Abstract
Purpose
There are two surgical procedures for proximal early gastric cancer (EGC): total gastrectomy (TG) and proximal gastrectomy (PG). This study aimed to compare the long-term outcomes of PG with those of TG.
Materials and Methods
Between January 2001 and December 2008, 170 patients were diagnosed with proximal EGC at Soonchunhyang University Cheonan Hospital, of which 64 patients underwent PG and 106 underwent TG. Clinicopathologic features, postoperative complications, blood chemistry data, changes in body weight, and oncological outcomes were analyzed and retrospectively compared between both groups.
Results
Tumor size was smaller and the number of retrieved lymph nodes was lower in the PG group. The postoperative complication rate was 10.9% in the TG group and 16.9% in the PG group. The incidence of Los Angeles grade C and D reflux esophagitis was significantly higher in the TG group. Hemoglobin level was higher and body weight loss was greater in the TG group at 2, 3, and 5 years postoperatively. The albumin levels at 3 and 5 years were lower in the TG group. There was no significant difference in the 5-year overall survival rates between the two groups (P=0.789).
Conclusions
Postoperative complications and oncologic outcomes were observed to be similar between the two groups. The PG group showed better laboratory data and weight loss than did the TG group. Moreover, severe reflux esophagitis occurred less frequently in the PG group than in the TG group. PG can be considered as an effective surgical treatment for proximal EGC.
Keywords: Stomach neoplasms, Proximal gastrectomy, Total gastrectomy
Introduction
Gastric cancer is one of the most common malignant tumors worldwide and the second most frequent cancer in Korea. Moreover, it is the most common cancer in men and the third most common in women.1 The early detection of early gastric cancer (EGC) has recently increased because of an increase in the use of esophagogastroduodenoscopy (EGD) as a health screening test.2 Various surgical approaches for gastric cancer, including minimally invasive surgery and function-preserving surgery, are being employed to improve the quality of life in gastric cancer patients. The incidence of gastric cancer in the upper stomach is high in Western countries. In Asia, the incidence of gastric cancer in the lower stomach is common, but an increasing incidence of proximal gastric cancer has been observed in recent years.3
Distal gastrectomy is regarded as the standard surgical treatment for EGC in the lower stomach. However, either total gastrectomy (TG) or proximal gastrectomy (PG) can be performed for the treatment of EGC in the upper stomach. Although PG has an advantage over TG in that the lower part of the stomach can be preserved with PG, it is rarely chosen because of the possibility of severe postoperative gastroesophageal reflux and the higher risk of cancer recurrence with PG than with TG.4,5 Jejunal interposition can be performed to reduce gastroesophageal reflux, which usually occurs after PG. However, the jejunal interposition procedure is quite complicated.
Many previous studies have compared the TG and PG procedures; however, there is no consensus regarding which procedure is superior. In Soonchunhyang University Hospital Cheonan, we usually perform the uncut Roux procedure after TG6 and esophagogastrostomy and pyloroplasty using Hegar's dilators after PG. This study aimed to compare the long-term postoperative results of TG with those of PG in patients who underwent both surgeries.
Materials and Methods
1. Patients
This study included patients who had a confirmed pathologic diagnosis of EGC after undergoing PG and TG for EGC in the upper stomach. The indication for PG is gastric cancer, with clinical stage T1N0M0, located in the upper third of the stomach. Patients with a superficial spreading type of gastric cancer detected on preoperative EGD were excluded. Although the gastric cancer lesions were located in the upper third of the stomach, TG was performed in patients who were considered unsuitable for PG because of the size or extent of the lesions. However, the final decisions regarding the surgical procedures depended on the surgeon. All surgical operations were conventional open procedures and were performed by a single surgeon at Soonchunhyang University Hospital Cheonan between January 2001 and December 2008. More than 5 years of follow-up was available for the enrolled patients. They were classified into two groups: 64 patients undergoing PG (PG group) and 106 patients undergoing TG with uncut Roux-en-y esophagojejunostomy (TG group).
