Abstract
Injectable bone implants have been widely used in bone tissue repairs including the treatment of comminuted bone fractures (CBF). However, most injectable bone implants are not suitable for the treatment of CBF due to their weak tissue adhesion strengths and minimal osteoinduction. Citrate has been recently reported to promote bone formation through enhanced bioceramic integration and osteoinductivity. Herein, a novel injectable citrate-based mussel-inspired bioadhesive hydroxyapatite (iCMBA/HA) bone substitute was developed for CBF treatment. iCMBA/HA can be set within 2–4 minutes and the as-prepared (wet) iCMBA/HA possess low swelling ratios, compressive mechanical strengths of up to 3.2±0.27 MPa, complete degradation in 30 days, suitable biocompatibility, and osteoinductivity. This is also the first time to demonstrate that citrate supplementation in osteogenic medium and citrate released from iCMBA/HA degradation can promote the mineralization of osteoblastic committed human mesenchymal stem cells (hMSCs). In vivo evaluation of iCMBA/HA in a rabbit comminuted radial fracture model showed significantly increased bone formation with markedly enhanced three-point bending strength compared to the negative control. Neovascularization and bone ingrowth as well as highly organized bone formation were also observed showing the potential of iCMBA/HA in treating CBF.
1. Introduction
Comminuted bone fractures (CBF) are recognized as one of the most difficult orthopedic conditions to treat 1, 2 due to the difficulty in bone reduction and fixation, which leads to bone segment displacement, deformed bone union, and non-union. 2 To improve reduction and fixation, open surgery with internal instrumentation fixation of large bone pieces has been implemented to provide sufficient stability in relatively simple bone fracture cases. However, for some complicated CBF with numerous bone pieces, open surgery is unable to offer sufficient fixation for those small bone pieces and consequently fails to reunite them for proper bone alignment and regular bone healing. Therefore, the development of an ideal method to mend together the broken bone pieces, maintain aligned bone reduction, and effectively fill the space between the scattered bone pieces against other connective tissue has become a significant clinical challenge.
To address the aforementioned issues, a variety of bone substitutes have been developed for bone regeneration. 3–20 Among them, injectable bioactive bone substitutes are preferred 6–9 and have been extensively studied. 10–20 However, the application of current injectable bioactive bone substitutes are limited by many shortcomings. For example, non-degradable or slow-degrading bone substitutes, such as commercially available poly (methyl methacrylate) (PMMA) and calcium phosphate cements (CPCs), compromise normal bone healing by inhibiting new bone and vascular ingrowth. 10–12 On the other hand, biodegradable hydrogel-based injectable bone composites, such as chitosan and gelatin-based hydrogels systems, 6–9, 19–22 show insufficient adhesive fixation strengths. Commercially available fibrin adhesives have been regarded as the gold standard for soft tissue adhesion, but their adhesion strengths are weak and diminish in wet conditions such as bleeding bone defects. We have recently shown that dopamine, a derivative of L-3, 4-dihydroxyphenylalanine (L-DOPA) that contributes to the strong under-water adhesive properties of marine mussels, 23 can be utilized to synthesize biomimetic injectable citrate-based mussel-inspired tissue bioadhesives (iCMBA), which possess much stronger adhesive strength to soft tissue when compared to commercially available fibrin sealant. 22 iCMBA composited with hydroxyapatite (HA) composites are expected to exhibit better adhesive properties to bone pieces, which is favorable to the bone fixation and healing process in CBF.
In addition to offering sufficient structural and mechanical support, an ideal bone substitute should possess osteogenic potential by incorporating osteoinductive elements. Citrate, an intermediate in the Kreb’s Cycle, is highly abundant in bone and closely associated with bone metabolism and formation. 24–26 Recent studies have shown that citrate plays an indispensible role in bone formation. Tran et al. were the first to experimentally find that citrate in degradable polymers greatly increased the alkaline phosphatase (ALP) and osterix (OSX) gene expression in C2C12 cells, a mouse myoblast cell line that is capable of differentiating into osteoblasts. 27 Hu et al. quantified the citrate content in native bone and discussed the indispensible role of citrate in the formation and regulation of the bone apatite nanocrystal structure. 28 Costello et al. discussed the speculated roles of citrate metabolism and production for the osteogenic differentiation of stem cells and bone formation. 29, 30 These latest findings are strong evidence that citrate should be involved in bone substitute design and has led to the development of a series of citrate-based composites for bone applications including poly (diol citrates)-HA (POC-HA), poly (ethylene glycol) maleate citrate-HA (PEGMC-HA), and citrate-based polymer blends-HA (CBPB-HA). 31–36
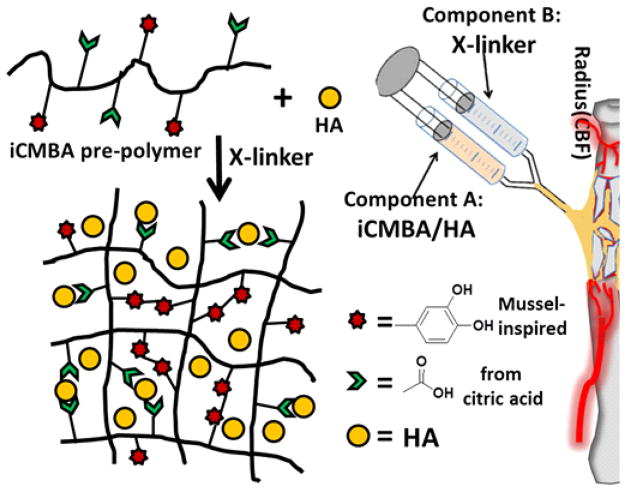
In the current study, iCMBA was composited with HA and used as an injectable bone substitute (Fig. 1). It is believed to have superior properties for CBF treatment due to the following properties afforded by a citrate and dopamine-containing composite: (1) excellent adhesion strength in wet conditions; (2) controlled degradability; (3) enhanced osteoinductive potential by the released citrate; 27 (4) calcium chelating ability of citrate; (5) minimal inflammatory response; 22 (6) instant hemostatic properties at surrounding tissue; 22 and (7) osteoconductive and osteointegration potential. The physical and mechanical properties, biocompatibility, and osteogenic potential of iCMBA/HA composites were studied and evaluated for CBF treatment in a rabbit model. Furthermore, citrate was exclusively investigated in vitro to explore its effect on the mineralization of osteoblastic committed human mesenchymal stem cells (hMSC).
Fig. 1.
Schematic representation for iCMBA/HA crosslinking process and iCMBA/HA injection procedure for treatment of comminuted bone fracture.
2. Experimental Section
2.1 Materials
All chemicals, cell culture medium, and supplements were purchased from Sigma-Aldrich (St. Louis, MO), and all chemicals were used as received, except where mentioned otherwise.
2.2 iCMBA/HA composites synthesis
Injectable iCMBA/HA composites were fabricated with iCMBA and HA powder (Fig. 1). Firstly, iCMBA pre-polymer was synthesized by polycondensation of citric acid (CA), poly(ethylene glycol) (PEG) and dopamine according to our previous work. 22 Briefly, CA and PEG were put in three-necked round-bottom flask and heated to 160 °C under stirring until melted. Then, under nitrogen gas flow, a calculated amount of dopamine was added to the mixture. The temperature was reduced to 140 °C and the reaction was continued under vacuum until the stir bar stopped turning at 60 rpm. Secondly, iCMBA-P200D0.3 (using PEG 200 and 0.3 eq. [to CA] of dopamine) was chosen to make iCMBA/HA composites. To do so, iCMBA-P200D0.3 was dissolved in deionized (DI) water (40 wt%) and then different amounts of HA were mixed with it to achieve various final composite formulations with 30, 50, and 70 wt% HA. Finally, calculated amounts of crosslinker solution (for 1 g pre-polymer, use 1 mL of PI [Sodium (meta)periodate, NaIO4] solution, PI solution was made in 4 or 8 wt% concentrations (in DI water) were added to the mixture to crosslink the composites.
2.3 Setting time
To control the crosslinking process, various calculated amounts of crosslinker solution (PI, in DI water) were added to the mixture. The amount of PI was determined to provide ample time for this composite preparation and injection without compromising its final mechanical properties. The setting time of the composites was determined as the time from addition of PI to the iCMBA-HA mixture until it was not flowable.
