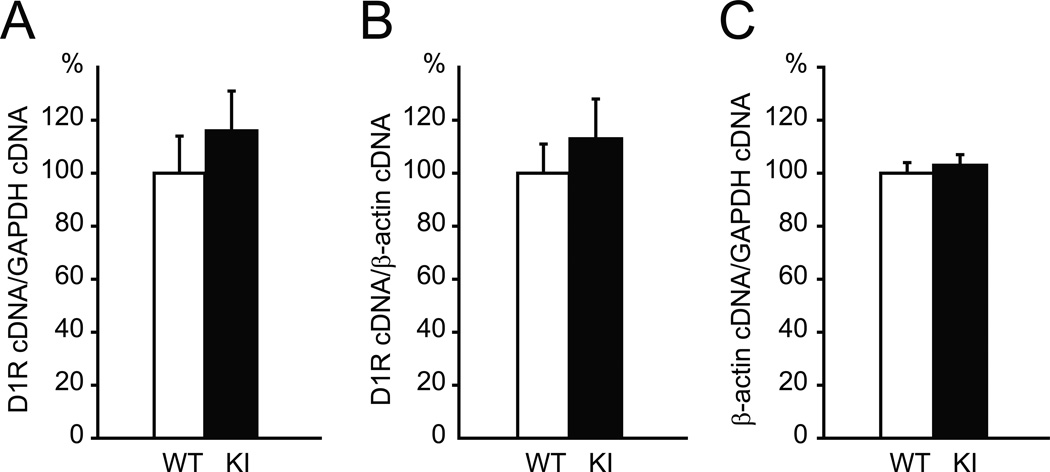
To determine whether the decreased D1R is due to changes in transcription, quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) was performed using striatal RNA. D1R mRNA level was compared between WT and Dyt1 KI mice by normalizing to either GAPDH or β-actin mRNA levels. No significant difference in D1R mRNA level was observed between WT and Dyt1 KI mice (GAPDH: WT: 100 ± 14 %, n = 5; KI: 116 ± 15 %, n = 5; p = 0.465; Fig. 3A; β-actin: WT: 100 ± 11 %, n = 5; KI: 113 ± 15 %, n = 5; p = 0.510; Fig. 3B). Since multiple controls are recommended in other papers [41, 42], we sought to determine the relative changes of mRNA transcripts between GAPDH and β-actin. There is no significant difference in β-actin mRNA level between WT and Dyt1 KI mice when normalized to GAPDH mRNA (WT: 100 ± 4 %, n = 5; KI: 103 ± 4 %, n = 5; p = 0.649; Fig. 3C), suggesting that there is no significant change in the mRNA levels of the house-keeping gene in the mouse striata. Amplification efficiencies for D1R, β-actin, GAPDH cDNAs were 98%, 97%, and 96%, respectively. Melt curve analysis detected a single sharp peak for each target at over 80°C, suggesting no obvious fluorescence derived from the primer dimers. The results suggest that Dyt1 ΔGAG heterozygous mutation does not affect striatal D1R mRNA level and the decreased D1R is not due to the transcription or stability of D1R mRNA. We previously reported similar findings of reduction of striatal D2R without alteration of striatal D2R mRNA in Dyt1 ΔGAG heterozygous KI mice [21].
Fig. 3.
Quantitative real-time PCR showed no significant difference of the striatal D1R mRNA level between Dyt1 ΔGAG KI and WT mice. (A) D1R mRNA levels normalized to GAPDH mRNA. (B) D1R mRNA levels normalized to β-actin mRNA. (C) β-actin mRNA levels normalized to GAPDH mRNA. Vertical bars represent means ± standard errors.
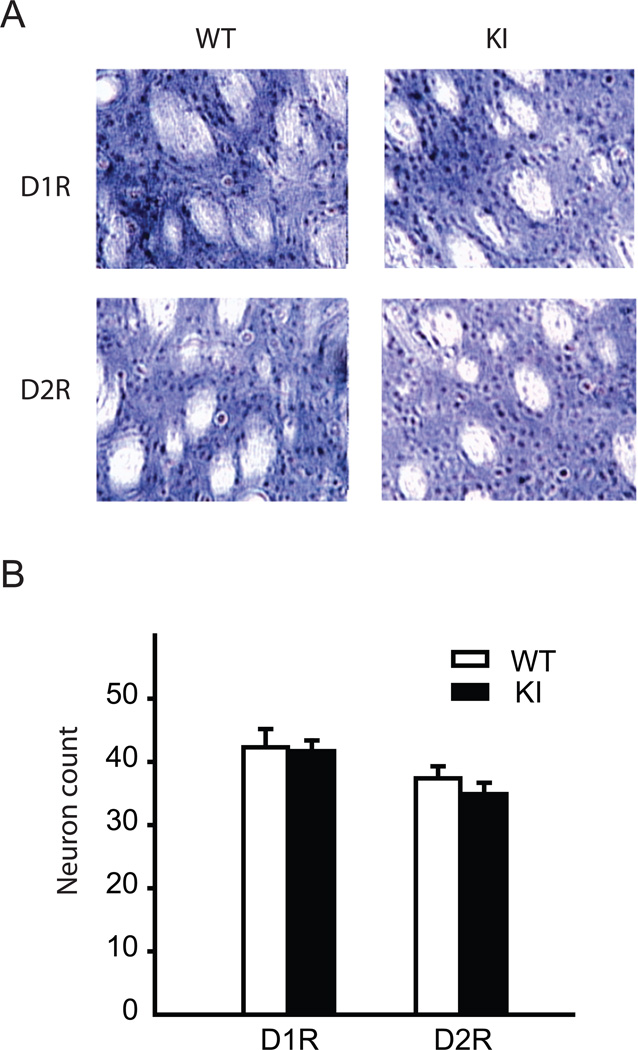
To analyze whether reduced striatal D1R and D2R binding activities were due to less striatal neurons expressing D1R and D2R, immunohistochemistry was performed using antibodies to D1R and D2R, respectively (Fig. 4A). The positive cells were stained in gray with DAB with nickel solution. The stained striatal neurons were counted in multiple sections of three mice from each genotype. The count of striatal neurons which contain D1R or D2R within a 100 µm × 100 µm square was not significantly changed in Dyt1 KI mice in comparison to WT mice (D1R: WT: 42.3 ± 2.9, n = 3; KI: 41.7 ± 1.7, n = 3; p = 0.83; D2R: WT: 37.4 ± 1.9, n = 3; KI: 34.9 ± 1.8, n = 3; p = 0.34; Fig. 4B). The results suggest that the reduced D1R and D2R in Dyt1 KI mice were not due to less striatal neuronal cells expressing D1R or D2R. Normal numbers of striatal neurons expressing D1R and D2R are consistent with the long-held notion that primary dystonia is a disorder of neuronal circuit abnormalities and not of neurodegeneration [17].
