Abstract
Objectives:
To assess clinical outcomes and patient satisfaction in patients with chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) or multifocal motor neuropathy (MMN) who were switched from intravenous immunoglobulin (IVIG) to subcutaneous immunoglobulin (SCIG).
Methods:
Eight consecutive patients, four with MMN and four with CIDP, already on long-term, hospital-based IVIG were switched to home-based SCIG. These patients were selected on the basis of their requirement for relatively low treatment doses, problems experienced with IVIG, and their willingness to switch to SCIG.
Results:
After a mean 33 [standard deviation (SD) 19] months receiving SCIG, 7 patients remained neurologically stable and 6 remained on a similar mean weekly immunoglobulin dose relative to their original intravenous dose. A good outcome was reported by 7 of the 8 patients: there were improvements in nausea and headache (n = 4), need to travel to hospital (n = 4), venous access problems (n = 3), immunoglobulin-induced neutropenia (n = 3), treatment wearing-off fluctuations (n = 2), IVIG-induced allergy requiring antihistamine/hydrocortisone (n = 1) and time taken off work (n = 1). The eighth patient required increasing doses of immunoglobulin to maintain strength but still wanted to continue SCIG. Seven patients completed a questionnaire: there was a very high overall satisfaction level with immunoglobulin treatment [mean 96 (SD 5), visual analogue scale (VAS) where 0 = very unsatisfied, 100 = very satisfied]; and very strong preference for subcutaneous over intravenous immunoglobulin (VAS mean 93 [SD 12] where 0 = prefer IVIG, 100 = prefer SCIG).
Conclusions:
In seven of the eight patients, SCIG gave improved tolerability and patient satisfaction with similar efficacy compared with IVIG.
Keywords: chronic inflammatory demyelinating polyradiculoneuropathy, intravenous immunoglobulins, multifocal motor neuropathy, subcutaneous immunoglobulins
Introduction
Compared with intravenous immunoglobulin (IVIG) treatment, subcutaneous immunoglobulin (SCIG) can more easily be self-administered at home and has a lower incidence of adverse effects relating to peak immunoglobulin levels.
In multifocal motor neuropathy (MMN), SCIG has been reported as a convenient alternative to IVIG [Harbo et al. 2009; Eftimov et al. 2009; Misbah et al. 2011]. In a small-scale study, a switch to SCIG was shown not to adversely affect quality-of-life assessed by the Short Form 36 (SF-36) survey [Harbo et al. 2009] and no major adverse events were reported during follow up over two years [Harbo et al. 2010].
In chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), SCIG has also been shown to be effective, although cohort sizes were small and only two studies have assessed patient satisfaction [Cocito et al. 2011; Markvardsen et al. 2013, 2014].
Here we report an assessment of clinical outcomes and patient satisfaction in a small group of patients with CIDP or MMN who were switched from IVIG to SCIG.
Case series
Methods
We introduced SCIG as a new clinical service available to suitable patients. This was a nonrandomized, partially prospective, observational study of patients switching from IVIG to SCIG as part of their routine clinical care, with subjective and objective assessment before and after the switch. We received institutional approval for this study as a service evaluation. We included consecutive patients with CIDP or MMN who (a) were already established on regular long-term IVIG, (b) were considered suitable for SCIG by the treating neurologist (R.H.), (c) were keen to switch and (d) required relatively low treatment doses (<25 g/week) of IVIG. The low dose requirement was because patients requiring lower infusion volumes would need fewer needle sites and/or shorter or less frequent infusions, and thus would be likely to find SCIG more convenient. In addition we wished to prioritize the most suitable patients for this new clinical service. All patients met European Federation of Neurological Societies (EFNS)/Peripheral Nerve Society (PNS) diagnostic criteria [Joint Task Force of the EFNS and the PNS, 2010a, 2010b]. All patients were under the care of the peripheral nerve service at King’s College Hospital, London.
All patients underwent weekly training in a hospital day ward to learn how to administer SCIG until they were independently competent. During the study, SCIG was administered at home, usually once per week (every 10 days in patient #6), using Crono SCIG infusion pumps. Over 1 hour, 20–25 ml was infused per needle site (2–4 needles were required; 2 simultaneously into the thighs or abdomen). The first SCIG dose was given 1 week after the last dose of IVIG and the initial weekly dose of SCIG was calculated as the dose of IVIG divided by the IVIG treatment interval in weeks, rounded up or down to the nearest whole number of vials. The dose of SCIG was subsequently adjusted by the treating neurologist (R.H.) according to clinical state, not following any particular protocol.
