Abstract
A total of 167 surgically resected primary invasive breast carcinomas and 63 metastatic lymph node lesions were analyzed for immunohistochemical (IHC) localization of the CD44+CD24−low breast cancer stem cell (CSC) markers, epithelial to mesenchymal transition (EMT) markers, and telomerase activity by double-staining IHC technique, in formalin-fixed, paraffin-embedded tissue, the results were validated by double-staining immunofluorescent and flow cytometry techniques. The results showed that CSCs with CD44+CD24−low phenotype were significantly increased in node-positive tumors, high-grade tumors, and ductal carcinoma in situ (DCIS). There was a high incidence of telomerase expression in metastatic lymph node lesion. There were considerably high number of tumor cells with EMT expression in metastatic lymph node lesion, and triple-negative tumor. The occurrence of EMT phenomena was usually accompanied by the co-existence of CSCs of CD44+CD24−low phenotype. There was no association between the existence of CSCs and detection of telomerase activity in tumor cells. Increased numbers of both CSCs of CD44+CD24−low phenotype and cells underwent EMT in DCIS lesion might be an initial step in the stromal invasion and propagation of breast cancer, and occurrence of EMT in the breast tumor associated with high prevalence of CSCs, promoting tumor invasiveness and metastasis.
Keywords: breast cancer stem cells, epithelial to mesenchymal transition, telomerase activity, breast cancer
Introduction
Breast carcinoma is the most common malignant tumor and the leading cause of carcinoma death in women, with more than 1,000,000 cases occurring worldwide annually. Human breast cancer is a truly complex disease with a large inter-tumoral and intra-tumoral heterogeneity resulting in highly variable clinical behavior and response to therapy. The maintenance of the heterogeneity of cells within a tumor is not fully understood. Possibly, every cell within a tumor may have a capacity to proliferate and form new tumors, although the likelihood for each cell is very low. Alternatively, only a small subset of cells with distinct characteristics has the capacity to maintain tumor growth,1 called cancer stem cells (CSCs), which are capable of both initiating tumor and sustaining tumor growth. Three novel concepts have emerged in breast cancer biology: the role of CSCs in tumor initiation and the involvement of an epithelial to mesenchymal transition (EMT) in the metastatic dissemination of cancer cells, along with the telomerase role in keeping the CSCs immortal, and avoiding senescence. The clinicopathologic significance of these three novel concepts in primary and metastatic breast carcinoma, and correlation among each other are the leading purpose of this research.
Cancer stem cells
Breast CSCs are a subset of tumor cells possessing distinct immunological markers; they have CD44+CD24−low phenotype, and they have been demonstrated by Al-Hajj et al.2 to have tumor-initiating properties in breast cancer. This tumorigenic phenotype has been associated with stem cell–like characteristics,3 with enhanced invasive properties,4 and radiation resistance.5 The concept of CSCs has led to new hypotheses about tumor growth. CSCs share similar properties with normal stem cells in terms of their capacity for self-renewal. They can self-renew and cause tumorigenesis, recurrence, and metastasis. Moreover, they can divide asymmetrically to generate differentiated cancer cells. Within the population of cancer cells, CSCs are the ones that can form new tumors, and their asymmetric division contributes to the heterogeneity.6 The CSC hypothesis posits that this minority cell population can fuel and drive tumor growth and remain in patients after conventional chemotherapy, which eradicates the rapidly growing nontumorigenic cells that constitute the major bulk of the tumor, and then it is unlikely to be curative and relapses would be expected. The hypothesis predicts that effective tumor eradication will require obtaining agents that can target CSCs while sparing normal stem cells. This explains why the CSC hypothesis is at the center of a rapidly evolving field that may play a pivotal role inchanging how basiccancer researchers, clinical investigators, physicians, and cancer patients view cancer.7 CD44+CD24−low cell-surface expression has been proposed as a marker for breast CSCs.2 A correlation of the CSCs to specific breast cancer subtypes has not yet been reported in human breast carcinoma. In the present study, we try to analyze these CSCs (CD44+CD24−low) in different variants of primary human breast carcinoma using double-staining immunohistochemistry (IHC) and immu-nofluorescence (IF) and to correlate the presence of CSCs in associated metastatic tumors. The prospective identification of these CSCs should allow the identification of molecules expressed in these cells that could be targeted to eliminate this crucial population of cancer cells, leading to more effective cancer therapies.2
Telomerase activity
Telomerase is the intracellular reverse transcriptase responsible for the elongation of chromosomal telomere after each cell division. Human chromosomes contain repeated TTAGGG DNA sequences at their ends called “Telomeres” that provide genomic stability. Human telomeres progressively shorten with ongoing cell division until they reach a critical length that induces senescence. Telomerase activity is required for lengthening of telomeres. Telomerase is an enzyme complex consisting of a human telomerase reverse transcriptase catalytic subunit (hTERT) and human telomerase RNA component for adding TTAGGG repeats to the end of the chromosome to maintain the length of the telomere. In normal body tissue, telomerase is detectable in proliferative germline cells, testicular seminiferous tubules, proliferating hematopoietic stem-like cells, activated lymphocytes, proliferative pre-menopausal endometrial cells, basal layer of cells in the epidermis, and in the crypts of the intestine. For detecting telomerase activity in a tissue specimen, the telomeric repeat amplification protocol (TRAP) assay is a relatively sensitive and specific method, but it can be used only on fresh tissue extracts and offers no information at the single-cell level. IHC allows detecting hTERT protein expression at an individual-cell level in human tissues. By polymerase chain reaction–based assay and TRAP, telomerase activity is detected in approximately 85%–90% of human cancer specimens and the levels of telomerase activity estimated by the TRAP assay vary among cancer tissues. These results suggest that human cancer cells in vivo do not always have, or absolutely require, telomerase reactivation.8 The hTERT protein can reliably be detected by IHC in formalin-fixed human tissues, but the choice of the antibody and tissue processing conditions are crucial hTERT protein localized in the nuclei, notably in the nucleoli of dividing cells. Telomerase-positive tumors showed significant heterogeneity of hTERT protein expression.9 It is noteworthy that telomerase activity has been detected in about 75%–90% of breast carcinoma in situ lesions, 88%–94% of invasive breast carcinoma, 5%–14% of tissues adjacent to breast cancer, and none of normal breast tissue. Studies from the University of Malaya10 show findings consistent with the above trend. In addition, mean telomere lengths of non-neoplastic breast tissues, fibroadenomas, and invasive breast carcinomas have been found to be 3.1, 1.9, and 1.0 kbp, respectively, supporting the concept of a crisis level of telomere shortening that triggers telomerase activation. As the average telomere length in breast cancer cells is substantially shorter than normal tissues, the probability that telomerase inhibitors could lead to arrest and death of cancer cells without adverse effects on normal tissues poses an attractive therapeutic approach.10 Triggering of telomerase activity along with stabilization of telomeres is consistent with the hypothesis that telomere maintenance is essential for attainment of immortality in CSCs and may be a rate-limiting step in cancer progression.11 Recent findings suggest that isolated CSC populations of CD44+CD24−low phenotype also exhibit telomerase activity and short telom-ere lengths similar to the parental cancer cell line.3 These results suggest that the cancer-initiating cells may be prime targets for telomerase inhibitor treatment as part of the therapeutic regimen for cancer.12
Epithelial to mesenchymal transition
The loss of epithelial differentiation and gain of the mesenchymal phenotype is called EMT. It was first recognized as a feature of embryo-genesis, which is vital for morphogenesis during embryonic development. EMT can be induced by disassembly of cell–cell junctions, including the downregulation and relocation of E-cadherin.13 This transition can also be triggered by a diverse range of stimuli including growth factor signaling, tumor–stromal interactions, and hypoxia. Recently, it has also been implicated in the progression of early-stage tumors into invasive malignancies.14 Vimentin is a marker of mesenchymal phenotype, while E-cadherin is the epithelial marker. The dissociation of the E-cadherin–mediated adherens junction is an initial step in EMT and may occur early or late in the growing carcinoma as a first step toward stromal invasion, lymphovascular permeation, and distant metastasis.15 EMT enhances tumor invasion metastasis by promoting loss of contact inhibition and increased cell motility.16 Increasing evidence suggests that tumor progression is critically involved with the acquisition of an EMT phenotype, which allows these cells to infiltrate surrounding tissues and promote distant metastasis. Progression of most carcinomas from stage to stage is associated with the acquisition of mesenchymal phenotype (vimentin expression), accompanied by the loss of epithelial marker expression (E-cadherin), leading to increased cell motility and invasion.13 Prospective identification of molecular characters of these tumor cells will allow the development of new therapeutic agents to eradicate the tumor completely, giving a new hope to improve the overall patient survival.
