Abstract
Amyloid aggregates of α-synuclein (αS) protein are the predominant species present within the intracellular inclusions called Lewy bodies in Parkinson’s disease (PD) patients. Among various aggregates, the low-molecular weight ones broadly ranging between 2 and 30 mers are known to be the primary neurotoxic agents responsible for the impairment of neuronal function. Recent research has indicated that the neurotransmitter dopamine (DA) is one of the key physiological agents promoting and augmenting αS aggregation, which is thought to be a significant event in PD pathologenesis. Specifically, DA is known to induce the formation of soluble oligomers of αS, which in turn are responsible for inducing several important cellular changes leading to cellular toxicity. In this report, we present the generation, isolation, and biophysical characterization of five different dopamine-derived αS oligomers (DSOs) ranging between 3 and 15 mers, corroborating previously published reports. More importantly, we establish that these DSOs are also capable of replication by self-propagation, which leads to the replication of DSOs upon interaction with αS monomers, a process similar to that observed in mammilian prions. In addition, DSOs are also able to cross-propagate amyloid-β (Aβ) aggregates involved in Alzheimer’s disease (AD). Interestingly, while self-propagation of DSOs occur with no net gain in protein structure, cross-propagation proceeds with an overall gain in β-sheet conformation. These results implicate the involvement of DSOs in the progression of PD, and, in part, provide a molecular basis for the observed co-existence of AD-like pathology among PD patients.
Keywords: α-synuclein, oligomers, propagation, prion, amyloid, Parkinson’s
Introduction
Parkinson’s (PD) and Alzheimer’s (AD) diseases are both progressive, neurodegenerative disorders characterized by pathogenic protein aggregation. In the case of PD, the aggregation of α-synuclein (αS) into fibrils leads to the formation of Lewy bodies and Lewy neurites within neuronal cells. Although fibrils were initially presumed to be the primary toxic agent in PD, recent evidence has highlighted the role of soluble αS oligomers in PD-associated toxicity (reviewed in Ref.1). AD is associated with the aggregation of amyloid-β (Aβ) into fibrils, which accumulate in extracellular inclusions known as senile plaques. As in PD, the formation of toxic Aβ oligomers has been shown to be a primary source of toxicity in AD (reviewed in Ref.2). In adddition to AD and PD, pathological similarities among many neurodegenerative diseases such as Huntington’s, Creutzfeldt-Jacob disease (CJD) and amyotrophic lateral sclerosis (ALS) have spurred speculations of a common molecular mechanism governing these individual pathologies. Accumulating evidence points to a prion-type mechanism as a plaussible molecular commonality among all amyloid diseases.3–6 This mechanism is based on replication and amplification of toxic assemblies formed by abnormal conformational variants via conversion of native protein molecules to abnormal assemblies of a specific molecular weight.7 In the context of conserved mechanisms of propagation, increasing evidence also points to a potential interplay between AD and PD pathologies; approximately 50% of AD patients develop significant Lewy bodies in addition to Aβ plaques,8 while up to 60% of PD patients suffer from varying degrees of Aβ pathology.9 Furthermore, the coexistence of toxic aggregate lesions of Aβ and αS seems to result in increased severity of either AD or PD relative to the individual pathologies.9,10 Only a handful of reports indicate interactions between Aβ and αS11,12 and their co-localization in vivo.13 However, some fundamental questions of whether interactions between Aβ and αS could lead to toxic soluble oligomer formation and whether soluble oligomers of one protein could laterally propagate similar oligomers of the other have yet to be fully explored.
We recently reported a specific oligomeric species of Aβ42 called large fatty acid-derived oligomers (LFAOs) that undergo replication by self-propagation to form nonfibrillar oligomers.14 Importantly, the off-pathway nature of these oligomers was speculatively attributed to their unique prion-like behavior. In the context of PD, oligomers of αS generated by the treatment of dompamine (DA) have displayed some unique molecular properties.15–18 Importantly, they seemed to be formed along a pathway different from one that leads to fibril formation.15 Therefore, in light of the emerging possibility that prion-type propagation may govern the amplification of toxic αS oligomers and the cross-propagation of Aβ, as well as the predicted off-pathway nature of dopamine-induced αS oligomers (DSOs), we investigated their self- and cross-propagation mechanisms. The data presented here indicated that DSOs are equally capable of initiating self-propation of αS oligomers and cross-propagation of Aβ, even at low seed concentrations. Intrestingly, while self-propagation occurs without a gain in overall structure, cross-propagation occurs in a classical disorder-to-order fashion to form β-sheet aggregates.
Results
DSOs are isolable as discrete species
As descibed in Materials and Methods, all recombinant αS purifications were stored as films prepared from hexafluoro-2-propanol (HFIP) solutions at −20°C. Before the start of each experiment, one or more HFIP films were subjected to fractionation by size exclusion chromotography (SEC) to ensure the removal of any preformed aggregates from the samples. A typical fractionation using a Superdex-200 column (GE Life Sciences) shows two major peaks, one between fractions 14 and 19 near the void volume, and the other between fractions 24 and 29 [Fig. 1(A)]. Silver stains of the peaks indicate aggregate-free monomers in fractions 27-30 [Fig. 1(B)], which were used in all the experiments presented in this report. The doublet band at ∼17 kDa for monomeric αS (14 kDa) is likely because of its intrinsically disordered nature, which is known to show exhibit low electrophoretic mobility in SDS-PAGE gels, leading to an apparent higher molecular weight band.19
Figure 1.

Purification of monomeric αS. (A) SEC chromatogram of αS purification. The dashed lines delineate fractions shown in (B). (B) Silver stain of SEC fractions 26–31.
