Abstract
INTRODUCTION
Knee osteoarthritis (OA) is the most common articular disease. Different methods are used to alleviate the symptoms of patients with knee OA, including analgesics, physical therapy, exercise prescription, and intra-articular injections (glucocorticoids, hyaluronic acid [HA], etc). New studies have focused on modern therapeutic methods that stimulate cartilage healing process and improve the damage, including the use of platelet-rich plasma (PRP) as a complex of growth factors. Due to the high incidence of OA and its consequences, we decided to study the long-term effect of intraarticular injection of PRP and HA on clinical outcome and quality of life of patients with knee OA.
METHOD
This non-placebo-controlled randomized clinical trial involved 160 patients affected by knee OA, grade 1–4 of Kellgren–Lawrence scale. In the PRP group (n = 87), two intra-articular injections at 4-week interval were applied, and in the HA group (n = 73), three doses of intra-articular injection at 1-week interval were applied. All patients were prospectively evaluated before and at 12 months after the treatment by Western Ontario and McMaster Universities Arthritis Index (WOMAC) and SF-36 questionnaires. The results were analyzed using SPSS 16.1 software (RCT code: IRCT2014012113442N5).
RESULTS
At the 12-month follow-up, WOMAC pain score and bodily pain significantly improved in both groups; however, better results were determined in the PRP group compared to the HA group (P < 0.001). Other WOMAC and SF-36 parameters improved only in the PRP group. More improvement (but not statistically significant) was achieved in patients with grade 2 OA in both the groups.
CONCLUSION
This study suggests that PRP injection is more efficacious than HA injection in reducing symptoms and improving quality of life and is a therapeutic option in select patients with knee OA who have not responded to conventional treatment.
Keywords: knee osteoarthritis, intra-articular injection, platelet-rich plasma, hyaluronic acid
Background
Knee osteoarthritis (OA) is a chronic progressive disease affecting more than 20% of people older than 45 years.1 According to the survey of the causes of productive work time loss in the United States, OA is the second most common cause of work performance loss after low back pain.2 With an increase in life expectancy, it is estimated that the need for knee arthroplasty would rise more than six times by 2030, causing significant economic burdens for pain control and rehabilitation of patients.3
The targets of OA treatment are pain decrement, function and mobility increment, prevention or correction of the deformity, and slowing the progression of the disease. There are numerous conservative treatments for knee OA that have short-term efficacy and have their own benefits and disadvantages.4 For example, Non steroidal anti-inflammatory drugs (NSAIDs) and intra-articular corticosteroid are common treatments of arthritis. Despite their low cost and easy access, these treatments have systemic adverse effects and may cause joint cartilage destruction and flare up of the osteoarthritic process.5 Also common treatments for cartilage tissue repair rarely achieve an ideal level of functional capacity for the patient.6
Because of the high costs of knee OA management, therapeutic options that are effective on tissue healing have been taken into consideration in recent years in order to prevent the progression of OA.7 Among these are growth factors that have been studied both in vitro and in vivo as effective factors for the healing of cartilage in OA with promising results.8–10 Growth factors are effective in chemotaxis, differentiation of mesenchymal stem cells, chondrocyte proliferation, and synthetic activities of osseous and cartilaginous cells; therefore, they have important roles in healing and remodeling of cartilage tissue.11
Platelet-rich plasma (PRP) is an autologous biologic treatment including patients’ own plasma, containing growth factors released from platelets and endogenous fibrin scaffold.12 The rationale for the use of PRP is to stimulate the natural healing cascade and tissue regeneration by a “supraphysiologic” release of platelet-derived factors directly at the site of treatment.4
Most studies believe that therapeutic PRP should have platelet concentrations four to six times greater than whole blood (200,000 mm−3). Some authors stated that the concentrations less than or greater than this amount may be ineffective or inversely lead to suppression of the healing process.13 PRP is classified into four categories, depending on leukocyte and fibrin contents: pure platelet-rich plasma (P-PRP); leukocyte-and platelet-rich plasma (L-PRP); pure platelet-rich fibrin (P-PRF); and leukocyte- and platelet-rich fibrin (L-PRF).14
The applications of biologic treatments such as PRP in musculoskeletal disorders are growing significantly. Although comparing PRP with other intra-articular and soft tissue injections has led to conflicting results, it seems that PRP has useful effects on healing and functional improvement of injured tissues.15–17
Viscosupplementation is another conservative method in OA management, which was approved by Food and Drug Administration for knee therapy in 1997 and was suggested by American college of Rheumatology (ACR) guideline as a therapeutic choice for pain decrement in knee OA in 2000.18