Fig. 4.
Normal numbers of the striatal neurons expressing D1R and D2R in Dyt1 ΔGAG heterozygous KI mice. (A) Representative images of immunohistochemistry with D1R and D2R antibodies. (B) Counting of striatal neurons stained with D1R and D2R antibodies. Dyt1 ΔGAG heterozygous KI mice did not have a reduced number of neurons expressing D1R and D2R, arguing against neurodegeneration as a cause for the decreased D1R and D2R binding activities, respectively. Vertical bars represent means ± standard errors.
3.2. Normal levels of other striatal polytopic membrane-associated proteins in Dyt1 KI mice
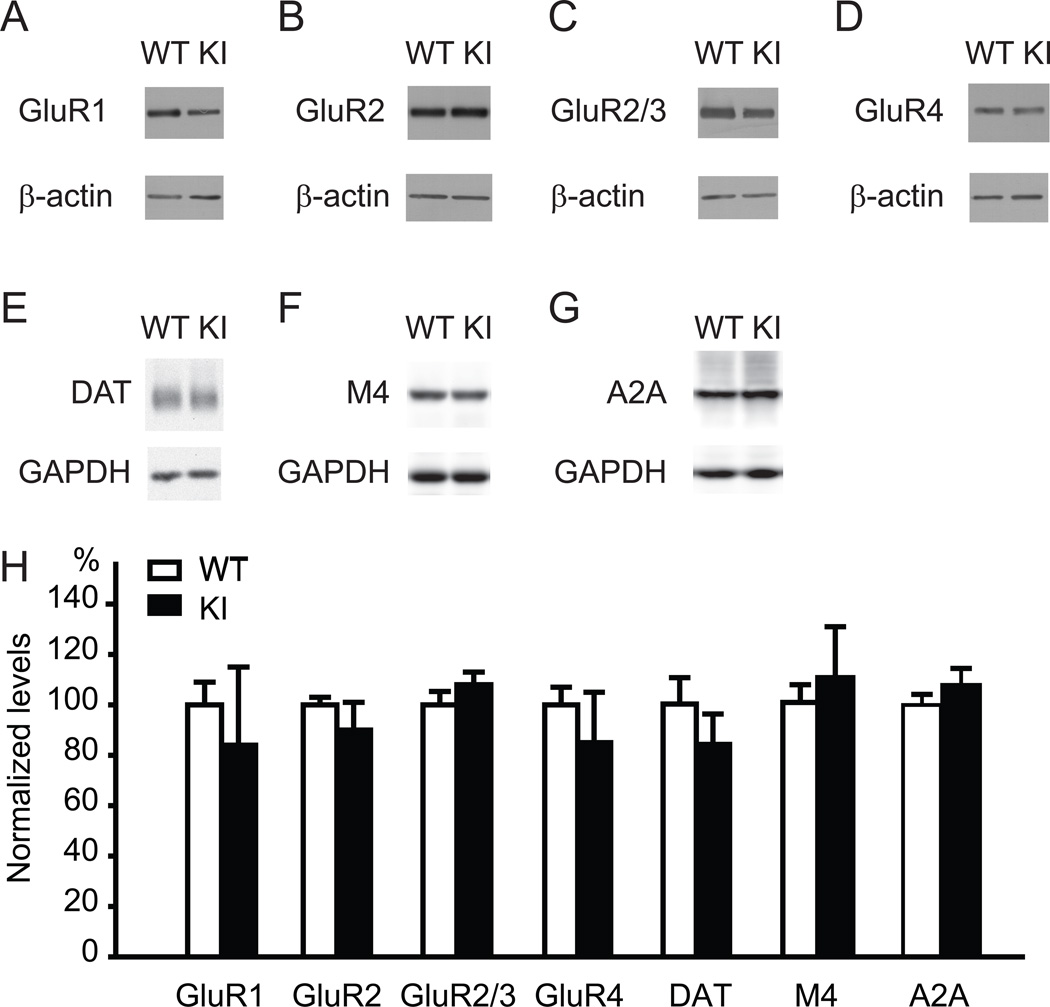
The previous studies using the cultured cells suggest that torsinA contributes to trafficking of polytopic membrane-associated proteins [15] and protein processing in the secretory pathway [16]. Since both D1R and D2R are polytopic membrane-associated proteins [43], the present results for D1R and our previous report for D2R [21] are consistent with their findings. To further analyze whether the levels of other polytopic membrane-associated proteins are also altered in Dyt1 ΔGAG heterozygous KI mice, the levels of striatal glutamate receptor GluR1, GluR2, GluR3 and GluR4 subunits were measured and standardized to the levels of β-actin by Western blot (Fig. 5A–D and H). There was no significant difference between Dyt1 ΔGAG heterozygous KI and WT littermate mice in the levels of striatal GluR1 (WT: 100 ± 9 %, n = 3; KI: 84 ± 31 %, n = 3; p = 0.647), GluR2 (WT: 100 ± 3 %, n = 3; KI: 90 ± 11 %, n = 3; p = 0.448), GluR2/3 (WT: 100 ± 5 %, n = 3; KI: 108 ± 5 %, n = 3; p = 0.297), or GluR4 (WT: 100 ± 7 %, n = 3; KI: 85 ± 20 %, n = 3; p = 0.523). We also measured other striatal polytopic membrane-associated proteins dopamine transporter, acetylcholine muscarinic M4 receptor and adenosine A2A receptor, which serve as makers for dopaminergic neurons, D1R-expressing medium spiny neurons and D2R-expressing medium spiny neurons, respectively. There was no significant difference between Dyt1 ΔGAG heterozygous KI and WT littermate mice in the levels of striatal dopamine transporter (WT: 100 ± 10 %, n = 4; KI: 84 ± 11 %, n = 4; p = 0.312; Fig. 5E and H), acetylcholine muscarinic M4 receptor (WT: 100 ± 7 %, n = 4; KI: 110 ± 20 %, n = 4; p = 0.637; Fig. 5F and H), or adenosine A2A receptor (WT: 100 ± 4 %, n = 5; KI: 108 ± 7 %, n = 7; p = 0.344; Fig.5G and H). The results suggest that Dyt1 ΔGAG mutation leads to specific reduction of striatal D1R and D2R rather than non-specific reduction of all polytopic membrane-associated proteins.
Fig. 5.
No significant difference of other striatal polytopic membrane-associated protein levels in Dyt1 ΔGAG heterozygous KI mice. The representative Western blot bands of striatal glutamate receptor subunits GluR1 (A), GluR2 (B), GluR2/3 (C) and GluR4 (D), dopamine transporter (E), acetylcholine muscarinic M4 receptor (F), adenosine A2A receptor (G), and their corresponding loading controls (β-actin or GAPDH). The quantified polytopic membrane-associated protein bands were standardized to the loading controls (H). Vertical bars represent means ± standard errors.
3.3. Impaired motor-skill transfer in Dyt1 KI mice
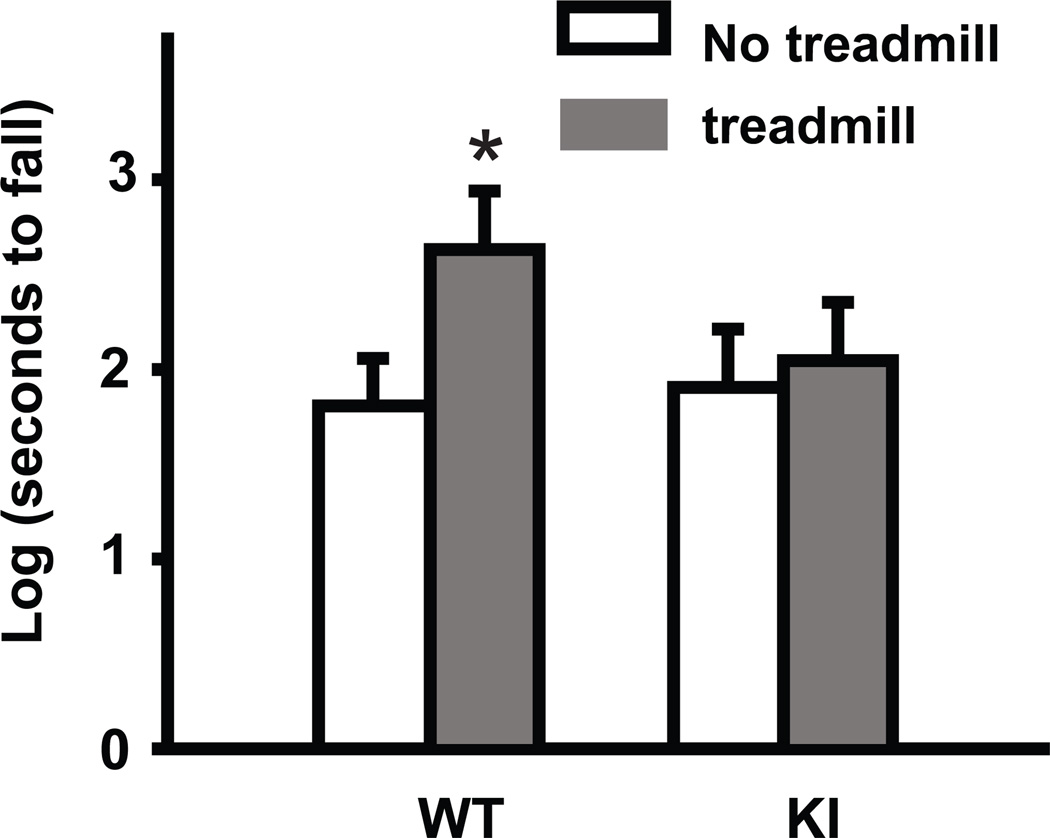
Impaired sequential motor learning has been reported in dystonia mutation carriers [44, 45]. To examine motor-skill learning in Dyt1 KI mice, we developed a novel skill transfer task. The motor task involved forcing mice to run on a treadmill with increasing speeds over a 2 weeks training period. After the 2 weeks training period, they were tested on an accelerated rotarod which additionally forced them to maintain an effective balance. WT mice that were trained on the treadmill were able to vastly improve their performance on the accelerated rotarod in comparison to untrained WT mice that were placed on the treadmill with the belt turned off (log-transformed latency to fall; WT no training: 1.81 ± 0.25; WT training: 2.63 ± 0.31; p = 0.049; Fig. 6). While treadmill training in Dyt1 KI mice did not affect the overall latency to fall in the following accelerated rotarod test (log-transformed latency to fall; KI no training: 1.91 ± 0.31; KI training: 2.05 ± 0.31; p = 0.75; Fig. 6). These results suggest that trained WT mice had enhanced learning abilities due to their previous training on the treadmill, while Dyt1 KI mice were unable to transfer their skills from the treadmill training to the accelerated rotarod task.
