Abstract
Purpose
Palliative gastrectomy and chemotherapy are important options for peritoneal seeding of gastric cancer. The treatment stage IV gastric cancer patient who respond to induction chemotherapy, is converted to gastrectomy (conversion therapy or conversion surgery). This study explored the clinical outcomes of gastric cancer patients with peritoneal seeding who had undergone conversion therapy.
Materials and Methods
Between 2003 and 2012, gastric cancer patients with peritoneal seeding, as determined by preoperative or intraoperative diagnosis were reviewed retrospectively. Clinicopathologic characteristics and clinical outcomes of patients with peritoneal seeding were analyzed.
Results
Forty-three patients were enrolled. Eighteen patients had undergone conversion surgery and 25 patients continued conventional chemotherapy. Among the 18 conversion patients, 10 received clinically curative resection. The median follow-up period was 28.5 months (range 8 to 60 months) and the total 3-year survival rate was 16.3%. The median survival time of the patients who received clinically curative conversion therapy was 37 months, and the 3-year survival rate was 50%. The median follow-up for non-curative gastrectomy patients was 18 months. No patient treated using chemotherapy survived to 3 years; the median survival time was 8 months. The differences in survival time between the groups was statistically significant (P<0.001).
Conclusions
In terms of survival benefits for gastric cancer patients with peritoneal seeding, clinically curative conversion therapy resulted in better clinical outcomes.
Keywords: Stomach neoplasms, Carcinomatosis, Chemotherapy, Gastrectomy
Introduction
The prevalence of early-stage gastric cancer has been increasing as the popularity of esophagogastroduodenoscopy increases. Various early stage treatments are available depending on the nature of disease progression. When gastric cancer continues to progress or recurs, peritoneal seeding is commonly observed during the pathological course, which can lessen treatment efficacy and portend a poor prognosis.
The survival time of gastric cancer patients with peritoneal seeding is reported to be 3 to 9 months.1,2,3 Management options, including systematic chemotherapy, peritonectomy, intra-peritoneal chemotherapy, and hyperthermic intraperitoneal chemotherapy have been used to improve the prognosis of patients with advanced gastric cancer and peritoneal seeding. None of these options have proven satisfactory.4,5 Palliative gastrectomy in advanced gastric cancer patients can reduce bleeding, perforation, and obstruction. The treatment strategy of stage IV gastric cancer patients who respond to induction chemotherapy is converted to gastrectomy (conversion therapy or conversion surgery). This conversion is made with a curative intent, in contrast to that in palliative surgery. Patients with stage IV advanced gastric cancer who undergo conversion surgery have good prognoses, with R0 resection predicting longer survival.6
The present study explored the clinical outcomes of conversion therapy in gastric cancer patients with peritoneal seeding.
Materials and Methods
In all, 43 patients in Yeungnam University Medical Center were diagnosed with advanced gastric cancer with peritoneal seeding between January 2003 and December 2012. Peritoneal seeding was diagnosed using laparoscopic exploration, which was performed when peritoneal seeding was suspected preoperatively by serosal invasion, thick peritoneum, or ascites apparent on abdominal computed tomography (CT) or positron emission tomography/CT (PET/CT). Elevated tumor marker levels were diagnosed by staging laparoscopy.
Patients initially received combination chemotherapy with 5-fluorouracil+cisplatin or titanium silicate-1+cisplatin. In cases of deterioration, chemotherapy was continued using docetaxel, paclitaxel, and irinotecan. Preoperative chemotherapy was performed for at least four cycles, with a mean therapy time of 5.7 months.
The response to chemotherapy was evaluated using gastroscopy and abdominal CT or PET/CT within 3 months after the first chemotherapy dose. Toxicity was graded according to the National Cancer Institute criteria. If patients experienced grade 3 or higher toxicity, chemotherapy was withheld until recovery and was restarted at the next lower dose level and/or modified, as appropriate to the toxicity.