Hyaluronic acid (HA) is a high molecular weight glucosamine comprising repeating units of acetyl glucosamine and d-acid glucoronic synthetized by synoviocytes, fibroblasts, and chondrocytes. It is available in synovial fluid and extracellular matrix and is responsible for viscoelastic and lubricant features of synovial fluid.19 A normal adult knee joint has 2 mL of synovial fluid containing 2.5–4 mg/mL of HA with mean molecular weight of 5–7 × 106 kD. In the OA setting, both concentration and molecular weight of endogenous HA decrease due to synovial fluid dilution secondary to effusion, abnormal synoviocyte production, and molecular fragmentation.16 Although the mechanism of intra-articular injection of HA in improving OA symptoms is not clearly known, it seems that it has some role in joint mechanical support and its metabolic effects, which causes endogenous HA synthesis, stimulation of chondrocyte metabolism, synthesis of cartilage matrix components, and inhibition of chondrodegenerative enzymes, as well as inflammatory process.20
Currently, there are numerous viscosupplements available with different molecular weights, preparation methods, dosing instructions, and biologic indices. However, clinical trials found no difference between these products.5 There are different studies with conflicting results about the efficiency of HA in knee OA. In a meta-analysis by Rutjes in 2012, HA injection in knee OA was accompanied by small and clinically irrelevant benefits and increased risk for serious side effects.21 Another meta-analysis by Miller and Block in 2013 showed that using US-approved HA in knee OA patients is safe and efficient.22
Despite wide clinical applications, evidences lack the amount and duration of efficiency of PRP as well as comparison with other intra-articular knee treatments. Moreover, HA is used frequently despite conflicting results, and for as long as we know, there have been few studies that compared PRP and HA in knee OA. Therefore, we decided to perform a study to compare the effect of these two therapeutic choices in the long term.
Methods
Eligibility and patient selection
Patients with knee OA (based on American College of Rheumatology criteria) in the age range of 40–70 years, with symptom duration of more than 3 months, confirmatory X-ray diagnosis (Kellgren–Lawrence grade 1–4) within the past 3 months, and who were available were included in our study.
Exclusion criteria included history of diabetes mellitus, immunodeficiency and collagen vascular disorders, history or presence of malignant disorders, infection or active wound in the knee area, recent history of severe trauma to the knee, autoimmune and platelet disorders, treatment with anticoagulant and antiplatelet medications 10 days before injection, use of NSAIDs 2 days before injection, history of knee intraarticular injections of corticosteroids during the past 3 weeks or use of systemic corticosteroids 2 weeks before PRP injections, hemoglobin measures of <12g/dL and platelet counts of <150,000/ml, history of vasovagal shock, pregnancy, or breastfeeding, and genu valgum/varum greater than 20 degrees, allergy to avian proteins, feathers and egg products or hypersensitivity to hyaluronate.
Our research was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences, and conducted in accordance with the principles of the Declaration of Helsinki. The evidence and the methods of PRP and HA injection as well as benefits and probable adverse effects of study participation were presented by a physiatrist. All the mentioned information was also given to the participants in a written form. All the participants who signed the written consent form were included in the study. Then Study participants attended a screening visit (visit 1) that included history taking, physical examination, laboratory testing (complete blood count with differential (CBC diff),erythrocyte sedimentation rate(ESR),C-reactive protein (CRP)), knee radiography (standing Anterior-posterior(AP) and lateral views), and survey of used medications and supplements. Then Patients were randomly (by using random numbers table) divided into two groups, PRP and HA.
Outcome measures
For all patients, Persian version of the short form-36 (SF-36) questionnaire of health survey for assessing the quality of life and Western Ontario and McMaster Universities Arthritis Index (WOMAC) questionnaire for assessing patients’ function were fulfilled by interview performed by a resident of physical and rehabilitation medicine.
The SF-36 is a patient-reported survey of patient health that yields information on physical health (comprising physical functioning, role-physical, bodily pain, and general health) and mental health (comprising vitality, social functioning, role-emotional, and mental health). Finally, the summary of the physical (PCS-36) and mental (MCS-36) components can be calculated. Each of these eight domains were scored from 0 to 100 with less scores indicating more disability.
WOMAC questionnaire is a tool used widely in studies of arthritis that includes five items for pain, two items for stiffness, and 17 items for assessing functional limitation. Each question is scored from 0 to 5 with fewer scores indicating less pain and better functional status.