DSOs were generated largely based on the method previously adopted by Cappai and colleagues17 but with some modifications as described in Materials and Methods. After 72 h of incubation with DA, an aliquot of the reaction was centrifuged at 18,000g for 20 min, and the resulting supernatant was subjected to SEC fractionation using a Superdex-200 size exclusion column [Fig. 2(A)]. The chromatogram showed two major peaks, with one broad peak between fractions 16 and 23 and a second one between fractions 24 and 28 [Fig. 2(A)]. An aliquot of the same reaction after 72 h subjected to electrophoresis and silver staining indicated the presence of several oligomeric species ranging between 22 and 250 kDa (∼2 and 18 mers) along with a band at 17 kDa corresponding to αS monomers [Fig. 2(B), lane P]. In addition, a 3.5 kDa band was also observed, which is likely a fragment arising from the reported autoproteolytic activity of αS.20 Electrophoretic analysis (SDS-PAGE) of SEC fractions 19–23 [Fig. 2(B)] indicated the presence of five relatively discrete DSOs as described in Table 1. In addition, aggregates in fractions 17 and 18, approximately corresponding to 8–18 and 5–15 mers or larger were obseved (data not shown). However, these samples were not included in our analyses because of their low concentration and the possibilty of them being protofibril-like high molecular weight aggregates. Based on further analysis, DSOs were found to be stable at 4°C for extended periods of time, with no detectable changes occurring within 72 h (Supporting Information Fig. S1). Only after 240 h did a slight increase in the molecular weight of gel bands become evident, along with the appearance of some high molecular weight species that failed to enter the gel (Supporting Information Fig S1). It is noteworthy that none of the DSOs exhibited high thioflavin-T (ThT) fluorescence (data not shown), which is indicative of the potential off-pathway characteritics of DSOs, as previously reported.16,17 Fractionated DSOs were also characterized using far-UV circular dichroism (CD) spectroscopy. All DSO fractions exhibited a random coil secondary structure virtually indistinguishable from fresh, monomeric αS [Fig. 2(C)]. Approximate size analysis using DLS based on an assumption of a spherical shape for these oligomers showed hydrodynamic diameters of 11.0, 10.3, 7.8, and 4.9 nm for DSO4–13, DSO3–10, DSO3–6, and DSO2–5, respectively, while monomeric αS showed a hydrodynamic radius of 3.8 nM [Fig. 2(D)].
Figure 2.

(A) SEC chromatogram for the isolation of DSOs. (B) Electrophoresis of DSOs prior to SEC fractionation (Lane P), as well as DSO fractions 19–23 (between the two dashed lines in A) probed with both silver (Ag) staining and western blotting with Syn211 antibody (WB). (C) CD spectra of freshly purified DSOs (dotted lines), all of which have a random coil conformation similar to monomeric αS (solid line). (D) Hydrodynamic diameters obtained from DLS for fractions 19 (very light grey), 20 (light grey), 21 (grey), and 22 (dark grey), as well as monomeric αS (black). The data presented are representative of at least three individual consistent experiments.
Table 1.
Molecular Weight Ranges and Corresponding Oligomeric Species for Fractionated DSOs Based on SDS-PAGE Data in Figure 2(B)
| Fraction | Molecular weight (kDa) | Oligomer species (n-mers) | Nomenclature |
|---|---|---|---|
| 19 | 56–182 | 4–13 | DSO4–13 |
| 20 | 42–140 | 3–10 | DSO3–10 |
| 21 | 33–70 | 3–6 | DSO3–6 |
| 22 | 28–56 | 2–5 | DSO2–5 |
| 23 | 28–42 | 2–3 | DSO2–3 |
DSOs have nonfibrillar morphologies
SEC-fractionated samples of DSOs were imaged using an atomic force microscope (AFM) in order to make morphological assessments. αS fibrils were imaged as a positive control. The DSO4–13 and DSO3–10 samples deposited onto mica substrates exhibited a homogeneous distribution of oblong punctate particles with average radius of 10 nm and average height of 1.2 nm (Fig. 3). We assume that the aggregates are flattened when deposited onto the mica substrate. More importantly, neither fibril-like nor large aggregates were observed on evaluation of the entire sample area, indicating that only small and discrete oligomeric species were formed. In contrast, αS fibrils showed elongated fibers with average half-width 18 nm and height of 7.6 nm (Fig. 3). Neither monomeric αS nor smaller DSO aggregates could be imaged with adequate clarity, indicating that the aggregate size for these fractions is smaller than the resolution capability, and no fibrillar structures were observed in these fractions (data not shown). Nevertheless DSO4–13 and DSO3–10 clearly displayed nonfibrillar morphologies, further indicative of an off-pathway nature of the aggregates.
Figure 3.

AFM images in height and amplitude modes for DSO4–13 and DSO3–10 (2 × 2 µm, z-scale: 0.8 nm), along with αS fibril control (2.5 × 2.5 µm, z-scale: 2.0 nm). The scale bar represents 500 nm.
DSOs can undergo replication by self-propagation without gain in structure
Previous reports15,17 indicate that DSOs are likely formed via an off-fibril formation pathway. Our own (not shown) and other’s data17 support this contention based on the low ThT binding ability of DSOs, as well as the temporal stability of their oligomeric form as observed by SDS-PAGE (Supporting Information Fig. S1). Recently, we established that some off-pathway oligomers of amyloid-β(1-42) (Aβ42) are capable of undergoing replication by self-propagation similar to that of mammilian prions.14 To determine whether off-pathway DSOs could exhibit self-propagation, 50 μM monomeric αS in 20 mM Tris with 50 mM NaCl and 0.01% NaN3 at pH 8.0 was incubated with 0.1% (molar percent; 50 nM) DSOs quiescent at 37°C in separate reactions. The molar % is expressed in terms of monomer concentration as there is no better handle on estimating DSO concentrations exclusively. The control αS reaction (50 µM) in the absence of DSO seeds failed to show any increase in ThT fluorescence even after 220 h of incubation at room temperature in quiescent conditions [▿; Fig. 4(A); inset]. In contrast, a similar control αS reaction agitated at 37°C showed a typical sigmoidal growth curve [▪; Fig 4(A)]. αS reactions seeded with 0.1% DSOs also failed to show any discernable increase in ThT intensities [Fig. 4(A); inset)]. Aliquots of the samples from the reactions removed after 72 and 144 h [indicated by arrows in Fig. 4(A)] were analyzed by CD. All samples including the control in the absence of DSO seeds remained in a random coil conformation, which is native to monomeric αS, even after 72 [Fig. 4(B)] and 144 h (data not shown). The lack of increase in ThT intensity and the nonemergence of β-sheet structure suggest the reactions were in a lag phase of aggregation.