EMT and CSCs
Morel et al.17 demonstrated that CD44+CD24−low breast CSC signatures could be generated from CD44lowCD24+ nontumorigenic mammary epithelial cells. Further, they also found that CD44+CD24−low cells displayed an EMT phenotype as characterized by the loss of E-cadherin expression and gain of vimentin expression. Mani et al.18 further demonstrated that the induction of non-tumorigenic, immortalized human mammary epithelial cells into EMT phenotype resulted in the loss of epithelial marker and the gain of mesenchymal markers concomitant with the acquisition of CD44+CD24low expression pattern and increased mammosphereforming ability as well as tumor-initiating capacity. Whereas, isolated CD44+CD24low stem-like cells from normal and neoplastic human mammary cells exhibited a mesenchymal morphology and expressed mesenchymal markers such as vimentin and fibronectin. Santisteban et al.19 observed that the induction of EMT by an immune response against an epithelial breast cancer led to the outgrowth of tumor in vivo. Interestingly, the resulting mesenchymal tumor cells had a CD44+CD24low phenotype with the ability to reestablish an epithelial tumor. More recently, Gupta et al.20 also found that the induction of EMT in transformed HMLER breast cancer cells by shRNA-mediated knock-down of E-cadherin expression displayed an increased population of CD44+CD24low, and these cells exhibited a ~100-fold enhanced mammosphere-forming ability compared to their epithelial phenotypic cells. Ultimately, these studies strongly suggest that the induction of EMT could generate cells of stem-like characteristic; however, the molecular process and changes responsible for such processes are not fully understood.15 It seems that the EMT help tumor cells in obtaining the ability to detach from the tumor mass, infiltrating into the adjacent stroma. This might also be the initial step for tumor spread and promoting its capability of invasion and distance metastasis. Metastatic tumor cells with a mesenchymal phenotype are believed to undergo reverse transition, ie, mesenchymal-to-epithelial transition (MET) at the site of metastasis. Its looks that this process is a crucial step by which metastatic tumor cells grow at thesecondary site to form the metastatic growth.15 In conclusion, the initiation and recurrence of tumors is believed to be strongly linked with the biology of CSCs, while stromal invasion and distant metastasis may be related to EMT phenotypic tumor cell, and accumulating evidence has shown that cells with an EMT phenotype induced by different factors are rich sources for cancer stem-like cells, suggesting the biological similarities between CSCs and EMT-phenotypic cells.13
The purpose of this study is to analyze and determine the prevalence and significance of CSCs, telomerase activity, and EMT, in primary breast cancers and their associated axillary lymph node metastases, and the association between the two. We are going to evaluate the expression of their markers by using IHC study and IF technique, to be validated by flow cytometry analysis.
Materials and Methods
A total of 167 surgically resected primary invasive breast carcinomas and 63 surgically removed axillary lymph nodes having secondary metastasis, for patients aged 28–92 years, were selected from the daily workflow of the Anatomical Pathology Unit of Queen Elizabeth Hospital, Kota Kinabalu, Sabah, Eastern Malaysia. This research was approved by the Institute for Medical Research of the Ministry of Health Malaysia, and was conducted in accordance with the principles of the Declaration of Helsinki. Specimens were obtained from patients who underwent surgery between January 2011 and March 2013. All the patient medical records were reviewed for clinical information, based on the request form sent by the surgeon along with the specimen. The most relevant clinical informations, including the type of surgery, age of the patient, tumor size, and location of the tumor in the relative breast, were stated according to the clinical information provided by the clinician in the request form. Meanwhile, hormone receptor status, Her2 status, nodal status, number of isolated lymph nodes, and the number of positive lymph nodes have been stated according to data provided in the patient’s original pathologic report. All archival hematoxylin/eosin–stained slides taken from the formalin-fixed paraffin-embedded tissue (FFPET) were reviewed, and diagnosis was confirmed. Other morphological and pathologic findings with certain clinicopathologic parameters, such as histological subtype, histological grade, using a modified Bloom–Richardson classification, lymphovascular permeation, lymphocytic infiltrate, tumor necrosis, and ER, PR, and Her2 status, were evaluated separately and have been stated according to data provided in the original patient report.
Immunohistochemistry
Five antibodies were used in this study to demonstrate their corresponding antigens. Table 1 shows their specification and optimal dilution factor. They were tested to find the optimal staining conditions. CD44, CD24, vimentin, and E-cadherin antigens were best retrieved by heat-induced Tris/EDTA (pH 9.0), at 95 °C for 20 minute, while telomerase antigen was retrieved by heat-induced sodium citrate 10 mM (pH 6.0), at 90 °C for 45 minute. Diluted CD44, CD24, vimentin, and E-cadherin antibodies were incubated for 20 minute at room temperature (RT), while telomerase antibody was incubated for 2 hours at RT. The most appropriate and representative paraffin block, from the archival paraffin block storage, containing a wide scope of tumor tissue, has been selected. From each paraffin block, we cut five sections, to evaluate five antigens (CD44, CD24, vimentin, E-cadherin, and telomerase). Four-micron-thick sections were obtained using a microtome, transferred onto labeled adhesive slides, and dried at 60 °C for 30 minutes in an oven, and the slides were deparaffinized by xylen and rehydrated through dropping alcohol series. The specific antigens were retrieved accordingly, by using Dako real Envision detection system, peroxidase/DAB+, rabbit/mouse, Code K5007. The five antigens were screened in all the selected cases. Paraffin sections of normal tonsillar tissue were used as a positive control for CD44, CD24, vimentin, E-cadherin, and telomerase. Mature, striated muscle tissue is used as negative control for CD44, CD24, E-cadherin, and telomerase, while appendix is used for vimentin. By using Dako Envision G|2 Doublestain System, Rabbit/Mouse (DAB+/Permanent Red), Code K5361, all cases that expressed CD44 were subjected for double-staining protocol to identify the CSCs (CD44+CD24−low) phenotype, while all the cases having vimentin-positive tumor cells and E-cadherin–negative tumor cells were subjected to double-staining protocol to identify the EMT phenomena.
Table 1.
Primary antibodies used in the study.
| S.N | ANTIBODY | DESCRIPTION | CODE | DILUTION |
|---|---|---|---|---|
| 1. | CD44 | Dako, monoclonal mouse antihuman | M 7081 | 1/100 |
| 2. | CD24 | Abcam, rabbit monoclonal | Ab110448 | 1/100 |
| 3. | Telomerase | Abcam, mouse monoclonal anti-telomerase reverse transcriptase antibody | Ab5181 | 1/50 |
| 4. | Vimentin | Dako, monoclonal mouse anti-vimentin, clone V9. | M 0725 | 1/100 |
| 5. | E-cadherin | Dako, monoclonal mouse anti-human E-cadherin clone NCH-38 | IR059 | 1/100 |
Scoring and interpretation of staining
Using light microscopy, stained tissue sections were reviewed. All unclear cases were discussed with another pathologist, the scoring and staining interpretation of each marker has been evaluated as follows:
-
CD44 staining
CD44 showed membranous staining; the staining expression is considered positive when it is complete circumferential staining, regardless of the intensity of staining, while incomplete circumferential staining is considered as negative.
This immunohistochemical evaluation was modified from Honeth et al method.1
-
CD24 staining
CD24 showed membranous and/or cytoplasmic staining.
The staining index positivity was evaluated as follows:- Negative, no staining detected
- Negative (low), incomplete circumferential, mild cytoplasmic stain.
- Positive, moderate staining
- Positive, strong staining
- CSC staining
CSCs, as determined by the phenotypic expression of CD44+CD24−low cells, show membranous circumferential permanent red staining of CD44 positivity, with negative or low brown staining of cell membrane and cytoplasm of CD24 (Fig. 1).
-
Telomerase staining
To evaluate telomerase activity as determined by anti-hTERT IHC staining, the proportion of immunopositive cells of interest is estimated, which show fine brown DAB nuclear staining accentuated in cell exhibiting active division (mitosis), which is considered as positive (Fig. 2).
-
Vimentin staining
Vimentin-positive cells show cytoplasmic, perinuclear staining considered as positive.
-
E-cadherin staining
E-cadherin stains the cell membrane in a complete circumferential pattern.
-
EMT staining
This phenomenon is demonstrated in epithelial tumor by the presence of tumor cells, determined by the phenotypic expression of vimentin+/E-cadherin−, which lose their E-cadherin positivity and gain vimentin positivity. They do not exhibit membranous circumferential red staining of E-cadherin expression but show brown cytoplasmic staining of vimentin positivity (Fig. 3).
Figure 1.

CSC double-staining, membranous circumferential permanent red staining of CD44, with negative or low brown staining of cell membrane and cytoplasm of CD24 (magnification ×200).
Figure 2.

Telomerase-positive ductal cells (nuclear brown staining) (magnification ×200).
Figure 3.

EMT, loss of E-cadherin red membranous staining with gain of brown cytoplasmic staining (magnification ×200).
