Abstract
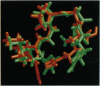
Molecular modeling and two-dimensional NMR techniques enable us to identify structural features in the third variable region (V3) loop of the human immunodeficiency virus (HIV) surface glycoprotein gp120, in particular the principal neutralizing determinant (PND), that remain conserved despite the sequence variation. The conserved structure of the PND is a solvent-accessible protruding motif or a knob, structurally isomorphous with the immunodominant knobs in the tandem repeat protein of human mucin 1 (MUC1) (a tumor antigen for breast, pancreatic, and ovarian cancer). We have replaced the mucin antigenic knobs by the PND knobs of the HIV MN isolate in a set of chimeric human MUC1/HIV V3 antigens. This produced multivalent HIV antigens in which PNDs are located at regular intervals and separated by extended mucin spacers. In this article we show by two-dimensional NMR spectroscopy that the multivalent antigens preserve the PNDs in their native structure. We also demonstrate by ELISA that the antigens correctly present the PNDs for binding to monoclonal antibodies or polyclonal antisera from HIV-infected patients.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chamat S., Nara P., Berquist L., Whalley A., Morrow W. J., Köhler H., Kang C. Y. Two major groups of neutralizing anti-gp120 antibodies exist in HIV-infected individuals. Evidence for epitope diversity around the CD4 attachment site. J Immunol. 1992 Jul 15;149(2):649–654. [PubMed] [Google Scholar]
- Chao B. H., Costopoulos D. S., Curiel T., Bertonis J. M., Chisholm P., Williams C., Schooley R. T., Rosa J. J., Fisher R. A., Maraganore J. M. A 113-amino acid fragment of CD4 produced in Escherichia coli blocks human immunodeficiency virus-induced cell fusion. J Biol Chem. 1989 Apr 5;264(10):5812–5817. [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Nishio J., Perryman S. Macrophage-tropic human immunodeficiency virus isolates from different patients exhibit unusual V3 envelope sequence homogeneity in comparison with T-cell-tropic isolates: definition of critical amino acids involved in cell tropism. J Virol. 1992 Nov;66(11):6547–6554. doi: 10.1128/jvi.66.11.6547-6554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza M. P., Durda P., Hanson C. V., Milman G. Evaluation of monoclonal antibodies to HIV-1 by neutralization and serological assays: an international collaboration. Collaborating Investigators. AIDS. 1991 Sep;5(9):1061–1070. doi: 10.1097/00002030-199109000-00001. [DOI] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Devine P. L., McKenzie I. F. Mucins: structure, function, and associations with malignancy. Bioessays. 1992 Sep;14(9):619–625. doi: 10.1002/bies.950140909. [DOI] [PubMed] [Google Scholar]
- Fontenot J. D., Finn O. J., Dales N., Andrews P. C., Montelaro R. C. Synthesis of large multideterminant peptide immunogens using a poly-proline beta-turn helix motif. Pept Res. 1993 Nov-Dec;6(6):330–336. [PubMed] [Google Scholar]
- Fontenot J. D., Tjandra N., Bu D., Ho C., Montelaro R. C., Finn O. J. Biophysical characterization of one-, two-, and three-tandem repeats of human mucin (muc-1) protein core. Cancer Res. 1993 Nov 15;53(22):5386–5394. [PubMed] [Google Scholar]
- Freed E. O., Myers D. J., Risser R. Identification of the principal neutralizing determinant of human immunodeficiency virus type 1 as a fusion domain. J Virol. 1991 Jan;65(1):190–194. doi: 10.1128/jvi.65.1.190-194.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendler S., Taylor-Papadimitriou J., Duhig T., Rothbard J., Burchell J. A highly immunogenic region of a human polymorphic epithelial mucin expressed by carcinomas is made up of tandem repeats. J Biol Chem. 1988 Sep 15;263(26):12820–12823. [PubMed] [Google Scholar]
- Ghiara J. B., Stura E. A., Stanfield R. L., Profy A. T., Wilson I. A. Crystal structure of the principal neutralization site of HIV-1. Science. 1994 Apr 1;264(5155):82–85. doi: 10.1126/science.7511253. [DOI] [PubMed] [Google Scholar]
- Gorny M. K., Xu J. Y., Gianakakos V., Karwowska S., Williams C., Sheppard H. W., Hanson C. V., Zolla-Pazner S. Production of site-selected neutralizing human monoclonal antibodies against the third variable domain of the human immunodeficiency virus type 1 envelope glycoprotein. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3238–3242. doi: 10.1073/pnas.88.8.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta G., Anantharamaiah G. M., Scott D. R., Eldridge J. H., Myers G. Solution structure of the V3 loop of a Thailand HIV isolate. J Biomol Struct Dyn. 1993 Oct;11(2):345–366. doi: 10.1080/07391102.1993.10508731. [DOI] [PubMed] [Google Scholar]
