Abstract
More than 20 years have passed without the launch of a new substance class for acute migraine therapy. Triptans were the latest class of substances which successfully passed all developmental stages with a significant antimigraine efficacy and a sufficient safety profile. New drugs with a better adverse event profile and at least similar efficacy are needed for migraine subjects who cannot tolerate triptans for attack treatment. Lasmiditan is a novel highly specific 5-HT1F receptor agonist currently in clinical trials for acute migraine therapy and devoid of vasoconstriction in coronary arteries as determined in a surrogate assay. In both phase II randomized, placebo-controlled trials in acute migraine the primary endpoint was met. For the intravenous formulation a clear dose-dependent effect on headaches could be determined. Lasmiditan tablets in doses of 50–400 mg show significant headache relief after 2 hours compared with placebo and improved accompanying symptoms. This substance is chemically clearly different from other antimigraine drugs, which is also reflected by its dose-dependent adverse event profile chiefly including dizziness, vertigo, paresthesia and fatigue. Adverse events are usually linked to the central nervous system. Future phase III clinical trials with an active triptan comparator or in a preferential trial design will allow a better comparison of lasmiditan and triptans. They will also determine whether lasmiditan will become available to the migraine patient.
Keywords: migraine, serotonin, receptor, pharmacology, clinical trials
Introduction
While there is room for new specific preventative antimigraine drugs, some believe that no further developments for the acute treatment are necessary. Migraine is sometimes seen as a lifestyle disease of the modern Western world based in part on the findings that migraine prevalence is highest in the technically most advanced societies such as the US and Europe [Fernández-de-Las-Peñas et al. 2010; Breslau and Rasmussen, 2001]. This view is supported by the observation that nonpharmacological treatments using muscle relaxation, sports or biofeedback techniques can reduce the frequency of attacks [Nicholson et al. 2011]. While there is little doubt that stress influences the generation of migraine attacks, the basis for this disorder is a recurrent dysfunction of the trigeminovascular system. This disorder significantly affects the life of affected people and is ranked among the most disabling diseases by the World Health Organization (WHO) [WHO, 2002].
Others might believe that we have a sufficient number of antimigraine drugs available. Indeed, the introduction of the triptans into the market in the early 1990s was a tremendous step forward for acute migraine treatment. Triptans are clearly an example for a success story in the eyes of affected people and physicians [Ferrari et al. 2001]. They are effective drugs and have significantly fewer side effects compared with ergot alkaloids [Tepper and Millson, 2003], which had been widely used for acute antimigraine therapy prior to the triptan era. There are several other acute antimigraine drugs available such as paracetamol, acetylsalicylic acid, metamizol, ibuprofen, indomethacin as monotherapy and in combination with each other or caffeine. Most of these drugs are available over the counter (OTC) including some of the triptans. Prices for triptans have come down over time in Europe, especially with the introduction of generic drugs after the end of patent protection. State-of-the-art acute migraine treatment is therefore accessible for everybody in the Western world.
Triptans are agonists of the 5-HT1B/D receptors and typically are used when subjects do not respond to the other OTC antimigraine drugs. Seven different oral triptans exist, but not all migraine patients are happy with them [Tfelt-Hansen and Olesen, 2012b]. In up to 25% of patients none of the triptans is effective and in other subjects they do not work consistently. Although they are safe drugs, triptans can cause uncomfortable side effects such as muscle pain, chest tightness and paresthesia. According to the American Migraine Prevalence and Prevention Study about 40% of episodic migraine patients still have unmet treatment needs [Lipton et al. 2011]. Two reasons were mentioned most frequently in this study: dissatisfaction with current migraine medication (15%) and headache related disability (19%).
The use of triptans is limited by their potential to constrict blood vessels via 5-HT1B receptors which are located on smooth muscle cells [Tfelt-Hansen et al. 2000]. Cardiovascular disease, uncontrolled hypertension, and certain forms of migraine (e.g. hemiplegic migraine) are conditions which do not allow the use of a triptan because of potential ischemia. Although the risk for cardiovascular events is very low, rare cases of stroke, myocardial infarction and arrhythmia have been reported [Abbrescia et al. 1997; O’Connor and Gladstone, 1995; Cavazos et al. 1994; Ribeiro et al. 2012]. Depression is a common comorbidity in migraine but the intake of serotonin reuptake inhibitors is a contraindication for triptan use due to the theoretical possibility of serotonergic syndrome [Rolan, 2012]. Together, these findings show that new acute antimigraine drugs with a different adverse event (AE) profile are needed. In particular, drugs without vasoactive properties are wanted for use in subjects with cardiovascular disease and older people, who are naturally at a higher risk for vascular events.