The proportion of immunopositive cells of interest for all the markers is estimated as follows:
Negative (0%)
Positive (1%–10%)
Positive (11%–40%)
Positive (41%–70%)
Positive (>70%)
IF technique
To evaluate and control the reliability of the immunohistochemical double staining of CSCs (CD44+CD24−low), IF double-staining with the same primary antibodies was performed in 10% of all cases. The double-staining protocol was run simultaneously. Detection of CD44 mouse antibodies was done by using goat polyclonal secondary antibody to mouse IgG (H+L) conjugated with blue fluorescent (FITC) Code (ab7086), which will stain the cell membrane green color, while detection of CD24 rabbit antibodies is done by using goat polyclonal secondary antibody to rabbit IgG (H+L) conjugated with green fluorescent (AMCA) Code (ab47052), which will stain the cytoplasm green color. CSCs, as determined by the phenotypic expression of CD44+CD24−low cells, show membranous circumferential green staining of CD44 positivity, with negative or low orange staining of cell membrane and cytoplasm of CD24 (Fig. 4).
Figure 4.

CSC IF double staining, membranous circumferential green staining of CD44, with negative or low orange staining of cell membrane and cytoplasm of CD24 (magnification ×100).
Flow cytometry analysis
Although the best flow cyto-metric histograms are obtained from fresh or frozen tissue samples, Hedley and coworkers21 developed a technique that permitted flow cytometry to be performed on FFPET sections. In order to sort tumor cells with CD44 and CD24 expression and measure CSCs with CD44+CD24−low using the flow cytometry from FFPET, single-cell suspensions need to be prepared. Various methods were described for dewaxing, rehydration, and disaggregation of FFPETS. A modified method (Hedley et al.21) to prepare single-cell suspension from FFPES was carried out as follows:
Two or more 50-μm sections were cut using a microtome, depending on the number of tumor cells in the tumor section.
Dewaxing: The sections were placed in 10-mL glass centrifuge tubes and dewaxed using two changes of xylene, 3 mL for 10 minutes at RT.
Rehydration: The sections were rehydrated in a descending ethanol range, a sequence of 3 mL of 100, 95%, 70%, and 50% ethanol for 10 minutes each, at RT.
Washing twice in distilled water.
Lysis of tissue section: The sections were minced by sharp seizer and incubated in 1 mL of 0.5% pepsin (0.5 mg pepsin/1 mL of 0.9% NaCl, adjusted to pH 1.5 with 2 N HCl). The tubes were placed in a water bath at 37 °C for 60 minutes, with intermittent vortex mixing each 10 minutes, and mincing with a pipette tip was needed.
Filtration: The resulting digest was filtered through a 70-mm nylon mesh, using Cell strainer 70 um white (Code LAB352350).
Washing: The pepsin was washed out by centrifugation at 400 g two times for 2 minutes using PBS, and the pellet was resuspended in Tris-buffered saline, pH 7.6, or PBS.
- Cells counting: Cell counts were then made using a hemocytometer provided that more than 105 cells/mL were present. Single-cell suspension is now ready for staining using the following antibodies and reagent to be run in flow cytometry:
- PMG anti-human CD44 PE (Code MAB555479)
- PE-labeled mouse IgG2b, KAPPA isotype CTL (Code MAB555743)
- Anti-human CD24 FITC (Code PMG555427)
- FITC-labeled mouse IgG2a, KAPPA isotype (Code MAB553456)
- Stain buffer, fetal bovine serum (Code MAB554656).
To evaluate and control the reliability of the CD44 and CD24 single staining, and immunohistochemical double staining of CSCs, a flow cytometric analysis with the same primary antibodies was performed in more than 10% of all cases, including 16 positive cases comprising CD44+CD24−low phenotypic tumor cells showing variable proportion of CSCs (CD44+CD24−low phenotype) and 4 negative cases devoid of any CSCs expressing CD44+CD24−low phenotype. Samples were analyzed on a BD FACSAria flow cytometer with filter configuration for the FITC/PE dye combination. The quality of the data depends on careful preparation of samples, instrument calibration, and exclusion of sources of artifact during data analysis. Combinations of fluorochrome-conjugated monoclonal antibodies against human CD44 (PE) and CD24 (FITC) or their respective isotype controls were added to the single-cell suspension at concentrations recommended by the manufacturer and incubated at 4 °C in the dark for 30–40 minutes. The labeled cells were analyzed on BD FACSAria.
After we have defined our classifier population (CD44+CD24−low), we measured variables that provide information about the state and proportion of CD44+CD24−low phenotype CSCs. In these examples we are examining expression of the CSC markers CD44 and CD24. Flow cytometric analysis allows us to detect the CSC populations according to different levels of the surface CSC markers CD44 and CD24.
Statistical analysis
To study the significant association between the variables, chi-square tests were employed. Under certain conditions, when it is not valid, Fisher’s exact test will be used to find out if there is any association between the variable. All statistical analysis was done using SPSS version 17.0 statistical software. In this study, significance level of 5% was considered statistically significant, that is, the statistical significance is set at P < 0.05.
Results
CSC prevalence
We analyzed CD44 and CD24 expression to identify the CSC phenotype (CD44+CD24−low) in the invasive breast cancer tissues, carcinoma in situ, and the metastatic lymph node lesion. CD44+CD24−low subpopulation tumor cell was detected in the in situ carcinomas and in the invasive tumor cells as well as in the metastatic lymph node lesions and in the normal epithelium, when the latter were observable in the examined sections. The CD44 staining was almost exclusively red membranous, with no or low brown cytoplasmic staining of CD24 (Fig. 1). Ductal carcinoma in situ (DCIS) component was identified in 38.3% (64/167). CSCs (as determined by the phenotypic expression of CD44+CD24−low cell) were detected in 75% (48/64) of cases, while they are negative in 25% (16/64) of the cases. They were significantly more prevalent in high-grade DCIS (P-0.002); also, the number of CSCs is higher than other histological subtypes. Moreover, there was significant increase in the number of CSCs within the DCIS component in contrast to its invasive counterpart (P-value 0.001). Overall, in 167 cases of invasive breast carcinoma, CD44+CD24−low sub-population tumor cells were expressed in 73.7% (123/167). The proportion of CSCs ranged from a few scattered cells to more than 70% of tumor cells bulk mass. Table 2 shows that the proportion of CSCs was 1%–10%, 11%–40%, 41%–70%, and over 70% of tumor cells in 34.14% (42/123), 39.83% (49/123), 18.7% (23/123), and 7.31 (9/123), respectively. However, there is no specific distinct morphological feature for the CSCs. In high-grade tumors containing numerous, pleomorphic, bizarre-looking tumor cells, the CSC subpopulation was included among normal-looking tumor cells. Neither the location of CSCs within the tumor tissue nor the arrangement of these cells among other tumor cells has any specific character or manner. Among the 123 cases in which we demonstrate the CSCs, 77.2% (95/123) were invasive ductal carcinoma NOS, 4.9% (6/123) were invasive lobular carcinomas (ILC), and 24.4% (30/123) were other histological subtypes. No significant differences were observed in the prevalence or percentage of CSCs in different breast cancer histological subgroups as shown in Table 2. The association between the CSC prevalence and classic prognostic factors, or independent variable and other clinicopathologic breast cancer parameters show that the CSC phenotype CD44+CD24−low is significantly correlated with tumor size. It was more prevalent in T4 subgroup (T4 any tumor size but associated with loco-regional invasion), P-0.049. The CSC phenotype CD44+CD24−low was significantly increased in node-positive tumors (P < 0.0001). There was significant correlation observed between tumor grade and CSC prevalence. They are more expressed in high-grade (III) tumors (P < 0.0001); also, these subpopulations of tumor cells are significantly more expressed in ER- and PR- negative tumors. But there was no significant correlation observed between CSC prevalence and lymphovascular permeation, Her2 status, and skin or nipple involvement. CSCs, as determined by the phenotypic expression of CD44+CD24−low, were detected in 63 instances of primary invasive breast cancer and their metastatic lymph node lesions from the same patient. CSCs were significantly more expressed in metastatic lymph node lesions (P < 0.000) in contrast to their primary tumors, as 74.6% (47/63) of the primary tumors only comprised the CSCs, while 79.4% (50/63) of the metastatic lymph node lesions comprised the CSCs as well as their proportion or percentage among the bulk of tumor cells, in which there was considerable and significant increase in the number of CSCs in metastatic lesion (P < 0.000). Table 3 shows that the CSCs were significantly associated with breast cancer classified according to hormonal receptors (P < 0.029). Most of the triple-negative tumors (88.5%, 23/26) were classified as CSC positive.
Table 2.
CSC proportion in each histological subtype.
| HISTOLOGICAL SUBTYPES | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CSCS% | NO. 167 | IDC 131 | ILC 7 | C 2 | A 3 | M 7 | T 3 | P 3 | S 2 | MP 2 | ME 2 | AD 1 | SQ 3 | N 1 | P-VALUE |
| 0% | N = 44 26.3% | 36 | 1 | 1 | 1 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.526 |
| 1%–10% | N = 42 26.1% | 33 | 3 | 1 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | |
| 11% – 4 0% | N = 49 29.3% | 41 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 2 | 1 | 0 | 1 | 0 | |
| 41%–70% | N = 23 13.8% | 17 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | |
| Above 70% | N = 9 5.3% | 4 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | |
Abbreviations: No, total number of cases; C, cribriform; A, apocrine; M, mucinous; T, tubular; P, papillary; S, secretory; MP, micropapillary; ME, medullary; AD, adenoid cystic; SQ, squamous; N, neuroendocrine.