2.4 Physical and mechanical properties of iCMBA/HA composites
The sol-gel content, an indication of non-crosslinked/crosslinked fractions of the composites, and swelling ratio was measured by the mass differential before and after incubation of the polymer network in a solvent as described in previous study. 22 The sol content (Table 1) and swelling ratio were then calculated using equations (1) and (2), respectively. Here Wi represents the initial dry weight of cross-linked hydrogel disk, Wd represents the weight of freeze-dried sample after the uncross-linked part being washed by 1,4-dioxane for 48 hrs, while Ws represents the network weight after leached and dried sample being suspended in water for 24 hrs. Degradation studies were conducted in PBS (pH 7.4) and at 37 °C using cylindrical disc specimens (7 mm in diameter, 2 mm thick) as described in previous study. 22 The mass loss was calculated by comparing the initial mass (W0) with the mass measured at the pre-determined time points (Wt ) using equation (3).
Table 1.
Set time of iCMBA/HA composites with various formulation
| Composite formulation | HA/composite ratio (dry w/w%) | PI to pre/polymer ratio (w/w%) | Measured set time (s) |
|---|---|---|---|
| iCMBA-P200 D0.3 HA30% | 30 | 4% | 247±13 |
| iCMBA-P200 D0.3 HA30% | 30 | 8% | 172±15 |
| iCMBA-P200 D0.3 HA50% | 50 | 4% | 238±9 |
| iCMBA-P200 D0.3 HA50% | 50 | 8% | 166±11 |
| iCMBA-P200 D0.3 HA70% | 70 | 4% | 231±10 |
| iCMBA-P200 D0.3 HA70% | 70 | 8% | 159±8 |
| Equation (1) |
| Equation (2) |
| Equation (3) |
The mechanical properties of iCMBA/HA composites were investigated by unconfined compressive testing. The measurements were conducted according to ASTM D695-10 on a MTS Insight 2 fitted with a 500 and 10KN load cell (Instron, Norwood, MA). Briefly, the cylindrical shaped samples (6 mm × 12 mm, diameter × height) were compressed at a rate of 1.3 mm/minute, and deformed to failure. Values were converted to stress-strain and the initial modulus was calculated from the initial slope of the curve (0–10% elongation except the modulus of the freeze-dried and air-dried samples of iCMBA-P200D0.3 PI8% HA70% [0–1%]). The mechanical tests were conducted on as-prepared samples (samples were sealed in vials for 2 hrs, 24 hrs and 48 hrs after preparation) as well as on samples completely dried naturally (air-dried for 3 days) or by lyophilization (freeze-dried). The structural difference between freeze-dried and air-dried samples was observed under scanning electron microscopy (SEM).
To test the adhesion strength of iCMBA/HA composites to the bone surface of chicken bone, a bone cut model was created by oblique cutting chicken bone into two parts and reunited with iCMBA (iC) and iCMBA/HA 70% (iCH70), using 8 wt% of PI solution as cross-linker. After 2 hours (wet condition, sealed with parafilm to keep wet) and 48 hours (dry condition), the load and shear strength of repaired chicken bones were tested on MTS system.
2.5 Mineralization of iCMBA/HA composites
To evaluate in vitro mineralization on iCMBA/HA composites, disk shaped scaffolds of the composites (iCMBA-P200D0.3 PI8% HA70%, iCH70) were immersed in simulated body fluid (SBF), which was prepared as described in the literature. 37 To accelerate the mineralization process, concentrated SBF was used, in which the concentration of inorganic ions was five times of those in human blood plasma (SBF-5X). The composite samples were immersed in 10 mL of SBF-5X and incubated at 37 °C for up to 5 days while the SBF was replaced every other day. At each predetermined time point the specimens (n = 5) were taken out, washed gently with DI water to remove any soluble inorganic ions from the surface of samples, and air-dried. Next, the specimens were sputter-coated with silver and examined by scanning electron microscope (SEM) using Hitachi 3000N (Hitachi, Pleasanton, CA). The elemental analysis of the mineralized composites was also conducted by Energy dispersive X-ray spectroscopy (EDX) to determine the composition and ratio of the elements present in the minerals formed on the composites surface. In addition to composites, iCMBA-P200D0.3 PI 8% without HA (iC) was also subjected to mineralization test to determine the possible role of HA in the mineralization of iCMBA/HA composites.
2.6 In vitro cell study
2.6.1 Cytotoxicity of sol content and degradation products
The cytotoxicity of sol content or leachable fraction and degradation products of iCMBA pre-polymer and iCMBA/HA composites were studied using MTT (methylthiazolyldiphenyl-tetrazolium bromide) assay against human mesenchymal stem cells (hMSCs, Lonza Walkersville Inc, US).
The sol content or leachable fraction of iCMBA and iCMBA/HA composites was obtained by incubating equal mass (0.5 g) composites specimen in 5 mL PBS (pH 7.4) for 24 hours. Next, three different solutions were prepared: 1X, 10X and 100X (1X was the solution of leached products with no dilution; 10X and 10X means 10 times and 100 times dilution of 1X by PBS, respectively). To each well of a 96-well cell culture plate, a 200 μL of solution of hMSC cells with a density of 5×104 cells/mL in complete Dulbecco’s modified eagle’s medium (DMEM, with 10% fetal bovine serum (FBS) and 1% anti-anti solution) was added and incubated for 24 hours at 37°C, 5% CO2. Next, 20 μL of sol content fraction with various concentrations of iCMBA and iCMBA/HA composites were added and incubated for another 24 hrs followed by MTT assay analysis as per the manufacturer’s protocol.
The cytotoxicity of degradation products was also evaluated. Equal weight (1g) of iCMBA and iCMBA/HA composite samples as well as poly (lactic-co-glycolic acid) (PLGA, used as control, LA/GA=50/50, Mw~60KDa, purchased from Polyscitech) were fully degraded in 10 mL of 0.2M NaOH solution, and the resultant solutions were diluted to three concentrations (1X, 10X and 100X) using PBS (pH 7.4), and used for cell culture (same cell density as sol content study, also 20 μL of degradation solution per well as sol content study described above) and subsequent MTT analysis.
All the above solutions were pH-neutralized and passed through a 0.2 μm filter prior to use for cell culture. The cell viability results were normalized to the viability of cells in complete DMEM medium.
2.6.2 Effect of iCMBA/HA degradation products on hMSCs’ osteogenic differentiation process
The effect of degradation products of iCMBA and iCMBA/HA composites on the proliferation (cell viability) and differentiation (ALP activity and calcium deposit formation) of hMSC during osteogenic differentiation process were studied with Live/Dead staining, ALP activity test, and Alizarin Red staining, respectively.
Briefly, iCMBA (iC) and iCMBA/HA70% (iCH70) composites were directly degraded in osteogenic (OG) media at 37°C with 5% CO2 to separately produce OiC and OiCH70 media. To eliminate the effect of HA particles in media on cell differentiation, OiCH media was created by filtering OiCH70 media with 0.22 μm filters. Osteogenic media were composed of completed DMEM supplemented with 10−7 M dexamethasone, 10−2 M β-glycerophosphate, 50 μM L-Ascorbic acid. Then hMSCs were cultured in those media mentioned above for 14 days using growth media (MG) as a negative control. Culture media were replaced every other day. Live/Dead staining (Life Technologies Inc., US) and scanning electron microscopy (SEM) scanning was conducted to assess the cell viability and morphology of differentiated hMSCs. Meanwhile, to test the effect of iCMBA/HA degradation products on osteoinduction and osteogenesis (ALP activity and calcium deposit formation), ALP activity test and Alizarin Red staining were conducted following standard protocols at three pre-determined time points (4, 7 and 14 days).
The adhesion, proliferation, and differentiation of a pre-osteoblast cell line, MC3T3 (ATCC) cultured on the composites were also studied. The details of experimental methods and results can be found in supporting document.