Interventions
For the process of PRP preparation and injection, participants were referred to the laboratory of Shahid Modarres Hospital. The PRP processing was done using the Rooyagen Kit (made by Arya Mabna Tashkis Corporation, RN:312569). The Rooyagen Kit uses a fully enclosed system that maintains sterility throughout the entire process and uses a dual spin system. In order to prepare PRP with concentrations of four to six times the average of normal values, 35–40 mL of blood was first collected from the patient’s upper limb cubital vein using an 18G needle, subsequently 5 mL of acid citrate dextrose solution-A was added to the sample as an anticoagulant. One milliliter of the blood sample was sent for complete blood count. The blood sample was then centrifuged for 15 minutes at 1600 rpm resulting in three layers: the lower layer made up of red blood cells, the intermediate layer is composed of white blood cells, and the upper layer is composed of plasma. The buffy coat layer and the plasma layer were later collected and centrifuged for another 7 minutes at 2800 rpm in order to concentrate platelets. The final product was 4–6 mL of PRP containing leukocytes. The PRP quantification and qualification procedure was performed using laboratory analyzer Sysmex KX 21 and swirling, and if approved, the injection was preceded. Local anesthetic agent was not injected. This was due to the fact that some resources stated that anesthetic agents not only could have toxic effects on chondrocytes but could also influence the activation of platelet by changing the pH of the environment.23 Instead, patients were given a single dose of acetaminophen-codeine 2 hours before the injection. It was also stated in some studies that a factor helpful for the activation of platelets is the contact with endogenous collagen.23 We did not use exogenous factors for the process of activation but let the platelets be in direct contact with the joint collagen to become active. The skin of the injection site was prepped and draped, and the liquid PRP was injected in a sterile condition using a 22G needle through the classic approach for intra-articular injection (lateral midpatellar in extended knee position or anteromedial in flexed knee position). After 15–20 minutes of rest, patients were asked to actively flex and extend their knees so that the PRP could spread evenly across the joint space before changing into gel. The second injection was administered 28 days (4 weeks) after the first injection with the same conditions.
In the second group, HA with Hyalgan® brand name was injected. Hyalgan manufactured by Fidia Farmaceutici S.p.A., Abano Terme, Italy, is a viscous solution consisting of a high molecular weight (500,000–730,000 Da) fraction of purified natural sodium hyaluronate in buffered physiological sodium chloride, having a PH of 6.8–7.5. The sodium hyaluronate is extracted from rooster combs. Hyalgan was supplied as a sterile, non-pyogenic solution in 2 mL pre-filled syringes containing 20 mg of sodium hyaluronate, 17 mg of sodium chloride, 0.1 mg of monobasic sodium phosphate, 1.2 mg of dibasic sodium phosphate, and up to 2cc water for injection.
Before the injections, the skin was prepped and draped. Hyalgan vials were injected immediately after opening using 20G needles through classic approach for knee intra-articular injections (anteromedial or lateral midpatellar) in sterile settings. At the end of the injections, patients were asked to flex and extend their knees several times. The second and third injections were administered at a one-week interval with the same conditions as for the first injection.
In both the groups, patients were discharged home after 10–15 minutes of rest and asked to follow instructions given to them in a written format: they were recommended to have relative rest for 24–48 hours after injections and limit weight-bearing over the injected joints. During this period, they were recommended to apply cold therapy three times a day for 10 minutes each time. Participants had permission to use 500 mg of acetaminophen without codeine (one tablet every 8 hours and maximally every 4 hours if pain continued). In the setting of persistent pain, they were prescribed acetaminophen with codeine (according to the patient’s needs). Also, patients were prohibited from using any other analgesics, NSAIDs, steroids, or medications influencing platelet count or function. They were generally recommended to continue their mild-to-moderate level of activities and increase their level gradually as tolerated.
For both groups, exercises were prescribed and instructed by a physical and rehabilitation medicine resident before the injections. Exercise therapy protocol consisted of multi-angle isometric strengthening exercises of the knee muscles (quadricpes femuris, hip adductors, and abductors) in addition to hamstring stretching exercises performed three times a day, each time holding for 10 seconds and repeating 10 times. These exercises were progressed to closed-chain isotonic exercises after one month.
For further contact with project executers, a 24-hour phone line was offered to participants and physical and rehabilitation medicine residents, and if needed, a physiatrist were responsive to any possible questions or problems.
All of the participants were followed-up at 4, 24, and 52 weeks after treatment. During this period, they were assessed for analgesic (acetaminophen) dose measure, joint pain, swelling, and stiffness. After 12 months, SF-36 and WOMAC forms were fulfilled again (IRCT2014012113442N5).
Statistical analysis
Final data before and after the treatment were imported and analyzed by SPSS v.16. Normality of the data was described by mean, and variance was evaluated using Shapiro–Wilk’s test. For comparing variables with normal distribution, paired t-test, independent t-test, and ANOVA’s test were used.
To evaluate non-normal variables, the non-parametric tests of Wilcoxon signed rank, Mann–Whitney, and Kruskal–Wallis were applied. Qualitative variables were expressed with frequency and percent. To evaluate the relationships between quantitative variables, correlation coefficients of Pearson and Spearman were used. Statistical significance was set at P < 0.05.