Clinical follow-up included two validated assessment tools, the Overall Neuropathy Limitation Scale (ONLS, 0 = normal, 11 = worst) to assess disability [Graham and Hughes, 2006], and Medical Research Council (MRC) sum score to assess muscle strength [Dyck et al. 2005], modified by the addition of the first dorsal interosseous muscle to give seven muscles on each side (70 = normal, 0 = worst). We also developed and applied a written questionnaire (unvalidated) prospectively to assess treatment satisfaction with SCIG. We developed our own satisfaction questionnaire as we were unaware of any validated questionnaires for SCIG administration. Other clinical data were obtained retrospectively from patients’ clinical notes.
Results
We included four patients with CIDP and four with MMN before and after the switch from IVIG to SCIG. Mean age was 57.4 [standard deviation (SD) 13.7] years. Patients had received IVIG for a mean 8.2 years (SD 6.6 years, range 1.1–19.3 years) before switching to SCIG. Patient characteristics are listed in Table 1.
Table 1.
Patient characteristics.
| Patient number | Gender | Age (years) | Diagnosis | Duration of previous IVIG treatment (years) | IVIG brand (immunoglobulin concentration) | Mean IVIG dose* (g/week) | SCIG brand (immunoglobulin concentration) | Mean SCIG dose$ (g/week) | Duration of follow up on SCIG (months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 65 | CIDP | 19.3 | Vigam® (5%) | 17.5 | Hizentra® (20%) | 17.0 | 21 |
| 2 | F | 51 | CIDP | 3.1 | Privigen® (10%) | 20.0 | Hizentra® (20%) | 22.0 | 20 |
| 3 | M | 66 | CIDP | 1.1 | Privigen® (10%) | 7.3 | Hizentra® (20%) | 8.0 | 21 |
| 4 | M | 49 | MMN | 13.3 | Vigam® (5%) | 20.0 | Hizentra® (20%) | 20.0 | 21 |
| 5 | F | 76 | CIDP | 13.2 | Flebogamma® (5%) | 12.5 | Vivaglobin® (16%)/ Hizentra (20%)/ Gammanorm (16.5%) | 13.2 | 64 |
| 6 | M | 58 | MMN | 2.8 | Sandoglobulin® (6%) | 20.0 | Subcuvia® (16%) | 13.4 | 51 |
| 7 | F | 63 | MMN | 8.9 | Sandoglobulin® (6%)/ Intratect® (5%)/ Flebogamma® (5%) | 24.0 | Subcuvia® (16%) | 24.0 | 50 |
| 8 | M | 31 | MMN | 3.8 | Flebogamma® (5%)/ Privigen® (10%) | 13.3 | Gammanorm® (16.5%) | 19.8 | 18 |
| Mean (SD) | na | 57.4 (13.7) | na | 8.2 (6.6) | na | 16.8 (5.4) | na | 17.2 (5.3) | 33 (19) |
Mean weekly IVIG dose immediately before switching.
Mean weekly SCIG dose at final follow up, mean 33 months after switching.
CIDP, chronic inflammatory demyelinating polyradiculoneuropathy; IVIG, intravenous immunoglobulin; MMN, multifocal motor neuropathy; na, not applicable; SCIG, subcutaneous immunoglobulin; SD, standard deviation.
The main reasons for wishing to switch from IVIG to SCIG were: adverse effects attributable to IVIG (neutropenia, n = 3; nausea or headache, n = 2; allergy requiring antihistamine or hydrocortisone, n = 1); unacceptable fluctuations in weakness as IVIG wore off (1 patient); poor intravenous access (2 patients); distance from home to hospital (2 patients); and missing work to attend hospital (1 patient). Several patients had more than one reason. Regarding the patients with neutropenia, the minimum recorded neutrophil count while on IVIG was 0.28 × 109/l in patient #6, 0.67 × 109/l in patient #3 and 1.34 × 109/l in patient #4; none had symptoms of sepsis attributed to neutropenia. All the above reasons were successfully resolved after switching.
At final clinical follow up, a mean 33 (SD 19) months after starting SCIG, all patients continued on regular SCIG. Seven of the eight patients reported a good outcome after switching to SCIG. There was substantial benefit, including where this had not been the main reason for switching, in nausea and headache (4 patients), travel convenience (4 patients), venous access problems (3 patients) and avoidance of wearing-off fluctuations (2 patients). Patients #3 and #4 reported minor neurological improvement. Although patient #4 had no objective outcomes measured, when last seen in the clinic he reported improved power in his upper limb and no longer had any end-of-dose fluctuation in weakness.
