Abstract
Sodium dodecyl sulfate (SDS) is a widely used anionic surfactant in industry and research settings, and is known to have a detrimental effect to the environment. The pathway of SDS degradation by bacteria is initiated by an alkylsulfatase and the oxidized product, 1-dodecanoic acid, subsequently enters into the β-oxidation pathway and is used as a carbon source. In this work, we solved the crystal structure of Escherichia coli uncharacterized protein YjcS and identified that it belongs to the Type III alkylsulfatase with a signal peptide (residues 1–29) at the N terminus. YjcS hydrolyzed SDS and the double mutant D184N-H185A located in the conserved HXHXDH catalytic motif abolished this activity.
Keywords: alkylsulfatase, hydrolase, metalloenzyme, X-ray crystallography, metallo-β-lactamase
Introduction
Sulfatases play key roles in regulating sulfation states, which determine the function of many physiological molecules.1–3 Sulfatases are a family of hydrolytic enzymes that catalyze the cleavage of the sulfate esters to liberate inorganic sulfate and the corresponding alcohol or aldehyde.4–7 Currently, there are at least three mechanistically distinct groups of sulfatases based on biochemical classification.8 Type I includes the formylglycine-dependent arylsulfatases and carbohydrate sulfatases, which are widespread from humans to bacteria, and are the best studied group among the three types.9,10 They undergo a unique post-translational modification to produce a formylglycine product in the serine or cysteine active site, which mediates the cleavage of the CO—S bond of sulfate esters.11,12 The Fe2+ α-ketoglutarate-dependent dioxygenase superfamily constitutes the Type II sulfatases. These enzymes require α-ketoglutarate as an electron acceptor.3,13 Alkylsulfatases, belong to the metallo-β-lactamase (MBL) superfamily and were first identified in 2006,8 and represent the Type III sulfatases. They usually exhibit a Zn2+-binding β-lactamase structure in the N-terminal sequence and the HXHXDH (X represent any residues) motif binding binuclear Zn2+ acts as the catalytic site that performs the degradation of sulfate esters. Currently, only two members of the Type III sulfatases, SdsA1 from Pseudomonas aeruginosa and Pisa1 from Pseudomonas sp., are verified. However, these two enzymes exhibit different substrate specificities. SdsA1 is a primary alkylsulfatase, which enables the bacterium to degrade primary alkylsulfate SDS, whereas Pisa1 is an inverting alkylsulfatase specific for secondary alkylsulfates.8,14 Recently, research attention has focussed on an effective bioremediation of detergents, especially SDS, because it is widely used in industrial settings and daily life, and is detrimental to the environment.15–18 The pathway of SDS degradation is well documented in which SDS degradation is initiated with alkylsulfatase and the product, 1-dodecanol (1DO), is subsequently oxidized to 1-dodecanoic acid. Finally, 1-dodecanoic acid enters into the β-oxidation pathway and is used as a carbon source.19
Here, we report the crystal structure of Escherichia coli uncharacterized protein YjcS at 1.75 Å resolution and show that this enzyme belongs to alkylsulfatases. Compared with SdsA1 and Pisa1, YjcS shows a similar catalytic mechanism with them. SDS hydrolysis experiments support the alkylsulfatase activity of YjcS and the addition of Ca2+ might slightly activate YjcS activity in vitro. Furthermore, the double mutant D184N-H185A lost enzyme activity.
Results and Discussion
YjcS is a secreted protein
The theoretical molecular weight (MW) of full-length YjcS is 73 kDa. However, SDS-PAGE analysis showed that the MW of the eluted protein after Ni-NTA purification was ∼60 kDa. Two proteins were expressed following induction. Here, SDS-PAGE showed a band representing a protein of ∼70 kDa and the second band representing a protein of ∼60 kDa. Thus, only the smaller protein is soluble and isolated during Ni-NTA purification [Fig. 1(A,B)]. This observation indicates that the larger species is overexpressed insoluble full-length YjcS and the smaller protein is likely to be a truncated YjcS species that is soluble. This identification was confirmed by the mass spectrometry after SDS-PAGE. Given the presence of an N-terminal signal peptide in alkylsulfatase SdsA1 and Pisa1, we postulated the smaller protein species is an N terminally truncated version of YjcS. To validate this hypothesis, we designed a truncated YjcS, termed T30, based on the SignalP 4.1 Server,20 which predicted the presence of a protease cleavage site between Ala29 and Lys30, that is, the signal peptide (Fig. 3). SDS-PAGE analysis of T30 and YjcS after Ni-NTA purification showed only one induced band was present in T30 and the eluted YjcS was smaller than T30 before TEV digestion [Fig. 1(B,C)]. After TEV digestion at 4°C overnight, the MW of YjcS remained the same; however, the digested T30 was close to YjcS [Fig. 1(C)]. The results of subsequent protein N-terminal sequencing of ∼60 kDa protein verified the cleavage site of the signal peptide. In summary, YjcS is a secreted protein with an N-terminal signal peptide that includes residues 1–29. In the process of secretion the signal peptide is cleaved resulting in soluble YjcS in the periplasm. However, the overproduction of the full-length YjcS is overburdening the secretion system, and then some protein will be produced cytoplasmically and will then be incorporated into inclusion bodies. The soluble protein has in fact been secreted to periplasm.
Figure 1.
