Abstract
6-Chlorotryptophan possesses unique bioactivity and can be used as a precursor for several bioactive compounds in medicinal chemistry. It was enantioselectively synthesized by condensing 6-chloroindole with racemic N-acetylserine, followed by enzymatic hydrolysis with l-aminoacylase (EC 3.5.1.14). The optical purity was examined by conducting high-performance liquid chromatography with a Cinchona alkaloid-based zwitterionic chiral stationary phase (CSP) [CHIRALPAK® ZWIX(+)], which bears a chiral trans-2-aminocyclohexanesulfonic acid moiety tagged at C-9 of the Cinchona alka-loid. The zwitterionic CSP enabled efficient enantiomeric separations of monosubstituted tryptophan derivatives 1-methyltryptophan, 5-methyltryptophan, 6-methyltryptophan, 5-methoxytryptophan, and 6-chlorotryptophan with a methanol/H2O (98/2) mobile phase containing formic acid (FA) and diethylamine (DEA) additives. The mobile phase contains 25–75 mM FA and 20–50 mM DEA, enabling good separation of the enantiomers of each tryptophan derivative (α > 1.25). Thus, the optical purity of the synthesized 6-chloro-l-tryptophan was easily determined (greater than 99.0%) using HPLC with the zwitterionic CSP.
Keywords: 6-chlorotryptophan, enantiomer, CHIRALPAK® ZWIX(+), high-performance liquid chromatography
Introduction
l-Tryptophan (l-Trp) is an essential amino acid and an important nutrient as it is a protein constituent in biological organisms. It is metabolized in the kynurenine (KYN) pathway, which is a major pathway of Trp metabolism in vivo,1–3 to produce neuroactive compounds such as kynurenic acid (KYNA) and quinolinic acid.
Monosubstituted Trp derivatives such as 6-chlorotryptophan (6-chloro-Trp) and 1-methyltryptophan (1-methyl-Trp) inhibit the enzyme activity of indoleamine 2,3-dioxygenase (IDO), a rate-limiting enzyme in the metabolism of l-Trp to l-KYN2 in the KYN pathway.4 Recently, studies on the inhibition of IDO have been carried out with a focus on the treatment of cancer, cataract, and Alzheimer’s disease5; therefore, a selective inhibitor of IDO is a desired novel drug candidate.6 Furthermore, the optical isomers of monosubstituted Trp derivatives, the l- and d-forms, should be considered, since both 1-methyl-l-Trp and 1-methyl-d-Trp exhibit IDO inhibition.7 In addition, optically active monosubstituted Trp derivatives can be used as starting materials for bioactive compounds such as lysergic acid and claviciptic acid.6,8,9 Thus, assessment of optical purity is required during the syntheses of optically pure monosubstituted Trp derivatives.
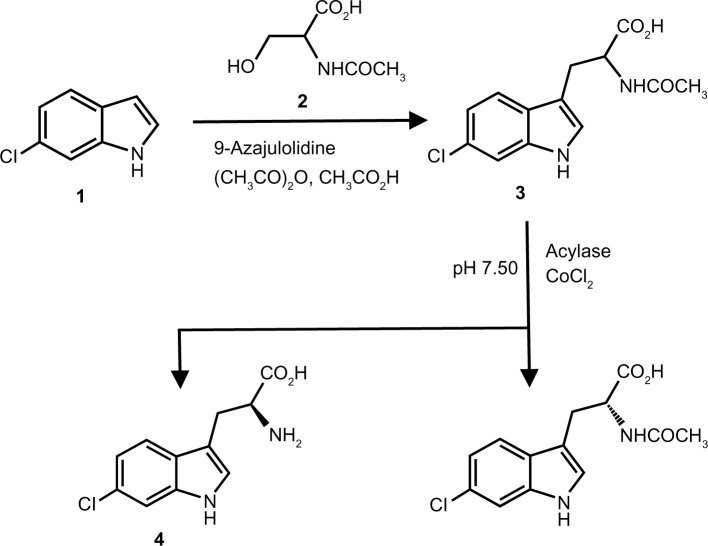
Recently, a novel chiral stationary phase (CSP) for high-performance liquid chromatography (HPLC), a Cinchona alkaloid-based zwitterionic CSP, was reported by Lindner’s group,10 and the packed chiral column [CHIRALPAK® ZWIX (+) or (−)] is currently commercially available from CPI Company, Daicel Co., Ltd. The zwitterionic CSP can afford enantiomeric separations of chiral acids, amines, and amino acids, including Trp, without further chemical derivatization.10,11 Using a mobile phase of aqueous methanol containing formic acid (FA) and diethylamine (DEA), enantiomeric separations of some Trp derivatives were reported recently by Zhang et al.11 Thus, in the present study, enantiomeric separations of 6-chloro-Trp and other monosubstituted Trp derivatives were examined using the zwitterionic CSP. The optical purity of 6-chloro-l-Trp obtained (according to Fig. 1) via enzyme kinetic resolution8 was also examined using the zwitterionic CSP.