Substances with a superior vascular profile compared with triptans and distinctly different AEs are of interest. Calcitonin gene related peptide (CGRP) antagonists, which seem to be suitable for acute and preventive migraine therapy, have been studied recently [Ho et al. 2010; Diener et al. 2011; Olesen et al. 2004]. Although one of them has advanced into phase III clinical trials, none of them is available for treatment. The development of telcagepant was stopped late in the development process because of elevated liver enzymes after repetitive intake. Olcagepant and BIBN44370 TA were put aside for other reasons. Newer generation CGRP receptor blockers such as the antibody AMG 334 are assessed in cinical trials not for acute but for preventative migraine treatment. Assessed in clinical trials in the near future. The development of CGRP receptor antagonists was hypothesis driven and so is the development of new selective 5-HT1F receptor agonists.
More recent experimental evidence suggests that migraine is a primary neuronal disease [Pietrobon and Moskowitz, 2012; Akerman et al. 2011]. Temporary neuronal dysfunction within the brain is believed to activate the trigeminal nerve system by noxious stimuli resulting in neuropeptide release (CGRP). As a consequence blood flow and vessel diameter may change in the meninges and cortex, thereby being probably an epiphenomenon. Based on the observation that 5-HT1F receptors are expressed on the trigeminal ganglion and secondary trigeminal neurons in the brainstem [Adham et al. 1997], the hypothesis was generated that the stimulation of the 5-HT1F receptor with a selective agonist inhibits central and peripheral neuronal activity [Mitsikostas et al. 1999] and the release of CGRP thereby terminating acute migraine. Drugs which fulfil these criteria and are devoid of vasoconstriction are called neurally acting antimigraine agents (NAAMAs). Lasmiditan is the first drug to be called so.
5-HT1F receptor agonists
Lasmiditan (formerly known as COL-144) is the newest, highly selective 5-HT1F receptor agonist and currently in clinical assessment. In addition to targeting peripheral 5-HT1F receptors, lasmiditan is centrally acting as it penetrates the blood–brain barrier (BBB). Lasmiditan is superior to other 5-HT1F receptor agonists such as LY-334370 because of a much higher selectivity for the 5-HT1F receptor, no activity at 5-HT1B/D receptors and extremely low affinity for the 5-HT1A receptor [Nelson et al. 2010]. In vivo binding studies showed a >470-fold selectivity for the 5-HT1F receptor than for the 5-HT1B/D receptor (Table 1). The receptor binding affinity of lasmiditan was tested on human recombinant 5-HT receptor subtypes. By using the [35S]GTPγS assay, a standard radioligand-binding assay, selective functional activity was demonstrated. Lasmiditan showed an activity profile clearly different to that from triptans, which is in line with its chemical structure. It does not contain the typical triptan indol core, which was also the chemical basis for LY334370 and other less selective 5-HT 1F receptor agonists [Yu, 2008]. Therefore, lasmiditan is chemically more different from triptans than its predecessors and a member of a novel class called ‘ditans’.
Table 1.
Binding affinity of lasmiditan (n > 5) at human 5-HT receptors [Nelson et al. 2010]; Ki (nM).
| 5-HT1A | 5-HT1B | 5-HT1D | 5-HT1F | 5-HT2A | 5-HT2B | 5-HT2C | 5-HT6 | 5-HT7 |
|---|---|---|---|---|---|---|---|---|
| 1053 ± 134 | 1043 ± 124 | 1357 ± 156 | 2.21 ± 0.22 | >5 µM | >2 µM | >3 µM | >4 µM | >3 µM |
Most notably, lasmiditan does not cause vasoconstriction in the rabbit saphenous vein in doses up to 100 µM [Nelson et al.2010]. This is a surrogate assay that predicts vasoconstriction in human coronary arteries based on a similar serotonin receptor (5-HT1) expression. LY334370, which is less selective for the 5-HT1F receptor than lasmiditan, also does not constrict the saphenous vein [Shepheard et al. 1999]. In contrast, triptans lead to a contractile response in this assay probably due to activation of the 5-HT1B receptor. In concentrations in which triptans cause a 50% vessel diameter constriction lasmiditan was without effect. Sumatriptan-induced vasoconstriction started at doses of 10−7 µM [Longmore et al. 1997; Cohen et al. 1997]. Based on this observation, which is of importance for the AE profile, lasmiditan should be studied in human coronary angiography to exclude coronary vasoconstriction beyond any doubt.
5-HT1F receptor protein cannot be found on endothelial or smooth muscle cells of cerebral vessels, which leads to the conclusion that this serotonin receptor subtype is not involved in the regulation of the vascular tone in the human brain [Neeb et al. 2010].