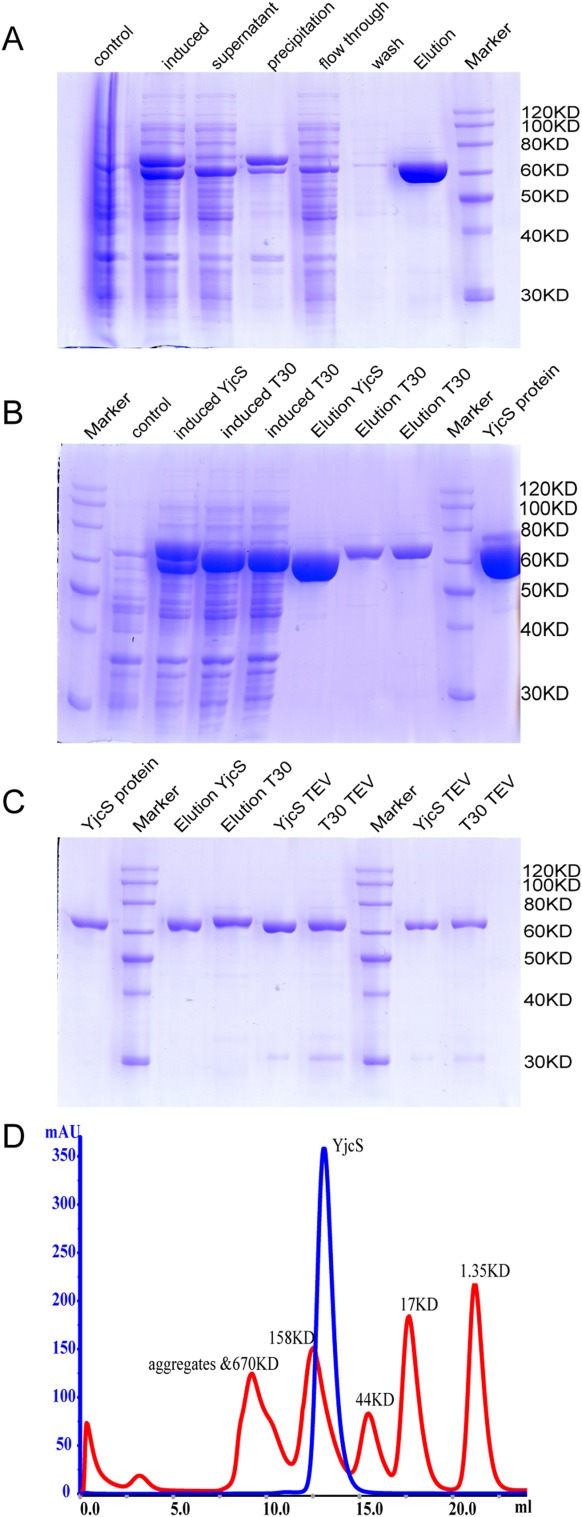
N-terminal signal peptide of YjcS is not required for solubility. (A) Expression and purification of full-length YjcS (pET28a-YjcS/BL21 (DE3) strain). (B) Expression and purification of full-length YjcS and truncation species T30 (pET28a-T30/BL21 (DE3) strain) in parallel. (C) The elution protein from (B) was cleaved by TEV protease overnight. All above control, high pure YjcS protein after size exclusion chromatography. (D) Molecular weight estimates of YjcS on the gel filtration chromatography column. Overlaid chromatograms are displayed with the molecular weight calibration standards.
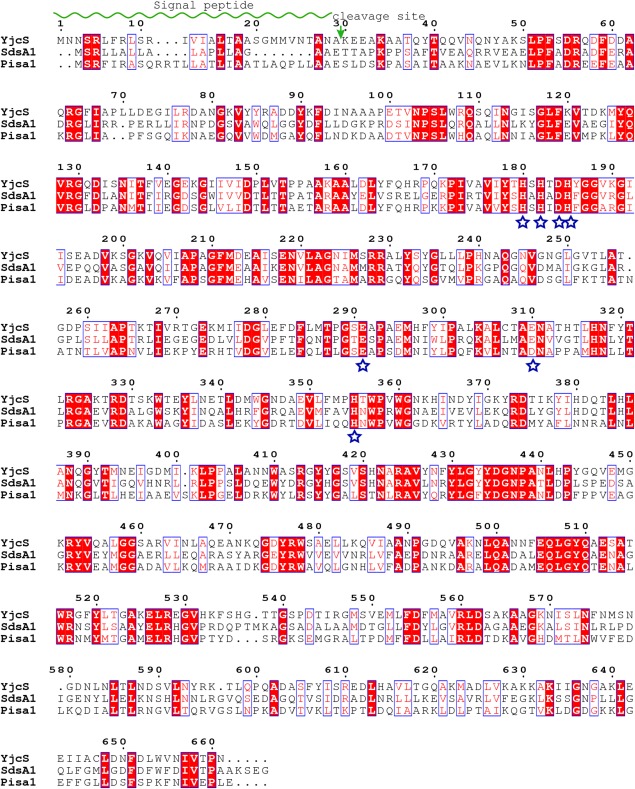
Figure 3.
Sequence alignment among alkylsulfatase members. The conserved residues in the catalytic motif are marked with blue stars. The signal peptide and its cleavage site are marked with green wave line and arrow, respectively.
Oligomerization state of YjcS in solution
Alkylsulfatases often function in the form of a dimer or tetramer.18 To investigate the oligomerization state of YjcS in solution, gel filtration chromatography was performed. The Superdex-200 10/300 column was calibrated with a gel filtration calibration kit (GE, high molecular weight proteins). Based on the elution times of the molecular markers, the MW of YjcS was determined to be 120 kDa [Fig. 1(D)], indicating that YjcS exists as a homodimer in solution, and that coincided with our solved crystal structure.
