Abstract
Gastric cancer is rare during pregnancy, and often advanced upon presentation. A Krukenberg tumor presents a diagnostic and therapeutic challenge in the pregnant patient. We present a case of a 38-year-old woman at 22 weeks' gestation who presented with worsening epigastric pain, and was found to have a left pelvic mass on ultrasound, which was confirmed by magnetic resonance imaging. She went into active labor and delivered a viable infant via vaginal delivery. An exploratory laparotomy revealed a large mass originating from her left ovary and diffuse thickening of the lesser curvature of the stomach. Frozen section investigation revealed the presence of signet cell adenocarcinoma. Subsequent upper endoscopy showed linitis plastica, while biopsy confirmed the presence of adenocarcinoma. In conclusion, the occurrence of gastric cancer in pregnancy is rare despite extremely common symptoms. The management poses a challenge because of the need for early treatment, and the continuation of the pregnancy.
Keywords: Krukenberg tumor, Stomach neoplasms, Pregnancy, Linitis plastica
Introduction
Gastric cancer is extremely rare during pregnancy, and often presents in advanced stages. A Krukenberg tumor refers to a malignancy in the ovary that has metastasized from a primary site, classically the gastrointestinal tract. We report a case of a Krukenberg tumor in a patient presenting with persistent abdominal pain, who subsequently developed preterm labor and underwent exploratory laparotomy and diagnosis.
Case Report
A 38-year-old pregnant Hispanic woman, gravida 4, para 4, with a gestational age of 22 weeks was referred to a tertiary center with complaints of intermittent abdominal pain for the past 3 months, and worsening symptoms over the past 3 to 4 weeks. The nonradiating pain originated in the epigastric region, was of moderate to severe intensity, and worsened upon food ingestion, with no obvious relieving factor. The patient also had nausea with a few intermittent episodes of bilious vomiting; however, she denied any hematemesis, melena, or hematochezia. During