Results
Study population
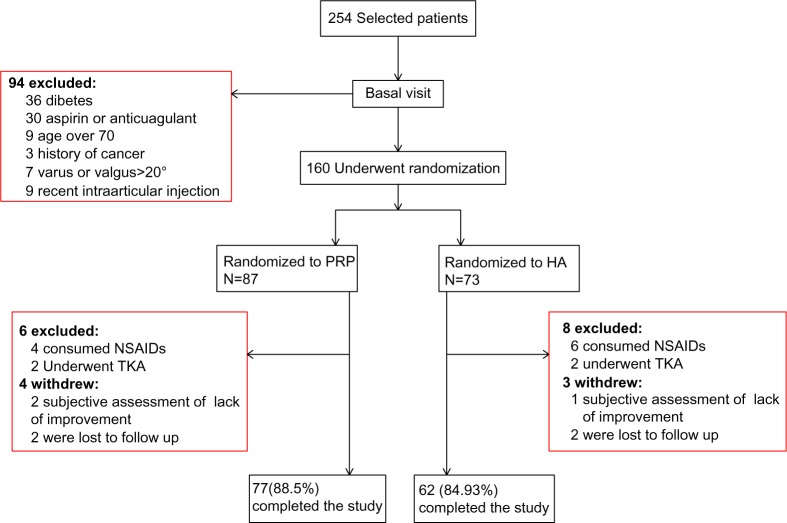
From the 254 potential participants who were candidates for intra-articular injection, 94 patients were not included due to lack of eligibility. Therefore, the study population included 160 subjects, men and women, with a mean (SD) age of 58.79 (8.66) years and BMI of 27.68 (4.44) kg/m2. A total of 87 subjects were randomized to the PRP group and 73 subjects to HA group. A total of 10 patients from the PRP group and 11 from the hyaluronic group were excluded from the hyaluronic group were excluded from the study (Fig. 1). The exclusion and withdrawal percentages did not differ significantly between the groups. Other characteristics of both groups and their pre-treatment comparison are demonstrated in Table 1.
Figure 1.
Patient disposition.
Table 1.
Baseline characteristics of the study subjects.
| VARIABLE | STUDY GROUP | P-VALUE | |
|---|---|---|---|
| PRP | HA | ||
| Age, mean ± SD | 56.85 ± 9.13 | 61.13 ± 7.48 | 0.01 |
| BMI, mean ± SD | 28.20 ± 4.63 | 27.03 ± 4.15 | 0.12 |
| Sex (%) | 69 8 | 47 15 | 0.029 |
| Female | 69 (89.6) | 47 (75.8) | |
| Male | 8 (10.4) | 15 (24.2) | |
| Grade of OA (%) | 0.11 | ||
| Grade1 | 6 | 0 | |
| Grade2 | 44 | 47 | |
| Grade3 | 38 | 37 | |
| Grade4 | 12 | 16 | |
| womac,mean,SD | |||
| Pain | 8.46 ± 4.17 | 6.91 ± 3.82 | 0.03 |
| Stiffness | 2.24 ± 1.76 | 1.88 ± 1.72 | 0.179 |
| Function | 28.91 ± 12.63 | 19.88 ± 12.32 | ,0.001 |
| Total | 39.5 ± 17.06 | 28.69 ± 16.69 | ,0.001 |
| SF-36,mean,SD | |||
| Physical functioning | 37.4 ± 24.92 | 43.66 ± 22.3 | 0.548 |
| Role limitations due to physical health | 28.83 ± 31.11 | 28.62 ± 36.17 | 0.33 |
| Pain | 49.9 ± 24.77 | 45.45 ± 20.5 | 0.184 |
| General health | 61.68 ± 25.72 | 61.37 ± 19.14 | 0.812 |
| PCS-36 | 178.14 ± 81.00 | 180.4 ± 68.52 | 0.832 |
| Emotional well being | 61.01 ± 26.86 | 57.74 ± 21.24 | 0.203 |
| Role limitations due to emotional problems | 50.64 ± 43.46 | 51.61 ± 46.13 | 0.978 |
| Vitality | 54.25 ± 24.95 | 54.43 ± 21.47 | 0.482 |
| Social functioning | 63.31 ± 28.41 | 60.64 ± 27.86 | 0.536 |
| MCS-36 | 229.22 ± 95.62 | 226.43 ± 97.39 | 0.93 |
PRP characteristic
Injected PRP in this study contained leukocytes (leukocyte-rich PRP) and platelet concentrations 5.2 ± 1.50 times and 4.8 ± 1.80 times the baseline values in the first and second preparations, respectively. The mean leukocyte count in PRP was 780.43 ± 1134.82 and 808.69 ± 825.38 in the first and second preparations, respectively.