Adverse effects of SCIG were generally mild and infrequent. Three patients had no adverse effects from SCIG. Patient #5 had no adverse effects on Vivaglobin® (16% SCIG), then when this product was discontinued by the manufacturer, she switched to Hizentra® (20% SCIG) but developed an urticarial rash and malaise with most infusions, so switched to Gammanorm® (16.5% SCIG) and had no more adverse effects. Patients #1 and #2 reported mild tiredness and patient #6 mild nausea, each for a few hours after SCIG, all less than six times per year.
Patient #8 (MMN) had a less good outcome but still preferred to remain on SCIG. His weakness worsened so he needed to increase his mean weekly SCIG dose by 44% and to have intermittent top-up doses of IVIG of 80 g approximately every 8–16 weeks. He had occasional tenderness at infusion sites. He developed painful perniosis (chilblains) of the toes of uncertain relationship to his treatment.
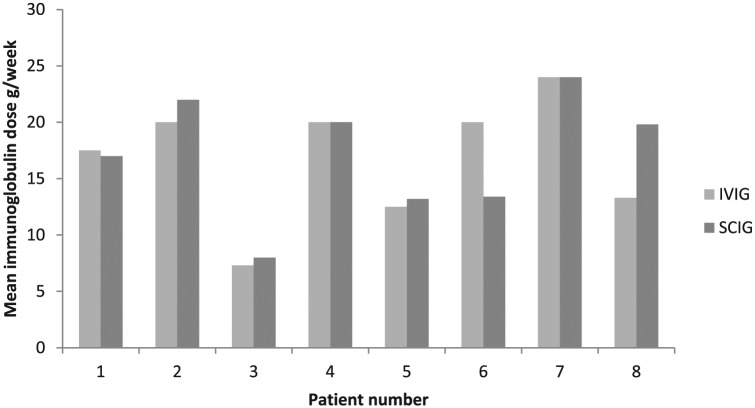
Compared with the calculated mean weekly dose of IVIG before switching, the mean weekly dose of SCIG at final follow up increased by a mean 5% (SD 22%) across the 8 patients (Figure 1). The dose remained within 10% of the previous dose in six of the eight patients; a greater change was observed in the remaining two patients. In patient #6, the mean weekly SCIG dose fell by 33% from 20 g/week to 13.4 g/week; no attempt had been made to reduce the IVIG dose before switching. The patient started SCIG on 19.2 g every week and remained stable; he was keen to extend the interval for convenience, and therefore with agreement from his neurologist, the dose was changed to 19.2 g every 10 days without clinical deterioration. In patient #8, the dose was increased by 49% from 13.3 g/week to 19.8 g/week due to clinical deterioration, though the patient regained his previous strength once on the new higher dose.
Figure 1.
Calculated mean weekly immunoglobulin dose (g/week) of IVIG (immediately before switching) and SCIG (at final follow up, mean 33 months after switching).
IVIG, intravenous immunoglobulin; SCIG, subcutaneous immunoglobulin.
MRC sum and ONLS scores before and after switch to SCIG
MRC sum scores were assessed by the treating neurologist before the switch to SCIG and a mean of 24.9 (SD 21.7) months following the switch; no patient worsened and a mean improvement in score of 0.7 (SD 0.8) was observed (Table 2). ONLS was also assessed before and after switching to SCIG administration [mean 31.8 (SD 17.7) months after switch]; a trivial mean worsening in score of 0.3 (SD 0.5) was noted. The worsening of ONLS in patient #1 was because of a clinically insignificant change in the upper limbs from ‘normal’ to ‘minor symptoms not affecting function’. Post-switch scores were not available in patient #4 who had emigrated to another country. Pre-switch ONLS scores were not available in another three patients who had started SCIG before this study began.
Table 2.
MRC sum and ONLS scores before and after switch to SCIG.
| Patient number | MRC sum score |
ONLS score |
||||||
|---|---|---|---|---|---|---|---|---|
| Score on IVIG pre switch | Months on SCIG until score reassessed | Score on SCIG | Change in score | Score* on IVIG pre switch | Months on SCIG until score reassessed | Score* on SCIG | Change in score | |
| 1 | 69.5 | 16 | 70.0 | 0.5 | 0 + 2 = 2 | 16 | 1 + 2 = 3 | 1 |
| 2 | 68.0 | 11 | 70.0 | 2.0 | 1 + 3 = 4 | 29 | 2 + 2 = 4 | 0 |
| 3 | 69.0 | 6 | 69.0 | 0 | 2 + 2 = 4 | 26 | 2 + 2 = 4 | 0 |
| 4 | 62.0 | – | – | – | 3 + 0 = 3 | – | – | – |
| 5 | 70.0 | 62 | 70.0 | 0 | – | 62 | 3 + 2 = 5 | – |
| 6 | 66.5 | 49 | 67.0 | 0.5 | – | – | – | – |
| 7 | 66.0 | 11 | 66.5 | 0.5 | – | – | – | – |
| 8 | 67.0 | 20 | 68.5 | 1.5 | 2 + 0 = 2 | 26 | 2 + 0 = 2 | 0 |
| Mean (SD) | 67.3 (2.6) | 25 (22) | 68.7 (1.5) | 0.7 (0.8) | 3.0 (1.0) | 32 (18) | 3.6 (1.1) | 0.3 (0.5) |
ONLS expressed as upper limb subscore + lower limb subscore = total ONLS score.