Fig. 6.
Motor-skill transfer deficits in Dyt1 ΔGAG heterozygous KI mice. WT mice that were trained on the treadmill had improvement on the accelerated rotarod in comparison to WT mice that were not previously trained. Dyt1 ΔGAG heterozygous KI mice, in contrast, did not have any benefit from the treadmill pre-training, suggesting a lack of motor-skill transfer. Vertical bars represent means ± standard errors. *p < 0.05.
4. Discussion
We previously reported that Dyt1 KI mice exhibited decreased striatal D2R levels [21], suggesting a malfunction of the indirect pathway in the basal ganglia circuits. Here, Dyt1 KI mice exhibited decreased striatal D1R levels, suggesting a malfunction of the direct pathway. Our results are consistent with the trends of decreased striatal D1R and D2R binding activities in a postmortem study of DYT1 dystonia patients [31]. The results may partially explain why deep brain stimulation in the globus pallidus internus, which is a component of both the direct and indirect pathways, is an effective surgical treatment for DYT1 dystonia [25–27]. Functional alteration of the D1R pathway was recently reported in another primary torsion dystonia caused by GNAL, coding for Gαolf that couples to D1R and regulates D1R pathway [46]. The present results also fit to a genetic mouse model with ablation of D1R-expressing neurons, that exhibits hind limb dystonia [47]. Moreover, the dystonic hamster dtsz exhibits decreased striatal D1R and D2R binding activities, suggesting a malfunction of the both direct and the indirect pathways [48]. These findings suggest that a functional alteration of the direct pathway may also contribute to pathophysiology of dystonia.
It should be noted that D1R knockout mice are growth retarded and die shortly after weaning age unless their diet is supplemented with hydrated food [49]. D1R D2R double mutant mice also exhibit lethal phenotype during the second or third week after birth [50]. This lethal phenotype is likely to be related to the altered feeding behavior and dysfunction of the gastrointestinal system. Therefore, partial functional alterations in the direct and the indirect pathways may contribute to the pathogenesis of dystonia, rather than complete loss of D1R and D2R functions. Development of drugs to compensate for the impaired direct and indirect pathways may rescue motor symptoms in DYT1 dystonia.
TorsinA contributes to trafficking of polytopic membrane-associated proteins [15] and protein processing in the secretory pathway in vitro [16]. Dyt1 KI mice exhibit decreased striatal D2R without significant alteration of striatal D2R mRNA [21]. Here, Dyt1 KI mice exhibit a normal number of D2R-expressiong striatal neurons, suggesting that the decreased striatal D2R is not due to loss of D2R-expressing neurons. Moreover, Dyt1 KI mice exhibited decreased striatal D1R without significant alterations in striatal D1R mRNA levels or number of striatal D1R-expressing neurons. The normal level of striatal M4 receptor, which is a marker for D1R-ecprssing medium spiny neurons, also supports normal numbers of D1R-ecprssing medium spiny neurons in the striatum. These results suggest that decreased D1R and D2R may be caused by translational or post-translational processes, such as modification, assembly, trafficking or stabilization. Since the torsinA level is reduced in Dyt1 KI mouse brains [36, 51–53], partial loss of torsinA function may cause trafficking deficits of striatal D1R and D2R and affect their stability. Other studies have shown that torsinA possesses molecular chaperon-like activities [8–10], the partial loss of molecular chaperon-like activity of torsinA may be responsible for D1R reduction at the posttranslational steps. We further analyzed whether the levels of other polytopic membrane-associated proteins are altered. Dyt1 KI mice exhibited a normal level of striatal dopamine receptor or glutamate receptor subunits, suggesting that Dyt1 ΔGAG mutation produces specific reduction of striatal D1R and D2R rather than non-specific reduction of all polytopic membrane-associated proteins. These results suggest that therapeutic strategy that non-specifically targets defective protein processing or secretion in general may not be desirable since torsinA may have target specificity. Further characterization of other torsinA target proteins will elucidate additional defected pathways in DYT1 dystonia.