Clinical outcomes of PRP and HA groups
Preliminary analyses showed that the WOMAC mean pain parameter was decreased meaningfully in both groups after 52 weeks of follow-up and this decrement was more in the PRP group (P < 0.001). The physical function, stiffness, and total WOMAC means were improved meaningfully only in the PRP group (Table 2).
Table 2.
WOMAC index scores during the study period.
| STUDY GROUPS AND TIME POINTS | WOMAC INDEX SCORES, MEAN (SD) | |||
|---|---|---|---|---|
| PAIN | STIFFNESS | PHYSICAL FUNCTION | TOTAL | |
| PRP group | ||||
| Baseline | 8.46 (4.17) | 2.2 (1.76) | 28.91 (12.63) | 39.5 (17.06) |
| week 52 | 4.03 (3.36) | 1.19 (1.4) | 13.19 (10.39) | 18.44 (14.35) |
| Change baseline vs week 52 | 4.39 (3.57) | 1.05 (1.78) | 15.77 (10.80) | 21.11 (14.18) |
| P value | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| Hyalgan group | ||||
| Baseline | 6.91 (3.82) | 1.88 (1.72) | 19.88 (12.32) | 28.69 (16.69) |
| week 52 | 5.08 (3.71) | 2.14 (1.66) | 19.51 (11.9) | 27.46 (16.36) |
| Change baseline vs week 52 | 1.11 (3.89) | 0.25 (2.11) | 0.3 (13.69) | 1.22 (18.65) |
| P value | 0.029 | 0.16 | 0.919 | 0.78 |
| Between group (P value) | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
With regard to results of SF-36 analysis, bodily pain scores changed meaningfully in both groups (P = 0.009 in HA group and P < 0.001 in PRP group). This improvement was more meaningful in the PRP group compared to the HA group (P < 0.001).
Individualized results of dimensions of physical health and mental health of the SF-36 questionnaire are detailed in Tables 3 and 4, respectively. With regard to PCS-36, mean scores in the PRP group improved significantly from 178.14 (81.00) at baseline to 255.96 (77.59) at the end of the study (mean change, P < 0.001), whereas in subjects given, HA scores increased from 180.4 (68.52) at baseline to 189.39 (103.73) at week 52 (mean change, P = 0.37).
Table 3.
Results of the physical health dimension of the SF-36.
| STUDY GROUPS AND TIME POINTS | SF-36 SCORES, MEAN (SD) | ||||
|---|---|---|---|---|---|
| PHYSICAL FUNCTIONING | ROLE-PHYSICAL | BODILY PAIN | GENERAL HEALTH | PCS-36 | |
| PRP group | |||||
| Baseline | 37.40 (24.92) | 28.83 (31.11) | 49.90 (25.77) | 61.68 (25.72) | 178.14 (81.00) |
| week 52 | 56.82 (25.68) | 53.98 (38.84) | 77.11 (19.56) | 68.60 (18.75) | 255.96 (77.59) |
| P value | 0.001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| Hyalgan group | |||||
| Baseline | 43.66 (22.30) | 28.62 (36.17) | 45.45 (20.5) | 61.37 (19.14) | 180.4 (68.52) |
| week 52 | 44.29 (28.14) | 33.46 (41.96) | 53.56 (27.89) | 60.73 (26.70) | 189.39 (103.73) |
| P value | 0.303 | 0.747 | 0.009 | 0.84 | 0.37 |
| Between group (P value) | 0.001 | 0.0001 | 0.0001 | 0.0008 | 0.0001 |
Table 4.
Results of the mental health dimension of the SF-36.
| STUDY GROUPS AND TIME POINTS | SF-36 SCORES, MEAN (SD) | ||||
|---|---|---|---|---|---|
| VITALITY | SOCIAL FUNCTIONING | ROLE-EMOTIONAL | MENTAL HEALTH | MCS-36 | |
| PRP group | |||||
| Baseline | 54.25 (24.95) | 63.31 (28.41) | 50.64 (43.46) | 61.01 (26.86) | 229.22 (95.62) |
| week 52 | 63.14 (26.66) | 79.38 (21.63) | 45.19 (39.03) | 70.25 (25.24) | 269.92 (91.48) |
| P value | 0.0001 | 0.0001 | 0.257 | 0.0001 | |
| Hyalgan group | |||||
| Baseline | 56.43 (21.47) | 60.64 (27.86) | 51.61 (46.13) | 57.74 (21.24) | 226.43 (97.39) |
| week 52 | 54.61 (26.07) | 63.3 (32.55) | 45.19 (39.03) | 56.45 (24.49) | 216.91 (100.9) |
| P value | 0.305 | 0.39 | 0.395 | 0.85 | |
| Between group (P value) | 0.0001 | 0.008 | 0.217 | 0.002 | 0.0001 |
With regard to MCS-36, mean scores in PRP group improved from 229.22 (95.62) at baseline to 269.92 (91.48) at the end of the study (mean change, P < 0.001), whereas in HA group, scores changed from 226.43 (97.39) at baseline to 216.91 (100.9) at week 52 (mean change P = 0.74).