Lasmiditan was studied in experimental animals in assays, which are believed to have predictive value for drug efficacy in acute migraine treatment. In one of these assays, the stimulus-induced expression of a proto-oncogene (c-fos) in the trigeminal brain stem complex is assessed in experimental animals. Several antimigraine drugs block induced c-fos expression and so does mechanical and chemical destruction of primary trigeminal afferents [Mitsikostas and Sanchez del Rio, 2001]. Because sumatriptan does not cross the BBB under physiological conditions, its mode of action in this model is most likely due to the binding to peripheral 5-HT1B/D receptors on trigeminal afferents. The 5-HT1F receptor agonist LY334370 (10 µg/kg) and others also abort c-fos expression. In line, oral doses of lasmiditan of 3 mg or higher successfully blocked c-fos expression without clear differences between doses. While lasmiditan 1 mg and placebo were without a significant effect on c-fos expression, doses of 10 mg and 100 mg were clearly superior to a dose of lasmiditan (1 mg) and placebo. Lasmiditan penetrates the BBB and could therefore block c-fos expression by activating centrally located 5-HT1F receptors on trigeminal neurons. Alternatively, 5-HT1F receptors outside the central nervous system (CNS) on primary trigeminal afferents or cell bodies within the trigeminal ganglion could mediate the effect of lasmiditan. Both mechanisms could also be active at the same time. In summary, lasmiditan is able to block the activation of second-order trigeminal neurons, which is a crucial mechanism in acute migraine pathophysiology.
By using the plasma protein leakage model it was demonstrated that 5-HT1F receptor agonists can reduce primary trigeminal afferent activation. In this model chemical, immunological or electrical stimulation of the trigeminal ganglion cause plasma protein leakage within minutes from veins within the dura mater of experimental animals [Markowitz et al. 1987]. This assay was believed for many years to predict the antimigraine efficacy of substances. While all currently available acute antimigraine drugs are efficacious in this model, not all substances that block leakage are effective acute antimigraine substances [Peroutka, 2005]. Strongest inhibition of protein leakage was found with the 5-HT1F agonist LY334370 followed in a rank-order by naratriptan > zolmitriptan > dihydroergotamine > sumatriptan > rizatriptan. For 5-HT1F receptor agonists it has been established that their potency to abort protein extravasation is correlated with their receptor affinity when given intravenously. In contrast, there was no correlation between potency and receptor affinity for the 5-HT1B and 5-HT1D receptor agonists [Phebus et al. 1997; Johnson et al. 1997].
Lasmiditan was successfully studied in this model. In one study comparing oral lasmiditan with oral rizatriptan, the latter was inferior to the selective 5-HT1F receptor agonist. Orally administered lasmiditan in doses of 0.0004 µg/kg completely aborted leakage while a rizatriptan dose of 5–10 µg/kg was necessary to achieve the same effect [Nelson et al. 2010]. Based on these findings it can be concluded that blockade of protein leakage is mediated by the activation of peripheral 5-HT1F receptors. This mechanism can contribute to the clinical antimigraine activity of lasmiditan. For a review please also see Mitsikostas and Tfelt-Hansen [Mitsikostas and Tfelt-Hansen 2012] or Lionetto and colleagues [Lionetto et al. 2012].
Human studies
Lasmiditan is still in development and phase III clinical trials for acute migraine treatment have not been performed to date. Four phase I studies in healthy individuals and two phase II clinical trials in subjects with acute migraine were successfully conducted. It should not be noted that another substance-LY 334370 was the first 5-HT1F receptor agonist which was studied successfully in acute migraine [Goldstein et al. 2001]. However, in effective doses this substance also expressed significant activity on other 5-HT1 receptors in contrast to lasmiditan.
In autumn 2012 new data confirmed cardiac safety for lasmiditan (COL MIG-105 study [CoLucid, 2012]). Lasmiditan did not cause QT prolongation in oral doses of 100 and 400 mg in healthy individuals in a comparator trial. In this study, the antibiotic moxifloxacin led to QT prolongation as this drug did in previous studies. Lasmiditan also did not cause arrhythmia or any pro-arrhythmic effects. These data add significantly to our knowledge of the safety profile of lasmiditan.
Lasmiditan has been evaluated for acute migraine treatment in two multicenter clinical trials. In a first proof of concept trial lasmiditan was administered intravenously, while in the second trial rapid disintegrating tablets were given.
The proof of concept trial was performed as a multicenter, double-blind, placebo-controlled phase II study [Ferrari et al. 2010]. Subjects with acute migraine attended to hospital or medical practice and lasmiditan was administered intravenously. It was the main goal of this study to determine a dose-response relationship using a group sequential adaptive treatment design. If successful then a specific, exclusive activation of 5-HT1F receptors would be sufficient to abort acute migraine. The sequential treatment design allows a fast reach of the most effective tolerable drug dose [Hall et al. 2005]. In this trial mainly females were included with a mean age of 40 years with one to eight migraine attacks per month. Subjects were not on prophylaxis. Placebo or lasmiditan was infused over 20 minutes and subjects were monitored for 4 hours after infusion for ECG, vital signs, AEs and headache and other migraine symptoms. The primary endpoint was headache response after 2 hours defined as improvement from moderate and severe headaches to mild or no headache.