routine prenatal care at another hospital, her symptoms were attributed to the underlying pregnancy. She was prescribed ranitidine, which failed to alleviate her symptoms. She denied any past medical problems and was not taking any medications prior to her pregnancy. Her prior pregnancies were full term, normal vaginal deliveries and were uneventful. She denied the use of alcohol, smoking, or illicit drugs.
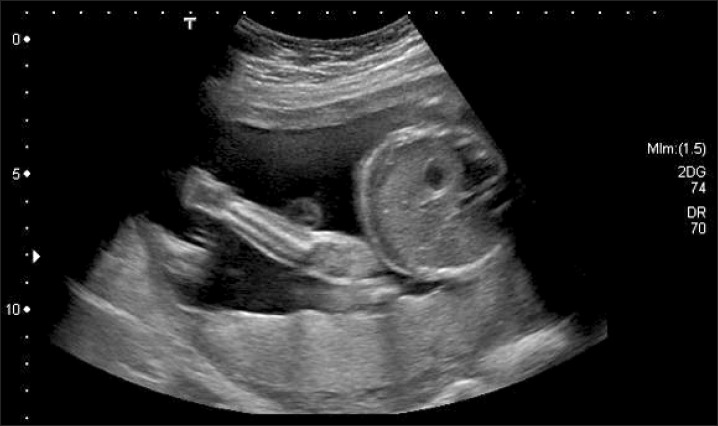
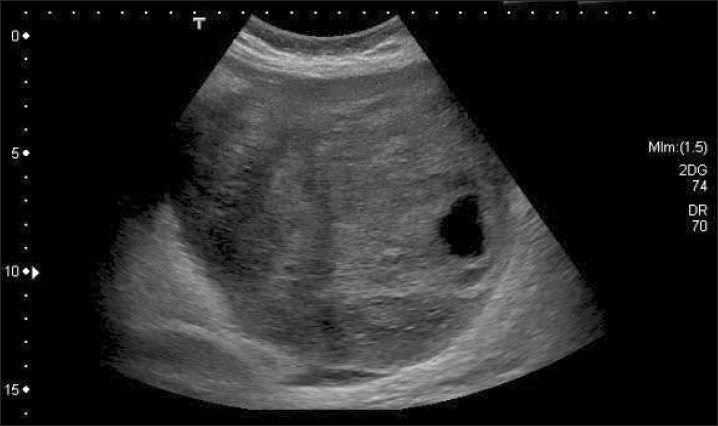
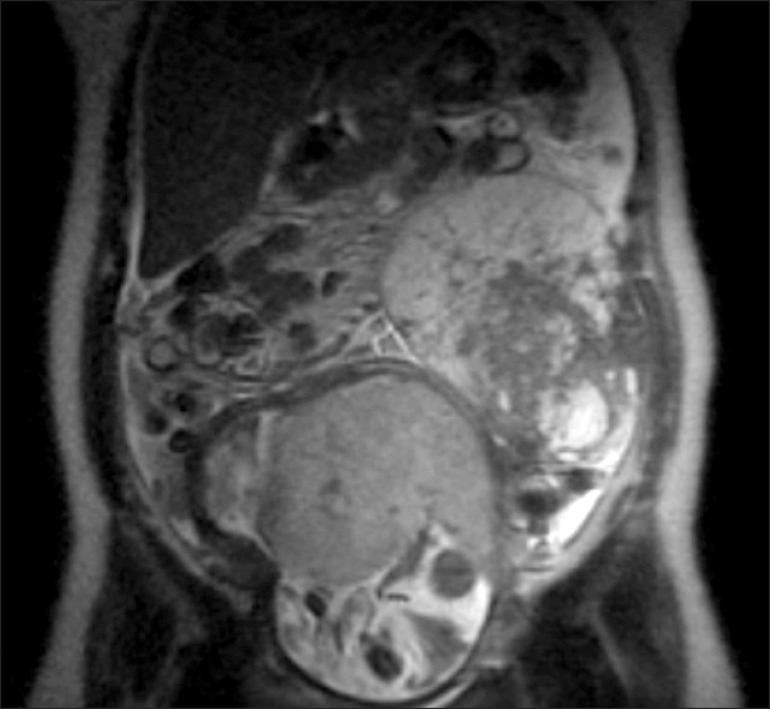
On examination she was afebrile, with a heart rate of 87 bpm, blood pressure of 123/87 mmHg, and was saturating well on room air. The physical exam was remarkable, indicating a gravid uterus at around 28 weeks' of gestation. She had mild epigastric tenderness and was found to have a tender mass from midline to the left flank. The patient had no pedal edema, and (I think the physical exam should be in a separate sentence from the labs.) laboratory findings revealed normal electrolytes and creatinine; hemoglobin was 11.5 g/dl, and platelets were 277 K/µl. While carcinoembryonic antigen (CEA) and cancer antigen 19-9 (CA 19-9) levels were normal, the cancer antigen 125 (CA 125) was found to be elevated to 846 U/ml. An ultrasound revealed a normal intrauterine pregnancy (Fig. 1), and a left adnexal mass of heterogeneous echodensity of ~13.4 cm in the diameter at its largest side, which was distinct from the uterine mass, displaying characteristics of an ovarian neoplasm (Fig. 2). Magnetic resonance imaging (MRI) was recommended for further evaluation, and confirmed a large pelvic mass arising from the left adnexa, with ascites and demonstrated edema of the mesentery and omentum (Fig. 3).
Fig. 1.
Ultrasound showing intrauterine pregnancy.
Fig. 2.
Ultrasound showing left adnexal mass of heterogenous echodensity measuring around 13.4 cm in the largest diameter which was separate from the uterine mass.
Fig. 3.
Magnetic resonance imaging showed large pelvic mass arising from the left adnexa, ascites and demonstrated edema of the mesentery and omentum.