Overall structure of YjcS
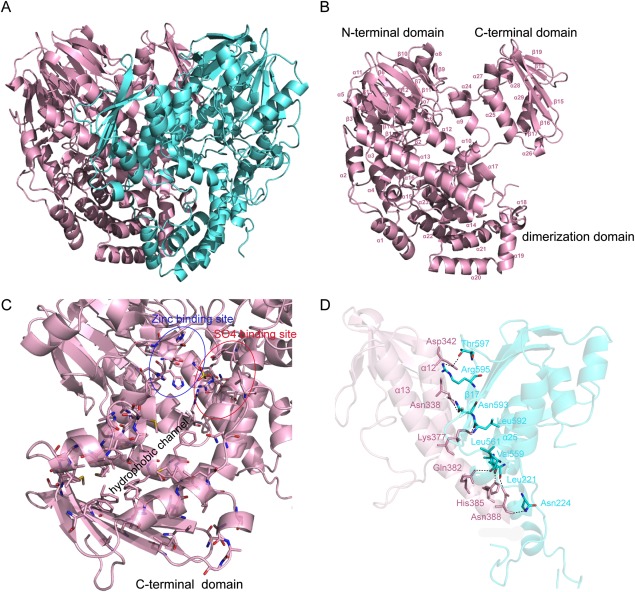
The crystal structure of YjcS was solved by the SAD method at 1.75 Å. YjcS is a homodimer in the crystallographic asymmetric unit (ASU) [Fig. 2(A)]. Each monomer consists of three domains: the N-terminal domain (residues 30–389) which adopts an αββα-sandwich conformation including 12 antiparallel β sheets in the center and an additional two β-strands antiparallel in the outer space; the dimerization domain (residues 390–534), which is composed of 10 helices (α14–α23) and encompasses the central region of YjcS, intricately intertwining the monomer; and the C-terminal domain (residues 542–664), which is represented by six α-helices located on one side of a five antiparallel β-sheet, and surrounds the central antiparallel β-sheets that are combined with three α-helices in the N-terminal domain of the other monomer [Fig. 2(A,B)]. The αββα-sandwich fold was first described for MBL,21 in addition to β-lactamases, the MBL superfamily members act as a different group of hydrolysis enzymes that participate in physiological processes, but almost all of them exhibit a binuclear metal center, similar to zinc β-lactamases with the HXHXDH motif as the catalysis site.22–24 Moreover, the C-terminal domain is structurally similar to several eukaryotic sterol-binding domains, as determined by the InterPro service online. The structure of YjcS has poorly defined regions, which are mainly located in two loop regions: residues 287–292, a part of the putative catalysis site, and residues 535–542, the loop linking the dimerization domain and the C-terminal domain. Although separated by the dimerization domain in terms of sequence, the N-terminal and C-terminal domains of YjcS are spatially directly adjacent [Fig. 2(A)]. Together they form a hydrophobic channel leading toward the putative active site pocket [Fig. 2(C)]. Thus, hydrophobic substrates may be recruited by the C-terminal domain or hydrophobic products presented.
Figure 2.
Structural analysis of YjcS. (A) Overview of the YjcS homodimer as a cartoon. Monomers are presented in cyan and in light pink. (B) A cartoon representation of a YjcS monomer. Secondary structure elements in this monomer are indicated and three structural domains, the N-terminal domain, the dimerization domain and the C-terminal domain, are also marked. (C) The hydrophobic channel formed by the C-terminal domain. The hydrophobic residues in the C-terminal domain and the residues, assumed to bind zinc and the sulfate group, are presented as sticks. (D) The interaction interface between the N-terminal domain and the C-terminal domain. The residues, forming hydrogen bonds, are presented as sticks and the hydrogen bonds are indicated by the dotted lines.
Interaction interface of YjcS
The total buried surface area of the YjcS homodimer is 5028.5 Å2/monomer, as calculated by the PDBe PISA server.25 The strong interactions that form the dimer interface include extensive hydrogen bonds and hydrophobic interactions from 10 α-helices in the dimerization domain. We were also surprised to observe that the N-terminal domain and the C-terminal domain contribute strongly to the stability of the structure, and 14 residues from each monomer are involved in the formation of hydrogen bonds between the N-terminal domain (α12 and α13) and the C-terminal domain (α25 and β17) [Fig. 2(D)].