Figure 1.
Synthetic route of 6-chloro-l-Trp.
Experimental
Chemicals
Trp, KYN, 3-hydroxykynurenine (3-hydroxy-KYN), 1-methyl-Trp, 6-chloro-Trp, 5-methyltryptophan (5-methyl-Trp), 6-methyltryptophan (6-methyl-Trp), and 5-methoxytryptophan (5-methoxy-Trp) were obtained from Nacalai Tesque, Inc. and Santa Cruz Biotechnology, Inc. HPLC-grade methanol (MeOH), acetonitrile (CH3CN), FA, special-grade ethyl acetate (EtOAc), DEA, cobalt(II) chloride hexahydrate (CoCl2·6H2O), and l-aminoacylase (AMANO) were obtained from Wako Pure Chemical Industries, Ltd. Water was used after purification using a WR600G instrument (Nihon Millipore K.K.). 6-Chloroindole was obtained from Tokyo Chemical Industry Co., Ltd. 9-Azajulolidine was obtained from Sigma Co., Ltd.
HPLC
The HPLC system comprised an intelligent pump (LC-10 AD), a UV detector (SPD-10 A), and CDS software ver. 6 (LAsoft Ltd.). All separations on the chiral column were performed at room temperature. The chiral column, featuring trans-2-aminocyclohexanesulfonic acid tagged at the C-9 position of the Cinchona alkaloid (quinine) via carbamate linkage and chemically bound onto silica gel [CHIRALPAK ZWIX(+); 250 × 4.0 mm i.d., 5 mm], was purchased from CPI Company, Daicel Co., Ltd. and connected to the HPLC system described above. For reversed-phase HPLC, the mobile phase comprised 0–100 mM FA and 0–100 mM DEA in aqueous MeOH [MeOH/H2O (98/2)], and flowed at a constant rate of 0.5 mL/minute. The detection wavelength was set at 254 nm. For analysis, 20 μL of each sample (2.0 nmol/10 μL in the mobile phase) was injected into the HPLC system. The degree of enantiomeric separation was evaluated using the resolutions (Rs) and separation factors (α):
where tR1, tR2 and w1, w2 are, respectively, the retention times and peak widths of the first and second eluted peaks, and k1 and k2 indicate capacity factors of the first and second eluted peaks.