Dyt1 KI mice lacked the accelerated performance improvement on the accelerated rotarod after treadmill training that was seen with WT mice. From this behavioral task perspective, we hypothesize that WT mice, and not Dyt1 KI mice, were able to use their skill in acclimating to the forced accelerated running of the treadmill while they contended with learning to balance on the accelerated rotarod. From the perspective of neuronal activity, this skill transfer could represent a prior priming of the neuronal apparatus involved in learning so that further learning is more easily achieved. The concept of motor-skill transfer is well-recognized in human behavioral studies and has been demonstrated in several different sequence learning and sensorimotor adaptation tests, most involving a variant of the joystick-aiming task in which subjects are asked to move a joystick to bring a cursor to a target on a monitor [54–57]. Subsequent trials challenged participants to adapt to a visuomotor rotation and require subjects to aim at a specified angle to hit the target. Interestingly, this skill transfer has been shown to be preserved in elderly human subjects while the learning of sequence tasks itself is affected by aging [58]. Motor-skill transfer is also the basis of intermanual skill acquisition which occurs when learning a new motor-skill using one hand helps subjects to achieve faster learning of the same skill in the opposite hand [59, 60]. In the present study, we developed a novel method to examine an ability of motor skill transfer and succeeded in detecting the deficits in Dyt1 KI mice.
Contribution of the direct pathway to motor-skill learning has been suggested in a pharmacological rat model injected with a D1R antagonist; SCH-23390 [61]. D1-neuron specific Gad1 conditional knockout mice, which are supposed to exhibit D1 expressing cells-specific deficits of GABA synthesis, show deficits in motor skills [62]. Moreover, the rate of motor learning is negatively correlated with the levels of DARPP-32 and D1 receptor mRNAs in mice [63]. These studies suggest contribution of the direct pathway to motor-skill learning. Finally, reversible blockade of primary motor cortex D1 and D2 receptors temporarily impairs skill acquisition [64]. Based on an analogy to other rodent models showing motor leaning deficits caused by functional manipulations in the direct pathway, the decreased D1R levels in Dyt1 KI mice may contribute, at least in part, to the motor skill transfer deficits.
Other studies showed differential contribution of the direct and the indirect pathways to motor leaning phases in rotarod test. D2R membrane expression was much higher in the dorsolateral or sensorimotor striatum (DLS) than the dorsomedial or associative striatum (DMS), whereas membrane expression of D1R was more uniform between DMS and DLS [65]. The task-related neuronal activity in the striatal regions differed during the acquisition and consolidation of a new skill. DMS or direct pathway is engaged during the early phase. On the other hand, DLS or indirect pathway is engaged during the late phase. In contrast with the above report, another study showed that specific lesion of D1R-expression neurons causes motor deficits during all phases in rotorod test, suggesting that the direct pathway contribute to overall motor performance [66]. Finally, specific lesion of D2R-expression neurons causes motor deficits only at the early phases in rotorod test, suggesting that the indirect pathway only contribute to motor learning in the early skill acquisition phases. Although these two reports showed quite different results, it is likely that both the direct and the indirect pathways contribute to different phases of motor skill learning. Future study of motor skill transfer experiments using Dyt1 conditional KO mice or knockdown mice targeting D1R- or D2R-positive neurons will elucidate the contribution of each pathway to the motor skill transfer deficits found in Dyt1 KI mice.
Highlights.
Decreased dopamine receptor 1 (D1R) activity and protein level in Dyt1 KI mice
D1R activity decrease is likely caused by altered protein processing or stability
Direct pathway of the basal ganglia circuits in DYT1 dystonia may also be affected
Development of a novel rodent skill transfer test using treadmill and rotarod tests
There is a specific motor skill transfer deficit attributed to ΔGAG mutation in mice
Acknowledgements
We thank Lisa Foster, Andrea McCullough and their staff for animal care, Viet Vo, Miki Jinno, Andrea Marshall, Mark P. DeAndrade, Jessica Artille and Kelly Dexter for their technical assistance. This work was supported by Tyler’s Hope for a Dystonia Cure, Inc (UF), National Institutes of Health grants (NS37409, NS47466, NS47692, NS54246, NS57098, NS65273, NS72782, NS74423, and NS82244), startup funds from the Lucille P. Markey Charitable Trust and Beckman Institute (UIUC), Department of Neurology (UAB), Dystonia Medical Research Foundation, and Bachmann-Strauss Dystonia and Parkinson Foundation, Inc.