2.6.3 Citrate release from iCMBA/HA composite
iCH70 was chosen as the representative for citrate release studies. Briefly, 0.1 g dried iCH70 composite was immersed into 10 mL PBS (pH 7.4) at 37°C, and at each pre-determined time points (2, 5, 7, 14, 21, 24, 28, and 30 days), 0.2 mL of PBS solution was removed and filtered (using 0.2 μm filters) to prepare samples for high-performance liquid chromatography (HPLC). 0.2 mL fresh PBS was replaced in the tube to maintain volume consistent at 10 mL. Determination of cumulative citrate release was carried out using a Shimadzu HPLC system equipped with a UV-visible PDA detector and a Phenomenex Kinetex C18 column at 40 °C. PBS with a pH value of 2.8 was used as the mobile phase with a flow rate of 1 mL/min. Detection of citrate was set at 210 nm. A calibration curve of citrate was collected at the same conditions.
2.6.4 Effect of citrate on calcium deposit formation
To test the effect of citrate on calcium deposit formation of osteogenic-differentiated and undifferentiated hMSC, Alizarin Red staining was conducted following well-established protocol. For proliferation, hMSCs were cultured in growth media (MG, completed DMEM). For osteogenic differentiation, hMSCs were cultured in osteogenic media (OG). A calculated volume of citrate was added into MG and OG separately to achieve a final citrate concentration of 20 μM in both media. hMSCs were cultured in those media separately and incubated at 37°C with 5% CO2. Media were replaced every other day. At 7, 14, and 21 days, the cells were stained with 2% Alizarin Red Staining solution (adjusted pH 4.0) to determine calcium deposit formation.
2.7 In vivo study
Due to its strong mechanical properties and biocompatibility in vitro, iCMBA-P200D0.3 PI 8% HA 70% (iCH70) composite was chosen for in vivo study.
2.7.1 Comminuted radial fracture model and iCH70 injection
All animal experiments were carried out in compliance with a protocol approved by Southern Medical University’s Animal Care and Use Committee (Guangzhou, China). Thirty-six New Zealand Rabbits (male, 3 kilogram on average) were randomly assigned into two groups: Blank control group and iCH70 group. All rabbits were anesthetized with 3% sodium pentobarbital (1.5 mL/kg) following previous protocol. 27 After shaving and disinfecting surgical area, 20 mm skin incisions were made at the sites 15mm below radius head of both fore limbs. Through the intermuscular space, the radius was exposed clearly with minimal tissue injury. To make a standard and reproducible comminuted radial facture, osteotomy was performed at two sites with a surgical electric saw to produce a 10 mm-length bone block. The bone blocks were cut into several segments (usually 3–4 fragments) with a bone rongeur. It’s worthy to note that the ulna was kept intact to provide sufficient biomechanical support for fractured radius (Fig. 1). Segmented bone pieces were re-aligned and kept in the space after iCH70 injection. The deep fascia and skin were sutured tightly and then analgesic and anti-bacterial ointments were given. For control, the same procedure was repeated and bone pieces were put back without other filling material inside. After rabbits were sacrificed with CO2 at pre-determined time points (4, 8, 12 weeks post-operation), their radial bone specimens were removed and prepared for the following assessments.
2.7.2 Computer tomography analysis for explants
Computer tomography (CT) analysis was conducted using a Micro-CT imaging system (ZKKS-MCT-Sharp-III scanner, Caskaisheng, CHINA) following standard and validated-precise protocols. 27 Briefly, the scanning system was set to 70 kV, 30 W, and 429 μA. A quantitative 3D histomorphometric evaluation (i.e., determination of bone mineral density and bone mineral content) was then performed on a rectangles volume of interest (VOI) using well-recognized methods. 38 Bone mineral content (BMC) and bone mineral density (BMD) were measured and the data were processed and analyzed using NIH-Image software (National Institute of Health, Bethesda, MD, USA).
2.7.3 Biomechanical test for explants
At pre-determined time points (4, 8, 12 weeks post-operation), rabbits were sacrificed and radius specimens were removed for three-point bending testing at the radial diaphysis following the previously described protocol. 39–42 Briefly, the two ends of radius were horizontally fixed on the Material Testing System platform (with a span of 2.5 cm between the supports) and a constant vertical compression load (5 mm/min) was applied to the midpoint of the fractured bone until a fracture occurred. The load and displacement data were recorded at 100 Hz. The maximum flexural strengths were calculated and compared between iCH70 group and blank control group.
2.7.4 Histological examination
For undecalcified sections, histological examination was performed at pre-determined time points (4, 8, 12 week post-operation) according to previous protocols. 41 After fixation and dehydration by series ethanol, the radius specimens were embedded in methyl methacrylate without decalcification. Next, 10 μm longitudinal sections were cut at the diaphysis of interest using a SP2500 microtome (Leica Microsystems, Wetzlar, Germany). Sections were then stained by the Masson Trichrome method. For decalcified sections, histological examination was performed at the 4-week time point for vascularization with hematoxylin and eosin (H&E) staining. Bone histomorphometric analysis was performed under a semiautomated digitizing image analyzer system for both types of sections. This system consisted of an Olympus BX51 microscope (Center Valley, PA, USA), a computer-coupled QImaging Retiga EXi camera (Surrey, Canada), and BioQuant Osteo 2009 software (Nashville, TN, USA).
2.8 Statistical analysis
All experiments were done in duplicates. The statistical results were based on the three experiments. All data are expressed as the mean ± standard deviation. The statistical significance between two sets of data was calculated using a Student’s t-test. Data were taken to be significant if a p<0.05 was obtained.
3. Results and Discussion
With the rapid advancement of orthopedic internal/external fixation instruments, the fixation of large bone segments in relatively simple fractures has shown great success. However, CBF remains an unresolved issue in achieving effective fixation of small and scattered bone pieces and maintaining bone alignment after complete bone reduction. (1, 13, 14) Adhesive biomaterials are considered a potential solution by offering the ability to stabilize small fractured bone pieces. Inspired by the adhesive strength of marine mussels and based on recent understanding of citrate’s role in bone formation, our group has developed a citrate-based iCMBA polymer, which shows strong adhesive properties in wet conditions. 22 For orthopedic applications, an adhesive iCMBA/HA bone composite was synthesized by combining iCMBA with osteoconductive HA particles and its performance with regards to intra-operational manipulation, mechanical strength, and bone formation was also investigated.
3.1 Rationale behind iCMBA/HA bone composites
Citrate has been well known as an essential intermediate of the Kreb’s Cycle in cellular metabolism. However, citrate hasn’t been given enough attention to its role in bone formation and mineralization although a few reports did find its close association with bone based on the evidence that citrate makes up about 5 wt% of the organic component in bone and over 90% of the body’s total citrate content is located in the skeletal system. 24–26 Until recently, a series of progress and increased attention to this “old” but curious natural chemical has been reported. 27, 28 Hu et al. confirmed that citrate has an indispensible effect in bone apatite nanocrystal structure as well as bone strength. 28 Tran et al. identified citrate to enhance bone-related gene expression such as alkaline phosphatase (ALP) and osterix (OSX) by C2C12 cells. 27 It is envisioned that citrate enhances osteoinduction and bone formation and should be included in bone substitute design.
Inspired by the adhesive strength of marine mussels, adhesive iCMBA were developed in our group by one-pot polymerization of citrate, poly(ethylene glycol) (PEG), and dopamine. After oxidation, dopamine showed stronger adhesion to biological surfaces such as skin or sheathes that connect with bone through the formation of covalent bonds with available nucleophile groups on these surfaces such as -NH2, -SH, -OH and -COOH groups. Dopamine can also directly interact with inorganic bone surface to provide adhesion strength. Although the iCMBA pre-polymer was initially applied for wound closure, its strong adhesion strength in wet tissue and fast degradation rate motivate us to apply it to bone substitute fabrication. In wound closure studies, iCMBA offer much stronger adhesion strength, especially in wet conditions when compared to fibrin sealants, which is regarded as gold standard for wound closure. Even more importantly, the fast degradability of ester bonds in the iCMBA backbone can potentially allow osteoblast migration and neovascularization in the bone scaffold in the early stages of bone healing. Based on the promising potential mentioned above, we have developed the iCMBA/HA bone substitute and applied it for CBF treatment in rabbit model.