In more detailed analyses, response to treatment in both groups were assessed based on age and the grade of OA according to total WOMAC score, PCS-36, and MCS−36. In both groups, patients with grade 2 OA responded better to treatment, but this improvement was not statistically meaningful (PRP P = 0.47, HA P = 0.60). There was also no meaningful difference between response to treatment among patients younger than 55 years and those older than 55 years.
Discussion
Our study was a single-center RCT conducted in Shahid Modarres Hospital from April 2012 to February 2014, and its results showed the long-term efficiency of PRP and HA injections in patients with knee OA who had pain. Obviously, PRP was more effective than HA and led to improvements in patients’ functions and quality of life.
Chang et al reviewed the effects of intra-articular PRP injection in knee OA compared to HA in a systematic review performed in 2014. The study demonstrated that PRP led to significant functional improvement in patients with knee cartilage pathology, whose effects last at least 12 months. Compared to patients receiving HA, patients in the PRP group had more and longer improvement. There were also better results among those patients with milder forms of OA than advanced ones.24 Similar results were obtained in another meta-analysis. Khoshbin et al found the PRP injection more efficient than HA and normal saline in mild-to-moderate OA in 2013.25
In another systematic review in 2014, it was stated that evidence often supported the use of PRP in knee OA. Different studies have shown that PRP effects in relieving pain and improving clinical symptoms decreases after 6 months. However, there is no evidence advocating PRP efficiency in traumatic or degenerative chondral pathology. Therefore, high-quality RCT studies are needed to compare PRP with placebo and also surgical treatments supplemented by PRP with operative management alone.26
In a meta-analysis held by Merchan in 2013, efficiencies of steroids, HA, and PRP were reviewed. Researchers suggested 3 to 5 weekly HA injections in knee OA before performing surgical treatment. Steroid injections had very short-term effects but PRP injections needed more studies to determine the grade and duration of efficiency.27
Kon et al conducted a study in 2011 comparing PRP with HA with low molecular weight (LWHA) and HA with high molecular weight (HWHA) in 150 patients. Treatment efficiency was evaluated using International Knee Documentation Committee (IKDC) and visual analogue scale (VAS) questionnaires at the beginning of treatment and 2 and 6 months later. Meaningful improvements in all parameters were observed in three groups after 2 and 6 months. Patient satisfaction of treatment in the PRP group was more than the two other groups (P = 0.04). At the end of 2 months, the PRP and the LWHA groups showed similar improvements and more than the HWHA group. However, after 6 months, PRP group had better results than the other two groups. Also patients in the PRP group, unlike in the LWHA group, had an ascending course of improvement between 2 and 6 months. The degree of improvement was related to OA intensity so that more improvement was achieved in patients with grade 0 OA than those with grade 1, 2, and 3 OA.8 In our study, patients with grade 2 OA showed more improvement than other grades, although this was not statistically meaningful, which can be explained by the low number of patients with grades 1 and 4 OA.
In another study by Vaquerizo et al in 2013, 96 patients in two groups underwent three sessions of PRGF injections or a single session of HA injection and were followed up for 48 weeks. The efficiency of PRGF in pain and stiffness decrement and physical performance improvement was more than that of HA. Also patients’ responses to PRGF in all scores including pain, stiffness, and physical performance in WOMAC, Lequesne, and OMERACT-OARSI (Outcome Measures in Rheumatology -Osteoarthritis Research Society International) were more meaningful than HA,28 which was consistent with our study.
Filardo et al conducted a study in 2012 to compare PRP and HA in the treatment of knee OA. A total of 109 patients (55 in HA group and 54 in PRP group) participated in that study. They were evaluated at the beginning, and at 2, 6, and 12 months after the treatment using KOOS, IKDC, and EQ-VAS questionnaires. PRP/HA were injected three times at one-week intervals between each session. At the end of the follow-up, significant improvements were observed in all parameters in both the groups. However, there were no meaningful differences between the groups in EQ-VAS and IKDC scores. The authors concluded that PRP does not have priority over HA in middle-aged patients with moderate OA and should not be applied as the first-line treatment.29 In the present study, patients in the PRP group demonstrated meaningful improvements in all parameters of WOMAC and SF-36 (except role limitation due to emotional problem) compared to the HA group after the first year. This difference can be due to the method of PRP preparation and its characteristics and also different evaluation tools being applied.