Table 3.
CSC prevalence versus hormonal receptor status.
| HORMONAL RECEPTOR EXPRESSION | TOTAL NO | CSC EXPRESSION/167 | ||
|---|---|---|---|---|
| NEGATIVE (N = 44) | POSITIVE (N = 123) | P-VALUE | ||
| Luminal A ER+, PR+, Her2− = 53 ER+, PR−, Her2− = 5 ER−, PR+, Her2− = 1 | 59 | 18 | 41 | |
| Luminal B ER+, PR+, Her2+ = 51 ER+, PR−, Her2− = 13 ER−, PR+, Her2− = 2 | 66 | 18 | 48 | |
| ER−, PR−, Her2+ (Her2 overexpression) | 16 | 5 | 11 | |
| ER−, PR−, Her2− Basal like (triple negative) | 26 | 3 | 23 | 0.029* |
Note:
Indicates significant association at 5% level of significance.
Telomerase activity prevalence
The expression of telomerase activity, as determined by the phenotypic expression of anti-hTER, was analyzed in all invasive breast cancer cases. Overall, in 167 cases of invasive breast carcinoma, telomerase activity was expressed in 68.3% (114/167). The proportion of tumor cells expressing telomerase ranged between a few scattered cells to more than 70% of tumor cells bulk mass. Among the 114 cases in which we detected the telomerase activity in tumor cells, 74.6% (85/114) were invasive ductal carcinoma, 6.1% (7/114) were ILC, and 25.4% (29/114) were other histological subtypes. All the cases of ILC expressed telomerase activity, so there were significant differences observed in the expression of telomerase activity between IDC and ILC, but there was no significant difference detected between histological subgroups of invasive ductal carcinoma (P < 0.526).
Telomerase was expressed in 59.4% (38/64) of the DCIS cases, while it was negative in 40.6% (26/64). The P-value was 0.757 indicating no significant association between DCIS grade and telomerase activity prevalence and proportion. Telomerase was expressed more frequently in invasive lesion compared to its DCIS component. There was significant correlation (P-value 0.001) in the expression of telomerase activity between the invasive tumor component 64% (41/64) and its DCIS lesion 57.8% (37/64). Moreover, there was significant increase in the number of tumor cells expressing telomerase activity within the invasive component in contrast to its DCIS counterpart (P-value 0.001). There was no significant correlation observed between telomerase activity and tumor grade, size, lymphovascular permeation, and skin or nipple involvement, and no significant association between telomerase activity with breast cancer classified according to hormonal receptors and Her2 status (P-0.633).
Telomerase was expressed in 63 primary invasive breast cancer and their metastatic lymph node lesions from the same patient. Telomerase was significantly more expressed in metastatic lymph node lesions (P < 0.001), as 73% (46/63) of the primary tumors only expressed the telomerase activity, while 74.6% (47/63) of the metastatic lymph node lesions exhibited telomerase activity, and there was considerable and significant increase in the number of cells expressing telomerase in metastatic lesion (P < 0.002).
EMT prevalence
We analyzed vimentin and E-cadherin expression to identify the EMT phenotype (vimentin+/E-cadherin−) tumor cells in the invasive breast cancer tissues, DCIS, and the metastatic lymph node lesion. The subpopulation of tumor cells that have undergone EMT, as determined by the expression of vimentin and loss of E-cadherin (vimentin+/E-cadherin−), was detected in the in situ carcinomas and in the invasive tumor cells, as well as in the metastatic lymph node lesion. Vimentin staining was almost exclusively brown cytoplasmic (perinuclear), while E-cadherin predominantly stained the cell membrane a red color (Fig. 3).
Overall, in 167 cases of invasive breast carcinoma, EMT was expressed in 27.54% (46/167). The proportion of tumor cells that underwent EMT ranged between a few scattered cells to 20% of tumor cells bulk mass; in the majority of positive cases, they constituted less than 10% of tumor cells. There are no specific distinct morphological features for the tumor cells with EMT phenotype; however, they were more prevalent at the periphery of tumors as single spindled cells or within small clusters of tumor cells. Among the 46 cases in which we demonstrated the tumor cells that have undergone EMT, 69.5% (32/46) were invasive ductal carcinoma NOS, 4.2% (2/46) ILC, and 26% (12/46) other histological subtype. There were no significant differences observed between the expression of EMT in tumor cells of IDC (NOS), other histological subtypes, and ILC.
Table 4 shows the prevalence of tumor cells that have undergone EMT, as determined by vimentin+/E-cadherin− phenotype in DCIS component related to its grade in details. EMT was observed in 35.9% (23/64) of cases. It was more expressed in high-grade DCIS. The P-value was 0.002, indicating significant association between DCIS grade and EMT expression. There was significant correlation (P-value 0.000) in the prevalence of tumor cells that underwent EMT, between the invasive tumor and its DCIS component. EMT was expressed more frequently in DCIS component in contrast to its invasive counterpart (Fig. 5).
Table 4.
EMT prevalence in DCIS component in relation to its grade.
| N = 64 | DCIS GRADE | P-VALUE | |||
|---|---|---|---|---|---|
| LOW (N = 12) | INTERMEDIATE (N = 19) | HIGH (N = 33) | |||
| EMT in DCIS | 0.002* | ||||
| 0% | 41 (64.1%) | 11 | 11 | 19 | |
| 1%–10% | 16 (25.0%) | 1 | 7 | 8 | |
| 11%–40% | 5 (7.8%) | 0 | 1 | 4 | |
| 41%–70% | 2(3.1%) | 0 | 0 | 2 | |
| Above 70% | 0 (0%) | 0 | 0 | 0 | |
Notes:
Indicates significant association at 5% level of significance. FIndicates P-value reported according to Fisher’s exact test.
Figure 5.

EMT tumor cell in DCIS component (magnification ×200).
Tables 5 and 6 compared the prevalence and proportion of tumor cells of an EMT phenotype (vimentin+/E-cadherin−) and tumor cells with vimentin+/E-cadherin+ expression in different histological subtypes of breast cancer. It seemed to us that the incidence and proportion of tumor cells expressing vimentin were more than the incidence and proportion of EMT phenotypic cells in all types of breast cancer; that is, EMT phenotypic tumor cells constitute a part of vimentin-positive tumor cells. In other words, not all the tumor cells that expressed vimentin had lost their E-cadherin expression. The association between the incidence of EMT expression and classic prognostic factors, or independent variable and other clinicopathologic breast cancer parameters, show that EMT expression was significantly more frequent in ER−, PR−, Her2− (P-0.01, P-0.003, P-0.006, respectively). There was no significant correlation observed between EMT expression and tumor grade, size, lymphovascular permeation, and skin or nipple involvement. There was significant association between EMT expression and breast cancer classified according to hormonal receptors and Her2 status. The frequency of tumor cells exhibiting EMT expression was significantly related to triple-negative subtype (P-0.000). The EMT was observed more frequently in triple-negative subtype, 69% (18/26), while Luminal B (ER+, PR+, and Her2+) subtype showed the lowest prevalence rate, 21.4% (9/42) of EMT expression. There was significantly higher incidence of tumor cells that have undergone EMT in metastatic lymph node lesions compared to the primary invasive tumor lesions (P-0.001). The number of tumor cells exhibiting EMT was considerably higher in metastatic lymph node lesion in contrast to their primary counterpart.
Table 5.
EMT expression versus vimentin expression.
| HISTOLOGICAL SUBTYPE | NUMBER OF CASES | EMTVIMENTIN+/E-CADHERIN− | VIMENTINVIMENTIN+/E-CADHERIN+ | ||
|---|---|---|---|---|---|
| POSITIVE | NEGATIVE | POSITIVE | NEGATIVE | ||
| IDC (NOS) | 131 | 32 | 99 | 36 | 95 |
| ILC | 7 | 2 | 5 | 2 | 5 |
| Others | 29 | 12 | 17 | 17 | 12 |
| Total number | 167 | 46 | 121 | 55 | 112 |
Table 6.
Proportion of cells expressed EMT versus vimentin.
| CELL PROPORTION | EMTVIMENTIN+/E-CADHERIN− | VIMENTINVIMENTIN+/E-CADHERIN+ |
|---|---|---|
| 0% | 121 | 112 |
| 1%–10% | 29 | 21 |
| 11% – 4 0% | 17 | 13 |
| 41%–70% | 0 | 8 |
| Above 70% | 0 | 13 |
| Total number of cases | 167 | 167 |
Correlation between CSC and telomerase activity
Overall, in the cases of invasive tumors that comprised CSCs (123/167), only 81 cases show co-expression with telomerase, while 33 cases from those cases of invasive tumor, which did not show CSC (44/167) expressed telomerase activity. This indicates that there was no association or significant correlation between the existence of CSCs and detection of telomerase activity in tumor cells (P-0.250).