Figure 4.
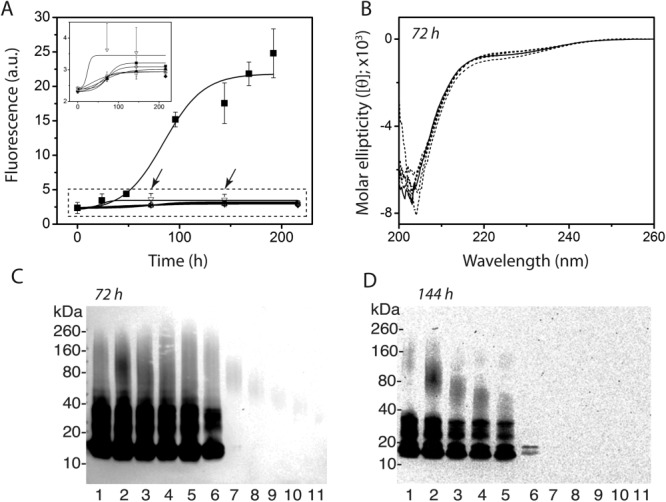
DSO self-propagation reactions. (A) ThT intensities of monomeric αS (50 μM) in 20 mM Tris with 50 mM NaCl incubated at 37°C agitated (▪), and quiescent (dashed box; inset) with 0.1 μM DSO4–13 (▪) DSO3–10 (○), DSO3–6 (▴), DSO2–5 (□), and in the absence of DSOs (▿) (n = 3). (B) The quiescent reactions in (A) after 72 h were analyzed by far-UV CD spectroscopy—αS in the absence of (solid line) and in the presence of (dashed lines) DSO seeds. C and D) Western blot analyses of supernatants of the reaction aliquots removed at 72 and 144 h from (A) (indicated by arrows) after centrifugation at 18,000g, using Syn211 monoclonal antibody. αS alone (lane 1) and with DSO4–13, DSO3–10, DSO3–6, DSO2–5, and DSO2–3 (lanes 2–6, respectively) at 72 h (C and D, respectively).
Aliquots of the reactions after 72 and 144 h were also subjected to immunoblotting to monitor the replication process [Fig. 4(C and D)]. Control αS sample in the absence of DSOs showed multiple oligomeric bands, one centered around 28–38 kDa and a faint band between 110 and 160 kDa [lane 1; Fig. 4(C and D)]. Despite the absence of indication of β-sheet structure in CD evaluation or lack of increase in ThT intensity by fluoresence studies, the reaction incubated with DSO4–13 showed an intense band centered at 85 kDa, that ranged between 60 and 180 kDa corresponding to 4–13 mers [lane 2; Fig. 4(C and D)] in addition to the 2–3mers that were also present in the control sample. The corresponding sample containing DSO4–13 seeds alone (0.1 μM) showed a band with much diminished intensity in the immunoblots [lane 7; Fig. 4(C and D)], suggesting that the increased intensity with DSO seeded monomers was because of amplification by self-propagation. Similar amplifcation of the band intensity for the molecular weight bands at 40–140, 30–70, and 28–60 kDa was observed for the reactions seeded with DSO3–10, DSO3–6 and DSO2–5 [lanes 3, 4, and 5, respectively; Fig. 4(C and D)], suggesting that all of these DSOs were able to propagate themselves in the presence of monomeric αS. However, the results with DSO2–3 remained inconclusive [lane 6, Fig. 4(C and D)], as low molecular weight bands corresponding to 2–3 mers were also observed in the unseeded control samples. An increase in DSO seed concentrations to 0.5% (molar percent, 0.5 µM) led to a substantial increase of all the band intensities in Western blots, which obscured the ability to discern any differences in the samples (data not shown).
A solution of 0.5% (molar percent) DSO seeds, however, was appropriate for a quantitative analysis of self-propagative replication by SEC. Therefore, after 72 h, the samples were subjected to fractionation via Superdex-200 SEC. The reaction seeded with DSO3–10 displayed an elution profile with a slight shift towards early fractionation of the self-propagated sample [solid line; Fig. 5(A)] as compared to DSO3–10 alone [dashed line; Fig. 5(A)], indicative of a slightly higher molecular mass of the self-propagated DSO. A similar shift towards higher molecular weight was recently reported for the self-propagation of Aβ oligomers.14 Reactions seeded with DSO3–6 and DSO2–5 also displayed a similar shift in their fractionations, while the one seeded with DSO2–3 did not show such a shift [Fig. 5(A)]. As a result of the lower yields obtained for DSO4–13 species, prohibitively high sample volumes were required for SEC analysis to ensure detection and hence, SEC data for the reaction seeded with DSO4–13 could not be obtained. The degree of amplification upon self-propgation was quantified by intergrating the peaks and normalizing against their respective seeds. By doing so, DSO3–10 showed a 1.5-fold increase in the amount of seed while DSO3–6 and DSO2–5 showed 1.1- and 1.05-fold increases, respectively [Fig. 5(B)]. This observation supports the western blot data, which also showed higher band intensities for DSO3–10 than those observed for DSO3–6 and DSO2–5 [Fig. 4(C)]. The combined data suggest that DSOs were able to effectively self-propagate with as low as 0.1% seeds (50 nM).
Figure 5.

Quantitation of DSO self-propagation. (A) Absorbance of SEC fractions at 280 nm for the self-propagation reactions in Figure 4(A). Reactions seeded with DSOs (solid lines) and control DSOs (dashed lines) are indicated. (B) Quantitative fold increases calculated by dividing the relative area under the curve (AUC) of the seeded samples by those of the control samples.