3.2 Physical, mechanical property, mineralization and cytotoxicity of iCMBA/HA
3.2.1 Setting time
In order to be suitable and practical for CBF applications, ease of handling and administration are important design requirements. In order to obtain a material which can be easily handled, an ideal setting (crosslinking) time is important because the expected bone substitutes should provide sufficient handling time during the transformation from a flowable liquid to a set material. It was reported that for self-setting bone cements, at least 1 minute should be allowed for clinicians to collect the paste and put it on a pallet knife or into syringes. 12 Thus, the setting time should be long enough (at least 2–3 min) to allow surgeons to collect and deliver the materials to bone defects before setting. iCMBA/HA composites was tested to be injectable using a cannula injection tool, which was used for femoral head injection in our previous study. 34 The setting time of our iCMBA/HA bone substitute is completely adjustable and can be easily tuned by regulating multiple factors (Table 1). iCMBA/HA composites were all prepared using iCMBA-P200D0.3 and varying amounts of HA and sodium periodate (PI). The setting time was varied between 159±8 seconds for iCMBA-P200D0.3 PI8% HA70% (iCH70) and 247±13 seconds for iCMBA-P200D0.3 PI 4% HA 30% with PI-to-pre-polymer ratios of 8% and 4%, respectively. Increasing the amount of HA in the composition slightly decreased the setting time, while an increase in PI concentrations accelerated the crosslinking reaction to shorten the setting time.
3.2.2 Sol/gel content, swelling ratios, and degradation properties
An ideal synthetic bone substitute should also possess acceptable sol/gel content, swelling ratio, and adequate degradation rates for new bone ingrowth and vascularization. 17 The sol contents of different iCMBA/HA composites were all at low percentages, ranging from 2.46±0.82% to 3.29±0.91% with no significant difference (p>0.05) between the samples with and without HA (Fig. 2A). Composite swelling data revealed that the degree of swelling was lowest for iCH70, followed by iCMBA/HA 50% (iCH50), iCMBA/HA 30% (iCH30) and pure iCMBA prepolymer (iC, no HA) with swelling ratios of 110±6, 249±31, 353±35, and 802±69%, respectively (Fig. 2B). Results from composite degradation show that the degradation rate decreased as the amount of HA increased in the composites. As shown in Fig. 2C, iCH70 exhibited the slowest degradation rate with complete degradation after 30 days of incubation in PBS at 37°C followed by iCH50 and iCH30. iC with no HA degraded much faster than composites with HA (Fig. 2C). The sol content of iCMBA/HA composites reached the expected crosslinking level (up to 97.54%) while swelling data showed that iCMBA/HA composites, especially iCH70, could maintain its structure with minimal swelling ratios. This indicates that iCMBA/HA composites can meet the requirements for an injectable bone substitute. More importantly, degradation studies show that iCH70 possesses an ideal degradation rate with a weight loss more than 25% after the one week and almost 100% after one month (Fig. 2C). It is already well known that relatively faster degradation rates can induce vascular ingrowth, which is significantly vital to cell migration, survival, and tissue formation. 17, 43 Relatively fast degradation rates may produce adequate space for vascularization and cell migration within the initial weeks after implantation, which is vital for capillary networks induced by the inflammatory processes and neovascularization of the scaffold. 44–46 Therefore, it is natural to believe that our iCMBA/HA bone substitute may allow for and promote neovascularization and cell migration into the composite through the space left after degradation (Fig. 2C).
Fig. 2.
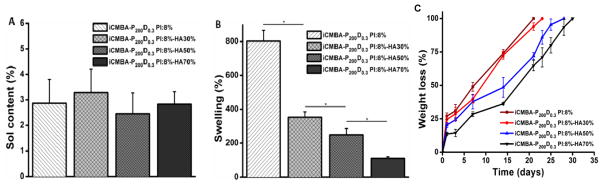
Soluble content (A), swelling ratio (B) and degradation profiles (C) of iCMBA/HA composites. *: p<0.05.
3.2.3 Mechanical Properties
The mechanical properties of iCMBA/HA composites for as-prepared (2, 24 and 48 hrs after preparation) and air- or freeze-dried samples are shown in Fig. 3. It can be seen that the compressive strength and modulus for iCMBA/HA was much higher than that of pure iCMBA (p<0.01), and increasing the amount of HA in the composition resulted in higher compressive strength and modulus. No differences in the compressive strength and modulus values of iCH30 and iCH50 as-prepared samples were observed (p>0.05) with compressive strengths around 1 MPa and modulus lower than 0.5 MPa. The compressive strength of both iCH30 and iCH50 showed nearly no distinct change when the curing time increased from 2 to 24 h (p>0.05), while the modulus showed a slight increase with the curing time (Fig. 3A and 3B). When the HA content in iCMBA/HA composites reached 70% of dry weight, the compressive strength of iCH70 reached 1.6±0.21 MPa 2 h after preparation, and the value increased to 2.6±0.29 and 3.2±0.28 MPa after 24 and 48 h, respectively, which was much higher than that of iCH30 and iCH50 (p<0.01) (Fig. 3A). The modulus of iCH70 also increased from 0.75±0.11 MPa for 2 h to 2±0.31 MPa for 24 h and the values were maintained 48 h after preparation. The compressive strain at failure (Fig. 3C) of iCMBA/HA composites with different HA ratios are all higher than that of pure iCMBA. The strain at failure of iCH30 and iCH50 was higher than 60% for 2 h samples, and decreased in response to an increase in time. The strain of iCH70 was higher than 50%, which indicates the soft nature of as-prepared iCMBA/HA composites. The stress-strain curves of the as-prepared composites 48 h after preparation are shown in Fig. 3D. After freeze-/air- drying, the compressive strength and modulus (Fig. 3E and 3F) for iCMBA/HA composites became much higher than that of the corresponding as-prepared samples. An increase in HA content resulted in higher mechanical strength. The compressive strength and modulus of freeze-dried/air-dried iCH70 reached 27.1±3.6 / 47.2±3.1 and 778.2±51.5 / 1525.6±172.9 MPa, respectively. Pores formed during the freeze-drying process, which was confirmed by SEM images of freeze-/air-dried iCH70 samples (Fig. 3G), contributed to the lower mechanical strength of freeze-dried composites compared to the corresponding air-dried composites.
Fig. 3.
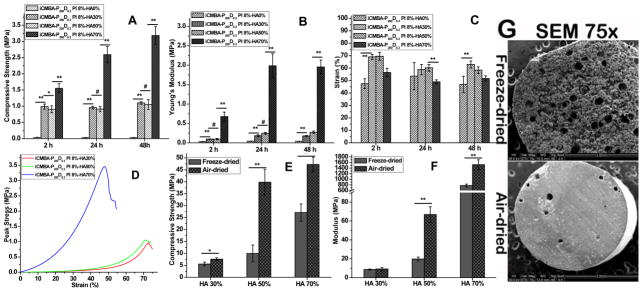
Mechanical properties and SEM images of iCMBA/HA composites. Compressive strength (A), modulus (B), and strain at failure (C) of as-prepared samples after 2, 24 and 48 hrs; Stress-strain curves (D) of iCMBA/HA composites measured through compressive mechanical testing of as-prepared samples 48 hrs after preparation. Compressive strength (E) and modulus (F) of freeze-dried and air-dried samples. # p>0.05, * p<0.05, ** P<0.01. (G) SEM images of freeze-dried and air-dried iCMBA-P200 D0.3-HA70% (iCH70) composites.