IVIG, intravenous immunoglobulin; MRC, Medical Research Council; ONLS, Overall Neuropathy Limitation Scale; SCIG, subcutaneous immunoglobulin; SD, standard deviation.
SCIG treatment satisfaction questionnaire
The SCIG treatment satisfaction questionnaire was self-completed by 7 patients (excluding patient #4 who had moved to another country), after having been on SCIG treatment for a mean 31 months (SD 22 months). Results are reported as mean (SD). The question ‘Overall how strong is your preference for IVIG or for SCIG?’ [(visual analogue scale VAS]; prefer IVIG = 0, prefer SCIG = 100) was scored as 93 (SD 12). ‘Please rate how easy (or difficult) it is to give yourself your treatment?’ (VAS; 0 = very difficult, 100 = very easy) was scored as 93 (SD 7). ‘How easy has it been for you to arrange holidays, social outings or other activities around your immunoglobulin treatment schedule?’ (VAS; 0 = very difficult, 100 = very easy) was scored as 89 (SD 22). ‘How would you rate your overall level of satisfaction with immunoglobulin treatment?’ (VAS; 0 = very unsatisfied, 100 = very satisfied) was scored as 96 (SD 5). Mean infusion duration was 126 (SD 33) minutes, usually once per week. Of the five patients who answered the question ‘Do you usually need the assistance of another person to carry out the infusions at home?’, four responded ‘No’ (patients #1, 2, 3, 7) and one (#5) reported that she required occasional assistance due to syringe stiffness.
Discussion and conclusion
In this nonrandomized study four patients with CIDP and four with MMN were switched from hospital-based IVIG to home-based SCIG for various reasons including adverse effects of IVIG.
Most of the patients remained clinically stable on the same mean weekly dose of immunoglobulin when switched from IVIG to SCIG, in contrast to a previous report that suggested the dose of SCIG may need to be increased [Harbo et al. 2010]. Most had a smooth transition despite not using the gradual switch protocol developed by Misbah and colleagues [Misbah et al. 2011]. Although the mean weekly SCIG dose was found to be within 10% of the previous dose for the majority of patients, marked changes were observed in two patients. It is possible that patient #6 had previously been overtreated on IVIG as there had been no prior attempts to reduce the dose. Patient #8’s increase may have been due to previous undertreatment on IVIG (80 g every 6 weeks) as a result of his reluctance to have IVIG administered more frequently.
Seven of the eight patients had a good outcome. The questionnaire showed high patient satisfaction with SCIG and a very strong preference for SCIG over IVIG. The reported reasons for switching were all successfully resolved after switching to SCIG, including fewer side effects, increased convenience and no wear-off effect.
These results are consistent with earlier studies demonstrating that SCIG is a viable alternative to IVIG administration. In one trial in CIDP, 20 (70%) of 29 patients who switched from IVIG to SCIG preferred to continue with SCIG at the end of the study [Markvardsen et al. 2013]. An open-label follow up to this study confirmed that SCIG preserved muscle strength and functional ability over 1 year [Markvardsen et al. 2014]. In a case series of five CIDP patients, four preferred SCIG administration at home [Cocito et al. 2011]. Case series of MMN patients also confirmed the suitability of SCIG as an alternative to IVIG, with no loss of efficacy [Eftimov et al. 2009; Harbo et al. 2009, 2010; Misbah et al. 2011]. Additionally, improvements in quality-of-life were reported in one study [Misbah et al. 2011].
It is possible that some of the CIDP patients may have remitted and may no longer require immunoglobulin treatment. Following this study, we have now implemented a policy of periodically attempting dose reduction to ensure that the patients are receiving the appropriate treatment.
A limitation of this study is the potential for bias as, compared with a general population of patients, the patients who were selected to switch required relatively low doses and were themselves keen to switch to SCIG.