Synthesis of 6-chloro-l-Trp
The synthesis of 6-chloro-l-Trp was carried out following procedures reported in previous papers.8 A mixture of 6-chloroindole (1) (37.8 mg, 0.25 mmol), N-acetylserine (2) (73.1 mg, 0.50 mmol), and 9-azajulolidine (11.7 mg, 0.075 mmol) in CHCl3 (1.0 mL) was stirred in the presence of acetic acid (0.09 mL) and acetic anhydride (0.08 mL) under argon overnight at room temperature. After the reaction was complete, 2 mL of H2O was added to the mixture, which was then basified with 30% NaOH in H2O and extracted with EtOAc (20 mL × 3). The aqueous phase was acidified with 35% HCl in H2O, and then extracted with EtOAc (25 mL × 3). The organic layer was washed with a saturated NaCl aqueous solution, dried with anhydrous MgSO4, and concentrated under vacuum. The residue was purified by silica-gel column chromatography to yield racemic N-acetyl-6-chloro-Trp (3) (25.6 mg, yield 36.5%). Subsequently, N-acetyl-6-chloro-Trp (25.6 mg) was dissolved in 1.0 M NaOH in H2O (0.5 mL). Acylase (3.12 mg), CoCl2·6H2O (1.26 mg), and 20 mM ammonium hydrogen phosphate (pH 7.50) (10.0 mL) were added, and the reaction mixture was incubated at 37°C for 40 hours. After the enzymatic reaction was complete, the reaction solution was acidified with 10% HCl in H2O and extracted with EtOAc (25 mL × 3). The aqueous phase was neutralized with 2% NaOH in H2O and concentrated in vacuo. The residue was purified by preparative-scale reversed-phase column chromatography with Purif-α2 (Shoko Scientific Co., Ltd.) to afford 6-chloro-l-Trp as white crystals (2.30 mg, yield 8.99%) (4), 1H NMR (D2O) δ 7.55 (d, 1H), 7.43 (s, 1H), 7.18 (s, 1H), 7.06 (d, 1H), 3.93–3.90 (t, 1H), and 3.35–3.14 (m, 2H).
Enantiomeric excess (ee, %) was calculated using the following equation:
where X1 and X2 are the peak areas of the first and second eluted enantiomers (X2 > X1).
Results and Discussion
Enantiomeric separation of Trp derivatives and the metabolites
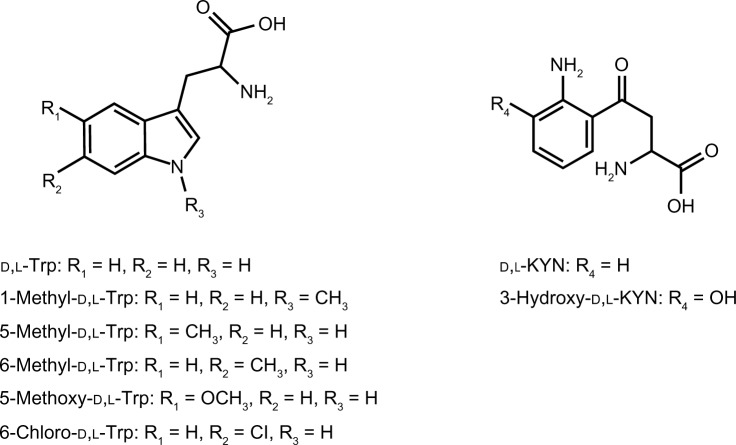
A Cinchona alkaloid-based zwitterionic CSP was used for an amphoteric compound, 6-chloro-Trp, because it has been reported that such a zwitterionic CSP10 [CHIRALPAK ZWIX(+)] was effective for the enantiomeric separation of racemic amphoteric compounds.11 In addition to 6-chloro-Trp, enantiomeric separations of several Trp derivatives and metabolites (Fig. 2) were tested. As the mobile phase, aqueous MeOH (MeOH/H2O = 98/2) containing FA and DEA was used, as reported in previous papers,10,11 and the effects of the FA and DEA concentrations in the aqueous MeOH mobile phase on the enantiomeric separation of Trp derivatives and metabolites were investigated.
Figure 2.
Chemical structures of Trp and their derivatives (1-methyl-d,l-Trp, 5-methyl-d,l-Trp, 6-methyl-d,l-Trp, 5-methoxy-d,l-Trp, and 6-chloro-d,l-Trp), and Trp metabolites (d,l-KYN and 3-hydroxy-d,l-KYN).