A total of 130 subjects were randomized to the following lasmiditan treatment groups: 2.5, 5, 10, 20, 30 or 45 mg (n = 88) or placebo (n = 42). The primary endpoint of this study was reached: there was a linear association between lasmiditan doses and headache response (p = 0.0126). In the lasmiditan group 54–75% of subjects showed a response versus 45% in the placebo group. Lasmiditan doses of 2.5 and 5 mg were not superior to placebo. All other doses were numerically superior to placebo. When analyzing these results one must bear in mind that this study was not designed to show superiority of one particular dose versus placebo. The onset of action was fast with doses of 20 mg and higher starting to separate from placebo after 20 minutes. This is a remarkable result, as subjects were further in to their migraine attack and pain was most likely more severe due to travel to hospital or practice. Usually antimigraine drugs work better when taken early, as shown in a trial with almotriptan [Goadsby et al. 2008]. ECGs, vital signs, hematology and clinical chemistry did not reveal pathological findings.
Because intravenous treatment is not practical and migraine is usually self-treated outside a medical environment, an oral formulation of lasmiditan was generated. Based on pharmacodynamic/pharmacokinetic (PD/PK) modeling from previous studies, oral doses of 100–400 mg were expected to have significant antimigraine activity [Liefaard et al. 2009].
In a larger multicenter, double-blind, placebo-controlled parallel-group, dose-ranging study rapidly disintegrating tablets of lasmiditan were used for acute migraine treatment [Färkkilä et al. 2012]. This formulation shows rapid absorption, oral bioavailability of 40% and linear PK (Figure 1) [Pilgrim et al. 2009]. Subjects with migraine with and without aura who were not on preventative drugs were studied. Each treatment group consisted of 64–82 mostly female subjects. Data from 310 subjects who received lasmiditan in doses of 50, 100, 200, 400 mg or placebo (n = 81) in an identical formulation were incorporated in to the final analysis. Subjects were randomized to one of the treatment groups in a 1:1:1:1:1 ratio. Based on the PD/PK modeling the 50 mg dose was expected to have no effect.
Figure 1.
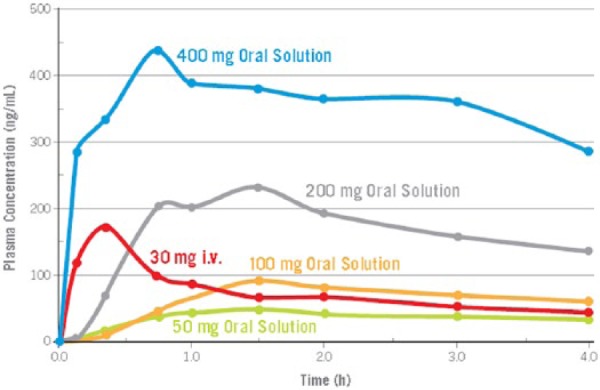
Time to reach peak plasma concentration after lasmiditan i.v. and different oral doses [Pilgrim et al. 2009].
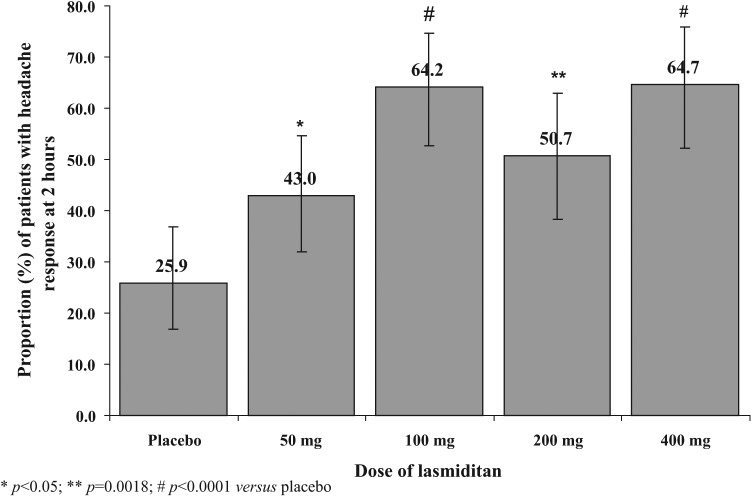
The primary endpoint of this study was a dose–response relationship for the improvement of migraine headache at 2 hours. For lasmiditan, a significant linear association between headache response rate and dose was observed (Figure 2). Every lasmiditan dose was superior to placebo for headache response (increasing doses: 43.0% [31.9–54.7], 64.2% [52.8–74.6], 50.7% [38.4–63.0], 64.7% [52.2–75.9] versus placebo 25.9% [18.8–36.9]). The lasmiditan 400 mg dose was also superior to the 50 mg dose (Pearson’s chi-square test, p < 0.03 for all comparisons).