DSOs can cross-propagate soluble aggregates of Aβ
It is well known that αS and Aβ can cross-seed each other’s fibril formation.21,22 Given the off-pathway nature of DSOs and their tendency to self-propagate to nonfibrillar aggregates, it is important to understand whether DSOs can cross-propagate nonfibrillar aggregates of Aβ. In order to investigate this, monomeric Aβ42 (15 µM in 20 mM Tris, 50 mM NaCl, 0.01% NaN3 at pH 8.0) was incubated with 6.7% (molar percent, 1 μM) DSO at 37°C under quiescent conditions. The control Aβ42 sample displayed a sigmoidal aggregation pattern with a lag time of ∼ 30 h [▪; Fig. 6(A)]. In contrast, incubations with DSOs resulted in increased lag times (∼ 60 h), as well as decreased ThT saturating intensities [Fig. 6(A)], suggesting all DSOs were able to inhibit Aβ42 fibril formaiton. Further analysis of the reactions after 72 h by Western blot using Aβ-specific antibody, Ab5, indicated the presence of high molecular weight aggregates that failed to enter the gel (consistent with fibrils) for control Aβ, along with a monomeric band [lane C, Fig. 6(B)]. Aβ incubations with DSOs also showed the presence of high molecular weight species centered between 160 and 260 kDa (35–60 mers; indicated by an arrow) in addition to the two bands observed in the control sample [lanes 1–5, Fig. 6(B)]. The 160–260 kDa band was most intense with DSO2–3 (lane 5) followed by DSO4–13 (lane 1). Interestingly, among the samples, the one seeded with DSO2–3 showed the least amount of fibrils and monomers, suggesting these DSO seeds preferentially promoted 160–260 kDa aggregates [lane 5, Fig. 6(B)]. Probing the reactions on Western blots using αS-specific, Syn211 antibody did not show any discernable difference from those observed for DSOs alone (data not shown), suggesting the oligomers are exclusively formed by Aβ. The reactions were also analyzed by far-UV CD as was done to monitor self-propagation of DSOs. The control Aβ showed a significant conformational change from random coil to partial β-sheet within 72 h [Fig. 6(C)], which was consistent with the ThT plot [Fig. 6(A)]. All the reactions seeded with DSOs also showed a conversion to β-sheet conformation within 72 h of incubation [Fig. 6(C)]. In order to obtain a better qualitative picture of the conformational change to β-sheet, the difference in ellipticity between 216 nm (β-sheet) and 200 nm (random coil) was plotted for each sample [Fig. 6(D)]. The value ‘0’ in Figure 6(D) represents approximately a 1:1 ratio of random coil to β-sheet strucutres. Thus, the control Aβ showed a loss of random coil structure but did not gain significant β-sheet structure [Fig. 6(D)]. All the reactions seeded with DSOs showed gain in β-sheet strucutres as compared to the control. Although three individual data sets showed different extents of β-sheet formation upon cross-propagation, qualitivative emergence of β-sheet structures was unambiguous. It also must be borne in mind that the individual contributions of Aβ and DSOs within the overall β-sheet structure could not be deciphered as the possibility of both inducing structural change in the other exists. Collectively, the data indicates that DSOs are able to cross-propagate Aβ oligomers with a promotion of β-sheet conformation.
Figure 6.
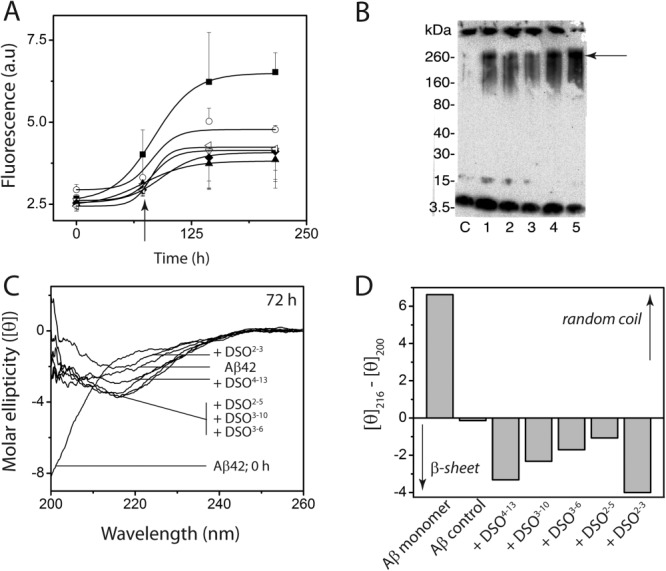
Cross-propagation of DSOs with Aβ42 monomer. (A) ThT aggregation reactions of 15 μM Aβ42 alone (▪) or co-incubated with 1 μM DSO4–13 (○), DSO3–10 (▴),DSO3–6 (▿), DSO2–5 (⋄), or DSO2–3 (◃) (n = 3). (B) Western blot of the reactions in (A) after 72 h aliquots probed by Aβ-specific monoclonal antibody Ab5 [indicated with an arrow in (A)]: Control Aβ42 (lane C), with DSO4–13, DSO3–10, DSO3–6, DSO2–5, and DSO2–3 (lanes 1–5), respectively. (C) CD spectra of cross-propagation reactions at 72 h. (D) Plots of difference in molar ellipticities at 216 and 200 nm obtained from (C).
Free-radical oxidation seems to be the likely mechanism of DSO formation
It has been suggested that the mechanism of DSO formation is dependent on the oxidation of DA, possibly because of subsequent oxidation of αS methionine residues resulting from DA oxidation,23 but ambiguity still remains regarding its precise mechanism of formation. In order to see whether DA oxidation is responsible for the formation of DSOs, DA oxidation in the presence of two reducing agents, Na2S2O3 and NaBH4, was monitored by UV-Vis spectroscopy. NaBH4 could effectively prevent the oxidation of DA, while Na2S2O3 clearly failed to do so [Fig. 7(A)]. αS was incubated alone and in DSO-generating conditions (4-fold molar excess of DA, 50 mM NaCl, agitated at 37°C) with and without the reducing agents. The samples were incubated for 72 h (the same time point chosen for SEC fractionation) and examined via western blotting [Fig. 7(B)], which showed that the sample incubated with Na2S2O3 [Fig. 7(B), Lane 5] promoted DSOs to a lesser extent than the one incubated with NaBH4 [Fig. 7(B), Lane 6] and the control [Fig. 7(B), Lane 4]. In other words, although NaBH4 prevented DA oxidation, it failed to inhibit DSO formation, which suggests that quinone formation is an unlikely mechanism of DA oxidation. On the other hand, although Na2S2O3 did not completely prevent DA oxidation based on UV data, it inhibited DSO formation to a substantial extent, indicating that free radical oxidation is a likely mechanism, which correlates with the prediction made previously.15,24
Figure 7.