Abbreviations
- ANOVA
analysis of variance
- EDTA
ethylenediaminetetraacetic acid
- DAT
dopamine transporter
- DOPAC
3, 4-dihydroxyphenylacetic acid
- D1R
dopamine receptor 1
- D2R
dopamine receptor 2
- Dyt1 KI mice
Dyt1 ΔGAG heterozygous knock-in mice
- LTD
long-term depression
- RT-PCR
reverse transcriptase polymerase chain reaction
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albanese A, Bhatia K, Bressman SB, Delong MR, Fahn S, Fung VS, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013;28:863–873. doi: 10.1002/mds.25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ozelius LJ, Hewett JW, Page CE, Bressman SB, Kramer PL, Shalish C, et al. The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nat Genet. 1997;17:40–48. doi: 10.1038/ng0997-40. [DOI] [PubMed] [Google Scholar]
- 3.Leung JC, Klein C, Friedman J, Vieregge P, Jacobs H, Doheny D, et al. Novel mutation in the TOR1A (DYT1) gene in atypical early onset dystonia and polymorphisms in dystonia and early onset parkinsonism. Neurogenetics. 2001;3:133–143. doi: 10.1007/s100480100111. [DOI] [PubMed] [Google Scholar]
- 4.Zirn B, Grundmann K, Huppke P, Puthenparampil J, Wolburg H, Riess O, et al. Novel TOR1A mutation p.Arg288Gln in early-onset dystonia (DYT1) J Neurol Neurosurg Psychiatry. 2008;79:1327–1330. doi: 10.1136/jnnp.2008.148270. [DOI] [PubMed] [Google Scholar]
- 5.Ritz K, Gerrits MC, Foncke EM, van Ruissen F, van der Linden C, Vergouwen MD, Bloem BR, Vandenberghe W, Crols R, Speelman JD, Baas F, Tijssen MA. Myoclonus-dystonia: clinical and genetic evaluation of a large cohort. J Neurol Neurosurg Psychiatry. 2009;80:653–658. doi: 10.1136/jnnp.2008.162099. [DOI] [PubMed] [Google Scholar]
- 6.Konakova M, Pulst SM. Dystonia-associated forms of torsinA are deficient in ATPase activity. J Mol Neurosci. 2005;25:105–117. doi: 10.1385/JMN:25:1:105. [DOI] [PubMed] [Google Scholar]
- 7.Kustedjo K, Deechongkit S, Kelly JW, Cravatt BF. Recombinant expression, purification, and comparative characterization of torsinA and its torsion dystonia-associated variant Delta E-torsinA. Biochemistry. 2003;42:15333–15341. doi: 10.1021/bi0349569. [DOI] [PubMed] [Google Scholar]
- 8.Burdette AJ, Churchill PF, Caldwell GA, Caldwell KA. The early-onset torsion dystonia-associated protein, torsinA, displays molecular chaperone activity in vitro. Cell Stress Chaperones. 2010;15:605–617. doi: 10.1007/s12192-010-0173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLean PJ, Kawamata H, Shariff S, Hewett J, Sharma N, Ueda K, et al. TorsinA and heat shock proteins act as molecular chaperones: suppression of alpha-synuclein aggregation. J Neurochem. 2002;83:846–854. doi: 10.1046/j.1471-4159.2002.01190.x. [DOI] [PubMed] [Google Scholar]
- 10.Caldwell GA, Cao S, Sexton EG, Gelwix CC, Bevel JP, Caldwell KA. Suppression of polyglutamine-induced protein aggregation in Caenorhabditis elegans by torsin proteins. Hum Mol Genet. 2003;12:307–319. doi: 10.1093/hmg/ddg027. [DOI] [PubMed] [Google Scholar]
- 11.Grundmann K, Reischmann B, Vanhoutte G, Hubener J, Teismann P, Hauser TK, et al. Overexpression of human wildtype torsinA and human DeltaGAG torsinA in a transgenic mouse model causes phenotypic abnormalities. Neurobiol Dis. 2007;27:190–206. doi: 10.1016/j.nbd.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Martin J, Bair T, Bode N, Dauer W, Gonzalez-Alegre P. Transcriptional and proteomic profiling in a cellular model of DYT1 dystonia. Neuroscience. 2009;164:563–572. doi: 10.1016/j.neuroscience.2009.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granata A, Watson R, Collinson LM, Schiavo G, Warner TT. The dystonia-associated protein torsinA modulates synaptic vesicle recycling. J Biol Chem. 2008;283:7568–7579. doi: 10.1074/jbc.M704097200. [DOI] [PubMed] [Google Scholar]
- 14.Granata A, Koo SJ, Haucke V, Schiavo G, Warner TT. CSN complex controls the stability of selected synaptic proteins via a torsinA-dependent process. EMBO J. 2011;30:181–193. doi: 10.1038/emboj.2010.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torres GE, Sweeney AL, Beaulieu JM, Shashidharan P, Caron MG. Effect of torsinA on membrane proteins reveals a loss of function and a dominant-negative phenotype of the dystonia-associated DeltaE-torsinA mutant. Proc Natl Acad Sci U S A. 2004;101:15650–15655. doi: 10.1073/pnas.0308088101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hewett JW, Tannous B, Niland BP, Nery FC, Zeng J, Li Y, et al. Mutant torsinA interferes with protein processing through the secretory pathway in DYT1 dystonia cells. Proc Natl Acad Sci U S A. 2007;104:7271–7276. doi: 10.1073/pnas.0701185104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breakefield XO, Blood AJ, Li Y, Hallett M, Hanson PI, Standaert DG. The pathophysiological basis of dystonias. Nat Rev Neurosci. 2008;9:222–234. doi: 10.1038/nrn2337. [DOI] [PubMed] [Google Scholar]