Figure 2.
Headache response 2 hours after oral lasmiditan (50–400 mg) or placebo.
Secondary endpoints also revealed benefits for lasmiditan (Table 2): a linear trend was observed between headache free rates at 2 hours and lasmiditan dose (Cochran–Armitage test, p = 0.0006). In contrast to the 100 mg dose both the 200 mg (18.8%) and 400 mg (27.9%) doses of lasmiditan were superior to placebo (p < 0.04 for both comparisons). The number of subjects who were pain free after 2 hours was a bit lower than in trials with some of the triptans, e.g. rizatriptan.
Table 2.
Secondary endpoints.
| Headache response at 2 h | Placebo (n = 81) |
Lasmiditan 50 mg (n = 79) |
Lasmiditan 100 mg (n = 81) |
Lasmiditan 200 mg (n = 69) |
Lasmiditan 400 mg (n = 68) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Response n; % [95% CI] | p- value | Response n; % [95% CI] | p- value | Response n; % [95% CI] | p-value | Response n; % [95% CI] | p-value | Response n;% [95% CI] | p-value | |
| Pain free at 2 h | ||||||||||
| Versus Placebo | 6; 7.4 [2.8–15.4] | n.a. | 10; 13.9 [7.2–23.5] | 0.286 | 8; 13.8 [7.1–23.3] | 0.190 | 10; 19.2 [10.6–30.5] | 0.0322 | 14; 28.4 [18.0–40.7] | 0.0007 |
| Rescue medication 2–24 h | ||||||||||
| Versus placebo | 55; 68.8 [57.4–78.7] | n.a. | 42; 54.5 [42.8–65.9] | 0.067 | 42; 51.9 [40.5–63.1] | 0.0285 | 41; 61.1 [48.5–72.9] | 0.3378 | 28; 41.8 [29.8–54.5] | 0.0010 |
| Patients global impression at 2 h | ||||||||||
| Versus placebo | 13; 16.0 [8.8–25.9] | n.a. | 18; 22.8 [14.1–33.6] | 0.281 | 29; 35.8 [25.4–47.2] | 0.0041 | 19; 27.5 [17.5–39.6] | 0.087 | 23; 34.3 [23.2–46.9] | 0.0099 |
| Clinical disability score at 2 h | ||||||||||
| Versus placebo | 81; 1.0 [1.7–2.2] | n.a. | 79; 1.5 [1.32–1.8] | 0.01 | 78; 1.35 [1.1–1.6] | 0.0002 | 66; 1.5 [1.2–1.7] | 0.0081 | 63; 1.46 [1.2–1.7] | 0.0039 |
p < 0.05 = statistically significant. CI, confidence interval.
The onset of action was fast. Lasmiditan reduced headache severity starting as early as 30 minutes in the 400 mg group versus placebo (Cochran–Mantel–Haenszel [CMH] mean score test, p = 0.0137). After 1 hour all but the lowest dose of lasmiditan (50 mg) were superior to placebo, and from 1.5 to 4 hours, all lasmiditan groups were superior. The therapeutic gain (2 h) for oral lasmiditan 100 mg was 38% (95% confidence interval [CI] 28–51%).
Patient global impression rating of much or very much better were obtained from more subjects in the lasmiditan groups than after placebo (linear association with dose; CMH correlation test, p = 0.0162), which indicates that not only headaches but also other symptoms improved. In line, lasmiditan led to an improvement of migraine associated symptoms such as phonophobia and photophobia and nausea.
In a recent manuscript Tfelt-Hansen compared headache relief of lasmidtian to LY 334360 and sumatriptan [Tfelt-Hansen and Olesen, 2012a]. The authors conclude that the absolute response to oral lasmiditan is similar to results with oral triptans. Further placebo-controlled studies with an active comparator are needed to validate this analysis. The number needed to treat (NNT) seems to be slightly lower than with sumatriptan 100 mg tablets which also points to similar efficacy of both drugs. However, since we have only data from one clinical trial with an oral formulation of the drug we should wait for results from further trials to be sure about the NNT.
Safety
One serious AE was reported in all published lasmiditan trials to date. One female experienced dizziness 30 min after the intake of a 200 mg lasmiditan disintegrating tablet. She had to be admitted to hospital. Bradycardia was seen at 1.5 and 4 h in the ECG but clinical assessment did not reveal any other pathological findings. She recovered completely and was discharged from hospital the next day.
In all studies the side effects of lasmiditan were clearly different from triptan AEs, thereby underscoring the different chemical nature of this drug. Typical triptan-associated complaints in acute migraine trials such as chest or neck pain, tightness or heaviness were rare and did not differ between placebo and active drug [Ferrari et al. 2010; Färkkilä et al. 2012]. AEs were dose-dependent across all lasmiditan trials (Table 3).
Table 3.