Effect of reduction on DSO generation. (A) UV spectra for DA at 0 and 72 h, as well as DA incubated with Na2S2O3 or NaBH4 for 72 h. (B) Syn211 western blot of 50 μM αS alone or with 5 mM Na2S2O3 or NaBH4 (lanes 1–3, respectively) and 50 μM αS co-incubated with 200 μM DA alone or with 5 mM Na2S2O3 or NaBH4 (lanes 4–6, respectively).
Discussion
DSOs formation is robust and occurs with no gain in structure
The results presented here corroborate the previously established report by Cappai17 that αS readily forms DSOs in the presence of DA. This report also validates the previous observation by Rekas and colleagues that DSOs can be isolated via SEC fractionation.15 However, one significant distinction between our results and that of Rekas and colleagues is that we could initiate the formation of DSOs with only fourfold molar excess of DA (200 µM) with a fourfold less concentrated αS monomer (50 µM) as compared to Rekas’s procedure (200 µM αS with 2 mM DA).
Perhaps the most intriguing aspect of our finding is that all DSOs display a random coil structure despite existing as aggregates of varied molecular sizes. Rekas and colleagues showed a decrease in random coil conformation for dimeric and trimeric DSOs, which was computed to reveal a corresponding increase in β-sheet content as compared to monomeric αS. This observation similar to a conformational ordering made by Cappai and co-workers.17 However, our data did not indicate any gain in β-sheet content for any of the DSOs, which exhibited random coil configurations scarcely distinguishable from monomeric αS. Given that purified DSOs seem to be off-pathway oligomeric species by virtue of failing to form fibrils even after extended periods of time, it is likely that cross-linking with DA traps αS in its extended, random coil conformation as reported previously.25,26 It is unlikely that random coil aggregates in the absence of cross-linking would represent the deep kinetic minima necessary to prevent fibril formation. However, oligomerization of intrinsically disordered proteins without gain in structure by a heterotypic, liquid–liquid demixing like mechanism has been proposed for poly-glutamine proteins involved in Huntington’s disease,27,28 and a gain in oligomerization state without a corresponding transition from disorder to order is emerging to be a realistic mechanism among intrinsically disordered proteins.
Replication by propagation mechanism implicate transmiscibility among αS aggregates
Oligomer toxicity has become one of the primary focal points in AD and PD research. As pointed out in the Introduction, increasing evidence points to a potential interplay between AD and PD pathologies; approximately 50% of AD patients exhibit significant Lewy bodies in addition to Aβ plaques,8 while up to 60% of PD patients suffer from varying degrees of Aβ pathology.9 Previous research in our lab has shown that off-pathway oligomers of Aβ42 called LFAOs are able to propagate their oligomeric state to monomeric Aβ42 in a manner analogous to prion propagation.14 DSOs also seem to be off-pathway oligomers of αS and are physiologically relevant. They have been demonstrated to prevent SNARE-mediated neuronal vesicle docking and may be responsible for the selective death of dopaminergic neurons in PD.18 In this report, we have established that DSOs are capable of efficient self-propagation, with only 0.1% parent DSO being sufficient to initiate the formation of DSO assemblies. Intriguing aspects of the self-propagation mechanism are: (a) Assuming that excess unbound DA is removed during fractionation by SEC, replication by self-propagation occurs in the absence of additional DA molecules—DSOs formed in vivo may therefore be able to persist and propagate even in the absence of excess DA. However, the possibility of residual free DA associated with DSOs initiating further DSO formation could not be ruled out, although one could presume such a mechanism would be unlikely because of substantially diminished concentrations of free DA present in the sample. (b) Replication proceeds with no gain in the overall structure, further providing evidence for a possible heterotypic mechanism reported previously for poly-glutamine aggregates27,28.
Similar to self-propagation, our demonstration that DSOs can cross-propagate their oligomeric state to Aβ42 monomer is, to our knowledge, the first of its kind, and it provides a potential mechanism for the pathological interplay of PD and AD, as soluble Aβ42 is known to exist intracellularly as a result of the cleavage of amyloid precurssor protein on the endoplasmic reticulum.29 The cross-propagation of off-pathway oligomeric states could lead not only to increased local toxicity, but the propagation of the disease state throughout the brain, as injections of both αS and Aβ42 have been shown to cause delocalized pathology in a manner analogous to prions.30 Interestingly, in contrast to self-propagation, cross-propagation with Aβ seems to proceed with a significant gain in β-sheet structure (Fig. 5), following a classical disorder-to-order transition upon propagation. The reason for such a difference in behavior is not clear at this time and will be the focus of future investigation in our lab.
Materials and Methods
Materials
Synthetic Aβ42 was procured from the Peptide Synthesis Facility at the Mayo Clinic (Rochester, MN). Ab5 monoclonal primary antibody was obtained from the Mayo Clinic (Jacksonville, FL), and Syn211 monoclonal primary antibody was obtained from Sigma® (St. Louis, MO). All other chemicals and equipment were purchased from VWR, unless otherwise indicated.