Physicians from the Maternal Fetal Medicine (high-risk obstetrics) and gynecology oncology departments discussed the findings with the patient and decided to continue the pregnancy while proceeding with surgical staging. However, a few days after admission and prior to the scheduled date for surgery, she had worsening abdominal pain and was found to be in active labor. The patient subsequently delivered a viable infant via vaginal delivery. Gestational age at birth was 23 weeks, with the female infant weighing 510 g and having Apgar scores of 2, 6, 7. There were no fetal anomalies and the infant was admitted to the neonatal intensive care unit for further care. Postpartum, the patient underwent an exploratory laparotomy, which revealed straw colored ascites upon entry and a large mass originating from the left ovary. The neoplasm had ruptured intraoperatively, and only the solid tumor was visible. The left ovary was processed for frozen section investigation, which revealed the presence of a Krukenberg tumor (Fig. 4). Subsequently, an abdominal examination noted that the omentum harbored marked reactive tissue, which indicated metastases, and a tumor was palpated along the lesser curvature of the stomach, which suggested a primary gastric cancer. Palliative gastrectomy was not performed.
Fig. 4.
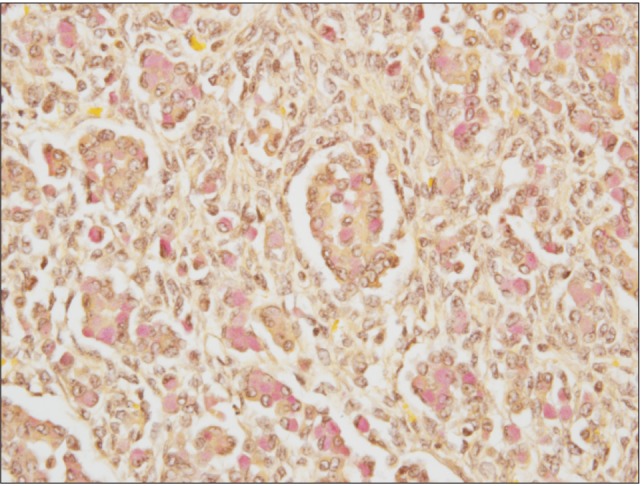
Ovarian tissue showing signet cell adenocarcinoma (mucicarmine stain positive, ×40).
Based on the intraoperative exams, an esophagogastroduodenoscopy was performed, which revealed patchy areas of erythema on the non-peristaltic stomach wall, with superficial ulceration along the lesser curvature (Fig. 5). Multiple biopsies were obtained that showed the presence of a poorly differentiated adenocarcinoma. The patient was diagnosed as having stage IV gastric cancer and was scheduled for palliative chemotherapy. She underwent multiple hospital admissions after the advanced-cancer diagnosis for reasons including development of submassive pulmonary embolism, seizures secondary to acute ischemic stroke, and hospital-associated pneumonia. She received 2 cycles of FOLFOX before succumbing to cancer.
Fig. 5.
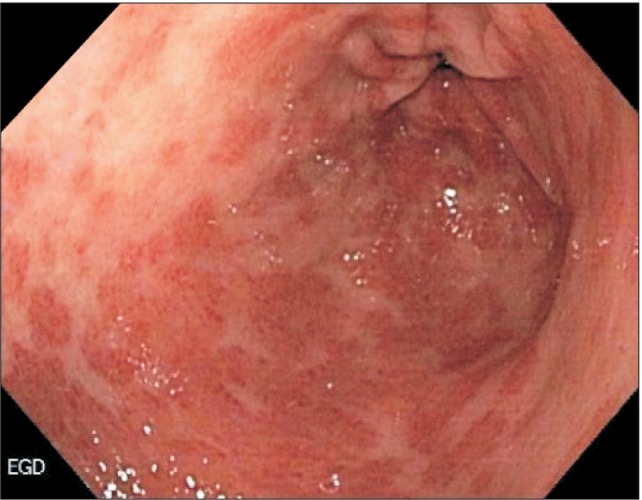
Esophagogastroduodenoscopy showing linitis plastica and multiple gastric erosions.