Treatment-emergent adverse events.
| Treatment-emergent adverse event, N (%) | Dose |
||||
|---|---|---|---|---|---|
| Placebo |
50 mg |
100 mg |
200 mg |
400 mg |
|
| (N = 19/86; 22%) | (N = 53/82; 65%) | (N = 59/82; 72%) | (N = 61/71; 86%) | (N = 59/70; 84%) | |
| Sensation of heaviness | 1 (1.2) | 4 (4.9) | 4 (4.9) | 7 (9.9) | 5 (7.1) |
| Nausea | 0 (0.0) | 4 (4.9) | 8 (9.8) | 2 (2.8) | 5 (7.1) |
| Paresthesia | 2 (2.3) | 2 (2.4) | 9 (11.0) | 12 (16.9) | 14 (20.0) |
| Somnolence | 2 (2.3) | 8 (9.8) | 10 (12.2) | 8 (11.3) | 8 (11.4) |
| Vertigo | 1 (1.2) | 8 (9.8) | 12 (14.6) | 12 (16.9) | 16 (22.9) |
| Fatigue | 2 (2.3) | 10 (12.2) | 17 (20.7) | 15 (21.1) | 16 (22.9) |
| Dizziness | 0 (0.0) | 19 (23.2) | 21 (25.6) | 27 (38.0) | 26 (37.1) |
In the latest phase II trial treatment-emergent adverse events (TEAEs) were reported by 65%, 72%, 86%, and 84% of subjects in the lasmiditan 50 mg, 100 mg, 200 mg, and 400 mg groups, respectively [Färkkilä et al. 2012]. After placebo 22% of subjects experienced a TEAE. CNS symptoms were the most frequent adverse events probably due to the good CNS permeability of lasmiditan. Dizziness and paresthesia were the chief complaints. Vertigo was also mentioned which could be of central or peripheral origin. 5-HT1F receptors are located in the cerebellum and the lateral vestibular nucleus. Activation of these by lasmiditan could contribute to the aforementioned unwanted effects.
The AEs were mostly mild or moderate [Färkkilä et al. 2010]. Between 19% and 44% of subjects reported severe AEs after lasmiditan tablets and 5.8% after placebo. Dizziness was the predominant severe AE. Fatigue was also more frequent with increasing lasmiditan doses (2% placebo; 12%, 21%, 21% and 23% for 50, 100, 200 and 400 mg lasmiditan) as was paresthesia.
In the intravenous study AEs were reported by 43% versus 65% of subjects after placebo or lasmiditan [Ferrari et al. 2010]. Limb heaviness, dizziness and paresthesia were most frequently mentioned. The number of AEs increased with increasing doses. Short-lasting dizziness was reported in 8–50% of subjects without a clear difference between placebo and COL-144 groups AEs were generally mild in this study. The number of AEs in the placebo group which was unexpectedly high might be due to the intravenous application mode.
Summary
In summary, lasmiditan is a new drug without vasoactive properties which is in clinical trials for the treatment of migraine. Published studies clearly show the superiority of lasmiditan over placebo in acute migraine. The side effect profile needs to be dissected out more precisely in future trials to find a dose with maximal efficacy but still tolerable side effects.
Lasmiditan is also an interesting substance which can help to further dissect out whether vasoconstriction is beneficial for acute migraine. Adding a triptan to lasmiditan in a comparator four-armed study would tell us whether triptan-mediated vasoconstriction enhances treatment response. If so then a drug with a nonvascular mechanism could help to clarify the meaning of vasoconstriction for acute migraine therapy.
Footnotes
Conflict of interest statement: UR has received honoraria for the participation in advisory boards, oral presentations or contributions to clinical trials from Pharm Allergan, Amgen, Almirall, Autonomic technologies, Astra Zeneca, Berlin Chemie, Böhringer Ingelheim, Co-Lucid, ElectroCore, Haas & Health, MSD Sharp and Dohme, Janssen Cilag, GSK, Pfizer. UR;HI and LN have no ownership interest and do not own stock in any pharmaceutical company. HI and LN have received honoraria from Pharm Allergan and Autonomic technologies.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Contributor Information
Uwe Reuter, Hochschulambulanz und Klinik für Neurologie, Charité-Universitätsmedizin Berlin, Chariteplatz 1, 10117 Berlin, Germany.
Heike Israel, Department of Neurology, Charite Universitätsmedizin Berlin, Berlin, Germany.
Lars Neeb, Department of Neurology, Charite Universitätsmedizin Berlin, Berlin, Germany.