Whether gastric cancer was worsening or responding to chemotherapy was judged by determining whether there was improvement in the primary tumor range and ulcer after gastroscopy and by determining changes in existing lymphadenopathy and the onset of new lesions using abdominal CT or PET/CT. If there was evidence of improvement in the primary tumor and the interior of the peritoneum, another laparoscopic exploration was performed to confirm the status of peritoneal seeding, as evaluated according to the Japanese Classification of Gastric Carcinoma.7 Changes in status from P2 to P1 or P0 or changes from P1 to P0 were indicative of an improved state of peritoneal seeding. After 12 chemotherapy cycles, diagnostic laparoscopy revealed a changed status from P2 to P1. Gastric lesions remained evident on esophagogastroduodenoscopy, because of which the decision for palliative gastrectomy with subsequent continued postoperative chemotherapy was made. If improvement was evident, additional gastrectomy was performed. If chemotherapy was ineffective or if the patients' conditions had deteriorated, the chemotherapy regimen was altered, as described above, and was continued instead of performing a gastrectomy. For all gastrectomy procedures, at least the D2 level of lymph node dissection was performed.8
A retrospective analysis was carried out based on the information recorded after surgery regarding clinicopathologic characteristics, including operation method, tumor size, Borrmann type, cell differentiation degree, Lauren type, invasion depth, and lymph node stage. IBM SPSS Statistics ver. 19.0 (IBM Co., Armonk, NY, USA) was used for statistical analyses including the chi-square test for cross-tabulation analysis and the Kaplan-Meier method for survival analysis. Significance was established using the log-rank test at a P-value<0.05.
Results
Of the 43 chemotherapy patients, 18 underwent gastrectomy (conversion therapy group) and 25 did not (chemotherapy group). The latter group of patients did not show any signs of improvement and did not subsequently undergo gastrectomy. Instead, chemotherapy was continued. The conversion therapy group showed improvement and gastrectomy was performed 2 to 12 months (average, 5.6 months) after chemotherapy initiation.
The group of 18 patients who had undergone gastrectomy had an equal number of men and women (age range 32 to 72 years; average age, 52.8±13.7 years). Of the 10 patients who underwent clinically curative gastrectomy, four underwent subtotal gastrectomy and six underwent total gastrectomy. The total gastrectomy cases included three of extended total gastrectomy (combined organ resections). In the eight patients in the non-curative gastrectomy group, the gastrectomy type included one subtotal gastrectomy and seven total gastrectomies, with the latter including four cases of extended total gastrectomy. The combined resected organs were the spleen, distal pancreas, transverse colon, and salpinx. The mean tumor size was 7.1±4.6 cm. In both groups, the most common histologic type poorly differentiated tumors. Most of the tumors were located in the middle area of the stomach. According to the current TNM staging criteria of the American Joint Committee on Cancer, stage IB was found in two patients (11.1%), IIA in two patients (11.1%), IIIA in three patients (16.7%), IIIB in two patients (11.1%), IIIC in one patient (5.6%), and IV in eight patients (44.4%) (Table 1).
Table 1.
Clinicopathologic characteristics of gastric cancer patients with peritoneal seeding who underwent gastrectomy (conversion surgery group)
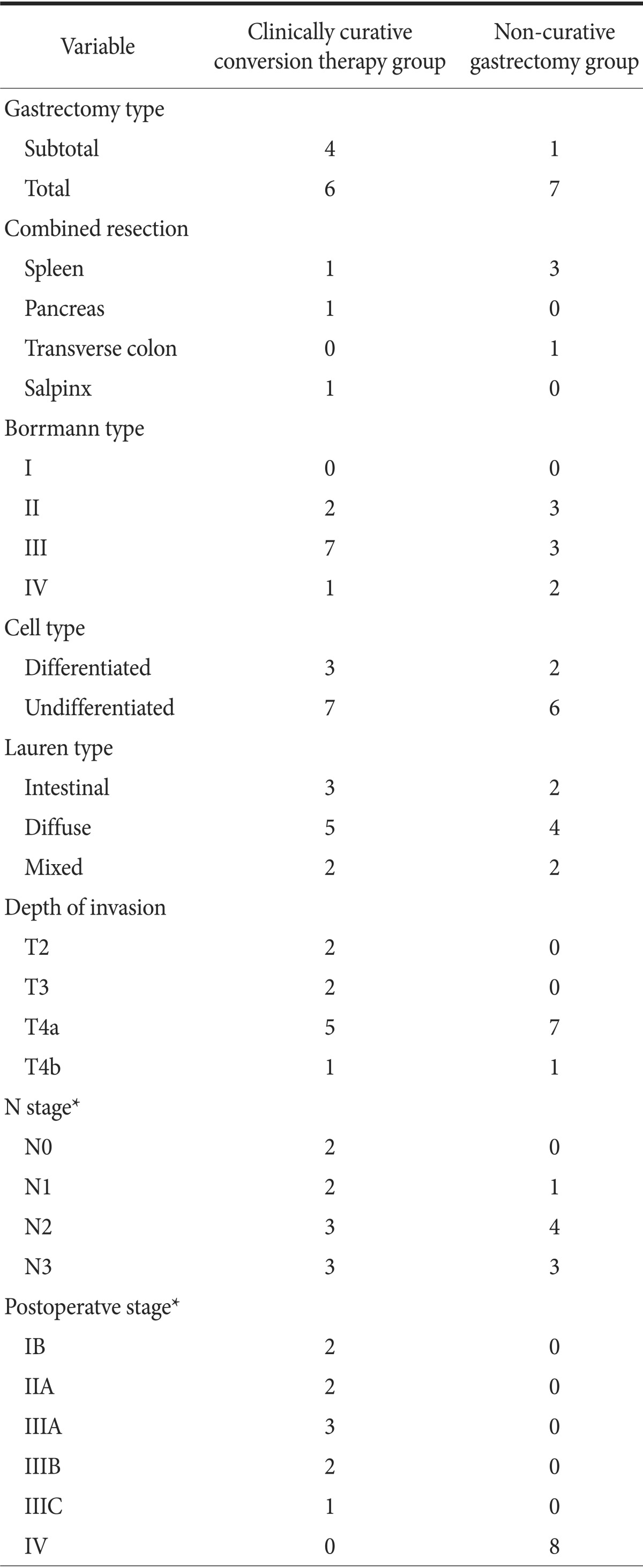
Values are presented as number. *According to the current TNM staging criteria of the American Joint Committee on Cancer.