2. Surgical procedures
1) Proximal gastrectomy
PG was performed by preserving the greater and lesser curvature side vessels including the right gastroepiploic vessels and right gastric vessels in the distal remnant stomach, and D1+ lymphadenectomy was performed.7 The gastric specimen was obtained by performing gastrectomy using a linear stapler after the distal esophagus was divided. Gastric tube reconstruction was performed as previously described.8 Next, 22- to 32-Fr Hegar's dilators were sequentially inserted at the incision site made on the anterior wall in the middle of the remnant stomach, and then, the dilators were passed through the pylorus, and pyloroplasty was completed. Esophagogastrostomy was performed through an identical incision site by using a 25-mm circular stapler. An anastomosis was made at the anterior wall of the stomach. Furthermore, interrupted sutures were placed between the stomach wall (posterior to and at the left site of the anastomosis) and tissues around the hiatus. The incision site on the stomach was closed with absorbable monofilament 3-0 sutures and reinforced with seromuscular sutures.
2) Total gastrectomy
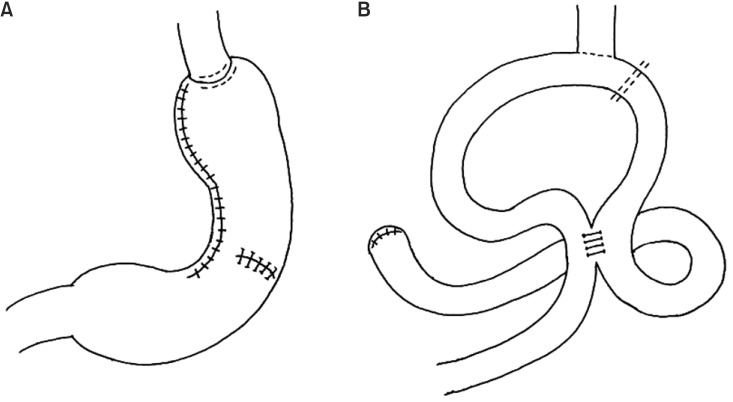
As demonstrated in a previous study,6 esophagojejunostomy was performed using a 25-mm circular stapler after TG. Next, the afferent loop directly beneath the esophagojejunostomy anastomosis site was closed using a TA stapler (linear noncutting stapler). Braun anastomosis was performed after interrupted suture reinforcement using 4-0 black silk was performed at the TA stapling site to prevent recanalization. D2 lymphadenectomy was performed in every patient undergoing TG (Fig. 1).7
Fig. 1.
Schematic representation of proximal (A) and total (B) gastrectomy.
3. Clinical characteristics
EGD was performed every 6 months for the first 3 years after surgery, after which it was performed every year. Reflux esophagitis was graded according to the Los Angeles (LA) classification, as follows: grade A or B esophagitis and grade C or D esophagitis. To compare the nutritional results between the PG and TG groups, serum markers (hemoglobin, total protein, albumin, and cholesterol) and body weight were assessed at 1-, 2-, 3-, and 5-year postoperative follow-ups. Vitamin B12 was also assessed at the same intervals.
For the assessment of postoperative complications, the incidences of intestinal obstruction, stricture, and leakage were investigated. A stricture was defined as clinically relevant if it caused the patient to undergo ballooning or endoscopic stent insertion. Intestinal obstruction and leakage were defined as clinically relevant if they caused the patient to undergo hospitalization and receive conservative and surgical treatment. Lastly, the overall survival was investigated and compared between both groups.
4. Statistical analysis
Student's t-test was used for analyzing the mean age, follow-up periods, operation time, and total protein, albumin, total cholesterol, hemoglobin, and vitamin B12 levels, and rate of body weight loss, and the chi-square test was used for analyzing sex, intestinal obstruction, stricture, leakage, and reflux esophagitis graded according to the LA classification. The 5-year overall survival rates were analyzed using the Kaplan-Meier method. Statistical analyses were performed using IBM SPSS Statistics ver. 19.0 (IBM Co., Armonk, NY, USA) and a value of P<0.05 was considered statistically significant.