Correlation between CSCs and EMT
It appeared that 91.4% (42/46) of the cases of invasive tumor that expressed EMT changes coincided with CSCs of CD44+CD24−low phenotype, but only 34%(42/123) of cases that showed CSCs co-expressed EMT changes, indicating that the occurrence of EMT phenomena was usually accompanied by the co- existence of CSCs of CD44+CD24−low phenotype.
Correlation between telomerase activity and EMT
Overall, of the cases of invasive tumor that comprised EMT changes (46/167), only 31 cases show co-expression with telomerase, while 83 cases from those cases of invasive tumor, which did not show EMT changes (121/167) expressed telomerase activity, indicating there was no association or significant correlation between the existence of EMT changes and detection of telomerase activity in tumor cells (P-0.255).
IF staining results
The IHC double staining of CSCs was validated by IF double-staining, which was performed on 10% of tumors that expressed CSCs (12/123). The results from both techniques were exactly the same (Table 7).
Table 7.
Percentages of CSCs detected by IHC versus percentage of CSCs highlighted by IF.
| S.N | NUMBER OF CD44+CD24−LOW DETECTED | |
|---|---|---|
| IHC TECHNIQUE | IF TECHNIQUE | |
| 1. | Negative | Negative |
| 2. | 0–10% of tumor cells | 0–10% of tumor cells |
| 3. | 0–10% of tumor cells | 0–10% of tumor cells |
| 4. | 11%–40% of tumor cells | 11%–40% of tumor cells |
| 5. | 11%–40% of tumor cells | 11%–40% of tumor cells |
| 6. | 11%–40% of tumor cells | 11%–40% of tumor cells |
| 7. | 41%–70% of tumor cells | 41%–70% of tumor cells |
| 8. | 41%–70% of tumor cells | 41%–70% of tumor cells |
| 9. | 41%–70% of tumor cells | 41%–70% of tumor cells |
| 10. | Above 70% of tumor cells | Above 70% of tumor cells |
| 11. | Above 70% of tumor cells | Above 70% of tumor cells |
| 12. | Above 70% of tumor cells | Above 70% of tumor cells |
Flow cytometry analysis results
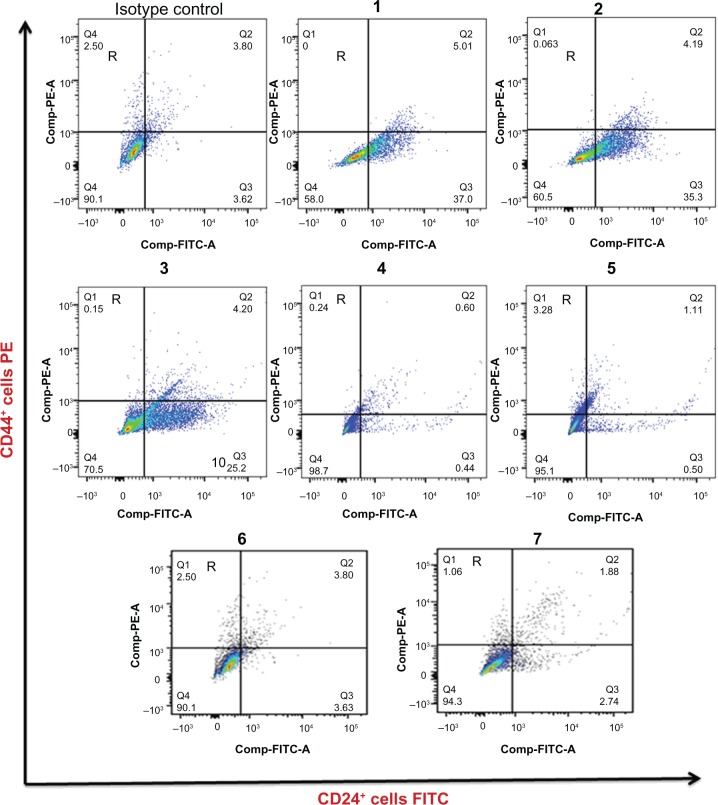
The single staining of CD44 and CD24 antigens along with IHC double staining of CSCs (CD44+CD24−low phenotype) was validated by flow cytometric analysis of 20 samples including a full scope of positive and negative cases. The results from both techniques were exactly compatible in terms of incidence of CD44 and CD24 expression and frequency of CSC occurrence, while the proportion of CSCs (CD44+CD24−low phenotype) detected by this technique is not concordant with the number of CSCs highlighted by IHC technique. The results obtained from running of negative cases (cases not showing CSCs) by this technique support the IHC findings (Figs. 6, 7, and 8.)
Figure 6.

Flow cytometric analysis of the expression of CD44 in four samples, in addition to unstained sample (negative control) and isotype control sample. CD44+CD24− cells were detected in all samples. Average percentage of cells is shown in each quadrant gated.
Figure 7.

Flow cytometric analysis of the expression of CD24 in four samples, in addition to unstained sample (negative control) and isotype control sample. CD44+CD24− cells were detected in all samples. Average percentage of cells is shown in each quadrant gated.
Figure 8.
Identification of CD44+CD24−low subpopulation in seven samples of breast cancer by flow cytometry. Cells in R correspond to CD44+CD24− cells.
Discussion
Cancer stem cells
Breast tumors are well known to be composed of phenotypically diverse groups of cells; however, it is clear now which of these cell types contribute to tumor development. They are a small subpopulation of cells within tumors that initiate the tumor and renew by themselves, as well as giving rise to a large population of differentiated progeny that constitute the bulk of the tumor mass but lack tumorigenic potential.22 In contrast to this notion, a previous hypothesis claimed that all tumor cell populations have the capacity to become tumorigenic through accumulation of mutations. Multiple studies indicate that breast CSCs have distinct immunological phenotype of CD44+/CD24−low,1,15,23,24 since Al-Hajj et al.2 identified and isolated these cells, which had the capacity to form tumors in immunocompromised mice.
In the present study, we have explored the dual expression of CD44 and CD24 in a sample of 167 cases of invasive breast cancer and 63 associated metastatic lymph node lesions, with specific regard to breast cancer histological subtypes. On the basis of this work, positivity for the cell-surface receptor CD44 staining was almost exclusively membranous, which is concordant with prior literature,1 and no or only low expression of the membrane/cytoplasmic protein CD24, which was predominantly stained in the cytoplasm in this study. Earlier publications have shown either membranous and/or cytoplasmic CD24 staining.25,26 Intracytoplasmic CD24 expression has been suggested to reflect overexpression of the protein or disturbance of the protein distribution or degradation in neoplastic cells.27 So it is reasonable to suspect expression of CD24 on the cell membrane in addition to cytoplasmic staining.
Overall, we saw a large heterogeneity of CSC expression patterns and prevalence between tumor subtypes, and also within the same tumor where the CSCs varied considerably in terms of proportion, location, and pattern of distribution, which was concordant with the findings of Honeth et al.1 This may be because of the diversity of the origin of the CSCs; in other words, if the CSCs were derived from multipotent tissue stem cells, they will give rise to more undifferentiated heterogeneous tumors of high CSC prevalence, but if the CSCs originated from more differentiated tissue stem cells, they will give rise to more differentiated tumors with less number of CSCs. This hypothesis is supported by Dontu et al.28 who proposed that mammary carcinogenesis results from the transformation of multiple stem cells and/or progenitor cells, and thus results in cellular heterogeneity within tumors.28
The present study demonstrates that the prevalence of breast CSC phenotype CD44+CD24−low was 73.7% (123/167) of all the tumors; in concordance with a previous study, Honeth et al.1 recorded 69% (165/240), but on the contrary to the findings of Al-Hajj et al.2 demonstrating the CSC phenotype CD44+CD24−low in all their breast cancer samples. This discordance could be because they depend mainly on metastatic tissues. Metastatic breast tumor cells have more number of breast CSCs as proved by our study (see later discussion).
To explore the significance of identifying breast CSCs on the patient clinical outcome, does CSC expression pattern as CD44+CD24−low and should its prevalence correlate with poor prognosis and metastasis?
The results from this clinical study showed that the breast CSC CD44+CD24−low expression pattern was associated with high-grade tumors, T4 tumor size, triple-negative tumors, and more expressed in node-positive tumors; that is, the expression of CSCs is associated with poor prognosis and predict lymph node metastasis; thus, the CSC is an independent, negative prognostic factor; ie, its absence indicates a favorable outcome, while its expression is a bad prognostic sign. There is a minor discordance with previous findings; however, analysis of the number of putative breast CSCs identified by IHC as CD44+CD24−low cells failed to identify a statistically significant association.29 Abraham et al.24 reported that CD44+CD24−low breast cancer was not associated with clinical outcome,24,30 while Ricardo et al.23 and Honeth et al.1 found that the poor prognosis of basal-like breast tumors, which are high-grade breast tumors, contained the higher percentage of CSCs. This controversy or discrepancy may result, in part, from technical errors, including usage of different antibodies and conditions for IHC staining and sample size variation. In other parts, it may result from impurity of CSCs.30,31
Accumulating evidence supports the concept of CSCs and its role in tumor initiation, progression, and sustaining tumor growth.32 Breast cancer cells with CD44+CD24−low subpopulations express higher levels of proinvasive genes and have highly invasive properties.4 In the current study, we noticed that breast CSCs were more prevalent and in significant numbers in DCIS compared to their invasive counterpart; moreover, the proportion and prevalence of CSCs was higher in high-grade DCIS. It seemed reasonable, therefore, to hypothesize that more tumor cells with stem cell phenotype are involved in DCIS lesions compared to invasive lesions. It is noteworthy to say that increased numbers of CSCs in DCIS lesions might be an initial step in tumor dissemination and invasion. Based on this finding, we arrive at the conclusion that these cells are crucial for tumor initiation, invasiveness, and propagation.