Two cases of intra-abdominal abscess and several cases of minor complications were treated with conservative care. There was no operation-related mortality.
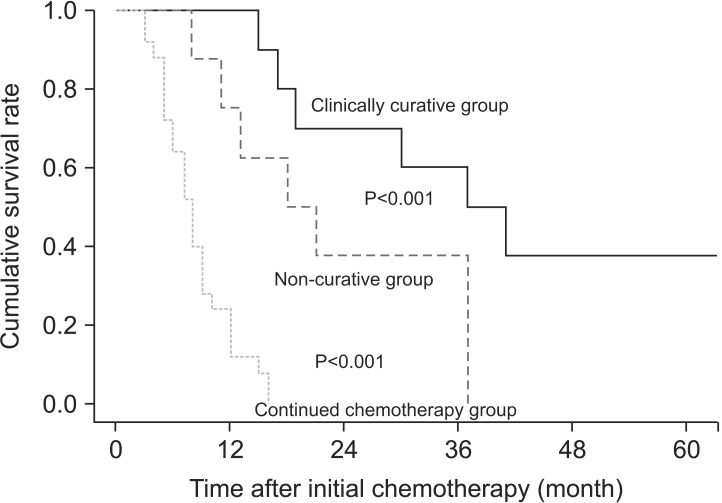
The median follow-up period was 28.5 months (range 8 to 60 months), and the total 3-year survival rate was 16.3%. The median survival time after the clinically curative conversion therapy patients was 37 months, the 2-year survival rate was 60%, and the 3-year survival rate was 50%. The median survival time, 2-year survival rate, and 3-year survival rate of the non-curative resected patients were 18 months, 37.5%, and 0%, respectively. No patient who received chemotherapy survived to 2 or 3 years, and the median survival time was 8 months. The differences between the groups were significant (P<0.001; Fig. 1).
Fig. 1.
Survival curves for gastric cancer patients with peritoneal seeding according to the treatment received.
Discussion
In recent years, although the number of early-stage gastric cancer patients is increasing, advanced cases are still detected. With peritoneal seeding, the prognosis can be poor.
Because of the poor prognosis, standard conventional chemotherapy is preferable. Intra-peritoneal chemotherapy with systemic intravenous chemotherapy, intra-peritoneal heat therapy with cytoreductive surgery, active peritonectomy, induction chemotherapy, and adjuvant chemotherapy have been recently found to improve the prognosis.9,10,11,12 Performing gastrectomy in patients with gastric cancer and peritoneal seeding has reported to increase response to chemotherapy and subsequently, improves patient prognosis.9,13,14 The present study compared the clinicopathologic characteristics and prognosis of patients according to gastrectomy after conventional chemotherapy (conversion therapy) and showed that clinically curative conversion therapy resulted in the best survival rate and prognosis, and that patients who underwent non-curative resected showed better outcomes than those who had continued chemotherapy.
Laparoscopic staging was very useful for detecting peritoneal seeding, as well as for precisely ascertaining the level of peritoneal seeding without the need for laparotomy. The accuracy of non-invasive diagnosis prior to surgery is 58% to 63%,15 and The sensitivity of laparoscopic exploration is greater than 90%.16 Accordingly, we conducted laparoscopic staging before laparotomy in patients with high serum tumor marker levels or who had clinical T4a and T4b preoperatively. We detected peritoneal seeding in nearly 40% of these patients.