Results
1. Patient characteristics
Patient characteristics are summarized in Table 1. There were no significant differences in sex, age, histological types, operation times, and follow-up periods between both groups. The results show that tumor size was larger and the number of retrieved lymph nodes was higher in the TG group. However, there was no significant difference in the number of metastatic perigastric lymph nodes between both groups. Although the number of retrieved lymph nodes was higher in the TG group, there was no significant difference in the incidence of positive lymph nodes between both groups: 10.9% in the PG group and 9.4% in the TG group (P=0.119).
Table 1.
Patient characteristics
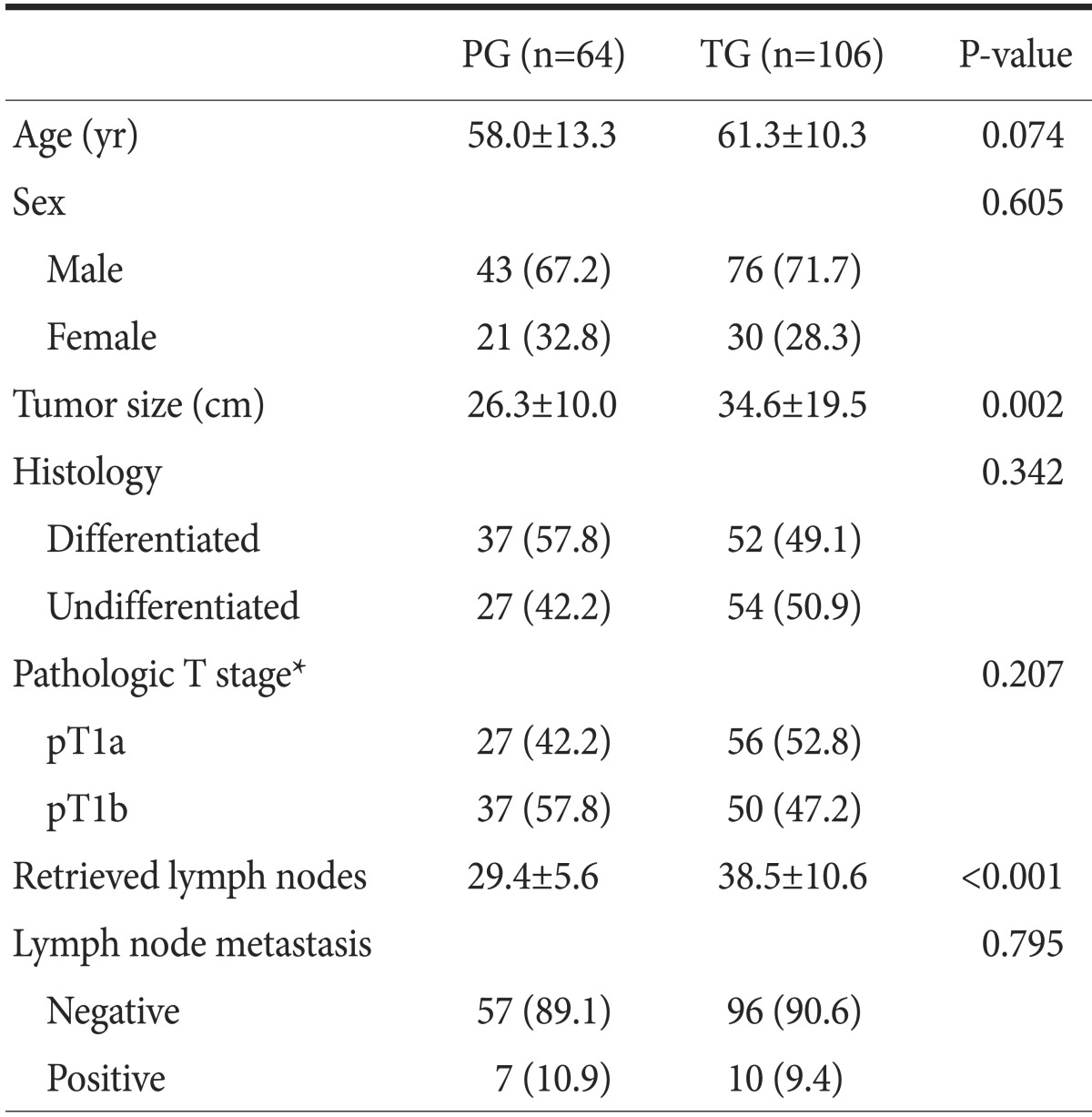
Values are presented as mean±standard deviation or number (%). PG = proximal gastrectomy; TG = total gastrectomy. *Pathologic T stage according to the 7th edition of the American Joint Committee on Cancer gastric cancer staging manual.