Table 1 shows the Rs and α values of Trp derivatives and metabolites using several concentrations of FA and DEA in aqueous MeOH. As shown in Table 1, the enantiomers of each Trp derivative as well as Trp can be separated effectively (α > 1.25). These results were consistent with those in previous studies.10,11 When the acidic additive (FA) concentration was greater than 40 mM in the mobile phase, the Rs values of Trp derivatives increased by 2.0 or more. In the presence of 50 mM FA, 25 mM basic additive (DEA) was optimal, because 50, 75, and 100 mM DEA yielded no elution of either enantiomer of Trp derivatives, while 0 mM DEA produced only a single peak at void volume (V0) in the chromatogram. However, a larger concentration of FA (75 mM) led to elution and separation of both enantiomers even in the presence of 50 mM DEA, likely owing to the counteracting effect between FA and DEA. Among the Trp derivatives, the Rs and α values of 1-methyl-d,l-Trp were relatively small, suggesting that methylation at the nitrogen atom of the indole ring greatly affected the enantiomer recognition of the CSP. In contrast, methylation at the 5- or 6-position of the indole ring afforded large Rs and α values. Since the methyl group is an electron-donating group, it was speculated that introduction of electron-donating groups to the 5- or 6-position of the indole ring increased enantiomer recognition. Substitution with methoxy or chloro groups at the 5- or 6-position of the indole ring resulted in a decreased enantiomer recognition compared to Trp. These tendencies were consistent with those reported in previous papers.10,11 Therefore, monosubstitution on the indole ring can greatly influence the enantiomer recognition of the CSP. Figure 3 shows representative chromatograms of 6-chloro-d, l-Trp with a mobile phase of MeOH/H2O containing 50 mM FA and 25 mM DEA. With regard to the elution order of enantiomers, the d-isomer of 6-chloro-Trp eluted earlier, similar to Trp and 1-methyl-Trp.11 The elution orders of other Trp derivatives, 5-methyl-Trp, 6-methyl-Trp, and 5-methoxy-Trp, were unclear because optically pure standards could not be obtained.
Table 1.
Separation factor (α) and resolution (Rs) of Trp, the derivatives, and Trp metabolites on the Cinchona alkaloid-based zwitterionic CSP.
| 25 mM FA |
25 mM DEA |
50 mM FA |
25 mM DEA |
50 mM FA |
20 mM DEA |
40 mM FA |
20 mM DEA |
60 mM FA |
20 mM DEA |
75 mM FA |
50 mM DEA |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α | Rs | α | Rs | α | Rs | α | Rs | α | Rs | α | Rs | |
| d,l-Trp | 1.45 | 2.57 | 1.51 | 5.04 | 1.50 | 5.14 | 1.50 | 4.87 | 1.49 | 5.02 | 1.47 | 4.21 |
| 1-Methyl-d,l-Trp | 1.28 | 1.52 | 1.27 | 2.92 | 1.27 | 2.64 | 1.27 | 2.51 | 1.26 | 2.61 | 1.27 | 2.14 |
| 5-Methyl-d,l-Trp | 1.52 | 2.96 | 1.56 | 5.39 | 1.55 | 5.43 | 1.56 | 5.15 | 1.54 | 5.40 | 1.53 | 4.79 |
| 6-Methyl-d,l-Trp | 1.59 | 3.65 | 1.59 | 5.74 | 1.58 | 5.98 | 1.58 | 5.59 | 1.63 | 6.08 | 1.58 | 5.03 |
| 5-Methoxy-d,l-Trp | 1.48 | 1.87 | 1.47 | 4.92 | 1.46 | 4.93 | 1.46 | 4.71 | 1.46 | 4.84 | 1.45 | 4.23 |
| 6-chloro-d,l-Trp | 1.33 | 2.22 | 1.38 | 3.99 | 1.38 | 4.37 | 1.39 | 3.96 | 1.39 | 4.09 | 1.36 | 3.49 |
| d,l-KYN | 1.26 | 1.36 | 1.28 | 2.55 | 1.27 | 2.72 | 1.27 | 2.46 | 1.29 | 2.78 | 1.27 | 2.22 |
| 3-Hydroxy-d,l-KYN | 1.26 | 1.18 | 1.25 | 2.43 | 1.26 | 2.67 | 1.26 | 2.31 | 1.27 | 2.71 | 1.26 | 2.20 |
Figure 3.