αS purification
The original αS plasmid in pTYB1 (New England Biolabs ®) was obtained from Dr. Shu-Hui Yen of the Mayo Clinic (Jacksonville, FL). The plasmid was transformed into Rosetta™ 2 pLysS E. coli (EMD Millipore®). Cells were grown in LB media until their optical density was between 0.6 and 0.8, at which point they were induced with 0.7 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) at 23°C for 20 h. The cells were then pelleted at 15,000g, and the supernatant was discarded. Cells were resuspended in lysis buffer containing 20 mM Tris, 100 mM NaCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 0.5% Triton™ X-100 at pH 8.5 and sonicated on ice using 20 s bursts of sonication followed by 60 s of rest for at least five cycles. The sonicated cells were centrifuged at 9300g for 35 min, and the supernatant was collected. Purification was then performed using the IMPACT™ system protocol (New England Biolabs®) at 4°C. Briefly, the supernatant was loaded onto a 10 mL chitin column pre-equilibrated with column buffer (20 mM Tris, 100 mM NaCl, 1 mM EDTA, 0.5% Triton™ X-100, pH 8.5). The column was then washed with 200 mL of column buffer, followed by a quick wash with 50 mL of cleavage buffer (20 mM Tris, 100 mM NaCl, 1 mM EDTA, 50 mM DTT, pH 8.5). The column was left at 4°C for two days, after which the protein was eluted in two 12 mL fractions using 24 mL of elution buffer (20 mM Tris, 100 mM NaCl, 1 mM EDTA, pH 8.5). The eluate was filtered using a 30 kDa nominal molecular weight limit (NMWL) Amicon® Ultra Centrifugal Filter (EMD Millipore®) to remove any residual intein tag. The filtrate was then dialyzed against nanopure water using a 10 kDa molecular weight cutoff (MWCO) Spectra/Por® 6 dialysis membrane to remove DTT and EDTA. The dialyzed solution was then evaporated in a Vacufuge Plus (Eppendorf). The resultant dry protein was resuspended in 4 mL of hexafluoro-2-propanol (HFIP), which was then evaporated to a thin film in the vacufuge. HFIP films were stored at −20°C until further use. To ensure only monomeric αS was used in experiments, HFIP films were purified by SEC. Films were dissolved in 35 mM NaOH and were left in this solution for approximately 15 minutes before being purified using a Superdex 200 10/300 GL SEC column (GE Life Sciences) on an ÄKTApurifier FPLC system (GE Life Sciences) in 20 mM Tris, pH 8.0. Concentrations were measured via UV absorbance with a molar extinction coefficient of 5600M−1cm−1 at 276 nm (expasy.org), and stored at 4°C. Fresh αS was used within five days of SEC purification to avoid the presence of preformed fibrils. The purification of Aβ42 monomers was done based on the protocol previously published.14,31
Generation of DSOs
αS (50 μM) was incubated with a fourfold molar excess of DA, along with 50 mM NaCl, in 20 mM Tris, pH 8.0. The solution was kept at 37°C with constant agitation for 72 h, after which it was centrifuged at 18,000g for 20 min. The supernatant (500 µL) was then fractionated on a Superdex 200 10/300 GL SEC column isocratically using the same buffer and at a flow rate of 0.2 mL/min, to obtain fractions of DSOs. The concentration of DSOs was estimated via UV spectroscopy using a molar extinction coefficient at 276 nm of 5600M−1cm−1 based on monomer concentration.
Self-propagation of DSOs
For self-propagation reactions, solutions containing 50 μM αS, 0.1 μM DSO, 50 mM NaCl, and 0.01% NaN3 in 20 mM Tris, pH 8.0 were incubated at 37°C in quiescent conditions. An αS control containing no DSO was incubated along with the experimental samples. DSO controls for each oligomer size were made with 0.1 μM DSO and no αS. These controls were kept at 4°C as their purpose was only to provide a measure of the initial amount of DSO included in the reaction. Samples were monitored by ThT fluorescence intermittently and analyzed via gel electrophoresis and western blotting with Syn211 primary antibody at 72 and 144 h.
DSO cross-propagation with amyloid-β monomer
To examine the cross-propagation of αS oligomers with Aβ42 monomers, 15 μM Aβ42 monomer was incubated with 50 mM NaCl, 0.01% NaN3, and 1 μM DSO, quiescent at 37°C, for 72 h. ThT fluorescence was monitored daily, and western blots using Ab5 primary antibody were used to visualize oligomer formation. A negative control for the primary antibody was performed using the same concentrations of oligomers in the absence of Aβ42 to ensure that no cross-reactivity was occurring.
Electrophoresis and western blotting
All the SDS-PAGE gels were run on Biorad Mini-PROTEAN® 4–20% precast gels. Silver stains were performed using the Pierce Silver Stain kit (Thermo Scientific). For Western blotting, following electrophoresis, the gels were electroblotted onto BioTrace™ NT nitrocellulose transfer membrane (Pall Corporation). The blots were then boiled in 1X PBS for 2 min, following which they were blocked overnight in blocking buffer containing 5% nonfat dry milk and 0.1% Tween®-20 in 1X PBS. Blots were incubated for 3 h in a solution containing 0.5 μg/mL of either Syn211 (for αS blots) or Ab5 (for Aβ blots) primary antibody in 10% (v/v) transfer buffer in 1X PBS. Blots were incubated in a solution of HRP conjugate anti-mouse secondary antibody containing 0.7 μg/mL antibody in 11% (v/v) transfer buffer in 1X PBS for 45 min, developed with Pierce ECL western blotting substrate (Thermo Scientific), and visualized on a GelDoc molecular imager (Biorad).
ThT fluorescence
ThT fluorescence assays were performed as previously reported.14,31 Briefly, 5 µM aliquots of reactions were diluted with 70 µL of 10 μM ThT in 20 mM Tris, pH 8.0 in a quartz microcuvette. ThT fluorescence was performed by exciting at 450 nm and monitoring the emission at 482 nm in kinetics mode for 1 min with excitation and emission slits were set at 10 nm. The average fluorescence value over the 1 min period was taken as the fluorescence value for a particular time point. ThT data were fitted to a Boltzman sigmoidal equation, shown below, where F is fluorescence intensity, A1 and A2 are fixed parameters, and t0.5 is the time required to reach half saturation of ThT fluorescence. Lag times for fitted curves were equal to t0.5 – dt.