2. Postoperative complications
The postoperative complication rate was 10.9% in the TG group and 16.9% in the PG group. Although this rate was found to be lower in the TG group, this difference was not significant. Moreover, no significant differences in the number of patients with obstruction, stricture, and leakage were observed between the two groups. The incidence of LA grade C and D reflux esophagitis was significantly higher in the TG group. After the surgery, there was no cancer recurrence, including remnant gastric cancer, in the PG group (Table 2).
Table 2.
Postoperative complications and severe reflux esophagitis
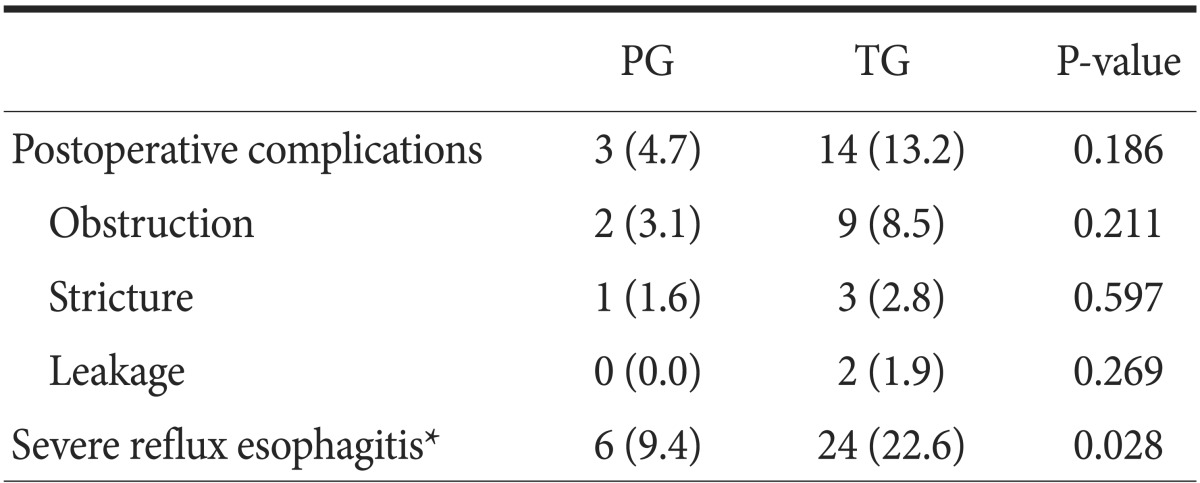
Values are presented as number (%). PG = proximal gastrectomy; TG = total gastrectomy. *Severe reflux esophagitis is defined as Los Angeles grade C or D reflux esophagitis.
3. Postoperative body weight and biochemical markers
Weight loss was greater in the TG group at 24, 36, and 60 months postoperatively. The total protein level was lower in the TG group than in the PG group at 2-year postoperative follow-up. The levels of albumin at 3- and 5-year follow-ups, hemoglobin and cholesterol at 2-, 3-, and 5-year follow-ups, and vitamin B12 in every year were higher in the PG group. However, there was no difference in the cholesterol levels between the groups (Table 3).
Table 3.