The adhesion property of bone cements to bone surfaces is also critical for bone regeneration, especially for CBF where fractured bone pieces should be glued by the adhesive materials. However, traditional bone cements such as PMMA have very weak bonding to bone because of the incompatible wetting properties between hydrophobic PMMA and hydrophilic bone. Thus, amphiphilic bone bonding glue was used to improve the bonding between PMMA cements and bone.47 Our iCMBA/HA composites by themselves offer substantial adhesive strength to bone thus eliminating the potential use of additional glue for bone bonding. To evaluate the adhesive strength of iC and iCH70 to the biological bone surface, both the tensile loads and shear strengths of iCH70 to the chicken bones were tested and compared (Fig. 4). From Fig. 4A, it can be seen that the tensile loads of repaired chicken bones with iCH70 were around 352 g in wet condition (2 hour after application) and 1280g in dry condition (48 hour after application), respectively. In wet condition, the shear strengths of chicken bones repaired by iCH70 and iC showed no significant difference (p>0.05), with shear strengths about 111±32.4 KPa and 110±17 KPa respectively (Fig. 4B). Although in dry condition (48 hours after application), the shear strengths of chicken bones repaired by iC (746±197 KPa) seems a little bit bigger than that repaired by iCH70 (680±68 KPa), there is also no significant difference between the two groups (p>0.05) (Fig. 4B). The mechanical studies show that iCH70 can offer sufficient cohesive and adhesive mechanical support for CBF at early bone healing stages, since internal or external fixation with instruments can provide major mechanical support for CBF.
Fig. 4.
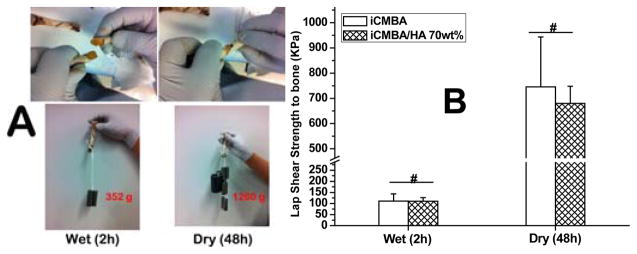
(A) Adhesion process of iCMBA/HA 70wt% (8 wt% PI) to beveled (beveling angle: 30°) chicken bone (top) and the strength show by hanging metal staff (low); (B) Wet (2h) and dry (48h) adhesion strengths of iCMBA (iCMBA-P200 D0.3, 40 wt% in DI water) and iCMBA/HA 70 wt% to chicken bones after applying equal volume of 8 wt% PI [Sodium (meta) periodate, NaIO4] solution.
3.2.4 Mineralization of iCMBA/HA composites
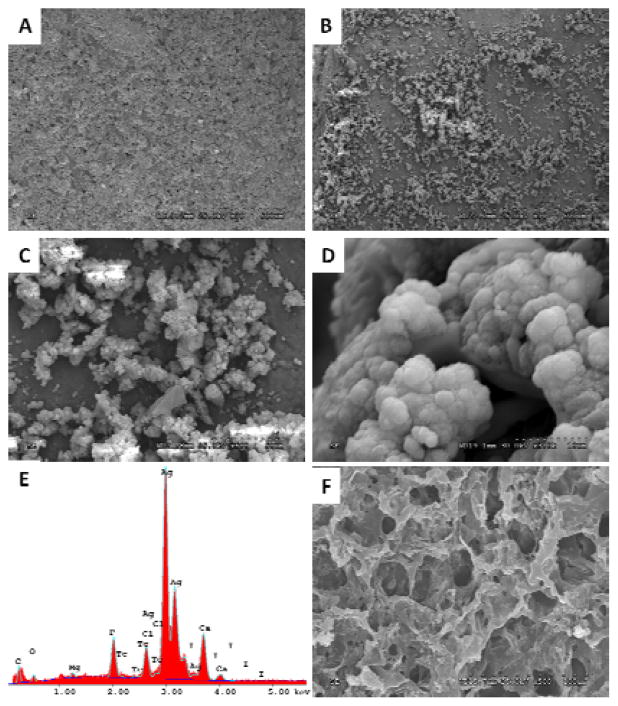
In order to investigate whether iCMBA/HA composites support biomineralization, the in vitro mineralization of iCMBA/HA composites in SBF-5X was conducted. After 1 day of incubation, no crystals on the surface of iCH70 samples were observed (Fig. 5A). As incubation time continued, crystals of calcium phosphate began to form and grow on the surface of iCMBA/HA composites (Fig. 5B–D). EDX analysis confirmed the existence of these crystals, in which the ratio of Ca/P was around 1.61 (Fig. 5E), which is in the range of the Ca/P ratio to HA (1.33–1.67). Interestingly, no crystal formation on the surfaces of pure iCMBA without HA was found during incubation in SBF-5X (Fig. 5F), which indicates that the inclusion of HA can promote the biomineralization process and in turn promote bone formation.
Fig. 5.
Mineralization of the iCMBA/HA composites. SEM images of iCMBA-P200D0.3 PI 8%/HA70% composites incubated in SBF-5X at 37°C for: (A) 1 day, and (B, C, and D) 5 days. E) EDX analysis of the surface of crystal-deposited composite. F) iCMBA-P200D0.3 PI 8% without HA, incubated in SBF-5X for 5 days at 37°C (no crystals are present).
3.2.5 Cytotoxicity of iCMBA/HA composites
The cytotoxicity of iCMBA/HA composites was estimated by conducting cytotoxicity studies of the soluble (leachable) content and degradation products of various iCMBA/HA composites using MTT (methylthiazolyldiphenyl-tetrazolium bromide) assay against human mesenchymal stem cells (hMSCs) (Fig. 6). The hMSC viability in the presence of soluble content of the composites (Fig. 6A) at 1X concentration was between of 72.51±2.90% (no HA) and 84.44±6.60% (50% HA), showing only minor cytotoxicity. The cell viability became much higher in diluted sol content solutions of 10X and 100X, and the cell viabilities of 100X were all higher than 90%. Furthermore, the degradation products (1X) showed a cell viability of at least 70.84±5.16% (no HA), which is comparable to that of widely used biodegradable polymer PLGA (67.35±5.99%) suggesting that the degradation products of all iCMBA composites did not induce significant cytotoxicity (Fig. 6B). The cell viability improved as the solution of degradation products was further diluted to 10X and 100X. In a separate study, we have also shown that iCH70 also supported the MC3T3 cell adhesion (Fig. S1) and proliferation (Fig. S2A). Based on the above results, iCH70 is considered as a promising iCMBA/HA formulation due to better physical (setting time, sol content, swelling and degradation rate) and mechanical properties with satisfying in vitro cytotoxicity.
Fig. 6.
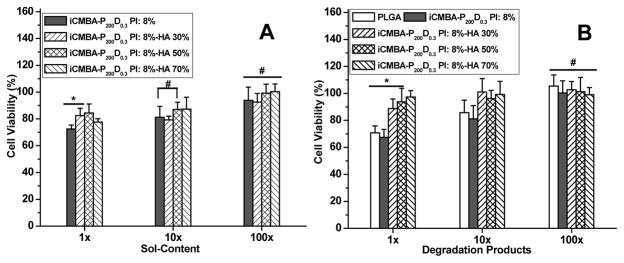
In vitro cytotoxicity of iCMBA/HA composites. Cytotoxicity study using hMSC cells by MTT assay for: A) sol content (leachable products), and B) Degradation products of iCMBA and iCMBA/HA composites. All data were normalized to cell viability in blank medium. # p>0.05, * p<0.05.
3.3 Proliferation and differentiation of hMSCs in the presence of iCMBA/HA, effect of citrate, and HA
3.3.1 Proliferation and differentiation of hMSCs in the presence of iCMBA/HA
To investigate and mimic the effect of HA and citrate (from iCMBA) on the proliferation and differentiation of hMSC during the release process of iCMBA/HA in practical situations when being applied in vivo, iCMBA/HA composites (using iCH70) and iCMBA (iC) were completely degraded in osteogenic media (OG) to produce OiC and OiCH70 media respectively. In order to further study the effects of HA, OiCH media was also created by filtering OiCH70 media with 0.22 μm filters. The proliferation and differentiation of hMSC in OiC, OiCH, and OiCH70 media was conducted and studied by Live/Dead assay, alkaline phosphatase (ALP) activity, and Alizarin Red staining using growth media (MG) and osteogenic media (OG) as controls (Fig. 7). From the Live/Dead images (Fig. 7A), it can be seen that hMSCs grew well for all the five groups. After 14 days, the morphology of hMSCs maintained an elongated shape, while the morphology of hMSCs changed after being cultured in OG, OiC, OiCH and OiCH70 media for 4 days. The cell densities of hMSCs cultured in OG, OiC, OiCH and OiCH70 media were all lower than that of hMSCs cultured in MG media. From one aspect, these results imply that hMSCs differentiated in OG and osteogenic media containing iCMBA and iCMBA/HA degradation products. The results of calcium deposit formation in cell culture are shown in Fig. 7B. In osteogenic media (OG), calcium deposits in hMSC cultures could be observed after 10 days, and the amount of calcium deposits increased over time in every group.