Does CSC prevalence have a significant correlation with tumor progression? Our finding did not support the tumor progression potential of these cells; in other words, CSC prevalence has no significant correlation with tumor progression from DCIS to IDC to increase tumor size. Tumor progression is independent of the prevalence of cancer stem tumor cells, in concordance with the finding of Abraham et al.24 who reported that there was no significant correlation between CD44+CD24−low tumor cell prevalence and tumor progression.24
The current study did not show any significant correlation between breast CSC prevalence and histological subtypes. In accordance with Abraham et al’s.24 report, there are no significant differences in the percentage of CSCs in breast cancer subgroups stratified by histopathologic characteristics and prognostic factors. Meanwhile, in our study, CSCs showed considerably high prevalence in triple-negative tumor, concordant with the findings of Ricardo et al.23 who showed significant association of breast CSC prevalence with basal-like tumor and vimentin-positive breast tumors, which usually have a triple-negative hormonal expression.
One purpose of this study was to evaluate if breast CSCs differed in the primary tumor and the metastatic lesions from the same patients. Pandit et al (2009) found a high proportion of breast CSCs with CD44+CD24−low expression in a model with high lymphatic metastatic ability. The expression of CD44+CD24−low cells showed significant difference in these sites. There was considerably high incidence of breast CSC expression in metastatic lymph node lesions compared to its primary tumor, in concordance with Abraham et al.24 who found high percentages of CD44+CD24−low tumor cells in metastatic and recurrent lesion. Moreover, Wei et al.33 conclude that detection of CD44+CD24−low CSCs in lymph node sections might help clinicians to determine the presence of lymph node metastasis. On the other hand, Guler et al.15 reported that the frequency of breast CSC phenotype does not differ in metastases relative to the primary breast cancer.
Telomerase activity
Telomerase has emerged as a near-universal marker of malignancy and has thus become an obvious diagnostic and therapeutic target. Significantly, a majority of human cancer cell lines and 85% of human cancers, encompassing a broad range of cancer types, possess telomerase activity.34
In the present study, we have explored the expression of telomerase activity, determined by the expression of hTERT in a sample of 167 cases of invasive breast cancer and 63 associated lymph node metastatic lesions. The anti-hTERT staining was detected in 68.3% (114/167) of the cases, almost exclusively nuclear, more strongly in the nucleolus, which is concordant with prior literature;9 however, Panizo et al.35 observed cytoplasmic staining also.
The results from this study showed that there was no significant correlation observed between telomerase activity and tumor grade, size, lymphovascular permeation, ER, PR, Her2, and skin or nipple involvement. These findings, however, are not in accordance with the report of Poremba et al.36 who reported that telomerase is associated with lower overall survival and it was an independent prognostic factor associated with worse prognosis.36
Whereas normal mammary tissue lacks significant telomerase activity, telomerase is expressed more frequently in invasive lesion compared to DCIS component.34 According to our study, telomerase activity was expressed in 64% (41/64) of the invasive tumor lesions and 57.8% (37/64) of its DCIS component, concordant with prior literature.37 Moreover, our study showed higher proportion of tumor cells exhibiting telomerase activity in invasive lesions compared to its DCIS component, in accordance with the finding of Yashima et al.38 who detected a progressive increase in the mean telomerase levels with the severity of histopathologic change: 14% in benign breast diseases, 92% in carcinoma in situ lesions, and 94% in invasive breast cancers. It is wise to say that these findings in addition to our further findings (discussed later) showed that telomerase activity was expressed more in metastatic lesions compared to primary tumors, making telomerase expression a marker of disease progression.
One purpose of this study was to evaluate if telomerase activity differed in the primary tumor and its counterpart metastatic lesions from the same patients. The expression of telomerase activity showed significant differences in these sites, and there was considerably high incidence of telomerase expression in metastatic lymph node lesions; moreover, a high number of tumor cells expressing telomerase was detected in later sites also. This finding is supported by Hiyama and Hiyama,39 who found that the level of telomerase activity observed in metastatic lesion was equivalent to or higher than those observed in analyzed primary lesion.
Telomerase expression was significantly associated with ILC rather than IDC or other histological subtypes. It seemed reasonable, therefore, to hypothesize that more tumor cells expressing telomerase activity are involved in ILC. This notion is supported by the findings of this study, in which the prevalence of telomerase activity in ILC was much higher than in IDC.
According to this study there was no association or significant correlation between the existence of breast CSCs (CD44+CD24−low phenotype) and the detection of telomerase activity in both the invasive and lymph node metastatic lesion.
Epithelial to mesenchymal transition
EMT is a complex cellular process by which epithelial cells undergo significant morphological changes characterized by a transition from an epithelial “cobblestone” appearance to a more elongated, mesenchymal phenotype.40 EMT involves loss of cell–cell adhesion, actin cytoskeleton reorganization, and the acquisition of mesenchymal features such as motility, invasiveness, and increased resistance to apoptosis.41
To demonstrate this phenomenon, we have applied a novel approach, using the double-staining IHC, to explore the dual expression of vimentin and E-cadherin in a sample of 167 cases of invasive breast cancer and 63 lymph node metastatic lesions. On the basis of this work, positivity for the vimen-tin staining was almost exclusively cytoplasmic and loss of expression of the membrane protein E-cadherin, which predominantly stained the cell membrane. Both staining patterns were concordant with prior literature.42,43
The current study did not show any significant correlation between the prevalence of tumor cells that have undergone EMT and histological subtypes of breast cancers, although the mesenchymal marker (vimentin) was more expressed in special subtypes of invasive carcinoma compared to IDC (NOS).
The results from this study showed that the EMT expression pattern vimentin+/E-cadherin− was associated with hormonal receptor and Her2 status and that the incidence of EMT was more in triple-negative tumor, whereas there was no association detected between EMT expression and tumor size, grade, lymphovascular invasion, and skin and nipple involvement.
Increasing evidence suggests that tumor progression is critically involved with the acquisition of an EMT phenotype, which allows tumor cells to acquire the capacity to infiltrate surrounding tissues.44,45 Progression of most carcinomas is associated with the acquisition of mesenchymal phenotype, which is accompanied by the loss of epithelial marker expression and upregulation of mesenchymal molecular markers, leading to increased cell motility and invasion.46 Our findings, however, related to this hypothesis, reflected by the data obtained from this study, were that EMT expression was more prevalent in DCIS lesion relative to its invasive component; moreover, the number of tumor cells that have undergone EMT in DCIS components was higher than its invasive lesion. It is wise to say that increased numbers of tumor cells that have undergone EMT in DCIS lesion are regarded as an initial step in the stromal invasion and propagation of breast cancer. Most of these tumor cells probably might undergo reversible MET that can convert the mesenchymal cancer cells back to epithelial phenotype to sustain tumor growth and heterogeneity,47 coming to the conclusion that the process of EMT is a vitally reversible process playing an important role in cancer invasiveness and metastasis, which is concordant with the prior literatures.44,48
One purpose of this study was to evaluate if EMT expression differed in the primary tumor and the metastatic lesions from the same patients. The prevalence of EMT tumor cell (vimentin+/E-cadherin−) along with the prevalence of tumor cells with mesenchymal marker (vimentin) expression (vimentin+/E-cadherin+) showed significant difference in these sites. There were considerably high number of tumor cells with EMT expression and vimentin expression in metastatic lymph node lesions. This finding is in accordance with the results reported by Guler et al.15 that vimentin was expressed more frequently in lymph node metastatic lesions and distant and locoregional metastases versus the primary site.
Vimentin expression is a rather rare finding in invasive breast cancer. A hypothesis proposes that vimentin-expressing breast carcinomas may derive from breast progenitor cells with bilinear (glandular and myoepithelial) differentiation potential.49 We found that vimentin was expressed in 32.93% (55/167) of the cases, while EMT was expressed in 29.3% (46/167) of the cases, that is, vimentin expression was not totally related to EMT. In other words, there was considerable number of tumor cells expressing both vimentin and E-cadherin, ie, some of the tumor cells still retained epithelial marker (E-cadherin) in addition to their expression of mesenchymal markers (vimentin). We hypothesize that vimentin expression in breast carcinoma may be due to one of the following:
Cancer cells that have undergone EMT.
Cancer cells derived from myoepithelial cell progenitors.