- 18.Oleas J, Yokoi F, Deandrade MP, Pisani A, Li Y. Engineering animal models of dystonia. Mov Disord. 2013;28:990–1000. doi: 10.1002/mds.25583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perlmutter J, Stambuk MK, Markham J, et al. Decreased (18F) spiperone binding in putamen in idiopathic focal dystonia. Journal of Neuroscience. 1997;17:843–850. doi: 10.1523/JNEUROSCI.17-02-00843.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asanuma K, Ma Y, Okulski J, Dhawan V, Chaly T, Carbon M, et al. Decreased striatal D2 receptor binding in non-manifesting carriers of the DYT1 dystonia mutation. Neurology. 2005;64:347–349. doi: 10.1212/01.WNL.0000149764.34953.BF. [DOI] [PubMed] [Google Scholar]
- 21.Dang MT, Yokoi F, Cheetham CC, Lu J, Vo V, Lovinger DM, et al. An anticholinergic reverses motor control and corticostriatal LTD deficits in Dyt1 DeltaGAG knock-in mice. Behav Brain Res. 2012;226:465–472. doi: 10.1016/j.bbr.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dang MT, Yokoi F, McNaught KS, Jengelley TA, Jackson T, Li J, et al. Generation and characterization of Dyt1 DeltaGAG knock-in mouse as a model for early-onset dystonia. Exp Neurol. 2005;196:452–463. doi: 10.1016/j.expneurol.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 23.Napolitano F, Pasqualetti M, Usiello A, Santini E, Pacini G, Sciamanna G, et al. Dopamine D2 receptor dysfunction is rescued by adenosine A2A receptor antagonism in a model of DYT1 dystonia. Neurobiol Dis. 2010;38:434–445. doi: 10.1016/j.nbd.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yokoi F, Dang MT, Li J, Standaert DG, Li Y. Motor deficits and decreased striatal dopamine receptor 2 binding activity in the striatum-specific Dyt1 conditional knockout mice. PLoS One. 2011;6:e24539. doi: 10.1371/journal.pone.0024539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toda H, Hamani C, Lozano A. Deep brain stimulation in the treatment of dyskinesia and dystonia. Neurosurg Focus. 2004;17:E2. doi: 10.3171/foc.2004.17.1.2. [DOI] [PubMed] [Google Scholar]
- 26.Coubes P, Roubertie A, Vayssiere N, Hemm S, Echenne B. Treatment of DYT1-generalised dystonia by stimulation of the internal globus pallidus. Lancet. 2000;355:2220–2221. doi: 10.1016/S0140-6736(00)02410-7. [DOI] [PubMed] [Google Scholar]
- 27.Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Lagrange C, Yelnik J, et al. Bilateral, pallidal, deep-brain stimulation in primary generalised dystonia: a prospective 3 year follow-up study. Lancet Neurol. 2007;6:223–229. doi: 10.1016/S1474-4422(07)70035-2. [DOI] [PubMed] [Google Scholar]
- 28.Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, et al. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–452. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Perlmutter JS, Mink JW. Dysfunction of dopaminergic pathways in dystonia. Adv Neurol. 2004;94:163–170. [PubMed] [Google Scholar]
- 31.Augood SJ, Hollingsworth Z, Albers DS, Yang L, Leung JC, Muller B, et al. Dopamine transmission in DYT1 dystonia: a biochemical and autoradiographical study. Neurology. 2002;59:445–448. doi: 10.1212/wnl.59.3.445. [DOI] [PubMed] [Google Scholar]
- 32.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Xu R, Sasaoka T, Tonegawa S, Kung MP, Sankoorikal EB. Dopamine D2 long receptor-deficient mice display alterations in striatum-dependent functions. J Neurosci. 2000;20:8305–8314. doi: 10.1523/JNEUROSCI.20-22-08305.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franklin K, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- 35.Benn CL, Farrell LA, Cha JH. Neurotransmitter receptor analysis in transgenic mouse models. Methods Mol Biol. 2004;277:231–260. doi: 10.1385/1-59259-804-8:231. [DOI] [PubMed] [Google Scholar]
- 36.Yokoi F, Yang G, Li J, Deandrade MP, Zhou T, Li Y. Earlier onset of motor deficits in mice with double mutations in Dyt1 and Sgce. J Biochem. 2010;148:459–466. doi: 10.1093/jb/mvq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stephens AS, Stephens SR, Morrison NA. Internal control genes for quantitative RT-PCR expression analysis in mouse osteoblasts, osteoclasts and macrophages. BMC Res Notes. 2011;4:410. doi: 10.1186/1756-0500-4-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 39.Yokoi F, Dang MT, Miller CA, Marshall AG, Campbell SL, Sweatt JD, et al. Increased c-fos expression in the central nucleus of the amygdala and enhancement of cued fear memory in Dyt1 DeltaGAG knock-in mice. Neurosci Res. 2009;65:228–235. doi: 10.1016/j.neures.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sunahara RK, Guan HC, O'Dowd BF, Seeman P, Laurier LG, Ng G, et al. Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature. 1991;350:614–619. doi: 10.1038/350614a0. [DOI] [PubMed] [Google Scholar]
- 41.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. Research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007;8:R19. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 44.Ghilardi MF, Carbon M, Silvestri G, Dhawan V, Tagliati M, Bressman S, et al. Impaired sequence learning in carriers of the DYT1 dystonia mutation. Ann Neurol. 2003;54:102–109. doi: 10.1002/ana.10610. [DOI] [PubMed] [Google Scholar]