Fig. 7.
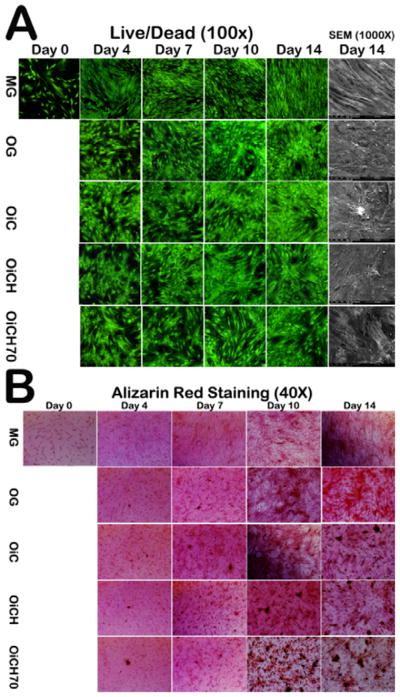
Live/Dead and SEM images (A) and Alizarin Red Staining (B) of hMSC treated with growth media (MG), osteogenic media (OG), osteogenic media with degradation products from iCMBA (OiC), iCH7 with HA filtered (OiCH) and iCH7 with HA in the media (OiCH70).
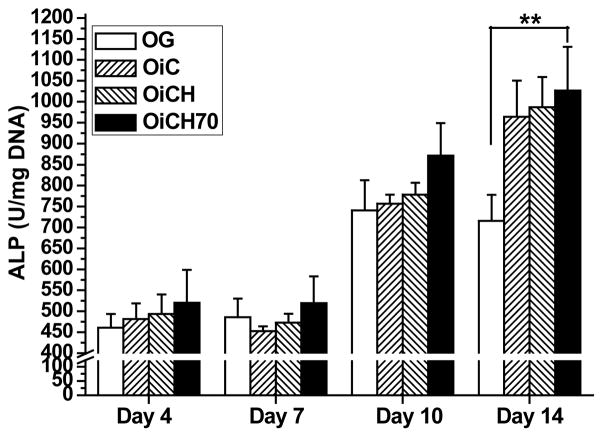
However, no calcium deposits were found in MG group in all time points. Increased calcium deposits were observed in OiC, OiCH and OiCH70 groups when compared to OG group, especially in OiCH70 group indicating that the inclusion of iCMBA and HA can promote calcium deposits formation by osteogenic differentiated hMSCs. The results of ALP activity are shown in Fig. 8. When hMSCs were induced into osteoblastic linage in osteogenic media, the ALP expression in each group increased over time. At 4, 7 and 10 days, no significant difference can be observed between the tested groups. However, after 14 days, the ALP expression of OiC, OiCH and OiCH70 groups increased and were all significantly higher than that of OG group in the corresponding time point (P<0.05), further indicating the positive effect of iCMBA and HA in hMSCs osteogenic differentiation. As shown in Fig. S2B, iCH70 also support the osteogenic differentiation of MC3T3 cultured on the composite surfaces verified by the increased ALP activities at 7 days.
Fig. 8.
ALP activity of osteogenic differentiated hMSC triggered by iCMBA/HA degradation products. **p<0.01.
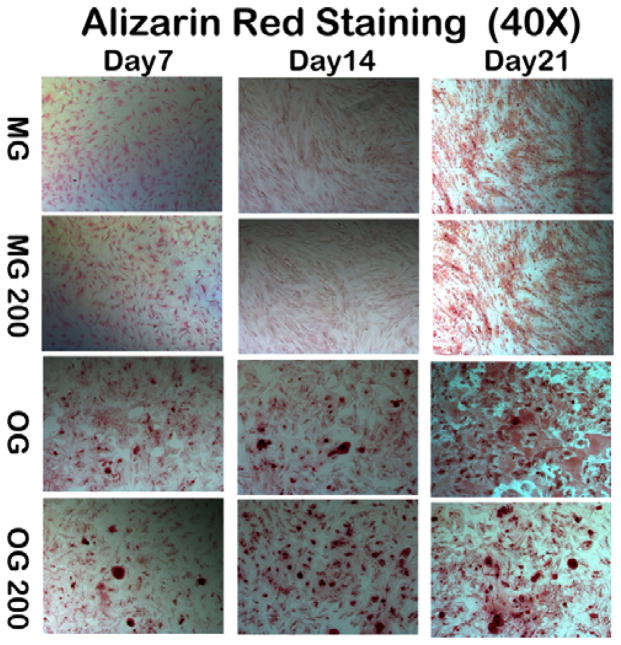
To further investigate the effect of citrate on calcium deposit formation of osteogenic-differentiated and undifferentiated stem cells, hMSCs were cultured in growth media (MG) and osteogenic media (OG) supplemented with citrate at a concentration of 200 μM with pure MG and OG media used as controls. After 7, 14 and 21 days, Alizarin Red staining was conducted to visualize calcium deposits (Fig. 9). When hMSC were cultured in growth media, no matter with or without 200 μM citrate supplementation, no calcium deposits were found at all time points. However, when hMSC were induced into osteocyte linage in osteogenic media, a few calcium deposits were seen by day 7, and the amount of calcium deposits rapidly increased with time, especially at day 14 and 21. With 200 μM citrate supplementation in OG media, more calcium deposits were observed after day 7 compared to the pure OG group with differences becoming more obvious with prolonged time.
Fig. 9.
Alizarin Red Staining for calcium deposit in hMSC cultures treated with growth media (MG), growth media with 200 μM citrate supplement (MG200), osteogenic media (OG), osteogenic media with 200 μM citrate supplement (OG200) at the 7th, 14th, and 21st day. Citrate in markedly enhanced calcium deposit formation of osteogenic differentiated hMSCs (Alizarin Red Staining, 40X).
3.3.2 Effect of citrate on calcium deposit formation
3.3.3 Citrate release from iCMBA/HA 70% (iCH70) composite
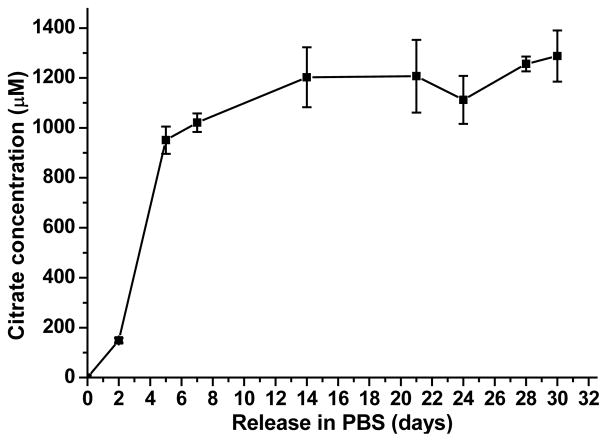
To mimic the citrate release process of iCMBA/HA in practical situation when being applied in vivo, iCH70 was chosen as the representative in citrate release studies. From Fig. 10, it can be seen that, after 2 days of release, the citrate concentration was already around 200 μM. The citrate concentrations increased to around 1000 μM with no significant increase during the later time points (to 30 days) indicating that it is possible to reach an effective concentration to promote calcium deposit formation when applied in vivo.
Fig. 10.
Cumulative citrate release in PBS (pH 7.4) at 37 °C.
3.4 Biological perfomance of iCMBA/HA in vivo
Due to the above advantages, iCH70 was chosen as a representative material for in vivo evaluation using reproducible comminuted radial facture model on rabbits. Computer tomography (CT) analysis, three-point bending test, and histological examination were conducted 4, 8, and 12 weeks post-operation.