Tumor derived from basal cell progenitor, which expresses both vimentin and E-cadherin and has the ability to differentiate to epithelial luminal cells and myoepithelial cells. In this study, we hypothesize that vimentin expression was either due to EMT or the tumor cells derived from basal cell progenitors, which can express both vimentin and E-cadherin, supported by the facts from the current study elucidating that not all the tumor cells expressing vimentin lose their E-cadherin (undergo EMT), but on the contrary, most tumor cells expressing vimentin do express E-cadherin also, leading to the conclusion that tumor cells that have undergone EMT constitute a minor percentage of the vimentin-positive tumor cells.
Correlation between CSCs and EMT
Cancer cells that have undergone EMT reportedly display the CD44+CD24−low phenotype.50 Accumulating evidence has shown that cells with an EMT phenotype induced by different factors are rich sources for cancer stem-like cells, suggesting the biological similarities between breast CSCs and EMT phenotypic cells. These reports strongly suggest that the induction of EMT could generate stem-like cells.13 Significantly, the induction of EMT in normal or neoplastic epithelial cell populations has been shown to result in the enrichment of cells with stem cell–like properties.18 The findings of this study are concordant with this hypothesis, showing that 91.3% (42/46) of the overall cases that expressed EMT phenomena also comprised CSCs of CD44+CD24−low phenotype, that is, induction of EMT in the breast tumor is associated with high prevalence of CSCs, promoting tumor invasiveness and propagation. Reversal of the process (MET) seems to occur in distant metastasis47 and invasive primary lesion, because we detected lower numbers of EMT tumor cells in the invasive primary lesion compared to DCIS compartment.
In our study, we did not notice any specific location within the tumor tissue to detect CSCs or EMT tumor cells, while Pang and Argyle51 detected CSCs in adjacent stroma surrounding the tumor tissue, and scattered EMT tumor cells in stroma, suggesting that these findings illustrate a direct link between the scattered CSCs in stroma and the EMT.
Correlation between CSCs and telomerase activity
According to this study, there was no association or significant correlation between the existence of CSCs and detection of telomerase activity in breast cancer tumor cells.
Correlation between EMT and telomerase activity
This study did not show any association or significant correlation between the existence of EMT changes and detection of telomerase activity in breast cancer tumor cells.
Flow cytometric study
The result from flow cytometric analysis shows compatible data with that obtained by IHC as concerns CSC incidence. Meanwhile, the proportion of CSCs (CD44+CD24−low phenotype) detected by both the techniques was discordant, which may be due to the following:
The single-cell suspension is prepared from more than one paraffin section, which is also much thicker than the section of IHC technique.
The single-cell suspension contained a variable number of non-neoplastic CD44+CD24−low phenotypic cells, such as normal ductal cells, histiocytes, and lymphocytes, in which it is difficult to discriminate them from the neoplastic CD44+CD24−low phenotypic cells using flow cytometry.
During mechanical and enzymatic treatment of the tissue section to prepare the single-cell suspension, plentiful neoplastic cells underwent crush and lysis.
Flow cytometry is a powerful, highly sensitive technique to detect the single CD44 and CD24 markers, but it is less specific to detect CSCs of CD44+CD24−low phenotype.
Conclusion
Identification and isolation of CSCs in breast cancer research became one of the priorities in this field, since it is now well accepted that these cells are driving the tumor and responsible for tumor invasiveness, heterogeneity, metastatic capacity, and therapy resistance. The present study aimed to evaluate the diagnostic importance and laboratory feasibility of detecting CSCs in the daily clinical anatomical pathology practice. CD44+CD24−low phenotype tumor cells seem to be related to CSCs with certain levels of differentiation and are confined to a distinct molecular subclass of breast cancer. Because we failed to detect breast CSCs in about 30% (44/167) of the cases, these findings suggest that tumor-initiating properties are not wholly confined to CD44+CD24−low cells and other new biomarkers need to be identified. In order to translate the concept of CSCs to clinical practice, we have to pay major attention to the prospective identification of breast CSCs, to recognize additional characteristics, and identify new techniques. It is noteworthy to say that increased number of tumor cells of CSCs and EMT phenotypes in DCIS lesions is an initial step in tumor dissemination and propagation. Based on this finding, we come to the conclusion that these cells are crucial for tumor initiation, invasiveness, and propagation. We conclude that the expression of breast CSCs har-boring CD44+CD24−low phenotype was associated with poor prognosis and predict lymph node metastasis, so these CSCs are independent, negative prognostic factors. Their absence indicates a favorable outcome, while their expression is a bad prognostic sign. It is worthy to suggest that breast CSCs of CD44+CD24−low phenotype should be included in future validation studies as a prognostic marker in breast cancer. Estimating the frequency of breast CSCs in different variants of breast cancer can predict the clinical course of the disease and give prognostic clues for different variants of breast cancer. The occurrence of high numbers of breast CSCs in lymph node metastatic lesions supports the conclusion that the greater expression of CSC markers in metastatic sites reflects the importance of CSCs in tumor metastasis.
Telomerase is an attractive target for diagnosis and therapy, since it is expressed in about 70%–90% of breast cancer. In the current study, we detected a progressive increase in the prevalence of telomerase activity with the progression of tumor: 57.8% in carcinoma in situ lesions and 64% in invasive breast cancers, in addition to its higher expression in metastatic lesions compared to primary lesions, making telomerase a marker of disease progression, associated with stepwise breast tumor progression.
The prospective assessment of telomerase within breast cancer may lead to its use as a diagnostic marker and improve the diagnostic accuracy. Estimation of the frequency of CSCs and telomerase activity in different variants of breast cancer can predict the clinical course of the disease and give prognostic clues for different variants of breast cancer. The expression of telomerase activity in a wide range of breast cancers justifies the role of this nuclear enzyme in keeping cancer cells immortal and representing an exciting therapeutic target by telomerase inhibitors. Although telomerase was expressed in all malignancies, its clinical significance in routine daily practice requires more prospective clinical studies.
Based on our novel approach to explore EMT phenomenon, our results demonstrate a clear variation in the occurrence of EMT phenomena in different subtypes of breast cancers, as well as in various stages of the disease. It was more prevalent in DCIS lesions, triple-negative breast tumors, and in metastatic lesions. According to these findings, EMT phenomena may play an important role in stromal invasiveness and metastatic progression of tumor in addition to their putative role in therapeutic refractoriness. Moreover, reversal of the process (MET) seems to occur in the invasive component and distant metastasis. Therefore, EMT-inducing events offer a clinically important therapeutic target, whereby inhibition of EMT may have a significant effect on disease outcome.
According to our findings, EMT occurrence is always co-existent with CSC subsistence, suggesting that EMT phenotype induced by different factors are rich sources for cancer stem-like cells, which raise the possibility of biological similarities between CSCs and EMT phenotypic cells. Our findings suggest that the increased proportion and prevalence of tumor cells with CD44+CD24−low and vimentin+/E-cadherin− phenotype in DCIS and metastatic lesions may play an important role in tumor invasiveness and aggressiveness, in addition to being a higher metastatic risk of the breast cancer. We conclude that inhibition of EMT occurrence and CSC elimination may have a significant effect on disease outcome, which could raise the possibility that these cells will be new targets for antitumor agents and breast cancer treatment.
Finally, we can conclude that the currently used markers and techniques to detect EMT and CSC tumor cells are not enough to identify these subtypes of tumor cells, because using these markers fails to detect breast CSCs in about 30% of the cases and fails to detect the dissociated singly dispersed CSCs and EMT cells in the stroma of the tumor. The clinical relevance on prognosis and therapy response must be further evaluated in a prospective trial.
Future study and recommendations
We recommend further future work to identify and detect the expression of more proteins representing CSCs and EMT markers in primary and metastatic breast lesions and inventing a new technique to demonstrate these distinct tumor cells, since it is difficult with the current markers and double-staining IHC technique to identify the dissociated and isolated tumor cells harboring the CSCs and EMT signature in FFPET, because these dissociated tumor cells share the same IHC expression with stromal histiocytes, endothelial cells, myofibroblasts, and activated lymphocytes, increasing the need for new additional particular markers for these tumor cells, in order to invent a new therapeutic agent that could target tumor cells with CSC and EMT phenotypes at various stages of differentiation, sparing normal stem cells and reducing side effects.