Dynamic light scattering
DLS measurements were made using a Zetasizer Nano S (Malvern, Worcestershire, UK) in a quartz microcuvette (Hellma). Samples were measured for 60 measurements of 30 s each with a pre-equilibration time of 60 s. Following measurement, sample number (%), and size (d.nm) were exported and plotted.
Circular dichroism
Far-UV CD measurements were made on a Jasco J-815 specropolarimeter (Jasco, MD). Samples in buffer (20 mM Tris, 50 mM NaCl, pH 8.0) were monitored in continuous scan mode from 260–190 nm in a 0.1 cm path-length quartz cuvette (Hellma). Acquisition parameters were 50 nm/min with 8 s response time, 1 nm bandwidth, and 0.1 nm data pitch. Data sets were averaged over two scans.
Atomic force microscopy
Oligomeric samples for AFM were prepared by diluting them approximately 100-fold to 25 nM in 20 mM Tris, pH 8.0. Monomer and fibril samples were diluted to 1 μM, also in 20 mM Tris, pH 8.0. For sample preparation, mica was cleaved with a razor blade and was covered with a freshly prepared, 100 μL solution of 3-aminopropyl-triethoxy silane (APTES) (500 μL APTES and 50 mL of 1 mM glacial acetic acid) for 20 min. The APTES solution was then decanted, and the mica was washed four times with 150 μL of DI water, dried with nitrogen gas, and stored in a desiccator for 1 h. The mica was then loaded with 150 μL of αS samples and was allowed to absorb for 20 min. The mica stubs were once again decanted, rinsed four times with DI water, dried with nitrogen gas and stored in the desiccator until imaged. Imaging was done under ambient conditions on a Dimension Icon with ScanAsyst AFM (Bruker) in tapping mode, using SCANASYST-AIR silicon probes (length 600 nm, nominal force constant 0.4 N/m, and resonance frequency 50–90 kHz) (Bruker Probes). The scan rate was held constant at 0.431 Hz and each image (512 × 512 data points) was flattened and analyzed using Nanoscope Analysis software. Multiple macroscopically separated areas were imaged for each sample, and representative height images are displayed.
Acknowledgments
The authors thank Mississippi INBRE funded by grants from the National Center for Research Resources (5P20RR016476-11) and the National Institute of General Medical Sciences (8 P20 GM103476-11) from the National Institutes of Health. VR also thanks American Heart Association (10GRNT4190124) for partial financial support. Support from the US Department of Education GAANN Fellowship Program under Award Number P200A090066 and the National Science Foundation Award Number OISE-1132079 is acknowledged by SEM. In addition, the authors wish to thank Drs. Cannon and Heinhorst, for use of the DLS instrument, Dr. Pritam Das (Mayo Clinic Jacksonville) for generously sharing Aβ antibodies and Dr. Shu-Hui Yen for gifting αS plasmid.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
References
- Kalia LV, Kalia SK, McLean PJ, Lozano AM, Lang AE. α-Synuclein oligomers and clinical implications for Parkinson disease. Ann Neurol. 2013;73:155–169. doi: 10.1002/ana.23746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed R, Lasagna-Reeves CA. Molecular mechanisms of amyloid oligomers toxicity. J Alzheimers Dis. 2013;33(Suppl 1):S67–78. doi: 10.3233/JAD-2012-129001. [DOI] [PubMed] [Google Scholar]
- Jucker M, Walker LC. Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Ann Neurol. 2011;70:532–540. doi: 10.1002/ana.22615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Coomaraswamy J, Bolmont T, Kaeser S, Schaefer C, Kilger E, Neuenschwander A, Abramowski D, Frey P, Jaton AL, M Meyer-Luehmann, J Coomaraswamy, T Bolmont, S Kaeser, C Schaefer, E Kilger, A Neuenschwander, D Abramowski, P Frey, AL Jaton, JM Vigouret, P Paganetti, DM Walsh, PM Mathews, J Ghiso, M Staufenbiel, LC Walker, M Jucker. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science. 2006;313:1781–1784. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- Prusiner SB. Cell biology. A unifying role for prions in neurodegenerative diseases. Science. 2012;336:1511–1513. doi: 10.1126/science.1222951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohr J, Watts JC, Mensinger ZL, Oehler A, Grillo SK, Dearmond SJ, Prusiner SB, Giles K. Purified and synthetic Alzheimer’s amyloid beta (Abeta) prions. Proc Natl Acad Sci U S A. 2012;109:11025–11030. doi: 10.1073/pnas.1206555109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryou C. Prions and prion diseases: fundamentals and mechanistic details. J Microbiol Biotechnol. 2007;17:1059–1070. [PubMed] [Google Scholar]
- Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ, LaFerla FM. Synergistic Interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. J Neurosci. 2010;30:7281–7289. doi: 10.1523/JNEUROSCI.0490-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotzbauer PT,CN, Campbell MC, Willis AW, Racette BA, Tabbal SD, Perlmutter JS. Pathologic accumulation of α-synuclein and Aβ in Parkinson disease patients with dementia. Arch Neurol. 2012;69:1326–1331. doi: 10.1001/archneurol.2012.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichney JM,GD, Salmon DP, Hofstetter CR, Hansen LA, Katzman R, Thal LJ. Cognitive decline is faster in Lewy body variant than in Alzheimer’s disease. Neurology. 1998;51:351–357. doi: 10.1212/wnl.51.2.351. [DOI] [PubMed] [Google Scholar]
- Ono K, Takahashi R, Ikeda T, Yamada M. Cross-seeding effects of amyloid beta-protein and alpha-synuclein. J Neurochem. 2012;122:883–890. doi: 10.1111/j.1471-4159.2012.07847.x. [DOI] [PubMed] [Google Scholar]