Active site
A DALI-based analysis in the PDB26 shows that SdsA1 (PDB ID: 2CG2) and Pisa1 (PDB ID: 2YHE) share high structural similarity to YjcS (Z score = 52, root mean square deviation [rmsd] = 1.6 and Z score = 50, rmsd = 2, respectively) and both of them are alkylsulfatases.8,14 The sequence alignment exhibits a conserved HXHXDH catalytic motif among these proteins (Fig. 3). These structural similarities raised the possibility that YjcS is an alkylsulfatase. In the crystal structures of SdsA1 and Pisa1, both proteins have a binuclear zinc center binding to the HXHXDH catalytic motif and a sulfate ion located nearby the zinc-binding site suggests a substrate site. However, we failed to observe zinc binding in the YjcS structure and only the sulfate ion was observed. By docking the zinc ions from SdsA1 and Pisa1 to the YjcS structure, we found that the same interaction partners of the zinc center [Fig. 4(A)] were present in the YjcS structure. This implies that YjcS shares similar catalytic machinery that consists of a binuclear zinc center coordinated by the conserved HXHXDH motif as observed for SdsA1 and Pisa1. In detail, residues Asp184, His185, Glu310, and His355 of YjcS matched residues Asp173, His174, Glu299, and His344 of SdsA1 or the residues Asp183, His184, Asp310, and His355 of Pisa1 in the zinc1-binding site, respectively [Fig. 3(A)]. Moreover, the interaction residues with zinc2 in SdsA1 are His169, His171, and Glu280 and in Pisa1 the residues are His179, His181, Glu291, and Asp310. These residues are structurally matched by residues His180, His182, and Glu310 in YjcS; however, a residue is lost because of the poor density of the loop region (residues 289–292) [Fig. 4(A)]. The overlapped sulfate ion in the putative substrate-binding site prompted us to investigate whether the substrate-binding model is shared with alkylsulfatases SdsA1 and Pisa1, although there are some differences among them [Fig. 4(B)]. The kinetic analysis of the alkylsulfate ester cleavage of wild-type Pisa1 and Tyr417 variants by ITC indicated the tyrosine side chain is critically involved in the correct positioning of the substrate’s sulfate group.14 Hence, the corresponding residue to Tyr416 in YjcS may also participate in substrate positioning, but further experiments should be performed to support this view. SdsA1 and Pisa1 share an active site histidine (His306 in SdsA1 and His317 in Pisa1) as a potential source for the protonation of the leaving group.14 In YjcS, the matched His317 may also act as a general acid catalyst, enhancing the leaving group ability of the sulfate group and C—O bond cleavage results in inversion at the carbon. Although SdsA1 and Pisa1 both operate through the same inverting mechanism, they catalyze hydrolysis of different sulfate esters. Docking SDS analogs 1-decane-sulfonic-acid (1DA) (PDB ID: 2CFU) and product 1DO (PDB ID: 2CFZ) from SdsA1 to the YjcS structure showed that both 1DA and 1DO are a suitable size for the hydrophobic channel at the putative substrate binding site [Fig. 4(C)] and this suggests that SDS is a candidate substrate of YjcS.
Figure 4.
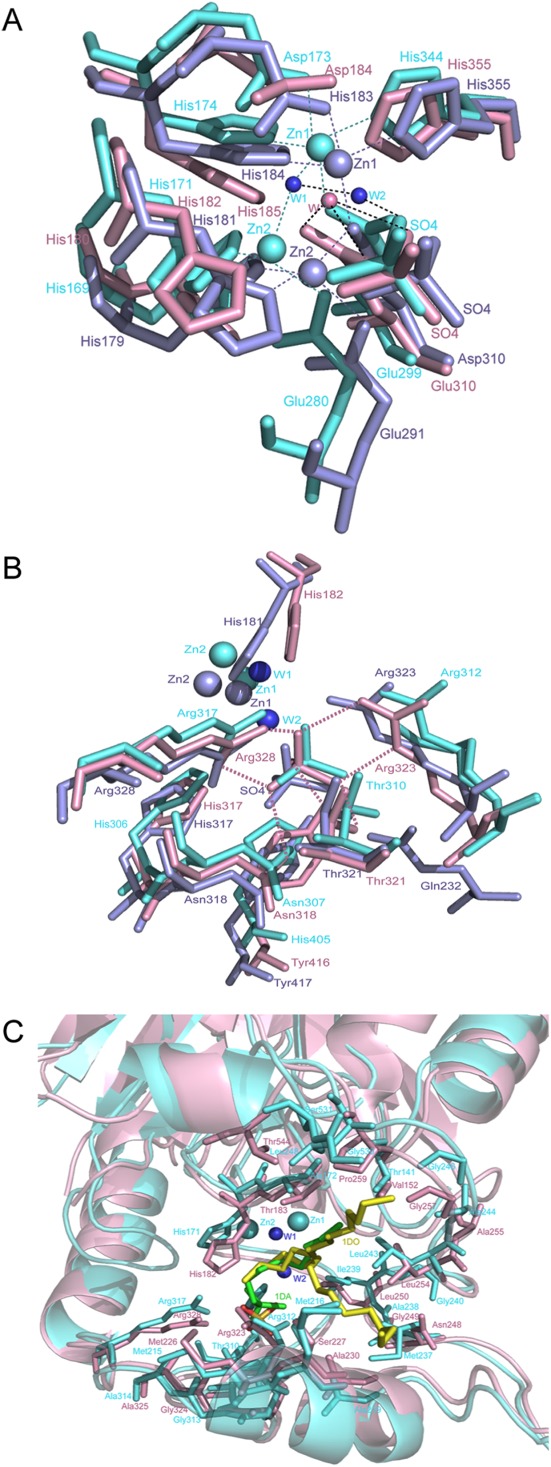
Active site of YjcS. (A) Superimposition of the zinc binding site. YjcS is in light pink, SdsA1 is in aquamarine, and Pisa1 is in light blue. Two water molecules from SdsA1 are shown as small spheres in tv_blue and the water from YjcS is in light pink. Hydrogen bonds are indicated by dotted lines in black and the zinc coordination bonds are shown as dotted lines and coloured in aquamarine or light blue. (B) Superimposition of the sulfate group binding site. Hydrogen bonds between the sulfate group and residues in YjcS are marked as light pink dotted lines. (C) Superimposition of the YjcS structure with SdsA1 containing 1DA (green) and 1DO (yellow). The hydrophobic residues located in the substrate-binding site are shown as sticks.