The total 3-year survival rate was 16.3% in this study, which is better than those previously reported of approximately 15%17 and 18%.18 In the present study, the 3-year survival rate of patients who had clinically curative conversion therapy was 50%. The rates exceeded 50% in one report17 and was only 12% in another.19 The present finding of a 0% 3-year survival rate in patients who continued alternative chemotherapy is in line with the result of a prior report.19 Despite differences in patients and research and treatment methods, present and previously reported survival rates are similar. Continued chemotherapy was not useful in increasing survival in peritoneal seeding patients, but successful conversion therapy was.
A previous study indicated that radical curability can be achieved using gastrectomy and that metastasis can be cured when the peritoneal seeding stage is P1.20 Improved prognosis also can be expected with gastrectomy when the peritoneal seeding is not more severe than P2.21 Induction chemotherapy in 61 patients with P3 required additional gastrectomy.17 Removal of the primary tumor may reduce the obstruction, bleeding, perforation, and ascites caused by the primary tumor and increase patient comfort.22
In the present study, the median survival time of patients who underwent non-curative resection was 18 months, and the 2-year survival rate was 37.5%. However, patients who continued chemotherapy did not survive to 2 years, and the median survival time was 8 months. The difference in survival rates between the non-curative gastrectomy and continued chemotherapy groups was significant. It is necessary to have clear plans for the methods, time of response tests, and period of chemotherapy because response to chemotherapy is an important variable even though chemotherapy is the primary treatment. It is important to judge the possible time of radical gastrectomy. The period of palliative chemotherapy for gastric patients with peritoneal seeding is thought to vary depending on the response to chemotherapy. Given the experience of the researchers in this study, some patients could have undergone gastrectomy after only two chemotherapy cycles because of their rapid response to treatment, while others who showed a more gradual improvement over the 12-month period could finally undergo the operation. In other studies, the number of chemotherapy cycles varied from 2 to 617 depending on the patients, and 3 to 7 cycles were carried out prior to gastrectomy.23 Patients who showed a complete response to chemotherapy experienced long-term survival after curative gastrectomy. Patients who showed a partial response to chemotherapy also had longer survival rates after non-curative gastrectomy than after continued chemotherapy. Thus, non-curative gastrectomy was helpful in increasing the survival rate of patients with peritoneal seeding. The procedure also increases the efficacy of chemotherapy by reducing tumor size. There are also immunological benefits in terms of lowered body metabolism and reduced level of cytokine, the immunosuppressant produced by tumors.
Although it is difficult to generalize the results of this study, performing gastrectomy actively when feasible is considered beneficial. To improve the prognoses of gastric cancer patients with peritoneal seeding, it is important to actively consider gastrectomy, depending on the response to induction chemotherapy. Palliative gastrectomy was also effective in improving survival. It will be necessary to compare the efficacy of continued chemotherapy and palliative gastrectomy in a well-designed, prospective, randomized clinical study to enable treatment guidance of gastric cancer patients with peritoneal seeding.
References
- 1.Gunderson LL, Sosin H. Adenocarcinoma of the stomach: areas of failure in a re-operation series (second or symptomatic look) clinicopathologic correlation and implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 1982;8:1–11. doi: 10.1016/0360-3016(82)90377-7. [DOI] [PubMed] [Google Scholar]
- 2.Landry J, Tepper JE, Wood WC, Moulton EO, Koerner F, Sullinger J. Patterns of failure following curative resection of gastric carcinoma. Int J Radiat Oncol Biol Phys. 1990;19:1357–1362. doi: 10.1016/0360-3016(90)90344-j. [DOI] [PubMed] [Google Scholar]