Discussion
The diagnosis of gastric cancer poses a challenge during pregnancy because of its extremely rare incidence, even while presenting with extremely common symptoms. Nausea and vomiting are common experiences during pregnancy, affecting 70% to 80% of all pregnant women.1 Gastric cancer presents with similar symptoms; however, it is rare even among other cancers that may occur during pregnancy. Smith et al.2 reported that the most frequent tumor types per 10,000 live singleton births were breast (1.3), thyroid (1.2), cervical (0.8), Hodgkin's disease and ovarian (each 0.5), acute and chronic leukemia (0.37), and lymphoma (0.28). Even in cases where the diagnosis is considered, confirmation through endoscopy and biopsy presents a dilemma: gastrointestinal endoscopy is inherently risky in pregnant patients because the fetus is particularly sensitive to maternal hypoxia and hypotension, either of which can lead to fetal demise.3 The American Society for Gastrointestinal Endoscopy recommends that the procedure be carried out only when there is a strong indication, and be postponed to the second trimester whenever possible.4
A Krukenberg tumor is an advanced presentation of gastric cancer and may be confused with other adnexal masses such as teratomas and corpus luteum cysts, which are more common during pregnancy. There are different clinical manifestations, as reported by Kiyokawa et al.5 who performed a clinicopathologic analysis of 120 Krukenberg tumors and found that abdominal swelling or pain usually accounted for the clinical presentation, while 17 patients had abnormal vaginal bleeding, 4 had virilization, and 4 had hirsutism without virilization. Ascites was present in 43% of the cases. Sixty-three percent of the tumors were documented to be bilateral. Two-thirds of the primary tumors were in the stomach; other primary sites in order of frequency were appendix, colon, breast, small intestine, rectum, gallbladder, and urinary bladder.
Among pregnant patients with Krukenberg tumors, Papantoniou et al.6 reported a case of excessive hirsutism during pregnancy, prompting clinical and laboratory investigation, which led to the diagnosis of a Krukenberg tumor. Similarly, Ozdegirmenci et al.7 reported a case of rapid onset of hirsutism and acne at 20 weeks' of gestation, and bilateral adnexal masses, which were thought to be pregnancy luteomas and were managed conservatively; however, upon onset of ascites and elevated tumor markers several months after delivery, the patient underwent exploratory laparotomy and was diagnosed with a Krukenberg tumor. The patient in our study presented with worsening abdominal pain, and was also found to have ascites. She had no evidence of virilization or hirsutism. Her tumor was unilateral and the primary cancer was in the stomach.
Given that the most common presentation is abdominal pain with or without ascites, imaging is essential in the workup. Ultrasonography and MRI are the modalities of choice for imaging of adnexal masses during pregnancy.8 Certain sonographic findings indicate a Krukenberg tumor. Shimizu and colleagues described the ultrasonographic appearance of the Krukenberg tumor in non-pregnant women. In their investigation, the tumors had distinct margins, an irregular hyperechoic solid pattern, and moth-eaten cyst formation.9
The role of tumor markers remains controversial. Pregnancy-associated pelvic masses are infrequently malignant, and the interpretation of these tumor markers varies with gestational age and comorbid conditions. Several of the tumor markers used to diagnose epithelial and non-epithelial ovarian cancers are difficult to interpret during pregnancy, because oncofetal antigens (e.g., alpha-fetoprotein, human chorionic gonadotropin, CEA, and CA 125) are involved in biological functions associated with fetal development, differentiation, and maturation. For instance, CA 125 is produced by normal tissues, including the endometrium, and may be elevated during early gestation and immediately following delivery10; however, markedly elevated CA 125 levels, which are more commonly observed during cancer, may serve as a tumor marker. Lower values may be pregnancy-related or may arise from inherently low CA 125 expression from the ovarian cancer; while CA 125 testing alone has low sensitivity and specificity, it may be used in combination with other findings.
The management remains a challenge because of the conflicting needs for immediate treatment, and the continuation of the pregnancy. A therapeutic plan should consider the gestational age, and should involve a multidisciplinary team comprising perinatal-obstetrics specialists and oncologists specializing in gastric cancers.11 Treatment should be individualized as there are no randomized controlled trials guiding therapy.
The overall prognosis of gastric cancer is often poor, given the delays in diagnosis and more advanced stages at presentation. In a study by Ueo et al.,12 of 61 pregnant Japanese women with gastric cancer, 59 cases (96.7%) were advanced, and resectability was consistently low (47.5%); only 20 (58.8%) patients underwent both obstetric treatment for the fetus and surgical treatment for the gastric cancer. The patients who received gastrectomy had a high incidence of in-hospital death (22.7%) and a poor prognosis, with a 21.1% 3-year survival rate.