- IF Tsigelny, L Crews, P Desplats, GM Shaked, Y Sharikov, H Mizuno, B Spencer, E Rockenstein, M Trejo, O Platoshyn, JX Yuan, E Masliah. Mechanisms of hybrid oligomer formation in the pathogenesis of combined Alzheimer’s and Parkinson’s diseases. PLoS One. 2008;3:e3135. doi: 10.1371/journal.pone.0003135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson ME,SM, Greimel S, Kuskowski M, Schneider JA, Bennett DA, Lesné SE. Soluble α-synuclein is a novel modulator of Alzheimer’s disease pathophysiology. J Neuro. 2012;32:10253–10266. doi: 10.1523/JNEUROSCI.0581-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Paslay LC, Lyons D, Morgan SE, Correia JJ, Rangachari V. Specific soluble oligomers of amyloid-β peptide undergo replication and form non-fibrillar aggregates in interfacial environments. J Biol Chem. 2012;287:21253–21264. doi: 10.1074/jbc.M112.355156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekas A, Knott RB, Sokolova A, Barnham KJ, Perez KA, Masters CL, Drew SC, Cappai R, Curtain CC, Pham CLL. The structure of dopamine induced α-synuclein oligomers. Eur Biophys J. 2010;39:1407–1419. doi: 10.1007/s00249-010-0595-x. [DOI] [PubMed] [Google Scholar]
- Conway KA, Rochet JC, Bieganski RM, Lansbury PT. Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science. 2001;294:1346–1349. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- Cappai R, Leck S-L, Tew DJ, Williamson NA, Smith DP, Galatis D, Sharples RA, Curtain CC, Ali FE, Cherny RA, JG Culvenor, SP Bottomley, CL Masters, KJ Barnham, AF Hill. Dopamine promotes alpha-synuclein aggregation into SDS-resistant soluble oligomers via a distinct folding pathway. FASEB J. 2005;19:1377–1379. doi: 10.1096/fj.04-3437fje. [DOI] [PubMed] [Google Scholar]
- Choi B-K, Choi M-G, Kim J-Y, Yang Y, Lai Y, Kweon D-H, Lee NK, Shin Y-K. Large α-synuclein oligomers inhibit neuronal SNARE-mediated vesicle docking. 2013. Proc Natl Acad Sci Early Edition: 201218424. [DOI] [PMC free article] [PubMed]
- Moussa CE1 WC, Rusnak M, Tomita Y, Sidhu A. Abnormal migration of human wild-type alpha-synuclein upon gel electrophoresis. Neuro Lett. 2004;371:239–243. doi: 10.1016/j.neulet.2004.09.004. [DOI] [PubMed] [Google Scholar]
- C Vlad, K Lindner, C Karreman, S Schildknecht, M Leist, N Tomczyk, J Rontree, J Langridge, K Danzer, T Ciossek, A Petre, ML Gross, B Hengerer, M Przybylski. Autoproteolytic fragments are intermediates in the oligomerization/aggregation of the Parkinson’s disease protein alpha-synuclein as revealed by ion mobility mass spectrometry. Chembiochem. 2011;12:2740–2744. doi: 10.1002/cbic.201100569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi H, Kusaka E, Hongo K, Mizobata T, Kawata Y. Amyloid fibril formation of α-synuclein is accelerated by preformed amyloid seeds of other proteins. J Biol Chem. 2005;280:38609–38616. doi: 10.1074/jbc.M508623200. [DOI] [PubMed] [Google Scholar]
- Ono K, Takahashi R, Ikeda T, Yamada M. Cross-seeding effects of amyloid β-protein and α-synuclein. J Neurochem. 2012;122:883–890. doi: 10.1111/j.1471-4159.2012.07847.x. [DOI] [PubMed] [Google Scholar]
- Leong SL, Pham CLL, Galatis D, Fodero-Tavoletti MT, Perez K, Hill AF, Masters CL, Ali FE, Barnham KJ, Cappai R. Formation of dopamine-mediated alpha-synuclein-soluble oligomers requires methionine oxidation. Free Radic Biol Med. 2009;46:1328–1337. doi: 10.1016/j.freeradbiomed.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Pham CL, Leong SL, Ali FE, Kenche VB, Hill AF, Gras SL, Barnham KJ, Cappai R. Dopamine and the dopamine oxidation product 5,6-dihydroxylindole promote distinct on-pathway and off-pathway aggregation of alpha-synuclein in a pH-dependent manner. J Mol Biol. 2009;387:771–785. doi: 10.1016/j.jmb.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Illes-Toth E, Dalton CF, Smith DP. Binding of dopamine to alpha-synuclein is mediated by specific conformational states. J Am Soc Mass Spectrom. 2013;24:1346–1354. doi: 10.1007/s13361-013-0676-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaglia M, Tosatto L, Munari F, Tessari I, de Laureto PP, Mammi S, Bubacco L. Dopamine quinones interact with alpha-synuclein to form unstructured adducts. Biochem Biophys Res Commun. 2010;394:424–428. doi: 10.1016/j.bbrc.2010.03.044. [DOI] [PubMed] [Google Scholar]
- Vitalis A, Wang X, Pappu RV. Atomistic simulations of the effects of polyglutamine chain length and solvent quality on conformational equilibria and spontaneous homodimerization. J Mol Biol. 2008;384:279–297. doi: 10.1016/j.jmb.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitalis A, Pappu RV. Assessing the contribution of heterogeneous distributions of oligomers to aggregation mechanisms of polyglutamine peptides. Biophys Chem. 2011;159:14–23. doi: 10.1016/j.bpc.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild-Bode C, Yamazaki T, Capell A, Leimer U, Steiner H, Ihara Y, Haass C. Intracellular generation and accumulation of amyloid beta-peptide terminating at amino acid 42. J Biol Chem. 1997;272:16085–16088. doi: 10.1074/jbc.272.26.16085. [DOI] [PubMed] [Google Scholar]
- Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, Akiyama H, Mann DMA, Hasegawa M. Prion-like spreading of pathological α-synuclein in brain. Brain. 2013;136:1128–1138. doi: 10.1093/brain/awt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Bullard RL, Patel P, Paslay LC, Singh D, Bienkiewicz EA, Morgan SE, Rangachari V. Non-esterified fatty acids generate distinct low-molecular weight amyloid-β (Aβ42) oligomers along pathway different from fibril formation. PLoS One. 2011;6:e18759. doi: 10.1371/journal.pone.0018759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