- 45.Carbon M, Argyelan M, Ghilardi MF, Mattis P, Dhawan V, Bressman S, et al. Impaired sequence learning in dystonia mutation carriers: a genotypic effect. Brain. 2011;134:1416–1427. doi: 10.1093/brain/awr060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fuchs T, Saunders-Pullman R, Masuho I, Luciano MS, Raymond D, Factor S, et al. Mutations in GNAL cause primary torsion dystonia. Nat Genet. 2013;45:88–92. doi: 10.1038/ng.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gantois I, Fang K, Jiang L, Babovic D, Lawrence AJ, Ferreri V, et al. Ablation of D1 dopamine receptor-expressing cells generates mice with seizures, dystonia, hyperactivity, and impaired oral behavior. Proc Natl Acad Sci U S A. 2007;104:4182–4187. doi: 10.1073/pnas.0611625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nobrega JN, Richter A, Tozman N, Jiwa D, Loscher W. Quantitative autoradiography reveals regionally selective changes in dopamine D1 and D2 receptor binding in the genetically dystonic hamster. Neuroscience. 1996;71:927–937. doi: 10.1016/0306-4522(95)00511-0. [DOI] [PubMed] [Google Scholar]
- 49.Drago J, Gerfen CR, Lachowicz JE, Steiner H, Hollon TR, Love PE, et al. Altered striatal function in a mutant mouse lacking D1A dopamine receptors. Proc Natl Acad Sci U S A. 1994;91:12564–12568. doi: 10.1073/pnas.91.26.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kobayashi M, Iaccarino C, Saiardi A, Heidt V, Bozzi Y, Picetti R, et al. Simultaneous absence of dopamine D1 and D2 receptor-mediated signaling is lethal in mice. Proc Natl Acad Sci U S A. 2004;101:11465–11470. doi: 10.1073/pnas.0402028101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goodchild RE, Kim CE, Dauer WT. Loss of the dystonia-associated protein torsinA selectively disrupts the neuronal nuclear envelope. Neuron. 2005;48:923–932. doi: 10.1016/j.neuron.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 52.Yokoi F, Dang MT, Li Y. Improved motor performance in Dyt1 DeltaGAG heterozygous knock-in mice by cerebellar Purkinje-cell specific Dyt1 conditional knocking-out. Behav Brain Res. 2012;230:389–398. doi: 10.1016/j.bbr.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yokoi F, Cheetham CC, Campbell SL, Sweatt JD, Li Y. Pre-synaptic release deficits in a DYT1 dystonia mouse model. PLoS One. 2013;8:e72491. doi: 10.1371/journal.pone.0072491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seidler RD. Multiple motor learning experiences enhance motor adaptability. J Cogn Neurosci. 2004;16:65–73. doi: 10.1162/089892904322755566. [DOI] [PubMed] [Google Scholar]
- 55.Krakauer JW, Ghez C, Ghilardi MF. Adaptation to visuomotor transformations: consolidation, interference, and forgetting. J Neurosci. 2005;25:473–478. doi: 10.1523/JNEUROSCI.4218-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zanone PG, Kelso JA. Coordination dynamics of learning and transfer: collective and component levels. J Exp Psychol Hum Percept Perform. 1997;23:1454–1480. doi: 10.1037//0096-1523.23.5.1454. [DOI] [PubMed] [Google Scholar]
- 57.Bock O, Schneider S, Bloomberg J. Conditions for interference versus facilitation during sequential sensorimotor adaptation. Exp Brain Res. 2001;138:359–365. doi: 10.1007/s002210100704. [DOI] [PubMed] [Google Scholar]
- 58.Seidler RD. Aging affects motor learning but not savings at transfer of learning. Learn Mem. 2007;14:17–21. doi: 10.1101/lm.394707. [DOI] [PubMed] [Google Scholar]
- 59.Parlow SE, Kinsbourne M. Asymmetrical transfer of training between hands: implications for interhemispheric communication in normal brain. Brain Cogn. 1989;11:98–113. doi: 10.1016/0278-2626(89)90008-0. [DOI] [PubMed] [Google Scholar]
- 60.Japikse KC, Negash S, Howard JH, Jr, Howard DV. Intermanual transfer of procedural learning after extended practice of probabilistic sequences. Exp Brain Res. 2003;148:38–49. doi: 10.1007/s00221-002-1264-9. [DOI] [PubMed] [Google Scholar]
- 61.Willuhn I, Steiner H. Motor-skill learning in a novel running-wheel task is dependent on D1 dopamine receptors in the striatum. Neuroscience. 2008;153:249–258. doi: 10.1016/j.neuroscience.2008.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heusner CL, Beutler LR, Houser CR, Palmiter RD. Deletion of GAD67 in dopamine receptor-1 expressing cells causes specific motor deficits. Genesis. 2008;46:357–367. doi: 10.1002/dvg.20405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qian Y, Chen M, Forssberg H, Diaz Heijtz R. Genetic variation in dopamine-related gene expression influences motor skill learning in mice. Genes Brain Behav. 2013;12:604–614. doi: 10.1111/gbb.12062. [DOI] [PubMed] [Google Scholar]
- 64.Molina-Luna K, Pekanovic A, Rohrich S, Hertler B, Schubring-Giese M, Rioult-Pedotti MS, et al. Dopamine in motor cortex is necessary for skill learning and synaptic plasticity. PLoS One. 2009;4:e7082. doi: 10.1371/journal.pone.0007082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yin HH, Mulcare SP, Hilario MR, Clouse E, Holloway T, Davis MI, et al. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci. 2009;12:333–341. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Durieux PF, Schiffmann SN, de Kerchove d'Exaerde A. Differential regulation of motor control and response to dopaminergic drugs by D1R and D2R neurons in distinct dorsal striatum subregions. Embo j. 2012;31:640–653. doi: 10.1038/emboj.2011.400. [DOI] [PMC free article] [PubMed] [Google Scholar]