The present authors had previously performed studies to evaluate the clinical application of PRP, and recorded safety and positive findings. It was a prospective study published in 2013 on 60 patients treated with two injections of PRP (1 every 4 weeks). Patients underwent clinical evaluation at the beginning and at 6 months of follow up. The clinical outcomes revealed a statistically relevant improvement in all the variables of WOMAC and SF-36.30
According to this study and similar studies, considering the side effects of analgesic and anti-inflammatory medications, PRP injection can be considered as a safe and useful therapeutic option in select patients with mild-to-moderate degrees of OA who fail to respond to current treatments including ADL modification, therapeutic exercise, and physical modalities.
Our study limitations included the lack of a placebo control group, not being blinded, and lack of objective evaluation of the effects of treatment on the morphology of the cartilage, soft tissue, and other intra- and peri-articular structures of the knee. Furthermore, considering the higher cost of PRP compared to other injection therapies such as HA and the need of special kit and a centrifuge devise for using PRP, use of this therapy should be considered wisely (cost-effectiveness and availability).
Conclusion
PRP is a novel option in knee OA management and an increasing number of clinical studies show promising results. However, despite its wide application in clinical practice and the positive findings reported, almost all these studies have used questionnaires and were based on subjective findings. Therefore, conducting a study based on objective findings such as MRI seems to be needed in this regard.
Acknowledgments
We appreciate Naser Aghayi, technical manager of Shahid Modarres Hospital Laboratory, and Dr. Mohammad Hossein Mohammadi, laboratory hematologist, for providing us necessary laboratory equipment and information, and also Zahra Razzaghi, statistician, for extraordinary cooperation with us in this study.
Footnotes
ACADEMIC EDITOR: Chuanju Liu, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: SAR, EG. Analyzed the data: EG, MB. Wrote the first draft of the manuscript: SAR, EG. Contributed to the writing of the manuscript: SMR. Agree with manuscript results and conclusions: MF, HH. Jointly developed the structure and arguments for the paper: SAR. Made critical revisions and approved final version: SMR, KA. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Lawrence RC, Felson DT, Helmick CG, National Arthritis, Data Workgroup, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States Part II. Arthritis Rheum. 2008;58(1):23–6. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 2.Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. JAMA. 2003;290(18):2443–54. doi: 10.1001/jama.290.18.2443. [DOI] [PubMed] [Google Scholar]
- 3.De La Mata J. Platelet rich plasma: a new treatment tool for the rheumatologist? Reumatol Clin. 2013;9(3):166–71. doi: 10.1016/j.reuma.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 4.McArthur BA, Dy CJ, Fabricant PD, Gonzalez DVA. Long term safety, efficacy, and patient acceptability of hyaluronic acid injection in patients with painful osteoarthritis of the knee. Patient Prefer Adherence. 2012;6:905–10. doi: 10.2147/PPA.S27783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kon E. Non-surgical management of early knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2012;20(3):436–49. doi: 10.1007/s00167-011-1713-8. [DOI] [PubMed] [Google Scholar]
- 6.Rayegani SR. Does intra articular platelet rich plasma injection improve function, pain and quality of life in patients with osteoarthritis of the knee? A randomized clinical trial. Orthop Rev. 2014;6(5405):112–7. doi: 10.4081/or.2014.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gobbi A, Karnatzikos G, Mahajan V, Malchira S. Platelet-rich plasma treatment in symptomatic patients with knee osteoarthritis: preliminary results in a group of active patients. Sports Health. 2012;4(2):162–72. doi: 10.1177/1941738111431801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kon E, Mandelbaum B, Buda R, et al. Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: from early degeneration to osteoarthritis. Arthroscopy. 2011;27(11):1490–501. doi: 10.1016/j.arthro.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Allan M, James W, Amy V. Treatment of tendon and muscle using platelet- richplasma. Clin Sports Med. 2009;28(1):113–25. doi: 10.1016/j.csm.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Rabago D, Best TM, Zgierska AE, Zeisig E, Ryan M, Crane D. A systematic review of four injection therapies for lateral epicondylosis: prolotherapy, polidocanol, whole blood and platelet rich plasma. Br J Sports Med. 2009;43(7):471–81. doi: 10.1136/bjsm.2008.052761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bir SC, Esaki J, Marui A, et al. Angiogenic properties of sustained release platelet-rich plasma: characterization in-vitro and in the ischemic hind limb of the mouse. J Vasc Surg. 2009;50(4):870.e–9.e. doi: 10.1016/j.jvs.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Sánchez M, Anitua E, Azofra J, Aguirre JJ, Andia I. Intra-articular injection of an autologous preparation rich in growth factors for the treatment of knee OA: a retrospective cohort study. Clin Exp Rheumatol. 2008;26:910–3. [PubMed] [Google Scholar]