- Hwang S. S., Boyle T. J., Lyerly H. K., Cullen B. R. Identification of the envelope V3 loop as the primary determinant of cell tropism in HIV-1. Science. 1991 Jul 5;253(5015):71–74. doi: 10.1126/science.1905842. [DOI] [PubMed] [Google Scholar]
- Jerome K. R., Barnd D. L., Bendt K. M., Boyer C. M., Taylor-Papadimitriou J., McKenzie I. F., Bast R. C., Jr, Finn O. J. Cytotoxic T-lymphocytes derived from patients with breast adenocarcinoma recognize an epitope present on the protein core of a mucin molecule preferentially expressed by malignant cells. Cancer Res. 1991 Jun 1;51(11):2908–2916. [PubMed] [Google Scholar]
- Kirkpatrick S., Gelatt C. D., Jr, Vecchi M. P. Optimization by simulated annealing. Science. 1983 May 13;220(4598):671–680. doi: 10.1126/science.220.4598.671. [DOI] [PubMed] [Google Scholar]
- LaRosa G. J., Davide J. P., Weinhold K., Waterbury J. A., Profy A. T., Lewis J. A., Langlois A. J., Dreesman G. R., Boswell R. N., Shadduck P. Conserved sequence and structural elements in the HIV-1 principal neutralizing determinant. Science. 1990 Aug 24;249(4971):932–935. doi: 10.1126/science.2392685. [DOI] [PubMed] [Google Scholar]
- Matthews T. J., Langlois A. J., Robey W. G., Chang N. T., Gallo R. C., Fischinger P. J., Bolognesi D. P. Restricted neutralization of divergent human T-lymphotropic virus type III isolates by antibodies to the major envelope glycoprotein. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9709–9713. doi: 10.1073/pnas.83.24.9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pau C. P., Lee-Thomas S., Auwanit W., George J. R., Ou C. Y., Parekh B. S., Granade T. C., Holloman D. L., Phillips S., Schochetman G. Highly specific V3 peptide enzyme immunoassay for serotyping HIV-1 specimens from Thailand. AIDS. 1993 Mar;7(3):337–340. doi: 10.1097/00002030-199303000-00005. [DOI] [PubMed] [Google Scholar]
- Putney S. D., Matthews T. J., Robey W. G., Lynn D. L., Robert-Guroff M., Mueller W. T., Langlois A. J., Ghrayeb J., Petteway S. R., Jr, Weinhold K. J. HTLV-III/LAV-neutralizing antibodies to an E. coli-produced fragment of the virus envelope. Science. 1986 Dec 12;234(4782):1392–1395. doi: 10.1126/science.2431482. [DOI] [PubMed] [Google Scholar]
- Rini J. M., Stanfield R. L., Stura E. A., Salinas P. A., Profy A. T., Wilson I. A. Crystal structure of a human immunodeficiency virus type 1 neutralizing antibody, 50.1, in complex with its V3 loop peptide antigen. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6325–6329. doi: 10.1073/pnas.90.13.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Guroff M., Brown M., Gallo R. C. HTLV-III-neutralizing antibodies in patients with AIDS and AIDS-related complex. Nature. 1985 Jul 4;316(6023):72–74. doi: 10.1038/316072a0. [DOI] [PubMed] [Google Scholar]
- Robey E., Axel R. CD4: collaborator in immune recognition and HIV infection. Cell. 1990 Mar 9;60(5):697–700. doi: 10.1016/0092-8674(90)90082-p. [DOI] [PubMed] [Google Scholar]
- Rose G. D., Gierasch L. M., Smith J. A. Turns in peptides and proteins. Adv Protein Chem. 1985;37:1–109. doi: 10.1016/s0065-3233(08)60063-7. [DOI] [PubMed] [Google Scholar]
- Rusche J. R., Javaherian K., McDanal C., Petro J., Lynn D. L., Grimaila R., Langlois A., Gallo R. C., Arthur L. O., Fischinger P. J. Antibodies that inhibit fusion of human immunodeficiency virus-infected cells bind a 24-amino acid sequence of the viral envelope, gp120. Proc Natl Acad Sci U S A. 1988 May;85(9):3198–3202. doi: 10.1073/pnas.85.9.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma M. H., Gupta G., Sarma R. H. Structure of a bent DNA: two-dimensional NMR studies on d(GAAAATTTTC)2. Biochemistry. 1988 May 3;27(9):3423–3432. doi: 10.1021/bi00409a045. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Nakagawa Y., Pendleton C. D., Houghten R. A., Yokomuro K., Germain R. N., Berzofsky J. A. Induction of broadly cross-reactive cytotoxic T cells recognizing an HIV-1 envelope determinant. Science. 1992 Jan 17;255(5042):333–336. doi: 10.1126/science.1372448. [DOI] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J. Report on the first international workshop on carcinoma-associated mucins. Int J Cancer. 1991 Aug 19;49(1):1–5. doi: 10.1002/ijc.2910490102. [DOI] [PubMed] [Google Scholar]
- de Lorimier R., Moody M. A., Haynes B. F., Spicer L. D. NMR-derived solution conformations of a hybrid synthetic peptide containing multiple epitopes of envelope protein gp120 from the RF strain of human immunodeficiency virus. Biochemistry. 1994 Mar 1;33(8):2055–2062. doi: 10.1021/bi00174a011. [DOI] [PubMed] [Google Scholar]
- di Marzo Veronese F., Reitz M. S., Jr, Gupta G., Robert-Guroff M., Boyer-Thompson C., Louie A., Gallo R. C., Lusso P. Loss of a neutralizing epitope by a spontaneous point mutation in the V3 loop of HIV-1 isolated from an infected laboratory worker. J Biol Chem. 1993 Dec 5;268(34):25894–25901. [PubMed] [Google Scholar]