Postoperative changes in biochemical markers and body weight
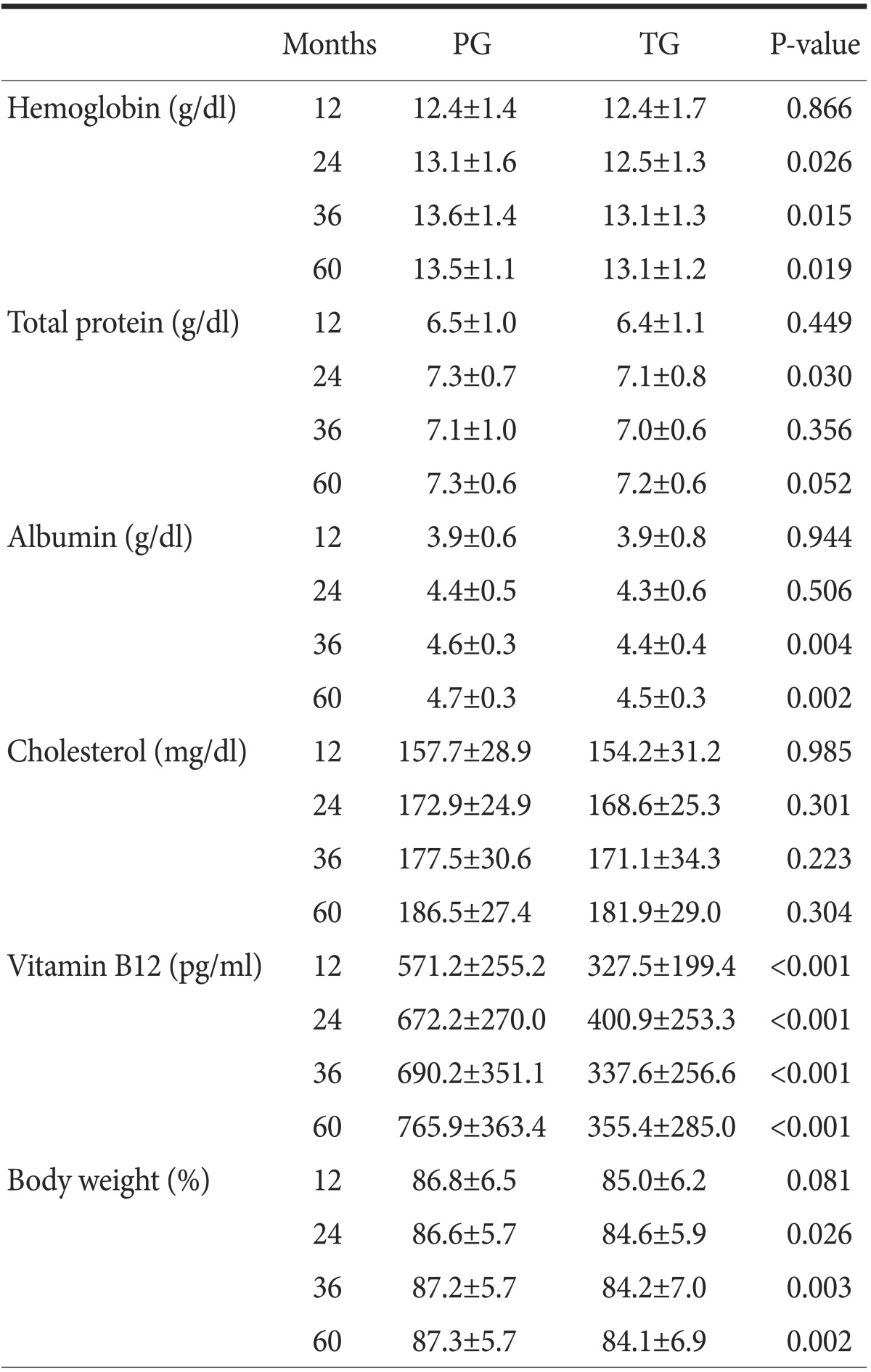
Values are presented as mean±standard deviation. PG = proximal gastrectomy; TG = total gastrectomy.