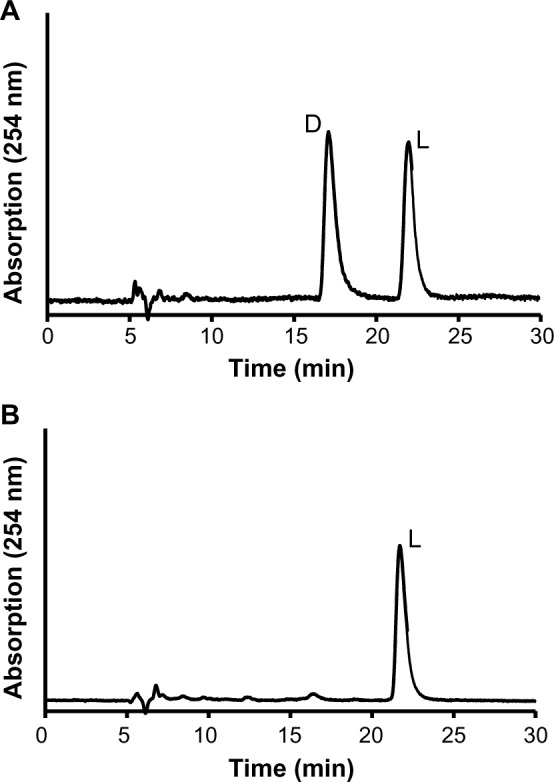
Chromatogram of a 6-chloro-d,l-Trp standard (A) and synthesized 6-chloro-l-Trp (B) on Cinchona alkaloid-based zwitterionic CSP with a mobile phase of 50 mM FA and 25 mM DEA in MeOH/H2O (98/2).
On the other hand, the enantiomers of Trp metabolites, KYN and 3-hydroxy-KYN, were separated effectively using 60 mM FA and 25 mM DEA. The l-isomers of KYN and 3-hydroxy-KYN eluted earlier on the column, and this was consistent with the results from a previous study.11
Evaluation of optical purity of synthesized 6-chloro-l-Trp
In the present study, we attempted the synthesis of 6-chloro-l-Trp, as shown in Figure 1.8 The substitution reaction of monosubstituted indole with N-acetylserine in the presence of acetic anhydride produced racemic N-acetyl-monosubstituted Trp.8 Although optically active N-acetylserine was employed, racemic N-acetyl-monosubstituted Trp was obtained owing to deprotonation at the asymmetric carbon of N-acetylserine during the reaction.8 Therefore, in order to obtain optically active monosubstituted l-Trp from racemic N-acetyl-monosubstituted Trp, optical resolution (kinetic resolution) using l-aminoacylase (EC 3.5.1.14) was required. Kinetic resolution using acylase provides only monosubstituted l-Trp via selective hydrolysis of N-acetyl-monosubstituted l-Trp. Utilizing these reactions, the synthesis of 6-chloro-l-Trp was attempted as shown in Figure 1. Finally, the synthesized 6-chloro-l-Trp was isolated, and its optical purity was investigated by HPLC with a Cinchona alkaloid-based zwitterionic CSP. As shown in Figure 3B, the 6-chloro-l-Trp peak was detected, while no detectable peak was observed at the expected retention time of 6-chloro-d-Trp. As a result, the ee value calculated from the peak areas was greater than 99.0%. Therefore, it was clear that 6-chloro-l-Trp with high optical purity was synthesized through the proposed synthetic route,8 and that HPLC with a Cinchona alkaloid-based zwitterionic CSP successfully evaluated the optical purity of 6-chloro-l-Trp synthesized in our laboratory, as revealed by the complete separation of 6-chloro-l- and d-Trp (Table 1).
Conclusion
Enantiomeric separation of monosubstituted Trp derivatives by HPLC was easily performed using a Cinchona alkaloid-based zwitterionic CSP, and no derivatization reactions of Trp derivatives were required. In the mobile phase, volatile constituents such as FA and DEA were used; therefore, highly sensitive and accurate mass-spectrometric detection of the enantiomers in biological samples would be possible. Thus, the optical purity of 6-chloro-l-Trp synthesized in our laboratory was successfully evaluated by conducting HPLC with the Cinchona alkaloid-based zwitterionic CSP.
Acknowledgments
The authors thank Dr. H. Yamada and Miss M. Seshimo, Toho University, for their kind advice and valuable discussion on this study.