References
- Abbrescia V., Pearlstein L., Kotler M. (1997) Sumatriptan-associated myocardial infarction: report of case with attention to potential risk factors. J Am Osteopath Assoc 97: 162–164. [DOI] [PubMed] [Google Scholar]
- Akerman S., Holland P., Goadsby P. (2011) Diencephalic and brainstem mechanisms in migraine. Nat Rev Neurosci 12: 570–584. [DOI] [PubMed] [Google Scholar]
- Breslau N., Rasmussen B. (2001) The impact of migraine: epidemiology, risk factors, and co-morbidities. Neurology 6: S4-S12. [DOI] [PubMed] [Google Scholar]
- Cavazos F., Caress P., Chilukuri V., Devlin T., Gray L., Hurwitz B.J., et al. (1994) Sumatriptan-induced stroke in sagittal sinus thrombosis. Lancet 343: 1105–1106. [DOI] [PubMed] [Google Scholar]
- Cohen M., Johnson K., Schenck K., Phebus L. (1997) Migraine therapy: relationship between serotonergic contractile receptors in canine and rabbit saphenous veins to human cerebral and coronary arteries. Cephalalgia 17: 631–638. [DOI] [PubMed] [Google Scholar]
- CoLucid (2012) CoLucid Pharmaceuticals Details Phase 3 Development Strategy for Lasmiditan to Address Major Unmet Needs in Acute Migraine Therapy. http://www.colucid.com/news_and_media/view_item/16-colucid_pharmaceuticals_details_phase_3_development_strategy_for_lasmiditan_to_address_major_unmet_needs_in_acute_migraine_therapy
- Diener H., Barbanti P., Dahlöf C, et al. (2011) BI 44370 TA, an oral CGRP antagonist for the treatment of acute migraine attacks: results from a phase II study. Cephalalgia 31: 573–584. [DOI] [PubMed] [Google Scholar]
- Fernández-de-Las-Peñas C., Hernández-Barrera V., Carrasco-Garrido P., et al. (2010) Population-based study of migraine in Spanish adults: relation to socio-demographic factors, lifestyle and co-morbidity with other conditions. J Headache Pain 11: 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari M., Färkkilä M., Reuter U., et al. (2010) Acute treatment of migraine with the selective 5-HT1F receptor agonist lasmiditan - a randomised proof-of-concept trial. Cephalalgia 30: 1170–1178. [DOI] [PubMed] [Google Scholar]
- Ferrari M., Roon K., Lipton R., et al. (2001) Oral triptans (serotonin 5-HT(1B/1D) agonists) in acute migraine treatment: a meta-analysis of 53 trials. Lancet 358: 1668–1675. [DOI] [PubMed] [Google Scholar]
- Färkkilä M., Diener H., Géraud G., et al. (2012) Efficacy and tolerability of lasmiditan, a new oral 5-HT1F receptor agonist for the acute treatment of migraine: a phase II randomised, placebo-controlled, parallel group dose ranging study. Lancet Neurol 11: 405–413. [DOI] [PubMed] [Google Scholar]
- Goadsby P., Zanchin G., Geraud G., et al. (2008) Early versus non-early intervention in acute migraine-’Act when Mild (AwM)’. A double-blind, placebo-controlled trial of almotriptan. Cephalalgia 28: 383–391. [DOI] [PubMed] [Google Scholar]
- Goldstein D., Roon K., Offen W., et al. (2001) Selective serotonin 1F (5-HT(1F)) receptor agonist LY334370 for acute migraine: a randomised controlled trial. Lancet 358: 1230–1234. [DOI] [PubMed] [Google Scholar]
- Hall D., Meier U., Diener H. (2005) A group sequential adaptive treatment assignment design for proof of concept and dose selection in headache trials. Contemp Clin Trials 26: 349–363. [DOI] [PubMed] [Google Scholar]
- Ho T., Edvinsson L., Goadsby P. (2010) CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol 6: 573–582. [DOI] [PubMed] [Google Scholar]
- Johnson K., Schaus J., Durkin M., et al. (1997) 5-HT1F receptor agonists inhibit neurogenicdural inflammation in guinea pigs. Neuroreport 8: 2237–2240. [DOI] [PubMed] [Google Scholar]
- Liefaard L., Drenth H., Pilgrim A., et al. (2009) Prediction of therapeutically effective dose of COL-144 based on relationship between plasma concentrations and headache response (poster). Cephalalgia 29: 24–29. [Google Scholar]
- Lionetto L., Negro A., Palmisani S., et al. (2012) Emerging treatment for chronic migraine and refractory chronic migraine. Expert Opin Emerg Drugs 17: 393–406. [DOI] [PubMed] [Google Scholar]
- Lipton R., Buse D., Serrano D., et al. (2011) Examination of unmet treatment needs among persons with episodic migraine: results of the American Migraine Prevalence and Prevention study. Eur J Neurol 18: 66–343. [DOI] [PubMed] [Google Scholar]