3.4.1 Micro CT
Micro-CT analysis data is shown in Fig. 11. Bone mineral content (BMC) and bone mineral density (BMD) values in the iCMBA/HA group (iCH70) at three pre-determined time points were 388±12 mg and 122±7 mg/cm3 (4 week), 417±30 mg and 133±6 mg/cm3 (8 week), 427±23 mg and 143±8 mg/cm3 (12 week), respectively, which were all higher than those of blank control group. The results in Fig. 11 indicate that at all time points, enhanced bone formation can be detected in the iCMBA/HA group compared to blank control group implying the beneficial effects of iCMBA/HA composites to bone healing in the early stages after injury.
Fig. 11.
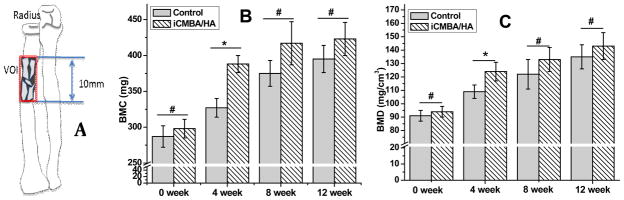
Micro/CT analysis of comminuted fracture area of radius bone. Volume of interest (VOI) in analysis (A), Bone mineral Content (B) and Bone mineral Density (C). *: significant difference (p<0.05), #: no significant difference (P>0.05).
3.4.2 Biomechanical test for explants
Biomechanical testing, an important index of the quality of bone healing, was assessed by three-point bending test (Fig. 12). The maximal loads of radial bone in iCMBA/HA group after 4, 8, and 12 weeks were 130±5, 150±4 and 178±7.8 N, respectively, which are significantly higher than that in blank control group (103±6, 125±5 and 146±7 N, respectively) at every predetermined time points (P<0.05).
Fig. 12.
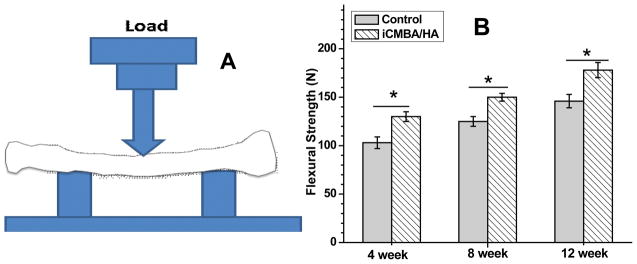
Three-point-bending test was performed of on MTS machine for comminuted fracture area of radius bone. Maximal flexural strength was recorded. *: significant difference (P<0.05).
3.4.3 Histological staining
Undecalcified histological assessment was conducted to assess new bone formation and microstructure in the area of interest (Fig. 13). Masson Trichrome staining results revealed: (1) iCH70 bone substitute degraded almost completely in 4 weeks with HA particles left scattered at the implant site; (2) At 12 week, HA particles were almost completely incorporated into new bone in the iCH70 bone substitute group and radial bone structure was found to be more organized compared to that in blank control group. Decalcified histological assessment was conducted to assess vascular formation and cell immigration in the area of interest (Fig. 13). H&E staining results (Fig. 13) revealed: in iCH70 group, the vascular formation was slightly higher, but generally comparable to that in blank control group at the 4-week time point.
Fig. 13.
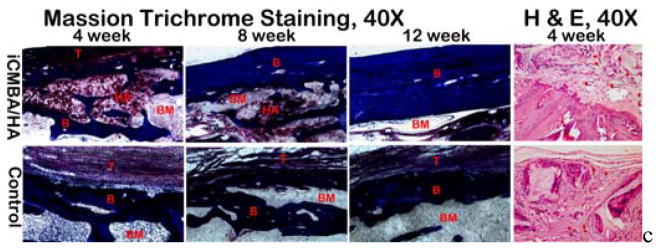
Massion Trichrome staining (undecalsified hard tissue sections at week 4, 8 and 12,) and hematoxylin and eosin (H&E) staining (decaclfied section at week 4) of iCMBA/HA and control groups. After the fast degradation of iCMBA/HA composites, hydroxyapatite particles were scattered around and consequently incorporated into the new bone formation, Thicker cortical bone with more organized microstructure were observed in iCMBA/HA group compared to control group (Massion Trichrome staining, 40X). Comparing to control group, parallel new vessel fomation was observed in iCMBA/HA group after 4 weeks of injection, indicates no inhibiting effect on neovasculation from iCMBA/HA (H&E staining, 40X). T: fibrous tissue; B: bone ; HA: hydroxyapatite particle; BM: Bone marrow cavity.
These three results indicate: (1) an increase in new bone formation and mineralization (calcium deposits) were found in iCH70 group, especially in first four weeks after injection; (2) higher-quality healing of comminuted radial bone was observed in the iCH70 group with higher maximal strengths in biomechanical test; (3) iCMBA/HA bone substitutes can promote bone mass formation and improve new-formed bone quality. The possible reasons for these findings could be found in the histological examination as following: (1) neovascularization was present deep into the bone substitutes with the help of proper degradation of the bone substitutes offering better blood supply to bring more nutrition for cell immigration and survival (Fig. 12); (2) the host bone could efficiently take in HA particles left by degrading polymer for new bone formation, which could speed up bone regeneration (see Fig. 10); and (3) more organized bone microstructure, especially cortical bone, was achieved due to better bone piece alignment (Fig. 13).
3.5 Citrate and HA contributions to calcium deposit formation
Mineralization is known to be a process controlled by multiple factors. Both citrate and HA in the iCMBA/HA bone substitute were supposed to play important roles in bone formation and mineralization and can explain the performance of iCMBA/HA for bone formation in vivo. For HA, we introduced it into bone material design to take advantage of its osteoconductivity and, based on the results of in vitro mineralization test of iCMBA/HA in SBF 5X, we confirmed its positive effect on mineralization (calcium deposit) on the surface of iCH70 (Fig. 5).
However, for citrate, except for its confirmed positive role in the expression of ALP, a protein marker of bone formation, 27 its role in mineralization has not been determined so far. We are the first to experimentally investigate citrate’s effect in calcium deposit formation of hMSC. Based on our Alizarin Red staining results, we confirmed that citrate promotes calcium deposit formation during osteogenic differentiation of hMSC. Specifically, for differentiated hMSC, exogenous citrate significantly promotes mineralization in the culture while it lost this function in undifferentiated hMSC. Therefore, we deem that citrate places positive effect on mineralization of differentiated hMSC in osteogenic stage.
4. Conclusions
We have confirmed that mussel-inspired citrate-based adhesive iCMBA/HA bone substitute may serve as an ideal candidate for CBF treatment as necessary supplement to instrumented fixation. On one hand, it shows favorable injectability and setting time, as well as suitable physical properties (sol content, swelling and degradation) and mechanical properties (cohesion and adhesion strength) in vitro. iCMBA/HA also possesses minimal cytotoxicity, and due to the ester-bond backbone of iCMBA pre-polymer, this citrate-based adhesive iCMBA/HA bone substitute degrades at a rate favorable to neovascularization and bone growth to ultimately provide a positive effect on increasing bone mass and bone strength recovery. The strong adhesive strength is a great benefit to maintain proper bone piece alignment, which is considered as one important factor for bone formation. On the other hand, we are the first to experimentally find that citrate, the distinctive degradation product of iCMBA/HA composites, significantly promotes mineralization and bone formation of differentiated hMSC in osteogenic media. Therefore, adhesive iCMBA/HA bone composites could potentially bridge the gap of osteoinduction property to parallel autograft in the future.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health (NIH) Awards (NIBIB EB012575, NCI CA182670, NHLBI HL118498), and National Science Foundation (NSF) Awards (DMR1313553, CMMI 1266116), and a National Natural Sciences Foundation of China (NSFC, grant 31228007).