Footnotes
Author Contributions
Conceived and designed the experiments: TF, H. Iizuka, H. Ichiba. Performed experiments and analyzed the data: AS, IF, SI, MO. Contributed to the writing of the manuscript: TF, H. Iizuka, H. Ichiba. Jointly developed the structure and arguments for the paper: HH, YY. Made critical revisions and approved the final version: HH, YY. All authors reviewed and approved the final manuscript.
ACADEMIC EDITOR: Giles Guillemin, Editor in Chief
FUNDING: This study was in part financially supported by a Grant-in-Aid for Scientific Research (C) (25460224) from the Japan Society for the Promotion of Science (JSPS) under the Ministry of Education, Culture, Sports, Science and Technology. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
REFERENCES
- 1.Macchiarulo A, Camaioni E, Nuti R, Pellicciari R. Highlights at the gate of tryptophan catabolism: a review on the mechanisms of activation and regulation of indoleamine 2,3-dioxygenase (IDO), a novel target in cancer disease. Amino Acids. 2009;37:219–29. doi: 10.1007/s00726-008-0137-3. [DOI] [PubMed] [Google Scholar]
- 2.Vecsei L, Szalardy L, Fulop F, Toldi J. Kynurenines in the CNS: recent advances and new questions. Nat Rev Drug Discov. 2013;12:64–82. doi: 10.1038/nrd3793. [DOI] [PubMed] [Google Scholar]
- 3.Myint AM. Kynurenines: from the perspective of major psychiatric disorders. FEBS J. 2012;279:1375–85. doi: 10.1111/j.1742-4658.2012.08551.x. [DOI] [PubMed] [Google Scholar]
- 4.Saito K, Chen CY, Masana M, Crowley JS, Markey SP, Heyes MP. 4-Chloro-3-hydroxyanthranilate, 6-chlorotryptophan and norharmane attenuate quinolinic acid formation by interferon-gamma-stimulated monocytes (THP-1 cells) Biochem J. 1993;291:11–4. doi: 10.1042/bj2910011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Pucchio T, Danese S, De Cristofaro R, Rutella S. Inhibitors of indoleamine 2,3-dioxygenase: a review of novel patented lead compounds. Expert Opin Ther Pat. 2010;20:229–50. doi: 10.1517/13543770903512974. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka M, Li X, Hikawa H, et al. Synthesis and biological evaluation of novel tryptoline derivatives as indoleamine 2,3-dioxygenase (IDO) inhibitors. Bioorg Med Chem. 2013;21:1159–65. doi: 10.1016/j.bmc.2012.12.028. [DOI] [PubMed] [Google Scholar]
- 7.Jia L, Schweikart K, Tomaszewski J, et al. Toxicology and pharmacokinetics of 1-methyl-d-tryptophan: absence of toxicity due to saturating absorption. Food Chem Toxicol. 2008;46:203–11. doi: 10.1016/j.fct.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokoyama Y, Hikawa H, Mitsuhashi M, Uyama A, Hiroki Y, Murakami Y. Total synthesis without protection: three-step synthesis of optically active clavicipitic acids by a biomimetic route. Eur J Org Chem. 2004;2004:1244–53. [Google Scholar]
- 9.Hurt CR, Lin R, Rapoport H. Enantiospecific synthesis of (R)-4-amino-5-oxo-1,3,4,5-tetrahydrobenz[cd]indole, an advanced intermediate containing the tricyclic core of the ergots. J Org Chem. 1999;64:225–33. doi: 10.1021/jo981723s. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann CV, Pell R, Lammerhofer M, Lindner W. Synergistic effects on enantioselectivity of zwitterionic chiral stationary phases for separations of chiral acids, bases, and amino acids by HPLC. Anal Chem. 2008;80:8780–9. doi: 10.1021/ac801384f. [DOI] [PubMed] [Google Scholar]
- 11.Zhang T, Holder E, Franco P, Lindner W. Zwitterionic chiral stationary phases based on cinchona and chiral sulfonic acids for the direct stereoselective separation of amino acids and other amphoteric compounds. J Sep Sci. 2014;37:1237–47. doi: 10.1002/jssc.201400149. [DOI] [PubMed] [Google Scholar]