YjcS is a SDS-hydrolyzing enzyme
E. coli BL21 (DE3) cells were shown to not grow on minimal medium agarose plates containing 0.1% (w/v) SDS (date not shown), indicating that the SDS was toxic to the bacteria cells. However, overexpression of YjcS in these cells rescued the cells, even when the dilution was 25-fold the initial concentration, and a distinct and white halo due to water-insoluble 1DO produced during the hydrolysis of SDS was subsequently visualized.8 In contrast, the overexpressed double mutant D184N-H185A bacteria strain only survived at the initial concentration [Fig. 5(A)]. However, the purified YjcS protein successfully degraded SDS in vitro, as measured by phenol red, and the existence of 1 mM Ca2+ was found to likely activate YjcS slightly compared with no extra adding metal ions [Fig. 5(B)], while other ions (Mg2+, Mn2+, Co2+, Ni2+, and Zn2+) abolished enzyme activity (date not shown). The YjcS double mutant D184N-H185A showed no activity, in agreement with the agarose plate assay. The above results indicate that YjcS hydrolyzes SDS and residues Asp184 and His185 located within the HXHXDH motif are important for enzymatic activity. However, it is surprising that the hydrolysis of SDS mediated by YjcS in vitro does not depend on the Zn2+, and even when adding 1 mM Zn2+ to the reaction system, the activity of YjcS was abolished. Similar results describing the activation function of Ca2+ have been reported in other enzymes. For example, aminopeptidase A, a zinc-metallopeptidase family M1, is also activated by Ca2+.27,28 Moreover, this Zn2+-containing Ca2+-activated aminopeptidase A is inhibited by Zn2+.29 Thus, further research on the mechanism of how YjcS is activated by Ca2+ and inhibited by Zn2+ is required. To identify whether the endogenous divalent metal ions bind to the active site of YjcS in the process of purification resulting in the alkylsulfatase activity in vitro, 1 mM EDTA was used to chelate the divalent metal ions of YjcS. But there was no obvious difference in the activity of YjcS. Nonetheless, we believe that YjcS coordinates metal ions as cofactors for catalytic activity because one sulfate binds to the active site in the structure of YjcS resulting in presumably an excess negative charge without metal ions being present.
Figure 5.
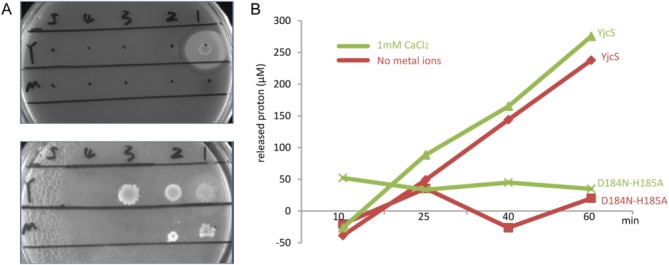
Functional properties of YjcS. (A) SDS hydrolysis of YjcS on minimal media agarose plates. Samples of wild-type YjcS (Y) and the double mutant D184N-H185A (M) were diluted sequentially by fivefold and plated at positions 1–5. The upper one is cultured at 37°C for 2 days and the lower one was cultured for 1 week. (B) The effects of Ca2+ and mutant on the enzyme activity of YjcS-mediated SDS hydrolysis in vitro. The released protons are calculated based on the standard curve of HCl.
Materials and Methods
Cloning, expression, and purification
The gene encoding YjcS was PCR-amplified from the E. coli DH5α strain using the primers yjcs−5′ and yjcs−3′ (Table 1). PCR fragments after restriction digestion treatment were conjugated into the pET28a (Novagen) vector with BamHI and Xhol restriction sites. The resulting pET28a-YjcS expression plasmid encodes YjcS with an N-terminal octa-His tag and a C-terminal hexa-His tag. The N-terminal octa-His tag fused with YjcS could be removed by cleavage using TEV protease.
Table 1.
Oligonucleotides Used for This Work
| Yjcs-5′ | CGCGGATCCATGAATAACTCTCGGTTATTCCGTTTGAG |
| yjcs-3′ | CCGCTCGAGATTTGGGGTTACGATATTCACCC |
| D184N-H185A-5′ | TCTACACTCACAGCCACACCAACGCCTATGGTGGCGTGA |
| D184N-H185A-3′ | GGTGTGGCTGTGAGTGTAGATAACGGCAACAATCG |
| T30-5′ | CCTGTATTTTCAGGGATCCAAAGAGGAAGCGAAAGCC |
| T30-3′ | GGATCCCTGAAAATACAGGTTTTCGCTGCTCCCATGG |
E. coli BL21 (DE3) cells were used for overexpression of YjcS. The cells were grown to an OD600 of ∼0.6 at 37°C and induced at 16°C for 18 h with isopropyl β-d-thiogalactopyranoside (IPTG) to a final concentration of 0.1 mM. Cell pellets were suspended in 50 mM HEPES (pH 8.0), 20% (w/v) sucrose, 500 mM NaCl, 10 mM imidazole, 0.1% Tween-20, 1 mM PMSF, 5 mM β-mercaptoethanol (β-ME), and 10 µg/mL DNase. The cell suspension was disrupted by a high-pressure homogenizer and then centrifuged at 30,000g for 50 min at 4°C. The supernatant was then loaded onto a Ni-NTA column that was pre-equilibrated with 50 mM HEPES (pH 8.0), 500 mM NaCl, 10 mM imidazole, and 1 mM β-ME buffer. The His-tagged protein was eluted in 50 mM HEPES (pH 8.0), 100 mM NaCl, 250 mM imidazole, and 1 mM β-ME. The N-terminal His-tag was removed by cleavage with TEV protease overnight at 4°C and further purified with Superdex-200 chromatography on an ÄKTA Prime system (GE Healthcare) to obtain highly pure YjcS-CHis. The gel filtration buffer contained 50 mM HEPES (pH 8.0), 100 mM NaCl, and 1 mM β-ME. The eluted fractions in all purification steps were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Selenomethionine-labeled (Se-Met) YjcS was overexpressed and purified using the same procedures described above; however, the medium was substituted by 1x M9 medium and seven essential amino acids were added at the mid-log phase before induction. To prevent oxidation of the selenium atoms, 5 mM dithiothreitol (DTT) was added to the final elution fraction containing Se-Met YjcS.