- 3.Wisbeck WM, Becher EM, Russell AH. Adenocarcinoma of the stomach: autopsy observations with therapeutic implications for the radiation oncologist. Radiother Oncol. 1986;7:13–18. doi: 10.1016/s0167-8140(86)80120-7. [DOI] [PubMed] [Google Scholar]
- 4.Yonemura Y, Fujimura T, Fushida S, Takegawa S, Kamata T, Katayama K, et al. Hyperthermo-chemotherapy combined with cytoreductive surgery for the treatment of gastric cancer with peritoneal dissemination. World J Surg. 1991;15:530–535. doi: 10.1007/BF01675656. [DOI] [PubMed] [Google Scholar]
- 5.Ryu KW, Mok YJ, Kim SJ, Kim CS. Prognostic factors in advanced gastric cancer with peritoneal carcinomatosis. J Korean Surg Soc. 2000;59:786–792. [Google Scholar]
- 6.Funaki H, Fujita J, Morioka E, Kaida D, Ohnishi T, Ohno Y, et al. Evaluation of conversion gastrectomy for treatment of Stage IV advanced gastric cancer. Gan To Kagaku Ryoho. 2013;40:1615–1617. [PubMed] [Google Scholar]
- 7.Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma: 2nd English edition. Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 8.Japanese Gastric. Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 9.Sugarbaker PH, Yonemura Y. Clinical pathway for the management of resectable gastric cancer with peritoneal seeding: best palliation with a ray of hope for cure. Oncology. 2000;58:96–107. doi: 10.1159/000012086. [DOI] [PubMed] [Google Scholar]
- 10.Ikeguchi M, Oka A, Tsujitani S, Maeta M, Kaibara N. Relationship between area of serosal invasion and intraperitoneal free cancer cells in patients with gastric cancer. Anticancer Res. 1994;14:2131–2134. [PubMed] [Google Scholar]
- 11.Sugarbaker PH. Peritonectomy procedures. Surg Oncol Clin N Am. 2003;12:703–727. doi: 10.1016/s1055-3207(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 12.Kuramoto M, Shimada S, Ikeshima S, Matsuo A, Yagi Y, Matsuda M, et al. Extensive intraoperative peritoneal lavage as a standard prophylactic strategy for peritoneal recurrence in patients with gastric carcinoma. Ann Surg. 2009;250:242–246. doi: 10.1097/SLA.0b013e3181b0c80e. [DOI] [PubMed] [Google Scholar]
- 13.Stern JL, Denman S, Elias EG, Didolkar M, Holyoke ED. Evaluation of palliative resection in advanced carcinoma of the stomach. Surgery. 1975;77:291–298. [PubMed] [Google Scholar]
- 14.Kim HI, Ha TK, Kwon SJ. Prognostic factors in gastric cancer patients with peritoneal carcinomatosis. J Gastric Cancer. 2010;10:126–132. [Google Scholar]
- 15.Hünerbein M, Rau B, Hohenberger P, Schlag PM. The role of staging laparoscopy for multimodal therapy of gastrointestinal cancer. Surg Endosc. 1998;12:921–925. doi: 10.1007/s004649900747. [DOI] [PubMed] [Google Scholar]
- 16.Song SC, Lee SL, Cho YK, Han SU. The role and efficacy of diagnostic laparoscopy to detect the peritoneal recurrence of gastric cancer. J Korean Gastric Cancer Assoc. 2009;9:51–56. [Google Scholar]
- 17.Yonemura Y, Bandou E, Sawa T, Yoshimitsu Y, Endou Y, Sasaki T, et al. Neoadjuvant treatment of gastric cancer with peritoneal dissemination. Eur J Surg Oncol. 2006;32:661–665. doi: 10.1016/j.ejso.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Glehen O, Gilly FN, Arvieux C, Cotte E, Boutitie F, Mansvelt B, et al. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 2010;17:2370–2377. doi: 10.1245/s10434-010-1039-7. [DOI] [PubMed] [Google Scholar]
- 19.Badgwell B, Cormier JN, Krishnan S, Yao J, Staerkel GA, Lupo PJ, et al. Does neoadjuvant treatment for gastric cancer patients with positive peritoneal cytology at staging laparoscopy improve survival? Ann Surg Oncol. 2008;15:2684–2691. doi: 10.1245/s10434-008-0055-3. [DOI] [PubMed] [Google Scholar]
- 20.Seo YJ, Bae JM, Kim SW, Kim SW, Song SK. Different clinical outcomes of stage iv gastric cancer according to the curability of surgery. J Korean Surg Soc. 2009;77:170–176. [Google Scholar]
- 21.Hioki M, Gotohda N, Konishi M, Nakagohri T, Takahashi S, Kinoshita T. Predictive factors improving survival after gastrectomy in gastric cancer patients with peritoneal carcinomatosis. World J Surg. 2010;34:555–562. doi: 10.1007/s00268-010-0396-5. [DOI] [PubMed] [Google Scholar]
- 22.Pollock RE, Roth JA. Cancer-induced immunosuppression: implications for therapy? Semin Surg Oncol. 1989;5:414–419. doi: 10.1002/ssu.2980050607. [DOI] [PubMed] [Google Scholar]
- 23.Yoon SY, Kim MG, Oh ST. The impact of preoperative chemotherapy on the surgical management of unresectable gastric cancer. J Korean Gastric Cancer Assoc. 2009;9:269–274. [Google Scholar]