- 13.Raeissadat SA, Sedighipour L, Rayegani SM, Bahrami MH, Bayat M, Rahimi R. Effect of platelet-rich plasma (PRP) versus autologous whole blood on pain and function improvement in tennis elbow: a randomized clinical trial. Pain Res Treat. 2014;2014:191525. doi: 10.1155/2014/191525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehrenfest D, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF) Trend Biotechnol. 2009;27(3):158–67. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Shetty VD, Dhillon M, Hegde C, Jagtap P, Shetty S. A study to compare the efficacy of corticosteroid therapy with platelet-rich plasma therapy in recalcitrant plantar fasciitis: a preliminary report. Foot Ankle Surg. 2014;20(1):10–3. doi: 10.1016/j.fas.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Raeissadat SA, Rayegani SM, Hassanabadi H, Rahimi R, Sedighipour L, Rostami K. Is platelet-rich plasma superior to whole blood in the management of chronic tennis elbow: one year randomized clinical trial. BMC Sports Sci Med Rehabil. 2014;18(6):12. doi: 10.1186/2052-1847-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scarpone M, Rabago D, Snell E, et al. Effectiveness of platelet-rich plasma injection for rotator cuff tendinopathy: a prospective open-label study. Global Adv Health Med. 2013;2(2):26–31. doi: 10.7453/gahmj.2012.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conduah AH. Managing joint pain in osteoarthritis: safety and efficacy of hylan G-F 20. J Pain Res. 2009;2:87–98. doi: 10.2147/jpr.s4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalman D. Effect of a natural extract of chicken combs with a high content of hyaluronic acid (Hyal-Joint®) on pain relief and quality of life in subjects with knee osteoarthritis: a pilot randomized double-blind placebo-controlled trial. Nutr J. 2008;7:3. doi: 10.1186/1475-2891-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vincent K. Hyaluronic acid (HA) viscosupplementation on synovial fluid inflammation in knee osteoarthritis: a pilot study. Orthop J. 2013;7:378–84. doi: 10.2174/1874325001307010378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rutjes AW. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med. 2012;157(3):180–91. doi: 10.7326/0003-4819-157-3-201208070-00473. [DOI] [PubMed] [Google Scholar]
- 22.Miller LE, Block JE. US-approved intra-articular hyaluronic acid injections are safe and effective in patients with knee osteoarthritis: systematic review and meta-analysis of randomized, saline-controlled trials. Clin Med Arthritis Musculoskelet Disord. 2013;6:57–63. doi: 10.4137/CMAMD.S12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mishra A, Woodall J, Jr, Vieira A. Treatment of tendon and muscle using platelet-rich plasma. Clin Sports Med. 2009;28(1):113–25. doi: 10.1016/j.csm.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Chang K. Comparative effectiveness of platelet-rich plasma injections for treating knee joint cartilage degenerative pathology: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2014;95:562–75. doi: 10.1016/j.apmr.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Khoshbin A, Leroux T, Wasserstein D, et al. The efficacy of platelet-rich plasma in the treatment of symptomatic knee osteoarthritis: a systematic review with quantitative synthesis. Arthroscopy. 2013;29(12):2037–48. doi: 10.1016/j.arthro.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Dold AP, Zywiel MG, Taylor DW, Dwyer T, Theodoropoulos J. Platelet-rich plasma in the management of articular cartilage pathology: a systematic review. Clin J Sport Med. 2014;24(1):31–43. doi: 10.1097/01.jsm.0000432855.85143.e5. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Merchan EC. Intra-articular injections of hyaluronic acid and other drugs in the knee joint. HSS J. 2013;9(2):180–2. doi: 10.1007/s11420-012-9320-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaquerizo V, Plasencia MÁ, Arribas I, et al. Comparison of intra-articular injections of plasma rich in growth factors (PRGF-Endoret) versus durolane hyaluronic acid in the treatment of patients with symptomatic osteoarthritis: a randomized controlled trial. Arthroscopy. 2013;29(10):1635–43. doi: 10.1016/j.arthro.2013.07.264. [DOI] [PubMed] [Google Scholar]
- 29.Filardo G, Kon E, Di Martino A, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskelet Disord. 2012;13:229. doi: 10.1186/1471-2474-13-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raeissadat SA, Rayegani SM, Babaee M, Ghorbani E. The effect of platelet-rich plasma on pain, function, and quality of life of patients with knee osteoarthritis. Pain Res Treat. 2013;2013:165967. doi: 10.1155/2013/165967. [DOI] [PMC free article] [PubMed] [Google Scholar]