4. Survival rates
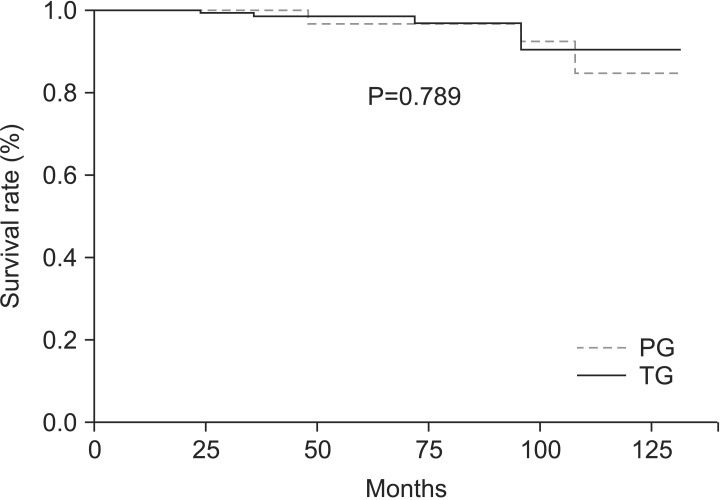
The 5-year overall survival rate was 95.3% in the TG group and 95.6% in the PG group. However, there was no significant difference in the survival rates between the groups (P=0.789) (Fig. 2).
Fig. 2.
Overall survival of patients in proximal gastrectomy (PG) and total gastrectomy (TG) groups. There were no significant differences in the overall survival rates of patients in PG and TG groups (5-year survival rates, 95.3% versus 95.6%, respectively; P=0.789).
Discussion
PG can be performed for the treatment of EGC in the upper stomach.9 Despite the advantage that PG can preserve the distal stomach, many surgeons choose to perform TG because of concerns regarding complications occurring after PG, such as reflux esophagitis, anastomosis site stricture, and lymph node metastasis along the lower part of the stomach. One study suggested that PG should not be performed because of the risk of serious postoperative complications such as gastroesophageal reflux and anastomotic leakage.3
In PG, various procedures such as reconstruction after gastrectomy are performed according to surgeon preference.10,11,12,13 However, many surgeons consider jejunal interposition to be a more complicated procedure than TG. We conducted PG by performing esophagogastrostomy using a circular stapler after pyloroplasty using Hegar's dilator. However, there was no significant difference in the operation time between TG and PG.
Severe reflux esophagitis following PG is a major cause of suffering and leads to quality-of-life deterioration in gastric cancer patients. In a study by Nakane et al.,14 patients who underwent PG with pyloroplasty showed better dietary intake, recovery of body weight, and gastric emptying than did those who underwent only PG. Moreover, the incidence of reflux gastritis or bile regurgitation was high in patients who underwent only PG. In our study, reflux esophagitis following EGD was observed in 60.9% of PG group patients and 61.3% of TG group patients. However, LA grade C or D severe reflux esophagitis occurred in 9.4% of PG group patients and 22.6% of TG group patients. The incidence of severe reflux esophagitis was lower in the PG group.
There were no differences in complications such as obstruction, stricture, and leakage between the two groups. Balloon dilatation, an effective treatment for anastomosis site stricture, was performed, and symptoms in all patients with stricture improved after PG or TG.15 Leakage did not occur in PG group patients, and 2 TG group patients with leakage recovered with conservative treatment. All patients with postoperative intestinal obstruction, except one who underwent band lysis procedure after TG, recovered with conservative treatment.
In PG, there might be limitations for performing lymphadenectomy of the nodes along the right gastroepiploic artery (No. 4d), suprapyloric lymph nodes (No. 5), and infrapyloric lymph nodes (No. 6) because the right gastric artery and right gastroepiploic artery should be preserved for blood supply to the remnant stomach. However, lymph node metastasis has been reported to rarely occur in the preserved sites in proximal EGC patients undergoing PG.16,17 In this study, no cancer recurrence, including lymph node recurrence, was reported during the follow-up period in the PG group. Even in cases where gastric cancer arises in the upper stomach, if the tumor extends from the distal to the middle parts of the stomach, the incidence of lymph node metastasis to No. 5 and No. 6 is reported to be high regardless of the depth of tumor invasion.18 In our study, the number of retrieved lymph nodes was lower in PG group patients than in TG group patients. However, there was no significant difference in the 5-year overall survival rates between the groups.