Crystallization and data collection
The hanging drop-vapor diffusion method was used for crystal screening by mixing 1 µL protein (10 mg/mL) with 1 µL of the reservoir solution. The primary crystals were shaped like a flake cluster and X-ray diffraction showed they were twin crystals. After optimization, including microcrystalline seeding,30 monocrystals suitable for data collection were obtained. The crystals were appeared at 20°C in the crystallization buffer containing 1.8M (NH4)2SO4, 2% PEG400, and 0.1M HEPES (pH 7.35). Before flash-cooling in liquid nitrogen, crystals were harvested into the crystallization solution with the addition of 20% (v/v) glycerol for cryo-protection. Diffraction data sets were collected at the beamline station BL17U of the Shanghai Synchrotron Radiation Facility (SSRF) and the temperature was held at 100 K by liquid nitrogen during data collection. All data were integrated, scaled, and merged using HKL2000.31
Structure determination and refinement
The crystal structure of YjcS was solved by the single wavelength anomalous diffraction (SAD) method using the data collected at a wavelength of 0.9791. Selenium sites were identified and the initial phases were calculated using the program ShelxD and ShelxE,32 respectively. Density modification was performed using the program Resolve33 to improve the initial phases and the improved phases were used for automatic model building in Buccaneer.34 About 90% of the residues of the sequence were traced using Buccaneer and those remaining were manually built using Coot.35 The structure was refined with the program Phenix.refine36 and checked with the program MolProbility.37 A summary of data collection and final refinement statistics are listed in Table 2. The program PyMOL (http://www.pymol.sourceforge.net/) was used to prepare structural figures.
Table 2.
Date Collection and Refinement Statistics
| Data collection | |
|---|---|
| Wavelength (Å) | 0.9791 |
| Space group | C2 |
| Unit-cell parameters (Å) | a = 199.550, b = 76.182, c = 103.681, β = 115.26° |
| Resolution (Å) | 1.75 (1.78–1.75) |
| Number of unique reflections | 139,777 (6874) |
| Completeness (%) | 98.8 (97.4) |
| Redundancy | 7.1 (6.3) |
| Mean I/ o’ (I) | 36.9 (5.2) |
| Molecules in asymmetric unit | 2 |
| Rmerge (%) | 15.1 (58.4) |
| Refinement | |
| Resolution range (Å) | 45.12–1.75 |
| Rwork/Rfree (%) | 18.4/21.6 |
| No. of residues/protein atoms | 1241/9729 |
| No. of water atoms | 1081 |
| Average B factor | |
| Main chain (A/B) | 17.20/17.27 |
| Side chain (A/B) | 19.48/19.53 |
| Waters | 25.77 |
| Ramachandran plot (%) | |
| Most favored | 96.6 |
| Allowed | 3.4 |
| R.m.s. deviations | |
| Bond lengths (Å) | 0.007 |
| Bond angles (°) | 1.076 |
Values in parentheses are for the highest resolution shell.
Site-directed mutagenesis of YjcS
Oligonucleotides used for the construction of the YjcS double mutant D184N-H185A and the truncated T30, in which the N-terminal residues 1–29 were deleted, are listed in Table 1. Plasmid pET28a-YjcS was used as the mutagenesis template. To obtain the mutants, the site-directed mutagenesis method with KOD DNA polymerase was performed. The introduced mutations were verified by sequencing.
SDS hydrolysis experiment on minimal media agarose plates
The minimal media agarose plates were prepared according to an earlier report.8 The pET28/BL21(DE3) strain, pET28a-YjcS/BL21(DE3) strain, and pET28a-D184N-H185A/BL21(DE3) strain were cultured separately in LB medium until the OD600 reached ∼0.6, and then 5 mL of the cells was separately harvested and re-suspended in sterilized water (100 µL). The 100 µL cell suspensions were regarded as the initial concentration. Samples of wild-type YjcS (Y) and the double mutant D184N-H185A (M) were diluted sequentially by fivefold and plated on the agarose plates with an additional 0.05 mM IPTG and no SDS or 0.1% (w/v) SDS, and grew at 37°C for 2 days.
Sulfatase activity assay of YjcS in vitro
The activity of the alkylsulfatase was assayed using a phenol red/HEPES indicator/buffer pair (100 µM phenol red, 0.2 mM HEPES pH 7.5), because the release of a proton accompanied by the hydrolysis of SDS led to a change in pH, which can be monitored using a spectrophotometer.8,38 The typical reaction system contained 0.2 mM HEPES (pH 7.5), 100 µM phenol red, 0.02% (w/v) SDS, and 0.77 µM YjcS at 37°C, and a decrease in absorbance at 557 nm was monitored.
To analyze the effects of divalent metal ions on alkylsulfatase activity of YjcS, various ions were added to the above reaction mixture. The final concentration of Mg2+ was 10 mM and other ions (Zn2+, Ca2+, Mn2+, Co2+, and Ni2+) were 1 mM. Standard curves were prepared by substituting SDS with equal amounts of HCl.
Accession numbers
Atomic coordinates and structure factors of YjcS have been deposited in the Protein Data Bank with code 4PDX.