- Longmore J., Hargreaves R., Boulanger C., et al. (1997) Comparison of the vasoconstrictor properties of the 5-HT1D-receptor agonists rizatriptan (MK-462) and sumatriptan in human isolated coronary artery: outcome of two independent studies using different experimental protocols. Funct Neurol 12: 3–9. [PubMed] [Google Scholar]
- Markowitz S., Saito K., Moskowitz M. (1987) Neurogenically mediated leakage of plasma protein occurs from blood vessels in dura mater but not brain. J Neurosci 7: 4129–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsikostas D., Sanchez del Rio M. (2001) Receptor systems mediating c-fos expression within trigeminal nucleus caudalis in animal models of migraine. Brain Res Brain Res Rev 35: 20–35. [DOI] [PubMed] [Google Scholar]
- Mitsikostas D., Sanchez del Rio M., Moskowitz M., Waeber C. (1999) Both 5-HT1B and 5-HT1F receptors modulate c-fos expression within rat trigeminal nucleus caudalis. Eur J Pharmacol 369: 271–277. [DOI] [PubMed] [Google Scholar]
- Mitsikostas D., Tfelt-Hansen P. (2012) Targeting to 5-HT1F receptor subtype for migraine treatment: lessons from the past, implications for the future. Cent Nerv Syst Agents Med Chem 12: 241–249. [DOI] [PubMed] [Google Scholar]
- Neeb L., Meents J., Reuter U. (2010) 5-HT1F Receptor agonists: a new treatment option for migraine attacks? Neurotherapeutics 7: 176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D., Phebus L., Johnson K., et al. (2010) Preclinical pharmacological profile of the selective 5-HT1F receptor agonist lasmiditan. Cephalalgia 30: 1159–1169. [DOI] [PubMed] [Google Scholar]
- Nicholson R., Buse D., Andrasik F., Lipton R. (2011) Nonpharmacologic treatments for migraine and tension-type headache: how to choose and when to use. Curr Treat Options Neurol 13: 28–40. [DOI] [PubMed] [Google Scholar]
- O’Connor P., Gladstone P. (1995) Oral sumatriptan-associated transmural myocardial infarction. Neurology 45: 2274–2276. [DOI] [PubMed] [Google Scholar]
- Olesen J., Diener H., Husstedt IW., et al. (2004) Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med 350: 1104–1110. [DOI] [PubMed] [Google Scholar]
- Peroutka S. (2005) Neurogenic inflammation and migraine: implications for the therapeutics. Mol Interv 5: 304–311. [DOI] [PubMed] [Google Scholar]
- Phebus L., Johnson K., Zgombick J., et al. (1997) Characterization of LY344864 as a pharmacological tool to study 5-HT1F receptors: binding affinities, brain penetration and activity in the neurogenic dural inflammation model of migraine. Life Sci 61: 2117–2126. [DOI] [PubMed] [Google Scholar]
- Pietrobon D., Moskowitz M. (2012) Pathophysiology of migraine. Annu Rev Physiol 75: 365–391. [DOI] [PubMed] [Google Scholar]
- Pilgrim A., Dussault B., Rupniak N., et al. (2009) COL-144, an orally bioavailable selective 5-HT1F receptor agonist for acute migraine therapy. Cephalalgia 29: 24–25. [Google Scholar]
- Ribeiro H., Batista A., Ferreira C., et al. (2012) Myocardial infarction after taking zolmitriptan. Rev Port Cardiol 31: 167–169. [DOI] [PubMed] [Google Scholar]
- Rolan P. (2012) Drug interactions with triptans: which are clinically significant? CNS Drugs 26: 949–957. [DOI] [PubMed] [Google Scholar]
- Shepheard S., Edvinsson L., Cumberbatch M., et al. (1999) Possible antimigraine mechanisms of action of the 5HT1F receptor agonist LY334370. Cephalalgia 19: 851–858. [DOI] [PubMed] [Google Scholar]
- Tepper S., Millson D. (2003) Safety profile of the triptans. Expert Opin Drug Saf 2: 123–132. [DOI] [PubMed] [Google Scholar]
- Tfelt-Hansen P., De Vries P., Saxena P. (2000) Triptans in migraine: a comparative review of pharmacology, pharmacokinetics and efficacy. Drugs 60: 1259–1287. [DOI] [PubMed] [Google Scholar]
- Tfelt-Hansen P., Olesen J. (2012a) The 5-HT1F receptor agonist lasmiditan as a potential treatment of migraine attacks: a review of two placebo-controlled phase II trials. J Headache Pain 13: 271–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tfelt-Hansen P., Olesen J. (2012b) Taking the negative view of current migraine treatments: the unmet needs. CNS Drugs 26: 375-382. [DOI] [PubMed] [Google Scholar]
- WHO (2002) World Health Report 2002: Reducing Risks, Promoting Healthy Life. Geneva: World Health Organization. [Google Scholar]
- Yu A. (2008) Indolealkylamines: biotransformations and potential drug-drug interactions. AAPS J. 10: 242–245. [DOI] [PMC free article] [PubMed] [Google Scholar]