Change in nutritional status is one of the most common and important associated problems, and it correlates with morbidity and mortality after gastrectomy in gastric cancer patients.19 Change in body weight is one of the indicators of the nutritional status of patients and can be evaluated easily.20 The PG group patients had a higher body weight than the TG group patients at the 2-, 3-, and 5-year postoperative follow-ups. In comparison of changes in body weight, PG group showed an increasing tendency whereas TG group showed a decreasing tendency. Moreover, the levels of the other indicators of nutritional status including total protein, albumin, and cholesterol, excluding hemoglobin, were higher in the PG group than in the TG group, although the TG group reported normal levels of most of the biochemical markers. Vitamin B12 deficiency is one of the metabolic complications following gastrectomy, especially total gastrectomy, and causes megaloblastic anemia, which results in quality-of-life deterioration.21,22 In our hospital, vitamin B12 is usually administered to patients who have undergone gastrectomy if the serum vitamin B12 level is <240 pg/ml. Although detailed data on vitamin B12 levels have not been included in this study, it should be noted that TG group patients had to be treated with vitamin B12 injections many times during the follow-up period (TG group vs. PG group, 5.06±3.19 vs. 0.13±0.13 times; P<0.001).
This study has some limitations. Because of the retrospective nature of this study, there was a selection bias between the PG and TG groups, such as in tumor size. In addition, only LA classification was used for grading postoperative esophageal reflux. Therefore, a precise comparison may not have been performed between the two groups.
In conclusion, postoperative oncologic outcomes were found to be similar between the PG and TG groups. Although the TG group had normal values of biochemical markers, the PG group showed better laboratory data and weight loss. Moreover, severe reflux esophagitis occurred less frequently in the PG group than in the TG group, and both groups had a similar incidence of postoperative late complications. Therefore, PG can be considered as an effective surgical treatment for proximal EGC.
References
- 1.Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013;45:1–14. doi: 10.4143/crt.2013.45.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sim YK, Kim CY, Jeong YJ, Kim JH, Hwang Y, Yang DH. Changes of the clinicopathological characteristics and survival rates of gastric cancer with gastrectomy: 1990s vs early 2000s. J Korean Gastric Cancer Assoc. 2009;9:200–206. [Google Scholar]
- 3.Ahn HS, Lee HJ, Yoo MW, Jeong SH, Park DJ, Kim HH, et al. Changes in clinicopathological features and survival after gastrectomy for gastric cancer over a 20-year period. Br J Surg. 2011;98:255–260. doi: 10.1002/bjs.7310. [DOI] [PubMed] [Google Scholar]
- 4.Hsu CP, Chen CY, Hsieh YH, Hsia JY, Shai SE, Kao CH. Esophageal reflux after total or proximal gastrectomy in patients with adenocarcinoma of the gastric cardia. Am J Gastroenterol. 1997;92:1347–1350. [PubMed] [Google Scholar]
- 5.Wen L, Chen XZ, Wu B, Chen XL, Wang L, Yang K, et al. Total vs. proximal gastrectomy for proximal gastric cancer: a systematic review and meta-analysis. Hepatogastroenterology. 2012;59:633–640. doi: 10.5754/hge11834. [DOI] [PubMed] [Google Scholar]
- 6.Lee MS, Nokes DJ, Hsu HM, Lu CF. Protective titres of measles neutralising antibody. J Med Virol. 2000;62:511–517. doi: 10.1002/1096-9071(200012)62:4<511::aid-jmv17>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 8.Shiraishi N, Adachi Y, Kitano S, Kakisako K, Inomata M, Yasuda K. Clinical outcome of proximal versus total gastrectomy for proximal gastric cancer. World J Surg. 2002;26:1150–1154. doi: 10.1007/s00268-002-6369-6. [DOI] [PubMed] [Google Scholar]