Conclusions
E. coli uncharacterized protein YjcS belongs to the alkylsulfatases, with an N-terminal signal peptide. Structural comparison and sequence alignment with members of the alkylsulfatases, SdsA1 and Pisa1, revealed that YjcS exhibits a conserved binuclear Zn2+ binding motif HXHXDH. Additionally, YjcS shares a common catalytic mechanism with SdsA1 and Pisa1, in which, His317 of YjcS as a potential source for the protonation of the leaving group and Tyr417 side chain of YjcS is critically involved in the correct positioning of the substrate’s sulfate group. SDS hydrolysis experiments verified the alkylsulfatase activity of YjcS. The double mutant D184N-H185A (residues that are part of the HXHXDH motif) was found to be inactive and did not hydrolyze SDS. The experiments on the effects of divalent metal ions on YjcS activity indicate the addition of Ca2+ might slightly activate YjcS activity in vitro.
Acknowledgments
The authors are grateful to the staff of the beamline station BL17U of Shanghai Synchrotron Radiation Facility (SSRF).
Glossary
- 1DA
1-decane-sulfonic-acid
- 1DO
1-dodecanol
- β-ME
β-mercaptoethanol
- DTT
dithiothreitol
- IPTG
isopropyl-β-d-thiogalactoside
- MBL
metallo-β-lactamase
- MW
molecular weight
- SAD
single wavelength anomalous diffraction
- SDS
sodium dodecyl sulfate
- Se-Met
Selenomethionine.
References
- Fitzgera JW, Payne WJ. Induction in a Pseudomonas species of sulfatases active on short chain alkylsulfates. Microbios. 1972;5:87. [PubMed] [Google Scholar]
- Beil S, Kehrli H, James P, Staudenmann W, Cook AM, Leisinger T, Kertesz MA. Purification and characterization of the arylsulfatase synthesized by Pseudomonas-aeruginosa pao during growth in sulfate-free medium and cloning of the arylsulfatase gene (Atsa) Eur J Biochem. 1995;229:385–394. doi: 10.1111/j.1432-1033.1995.0385k.x. [DOI] [PubMed] [Google Scholar]
- Kahnert A, Kertesz MA. Characterization of a sulfur-regulated oxygenative alkylsulfatase from Pseudomonas putida S-313. J Biol Chem. 2000;275:31661–31667. doi: 10.1074/jbc.M005820200. [DOI] [PubMed] [Google Scholar]
- Bojarova P, Williams SJ. Sulfotransferases, sulfatases and formylglycine-generating enzymes: a sulfation fascination. Curr Opin Chem Biol. 2008;12:573–581. doi: 10.1016/j.cbpa.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Sinha DN, Gupta PC, P Gangadharan. Tobacco use among students and school personnel in India. Asian Pac J Cancer Prev. 2007;8:417–421. [PubMed] [Google Scholar]
- Kertesz MA. Riding the sulfur cycle—metabolism of sulfonates and sulfate esters in gram-negative bacteria. FEMS Microbiol Rev. 2000;24:135–175. doi: 10.1016/S0168-6445(99)00033-9. [DOI] [PubMed] [Google Scholar]
- Hanson SR, Best MD, Wong CH. Sulfatases: structure, mechanism, biological activity, inhibition, and synthetic utility. Angew Chem. 2004;43:5736–5763. doi: 10.1002/anie.200300632. [DOI] [PubMed] [Google Scholar]
- Hagelueken G, Adams TM, Wiehlmann L, Widow U, Kolmar H, Tummler B, Heinz DW, Schubert WD. The crystal structure of SdsA1, an alkylsulfatase from Pseudomonas aeruginosa, defines a third class of sulfatases. 2006;103:7631–7636. doi: 10.1073/pnas.0510501103. Proc Natl Acad Sci USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boltes I, Czapinska H, Kahnert A, von Bulow R, Dierks T, Schmidt B, von Figura K, Kertesz MA, Uson I. 1.3 A structure of arylsulfatase from Pseudomonas aeruginosa establishes the catalytic mechanism of sulfate ester cleavage in the sulfatase family. Structure. 2001;9:483–491. doi: 10.1016/s0969-2126(01)00609-8. [DOI] [PubMed] [Google Scholar]
- Benjdia A, Martens EC, Gordon JI, Berteau O. Sulfatases and a radical S-adenosyl-L-methionine (AdoMet) enzyme are key for mucosal foraging and fitness of the prominent human gut symbiont, Bacteroides thetaiotaomicron. J Biol Chem. 2011;286:25973–25982. doi: 10.1074/jbc.M111.228841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berteau O, Guillot A, Benjdia A, Rabot S. A new type of bacterial sulfatase reveals a novel maturation pathway in prokaryotes. J Biol Chem. 2006;281:22464–22470. doi: 10.1074/jbc.M602504200. [DOI] [PubMed] [Google Scholar]
- Marquordt C, Fang Q, Will E, Peng J, von Figura K, Dierks T. ) Posttranslational modification of serine to formylglycine in bacterial sulfatases. Recognition of the modification motif by the iron-sulfur protein AtsB. J Biol Chem. 2003;278:2212–2218. doi: 10.1074/jbc.M209435200. [DOI] [PubMed] [Google Scholar]
- Muller I, Kahnert A, Pape T, Sheldrick GM, Meyer-Klaucke W, Dierks T, Kertesz M, Uson I. Crystal structure of the alkylsulfatase AtsK: insights into the catalytic mechanism of the Fe(II) alpha-ketoglutarate-dependent dioxygenase superfamily. Biochemistry. 2004;43:3075–3088. doi: 10.1021/bi035752v. [DOI] [PubMed] [Google Scholar]
- Knaus T, Schober M, Kepplinger B, Faccinelli M, Pitzer J, Faber K, Macheroux P, Wagner U. Structure and mechanism of an inverting alkylsulfatase from Pseudomonas sp. DSM6611 specific for secondary alkyl sulfates. FEBS J. 2012;279:4374–4384. doi: 10.1111/febs.12027. [DOI] [PubMed] [Google Scholar]