Krukenberg tumors are rare during pregnancy, but generally portend a grave prognosis. Diagnosis is difficult because presenting symptoms are often attributed to the pregnancy and there are inherent risks to maternal and fetal outcomes when pursuing invasive testing. Worsening abdominal pain, new onset ascites, persistent hyperemesis gravidarum, and virilization should prompt the astute physician to pursue alternative diagnoses. Ultrasound and MRI are useful tools in characterizing adnexal masses, and may be used in conjunction with tumor markers. Timely diagnosis may improve individual outcomes.
Acknowledgments
The authors appreciate Gabor Tarjan, MD (Department of Pathology, John H. Stroger Jr. Hospital of Cook County, Chicago, IL 60612).
References
- 1.Lee NM, Saha S. Nausea and vomiting of pregnancy. Gastroenterol Clin North Am. 2011;40:309–334. doi: 10.1016/j.gtc.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith LH, Dalrymple JL, Leiserowitz GS, Danielsen B, Gilbert WM. Obstetrical deliveries associated with maternal malignancy in California, 1992 through 1997. Am J Obstet Gynecol. 2001;184:1504–1512. doi: 10.1067/mob.2001.114867. discussion 1512-1513. [DOI] [PubMed] [Google Scholar]
- 3.Kammerer WS. Nonobstetric surgery in pregnancy. Med Clin North Am. 1987;71:551–560. doi: 10.1016/s0025-7125(16)30858-6. [DOI] [PubMed] [Google Scholar]
- 4.ASGE Standard of Practice Committee. Shergill AK, Ben-Menachem T, Chandrasekhara V, Chathadi K, Decker GA, et al. Guidelines for endoscopy in pregnant and lactating women. Gastrointest Endosc. 2012;76:18–24. doi: 10.1016/j.gie.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 5.Kiyokawa T, Young RH, Scully RE. Krukenberg tumors of the ovary: a clinicopathologic analysis of 120 cases with emphasis on their variable pathologic manifestations. Am J Surg Pathol. 2006;30:277–299. doi: 10.1097/01.pas.0000190787.85024.cb. [DOI] [PubMed] [Google Scholar]
- 6.Papantoniou N, Belitsos P, Hatzipapas I, Rodolakis A, Papaspyrou I, Antsaklis A. Excessive hirsutism in pregnancy because of Krukenberg tumor. J Matern Fetal Neonatal Med. 2012;25:869–871. doi: 10.3109/14767058.2011.592879. [DOI] [PubMed] [Google Scholar]
- 7.Ozdegirmenci O, Kayikcioglu F, Haberal A, Ozfuttu A. Krukenberg tumor mimicking pregnancy luteoma. Gynecol Endocrinol. 2007;23:482–485. doi: 10.1080/09513590701532401. [DOI] [PubMed] [Google Scholar]
- 8.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin. Management of adnexal masses. Obstet Gynecol. 2007;110:201–214. doi: 10.1097/01.AOG.0000263913.92942.40. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu H, Yamasaki M, Ohama K, Nozaki T, Tanaka Y. Characteristic ultrasonographic appearance of the Krukenberg tumor. J Clin Ultrasound. 1990;18:697–703. [PubMed] [Google Scholar]
- 10.Sarandakou A, Protonotariou E, Rizos D. Tumor markers in biological fluids associated with pregnancy. Crit Rev Clin Lab Sci. 2007;44:151–178. doi: 10.1080/10408360601003143. [DOI] [PubMed] [Google Scholar]
- 11.Sakamoto K, Kanda T, Ohashi M, Kurabayashi T, Serikawa T, Matsunaga M, et al. Management of patients with pregnancy-associated gastric cancer in Japan: a mini-review. Int J Clin Oncol. 2009;14:392–396. doi: 10.1007/s10147-009-0903-6. [DOI] [PubMed] [Google Scholar]
- 12.Ueo H, Matsuoka H, Tamura S, Sato K, Tsunematsu Y, Kato T. Prognosis in gastric cancer associated with pregnancy. World J Surg. 1991;15:293–297. doi: 10.1007/BF01659068. discussion 298. [DOI] [PubMed] [Google Scholar]