- 9.Kitamura K, Yamaguchi T, Nishida S, Yamamoto K, Ichikawa D, Okamoto K, et al. The operative indications for proximal gastrectomy in patients with gastric cancer in the upper third of the stomach. Surg Today. 1997;27:993–998. doi: 10.1007/BF02385777. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura M, Nakamori M, Ojima T, Katsuda M, Iida T, Hayata K, et al. Reconstruction after proximal gastrectomy for early gastric cancer in the upper third of the stomach: an analysis of our 13-year experience. Surgery. 2014;156:57–63. doi: 10.1016/j.surg.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Seshimo A, Miyake K, Amano K, Aratake K, Kameoka S. Clinical outcome of esophagogastrostomy after proximal gastrectomy for gastric cancer. Hepatogastroenterology. 2013;60:616–619. doi: 10.5754/hge12782. [DOI] [PubMed] [Google Scholar]
- 12.Hoshikawa T, Denno R, Ura H, Yamaguchi K, Hirata K. Proximal gastrectomy and jejunal pouch interposition: evaluation of postoperative symptoms and gastrointestinal hormone secretion. Oncol Rep. 2001;8:1293–1299. doi: 10.3892/or.8.6.1293. [DOI] [PubMed] [Google Scholar]
- 13.Hoshikawa T, Denno R, Yamaguchi K, Ura H, Hirata K. Chronic outcome of proximal gastrectomy with jejunal pouch interposition in dogs. J Surg Res. 2003;112:122–130. doi: 10.1016/s0022-4804(03)00126-4. [DOI] [PubMed] [Google Scholar]
- 14.Nakane Y, Michiura T, Inoue K, Sato M, Nakai K, Ioka M, et al. Role of pyloroplasty after proximal gastrectomy for cancer. Hepatogastroenterology. 2004;51:1867–1871. [PubMed] [Google Scholar]
- 15.Shemesh E, Czerniak A. Comparison between Savary-Gilliard and balloon dilatation of benign esophageal strictures. World J Surg. 1990;14:518–521. doi: 10.1007/BF01658680. discussion 521-522. [DOI] [PubMed] [Google Scholar]
- 16.Okamura T, Tsujitani S, Korenaga D, Haraguchi M, Baba H, Hiramoto Y, et al. Lymphadenectomy for cure in patients with early gastric cancer and lymph node metastasis. Am J Surg. 1988;155:476–480. doi: 10.1016/s0002-9610(88)80116-8. [DOI] [PubMed] [Google Scholar]
- 17.Kitamura K, Nishida S, Yamamoto K, Ichikawa D, Okamoto K, Taniguchi H, et al. Lymph node metastasis in gastric cancer in the upper third of the stomach: surgical treatment on the basis of the anatomical distribution of positive node. Hepatogastroenterology. 1998;45:281–285. [PubMed] [Google Scholar]
- 18.Kaibara N, Nishimura O, Nishidoi H, Kimura O, Koga S. Proximal gastrectomy as the surgical procedure of choice for upper gastric carcinoma. J Surg Oncol. 1987;36:110–112. doi: 10.1002/jso.2930360207. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe M, Iwatsuki M, Iwagami S, Ishimoto T, Baba Y, Baba H. Prognostic nutritional index predicts outcomes of gastrectomy in the elderly. World J Surg. 2012;36:1632–1639. doi: 10.1007/s00268-012-1526-z. [DOI] [PubMed] [Google Scholar]
- 20.Ryan AM, Healy LA, Power DG, Rowley SP, Reynolds JV. Short-term nutritional implications of total gastrectomy for malignancy, and the impact of parenteral nutritional support. Clin Nutr. 2007;26:718–727. doi: 10.1016/j.clnu.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Hu Y, Kim HI, Hyung WJ, Song KJ, Lee JH, Kim YM, et al. Vitamin B(12) deficiency after gastrectomy for gastric cancer: an analysis of clinical patterns and risk factors. Ann Surg. 2013;258:970–975. doi: 10.1097/SLA.0000000000000214. [DOI] [PubMed] [Google Scholar]
- 22.Adachi S, Kawamoto T, Otsuka M, Todoroki T, Fukao K. Enteral vitamin B12 supplements reverse postgastrectomy B12 deficiency. Ann Surg. 2000;232:199–201. doi: 10.1097/00000658-200008000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]