Footnotes
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/b000000x/
Notes and references
- 1.Stöhr E, Holz U. Orthopade. 2000;29:342. doi: 10.1007/s001320050454. [DOI] [PubMed] [Google Scholar]
- 2.Teasdall R, Savoie FH, Hughes JL. Clin Orthop Relat Res. 1993;292:37. [PubMed] [Google Scholar]
- 3.Taylor D, Hazenberg JG, Lee TC. Nat Mater. 2007;6:263. doi: 10.1038/nmat1866. [DOI] [PubMed] [Google Scholar]
- 4.Galperin A, Oldinski RA, Florczyk SJ, Bryers JD, Zhang M, Ratner BD. Adv Healthcare Mater. 2013;2:872. doi: 10.1002/adhm.201200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan H, Fernandes Habibovic HP, de Boer J, Barradas AM, de Ruiter A, Walsh WR, van Blitterswijk CA, de Bruijn JD. Proc Natl Acad Sci USA. 2010;107:13614. doi: 10.1073/pnas.1003600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandal BB, Grinberg A, Gil ES, Panilaitis B, Kaplan DL. Proc Natl Acad Sci USA. 2012;109:7699. doi: 10.1073/pnas.1119474109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Annabi N, Tamayol A, Uquillas JA, Akbari M, Bertassoni LE, Cha C, Camci-Unal G, Dokmeci MR, Peppas NA, Khademhosseini A. Adv Mater. 2014;26:85. doi: 10.1002/adma.201303233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levengood SKL, Zhang M. J Mater Chem B. 2014;2:3161. doi: 10.1039/C4TB00027G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ni P, Ding Q, Fan M, Liao J, Qian Z, Luo J, Li X, Luo F, Yang Z, Wei Y. Biomaterials. 2014;35:236. doi: 10.1016/j.biomaterials.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Chen B, Li Y, Xie D, Yang X, Zheng Z. Eur Spine J. 2011;20:1272. doi: 10.1007/s00586-011-1744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugawara A, Asaoka K, Ding SJ. J Mater Chem B. 2013;1:1081. doi: 10.1039/c2tb00061j. [DOI] [PubMed] [Google Scholar]
- 12.Dorozhkin SV. Int J Mater Chem. 2011;1:1. [Google Scholar]
- 13.Guo J, Nguyen DY, Tran RT, Xie Z, Bai X, Yang J. Natural and Synthetic Biomedical Polymers. 2014:259. [Google Scholar]
- 14.Solari MG, Spangler E, Lee A, Wollstein R. Hand Surg. 2011;16:223. doi: 10.1142/S0218810411005412. [DOI] [PubMed] [Google Scholar]
- 15.Wu Y, Wang L, Guo B, Ma PX. J Mater Chem B. 2013;2:3674. doi: 10.1039/c3tb21716g. [DOI] [PubMed] [Google Scholar]
- 16.Cipitria A, Lange C, Schell H, Wagermaier W, Reichert JC, Hutmacher DW, Fratzl P, Duda GN. J Bone Miner Res. 2012;27:1275. doi: 10.1002/jbmr.1589. [DOI] [PubMed] [Google Scholar]
- 17.D’Este M, Eglin D. Acta Biomater. 2013;9:5421. doi: 10.1016/j.actbio.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 18.Xia Z, Villa MM, Wei M. J Mater Chem B. 2014;2:1998. doi: 10.1039/C3TB21595D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kretlow JD, Young S, Klouda L, Wong M, Mikos AG. Adv Mater. 2009;21:3368. doi: 10.1002/adma.200802009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pompili A, Caroli F, Carpanese L, Caterino M, Raus L, Sestili G, Occhipinti E. J Neurosurg. 1998;89:236. doi: 10.3171/jns.1998.89.2.0236. [DOI] [PubMed] [Google Scholar]
- 21.Bae KH, Wang LS, Kurisawa M. J Mater Chem B. 2013;1:5371. doi: 10.1039/c3tb20940g. [DOI] [PubMed] [Google Scholar]
- 22.Mehdizadeh M, Weng H, Gyawali D, Tang L, Yang J. Biomaterials. 2012;33:7972. doi: 10.1016/j.biomaterials.2012.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu Q, Danner E, Waite JH, Israelachvili JN, Zeng H, Hwang DS. J R Soc Interface. 2013 doi: 10.1098/rsif.2012.0759. doi:10:20120759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leimgruber C. Divulg Cult Odontol. 1965;103:3. [PubMed] [Google Scholar]
- 25.Hartles RL. Adv Oral Biol. 1964;1:225. doi: 10.1016/b978-1-4832-3117-4.50014-0. [DOI] [PubMed] [Google Scholar]
- 26.Davies E, Müller KH, Wong WC, Pickard CJ, Reid DG, Skepper JN, Duer MJ. Proc Natl Acad Sci USA. 2014;111:E1354. doi: 10.1073/pnas.1315080111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tran RT, Wang L, Zhang C, Huang M, Tang W, Zhang C, Zhang Z, Jin D, Banik B, Brown JL, Xie Z, Bai X, Yang J. J Biomed Mater Res A. 2014;102:2521. doi: 10.1002/jbm.a.34928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu YY, Rawal A, Schmidt-Rohr K. Proc Natl Acad Sci USA. 2010;107:22425. doi: 10.1073/pnas.1009219107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costello LC, Franklin RB. J Regen Med Tissue Eng. 2013;2:1. doi: 10.7243/2050-1218-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Costello LC, Franklin RB, Reynolds MA, Chellaiah M. Open Bone J. 2012;4 doi: 10.2174/1876525401204010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang J, Webb AR, Ameer GA. Adv Mater. 2004;16:511. [Google Scholar]
- 32.Qiu H, Yang J, Kodali P, Koh J, Ameer GA. Biomaterials. 2006;27:5845. doi: 10.1016/j.biomaterials.2006.07.042. [DOI] [PubMed] [Google Scholar]
- 33.Mehdizadeh M, Yang J. Macromol Biosci. 2013;13:271. doi: 10.1002/mabi.201200332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gyawali D, Nair P, Kim PH, Yang J. Biomater Sci. 2013;1:52. doi: 10.1039/C2BM00026A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo J, Xie Z, Tran RT, Xie D, Jin D, Bai X, Yang J. Adv Mater. 2014;26:1906. doi: 10.1002/adma.201305162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo Y, Tran RT, Xie D, Wang Y, Nguyen DY, Gerhard E, Guo J, Tang J, Zhang Z, Bai X, Yang J. J Biomed Mater Res A. 2014 doi: 10.1002/jbm.a.35228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oyane A, Kim HM, Furuya T, Kokubo T, Miyazaki T, Nakamura T. J Biomed Mater Res A. 2003;65:188. doi: 10.1002/jbm.a.10482. [DOI] [PubMed] [Google Scholar]
- 38.Kallai I, Mizrahi O, Tawackoli W, Gazit Z, Pelled G, Gazit D. Nat Protoc. 2011;6:105. doi: 10.1038/nprot.2010.180. [DOI] [PubMed] [Google Scholar]
- 39.Chen B, Li Y, Yang X, Xie D. J Orthop Sci. 2012;17:70. doi: 10.1007/s00776-011-0167-7. [DOI] [PubMed] [Google Scholar]
- 40.Chen B, Li Y, Yang X, Xie D. Calcif Tissue Int. 2013;93:481. doi: 10.1007/s00223-013-9765-z. [DOI] [PubMed] [Google Scholar]
- 41.Leppänen O, Sievänen H, Jokihaara J, Pajamäki I, Järvinen TLN. J Bone Miner Res. 2006;21:1231. doi: 10.1359/jbmr.060511. [DOI] [PubMed] [Google Scholar]
- 42.Chen B, Xie D, Zheng Z, Lu W, Ning C, Li Y, Li F, Liao W. Osteoporos Int. 2011;22:265. doi: 10.1007/s00198-010-1186-5. [DOI] [PubMed] [Google Scholar]
- 43.Santos MI, Reis RL. Macromol Biosci. 2010;10:12. doi: 10.1002/mabi.200900107. [DOI] [PubMed] [Google Scholar]
- 44.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Nat Med. 2001;7:430. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 45.Winet H, Bao JY, Moffat R. J Bone Miner Res. 1990;5:19. doi: 10.1002/jbmr.5650050106. [DOI] [PubMed] [Google Scholar]
- 46.Browne S, Pandit A. J Mater Chem B. 2014;2:6692. doi: 10.1039/c4tb00863d. [DOI] [PubMed] [Google Scholar]
- 47.Smeets R, Endres K, Stockbrink G, Hanken H, Hermanns B, Sachweh, Marx R, Heiland M, Blessmann M, Wolff K-D, Kolk A. J Biomed Mater Res A. 2013;101A:2058. doi: 10.1002/jbm.a.34505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.