We have not attempted to calculate the cost difference between SCIG and IVIG as it depends on a number of factors. The immunoglobulin cost per gram varies in different countries and different hospitals, but is typically slightly more expensive for SCIG than IVIG. SCIG avoids the cost of admission to a hospital day unit with associated staff costs and the costs of the patient taking a day off work, but requires the involvement of a home care company for equipment and delivery. Overall, the cost difference is small and it is not clear which is cheaper.
In summary, in seven of the eight patients SCIG had similar efficacy and better tolerability compared with IVIG. Patient satisfaction was greatly improved after the switch to SCIG, with no marked change in weakness or disability. However, there is a need for randomized controlled trials to confirm these results.
Acknowledgments
Editorial assistance was provided by Meridian HealthComms, supported by an unrestricted educational grant from CSL Behring.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: R.H. has received payment from CSL Behring UK Ltd, Baxter Healthcare Ltd and Grifols. None of these companies had any role in the design of the study, or in the collection, analysis and interpretation of the data, or in the decision to approve publication of the finished manuscript.
Contributor Information
Robert D. M. Hadden, Consultant Neurologist and Honorary Senior Lecturer, King’s College Hospital, Denmark Hill, London, SE5 9RS, UK
Fabrizio Marreno, Specialist Nurse Department of Neurology, King’s College Hospital, London, UK.
References
- Cocito D., Serra G., Falcone Y., Paolasso I. (2011) The efficacy of subcutaneous immunoglobulin administration in chronic inflammatory demyelinating polyneuropathy responders to intravenous immunoglobulin. J Peripher Nerv Syst 16: 150–152. [DOI] [PubMed] [Google Scholar]
- Dyck P., Boes C., Mulder D., Millikan C., Windebank A., Dyck P., et al. (2005) History of standard scoring, notation, and summation of neuromuscular signs. A current survey and recommendation. J Peripher Nerv Syst 10: 158–173. [DOI] [PubMed] [Google Scholar]
- Eftimov F., Vermeulen M., de Haan R., van den Berg L., van Schaik I. (2009) Subcutaneous immunoglobulin therapy for multifocal motor neuropathy. J Peripher Nerv Syst 14: 93–100. [DOI] [PubMed] [Google Scholar]
- Graham R., Hughes R. (2006) A modified peripheral neuropathy scale: the Overall Neuropathy Limitations Scale. J Neurol Neurosurg Psychiatry 77: 973–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbo T., Andersen H., Hess A., Hansen K., Sindrup S., Jakobsen J. (2009) Subcutaneous versus intravenous immunoglobulin in multifocal motor neuropathy: a randomized, single-blinded cross-over trial. Eur J Neurol 16: 631–638. [DOI] [PubMed] [Google Scholar]
- Harbo T., Andersen H., Jakobsen J. (2010) Long-term therapy with high doses of subcutaneous immunoglobulin in multifocal motor neuropathy. Neurology 75: 1377–1380. [DOI] [PubMed] [Google Scholar]
- Joint Task Force of the EFNS and the PNS (2010a) European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy. Report of a Joint Task Force of the European Federation of Neurological Societies and the Peripheral Nerve Society—First Revision. J Peripher Nerv Syst 15: 1–9. [DOI] [PubMed] [Google Scholar]
- Joint Task Force of the EFNS and the PNS (2010b) European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of multifocal motor neuropathy. Report of a Joint Task Force of the European Federation of Neurological Societies and the Peripheral Nerve Society—First Revision. J Peripher Nerv Syst 15: 295–301. [DOI] [PubMed] [Google Scholar]
- Markvardsen L., Debost J., Harbo T., Sindrup S., Andersen H., Christiansen I., et al. (2013) Subcutaneous immunoglobulin in responders to intravenous therapy with chronic inflammatory demyelinating polyradiculoneuropathy. Eur J Neurol 20: 836–842. [DOI] [PubMed] [Google Scholar]
- Markvardsen L., Harbo T., Sindrup S., Christiansen I., Andersen H., Jakobsen J, et al. (2014) Subcutaneous immunoglobulin preserves muscle strength in chronic inflammatory demyelinating polyneuropathy. Eur J Neurol 21: 1465–1470. [DOI] [PubMed] [Google Scholar]
- Misbah S., Baumann A., Fazio R., Dacci P., Schmidt D., Burton J., et al. (2011) A smooth transition protocol for patients with multifocal motor neuropathy going from intravenous to subcutaneous immunoglobulin therapy: an open-label proof-of-concept study. J Peripher Nerv Syst 16: 92–97. [DOI] [PubMed] [Google Scholar]