Footnotes
ACADEMIC EDITOR: Dama Laxminarayana, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: JM, OM, AAW, ATS. Analyzed the data: JM, DVJ. Wrote the first draft of the manuscript: JM. Contributed to the writing of the manuscript: JM, OM, AAW. Agree with manuscript results and conclusions: JM, OM, AAW, ATS, DVJ. Jointly developed the structure and arguments for the paper: JM, OM, AAW. Made critical revisions and approved final version: JM, OM, AAW. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Honeth G, Bendahl PO, Ringnér M, et al. The CD44+/CD24− phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008;10:R53. doi: 10.1186/bcr2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ponti D, Costa A, Zaffaroni N. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–11. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 3.Sheridan C, Kishimoto H, Fuchs RK, et al. CD44+/CD24− breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res. 2006;8(5):R59. doi: 10.1186/bcr1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phillips TM, Singh SK, Hawkins C, et al. The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98:1777–85. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 5.Hwang-Verslues WW, Chang KJ, Lee EY, Lee WH. Breast cancer stem cells and tumor suppressor genes. J Formos Med Assoc. 2008;107:10. doi: 10.1016/S0929-6646(08)60188-6. [DOI] [PubMed] [Google Scholar]
- 6.Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells – perspectives on current status and future directions: AACR workshop on cancer stem cells. Cancer Res. 2006;66(19):9339–44. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 7.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Erratum: Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiyama E, Hiyama K, Yokoyama T, Shay JW. Immunohistochemical detection of telomerase (hTERT) protein in human cancer tissues and a subset of cells in normal tissues. Neoplasia. 2001;3:17–26. doi: 10.1038/sj.neo.7900134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan P, Benhattar J, Seelentag W, Stehle JC, Bosman FT. Immunohistochemical localization of hTERT protein in human tissues. Histochem Cell Biol. 2004;121:391–7. doi: 10.1007/s00418-004-0645-5. [DOI] [PubMed] [Google Scholar]
- 10.Looi LM. Telomerase in breast cancer. Malays J Pathol. 2007;29(suppl A) [Google Scholar]
- 11.Shay JW. Aging and cancer: are telomeres and telomerase the connection? Mol Med Today. 1995;1:378–84. doi: 10.1016/s1357-4310(95)93872-9. [DOI] [PubMed] [Google Scholar]
- 12.Dikmen ZG, Ozgurtas T, Gryaznov SM, Herbert BS. Targeting critical steps of cancer metastasis and recurrence using telomerase template antagonists. Biochim Biophys Acta. 2009;1792:240–7. doi: 10.1016/j.bbadis.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 13.Kong D, Li Y, Wang Z, Sarkar FH. Cancer stem cells and epithelial-to-mesenchymal transition (EMT)-phenotypic cells: are they cousins or twins? Cancers (Basel) 2011;3:716–29. doi: 10.3390/cancers30100716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 15.Guler G, Balci S, Costinean S, et al. Stem cell-related markers in primary breast cancers and associated metastatic lesions. Modern Pathol. 2012;25:949–55. doi: 10.1038/modpathol.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–26. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- 17.Morel AP, Lièvre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santisteban M, Reiman JM, Asiedu MK. Immune-induced epithelial to mesenchymal transition in vivo generates breast cancer stem cells. Cancer Res. 2009;69:2887–95. doi: 10.1158/0008-5472.CAN-08-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta PB, Onder TT, Jiang G, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–59. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hedley DW, Friedlander ML, Taylor IW, Rugg CA, Musgrove EA. Method for analysis of cellular DNA content of paraffin-embedded pathological material using flow cytometry. J Histochem Cytochem. 1983;31:1333–5. doi: 10.1177/31.11.6619538. [DOI] [PubMed] [Google Scholar]
- 22.Ailles LE, Weissman IL. Cancer stem cells in solid tumors. Curr Opin Biotechnol. 2007;18:460–6. doi: 10.1016/j.copbio.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Ricardo S, Vieira AF, Gerhard R, et al. Breast cancer stem cell markers CD44, CD24 and ALDH1: expression distribution within intrinsic molecular subtype. J Clin Pathol. 2011;64:937–46. doi: 10.1136/jcp.2011.090456. [DOI] [PubMed] [Google Scholar]
- 24.Abraham BK, Fritz P, McClellan M, Hauptvogel P, Athelogou M, Brauch H. Prevalence of CD44+/CD24−/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin Cancer Res. 2005;11:1154–9. [PubMed] [Google Scholar]
- 25.Fogel M, Friederichs J, Zeller Y, et al. CD24 is a marker for human breast carcinoma. Cancer Lett. 1999;143:87–94. doi: 10.1016/s0304-3835(99)00195-0. [DOI] [PubMed] [Google Scholar]
- 26.Surowiak P, Dziegiel P, Zabel M, Matkowski R, Kornafel J. Multivariate analysis of oestrogen receptor alpha, pS2, metallothionein and CD24 expression in invasive breast cancers. Br J Cancer. 2006;95:339–46. doi: 10.1038/sj.bjc.6603254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kristiansen G, Schlüns K, Yongwei Y, Denkert C, Dietel M, Petersen I. CD24 is expressed in ovarian cancer and is a new independent prognostic marker of patient survival. Am J Pathol. 2002;161:1215–21. doi: 10.1016/S0002-9440(10)64398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dontu G, El-Ashry D, Wicha MS. Breast cancer, stem/progenitor cells and the estrogen receptor. Trends Endocrinol Metab. 2004;15:193–7. doi: 10.1016/j.tem.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Campbell LL, Polyak K. Breast tumor heterogeneity: cancer stem cells or clonal evolution? Cell Cycle. 2007;6:2332–8. doi: 10.4161/cc.6.19.4914. [DOI] [PubMed] [Google Scholar]
- 30.Kim HJ, Kim MJ, Ahn SH, et al. Different prognostic significance of CD24 and CD44 expression in breast cancer according to hormone receptor status. Breast. 2011;20:78–85. doi: 10.1016/j.breast.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–68. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 32.Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3:895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- 33.Wei W, Hu H, Tan H, Chow LW, Yip AY, Loo WT. Relationship of CD44+CD24−/low breast cancer stem cells and axillary lymph node metastasis. J Transl Med. 2012;10(suppl 1):S6. doi: 10.1186/1479-5876-10-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herbert B-S, Wright WE, Shay JW. Telomerase and breast cancer. Breast Cancer Res. 2001;3:146–9. doi: 10.1186/bcr288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panizo A, Echegoyen, Merino JM. Immunohistochemical determination of telomerase expression in tumoral and non-tumoral breast tissue. Available at http://conganat.uninet.edu/IVCVHAP/CONFERENCIAS/Merino/index.html.
- 36.Poremba C, Heine B, Diallo R, et al. Telomerase as a prognostic marker in breast cancer: high-throughput tissue microarray analysis of hTERT and hTR. J Pathol. 2002;198(2):181–9. doi: 10.1002/path.1191. [DOI] [PubMed] [Google Scholar]
- 37.Bacchetti S, Shay JW. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–91. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 38.Yashima K, Milchgrub S, Gollahon LS, et al. Telomerase enzyme activity and RNA expression during the multistage pathogenesis of breast carcinoma. Clin Cancer Res. 1998;4:229–34. [PubMed] [Google Scholar]
- 39.Hiyama E, Hiyama K. Telomerase activity in human breast tumors. J Natl Cancer Inst. 1996;88:2–17. doi: 10.1093/jnci/88.2.116. [DOI] [PubMed] [Google Scholar]
- 40.Dumont N, Wilson MB, Crawford YG, Reynolds PA, Sigaroudinia M, Tlsty TD. Sustained induction of epithelial to mesenchymal transition activates DNA methylation of genes silenced in basal-like breast cancers. Proc Natl Acad Sci U S A. 2008;105:14867–72. doi: 10.1073/pnas.0807146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–73. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 42.Herrmann H, Aebi U. Intermediate filaments and their associates: multi-talented structural elements specifying cytoarchitecture and cytodynamics. Curr Opin Cell Biol. 2000;12:79–90. doi: 10.1016/s0955-0674(99)00060-5. [DOI] [PubMed] [Google Scholar]
- 43.Bishop PW. An immunohistochemical vade mecum. 2009. Available at www.e-immunohistochemistry.info.
- 44.Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Migrating cancer stem cells – an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5:744–9. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 45.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–81. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hollier BG, Evans K, Mani SA. The epithelial-to-mesenchymal transition and cancer stem cells: a coalition against cancer therapies. J Mammary Gland Biol Neoplasia. 2007;14:29–43. doi: 10.1007/s10911-009-9110-3. [DOI] [PubMed] [Google Scholar]
- 47.Chaffer CL, Thompson EW, Williams ED. Mesenchymal to epithelial transition in development and disease. Cells Tissues Organs. 2007;185:7–19. doi: 10.1159/000101298. [DOI] [PubMed] [Google Scholar]
- 48.Hugo H, Ackland ML, Blick T, et al. Epithelial-mesenchymal and mesenchymal-epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–83. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 49.Korsching E, Packeisen J, Liedtke C, et al. The origin of vimentin expression in invasive breast cancer: epithelial-mesenchymal transition, myoepithelial histogenesis or histogenesis from progenitor cells with bilinear differentiation potential? J Pathol. 2005;4:451–7. doi: 10.1002/path.1797. [DOI] [PubMed] [Google Scholar]
- 50.Bhat-Nakshatri P, Appaiah H, Ballas C, et al. SLUG/SNAI2 and tumor necrosis factor generate breast cells with CD44+/CD24−phenotype. BMC Cancer. 2010;10:411. doi: 10.1186/1471-2407-10-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pang LY, Argyle D. Cancer stem cells and telomerase as potential biomarkers in veterinary oncology. Vet J. 2010;185:15–22. doi: 10.1016/j.tvjl.2010.04.008. [DOI] [PubMed] [Google Scholar]