- Davison J, Brunel F, Phanopoulos A, Prozzi D, Terpstra P. Cloning and sequencing of Pseudomonas genes determining sodium dodecyl-sulfate biodegradation. Gene. 1992;114:19–24. doi: 10.1016/0378-1119(92)90702-q. [DOI] [PubMed] [Google Scholar]
- Shukor MY, Husin WSW, Rahman MFA, Shamaan NA, Syed MA. Isolation and characterization of an SDS-degrading Klebsiella oxytoca. J Environ Biol. 2009;30:129–134. [PubMed] [Google Scholar]
- Chaturvedi V, Kumar A. Isolation of a strain of Pseudomonas putida capable of metabolizing anionic detergent sodium dodecyl sulfate (SDS) Iranian J Microbiol. 2011;3:47–53. [PMC free article] [PubMed] [Google Scholar]
- Long M, Ruan L, Li F, Yu Z, Xu X. Heterologous expression and characterization of a recombinant thermostable alkylsulfatase (sdsAP) Extromophiles. 2011;15:293–301. doi: 10.1007/s00792-011-0357-4. [DOI] [PubMed] [Google Scholar]
- Thomas ORT, White GF. Metabolic pathway for the biodegradation of sodium dodecyl-sulfate by Pseudomonas Sp-C12b. Biotechnol Appl Biochem. 1989;11:318–327. [PubMed] [Google Scholar]
- Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Meth. 2011;8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- Carfi A, Pares S, Duee E, Galleni M, Duez C, Frere JM, Dideberg O. The 3-D structure of a zinc metallo-beta-lactamase from Bacillus cereus reveals a new type of protein fold. EMBO J. 1995;14:4914–4921. doi: 10.1002/j.1460-2075.1995.tb00174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron AD, Ridderstrom M, Olin B, Mannervik B. Crystal structure of human glyoxalase II and its complex with a glutathione thiolester substrate analogue. Structure. 1999;7:1067–1078. doi: 10.1016/s0969-2126(99)80174-9. [DOI] [PubMed] [Google Scholar]
- Frazao C, Silva G, Gomes CM, Matias P, Coelho R, Sieker L, Macedo S, Liu MY, Oliveira S, Teixeira M, AV Xavier, C Rodrigues-Pousada, MA Carrondo, J LeGall. Structure of a dioxygen reduction enzyme from Desulfovibrio gigas. Nat Struct Biol. 2000;7:1041–1045. doi: 10.1038/80961. [DOI] [PubMed] [Google Scholar]
- Liu D, Lepore BW, Petsko GA, Thomas PW, Stone EM, Fast W, Ringe D. Three-dimensional structure of the quorum-quenching N-acyl homoserine lactone hydrolase from Bacillus thuringiensis. 2005;102:11882–11887. doi: 10.1073/pnas.0505255102. Proc Natl Acad Sci USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372:774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Holm L, Rosenstrom P. Dali server: conservation mapping in 3D. Nucleic Acids Res. 2010;38:W545–549. doi: 10.1093/nar/gkq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto M, Goto Y, Maruyama M, Hattori A. Biochemical and enzymatic properties of the M1 family of aminopeptidases involved in the regulation of blood pressure. Heart Failure Rev. 2008;13:285–291. doi: 10.1007/s10741-007-9064-8. [DOI] [PubMed] [Google Scholar]
- Danielsen EM, Noren O, Sjostrom H, Ingram J, Kenny AJ. Proteins of the kidney microvillar membrane. Aspartate aminopeptidase: purification by immunoadsorbent chromatography and properties of the detergent- and proteinase-solubilized forms. Biochem J. 1980;189:591–603. doi: 10.1042/bj1890591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen KS, Auld DS. Carboxypeptidase-a—mechanism of zinc inhibition. Biochemistry. 1989;28:9620–9625. doi: 10.1021/bi00451a012. [DOI] [PubMed] [Google Scholar]
- Bergfors T. Seeds to crystals. J Struct Biol. 2003;142:66–76. doi: 10.1016/s1047-8477(03)00039-x. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Method Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Rogazinskaya OV, Milovidova SD, Sidorkin AS, Plaksitsky AB, Goliandrin YV, Ponomareva NY. Electron emission from TGS crystals grown on seeds exposed to alpha particles. Ferroelectrics. 2007;360:246–249. [Google Scholar]
- Terwilliger TC. Maximum-likelihood density modification. Acta Cryst. 2000;D56:965–972. doi: 10.1107/S0907444900005072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowtan K. The Buccaneer software for automated model building. 1. Tracing protein chains. Acta Cryst. 2006;D62:1002–1011. doi: 10.1107/S0907444906022116. [DOI] [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Cryst. 2010;D66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, AJ McCoy, NW Moriarty, R Oeffner, RJ Read, DC Richardson, JS Richardson, TC Terwilliger, PH Zwart. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Cryst. 2010;D66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen VB, Arendall WB, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Cryst. 2010;D66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PW, Stone EM, Costello AL, Tierney DL, Fast W. The quorum-quenching lactonase from Bacillus thuringiensis is a metalloprotein. Biochemistry. 2005;44:7559–7569. doi: 10.1021/bi050050m. [DOI] [PubMed] [Google Scholar]