Abstract
Autologous stem cell transplantation (ASCT) is a widely used procedure for AIDS-related lymphomas, and it represents an opportunity to evaluate strategies curing HIV-1 infection. The association of autograft HIV-DNA load with peripheral blood HIV-1 reservoir before ASCT and its contribution in predicting HIV-1 reservoir size and stability during combination antiretroviral therapy (cART) after transplantation are unknown. Aiming to obtain information suggesting new functional cure strategies by ASCT, we retrospectively evaluated HIV-DNA load in autograft and in peripheral blood before and after transplantation in 13 cART-treated HIV-1 relapse/refractoring lymphoma patients. Among them seven discontinued cART after autograft infusion. HIV-DNA was evaluated by a sensitive quantitative real-time polymerase chain reaction (PCR). After debulking chemotherapy/mobilization, the autograft HIV-1 reservoir was higher than and not associated with the peripheral HIV-1 reservoir at baseline [median 215 HIV-DNA copies/106 autograft mononuclear cells, range 13–706 vs. 82 HIV-DNA copies/106 peripheral blood mononuclear cells (PBMCs), range 13–479, p=0.03]. After high dose chemotherapy and autograft infusion, HIV-DNA levels reached a plateau between month 6 and 12 of follow-up. No association was found between peripheral HIV-DNA levels at baseline and after infusion in both cART interrupting and not interrupting patients. Only in the last subgroup, a stable significant linear association between autograft and peripheral blood HIV-1 reservoir emerged from month 1 (R2=0.84, p=0.01) to month 12 follow-up (R2=0.99, p=0.0005). In summary, autograft HIV-1 reservoir size could be influenced by the mobilization phase and predicts posttransplant peripheral HIV-1 reservoir size in patients on continuous cART. These findings could promote new research on strategies reducing the HIV-1 reservoir by using the ASCT procedure.
Introduction
The advent of combination antiretroviral therapy (cART) and the possibility of reaching chronic suppression of HIV-1 replication have deeply changed the natural history of HIV-1 infection, allowing many infected persons to live much longer.1 However, managing lifelong cART is challenged because of necessary continuous adherence, possible toxic effects, and age-associated illnesses.2,3 The last considerations have encouraged research finalized to eradicate HIV-1 reservoirs.4 This line of research is fuelled by clinical reports suggesting the possibility of complete virus eradication following hematopoietic stem cell transplantation (HSCT) with an allogeneic homozygous CCR5 (Δ32) donor5 and by studies on elite suppressors, i.e., patients naturally controlling viral replication under the detection limits of clinical assays.6
Studies on the last subjects and secondary controllers (treated during chronic HIV infection) evidenced that undetectable viremia was associated with a very low HIV-1 peripheral blood reservoir.7,8 Although anti-HIV immune responses are likely to be one of the key elements at the base of virus control in these patients, these findings suggested that reducing the pool of infected cells might be crucial for a successful control of viral replication without therapy.
Among HIV-1 curing approaches, HSCT has been proposed, in association with other new or current therapies, as it allows management of the graft and it provides reconstitution of renewed immunological responses following development of hematopoiesis.9 In the setting of allogeneic transplantation with CCR5 wild-type donors, immune reconstitution during cART, followed by reduction in reservoir, has recently been reported.10,11 Nonetheless, the absence of clear evidence concerning the relative contribution of HIV-specific and nonspecific immune responses in the reduction in the residual reservoir in the setting of optimal antiretroviral therapy12 suggested that dilution of the HIV-1 reservoir within donor uninfected cells, rather than the selective killing of infected cells, likely played an important role in the dynamics of the reduction in HIV-DNA levels posttransplant in those patients who underwent analytical cART interruption 2.6–4.3 years following HSCT.
Autologous stem cell transplantation (ASCT) by itself cannot significantly reduce the HIV-1 reservoir13–15; in fact, infected lymphomonocytes are infused together with progenitor cells, hence, both the reservoir and the rest of the immune system are reconstituted. However, because it is a strengthened approach for AIDS-related lymphomas and more widely used than allogeneic transplantation, it offers a unique opportunity to evaluate strategies finalized to cure HIV-1 infection. This notion is in part supported by recent data in the cART-treated nonhuman-primate model16 suggesting that total body irradiation followed by immediate infusion of a clean autograft (obtained by cryopreservation of hematopoietic stem cells prior to infection of the host) led to a decrease of SHIV reservoir size during a period of 40–75 days preceding antiretroviral therapy interruption.
Modifying autograft characteristics by inserting genetically engineered and HIV-1-resistant CD34+ progenitors has proven to be safe, but limited by the low efficient engraftment of transformed cells.17,18 Alternatively, reducing the HIV-1 reservoir through cART and selection/depletion of cells from the autograft could be a hypothetical strategy needing further investigation, especially in light of evidence showing that highly purified CD34+ cells in successfully cART-treated patients might be uninfected.19–21
Among those few studies in which measurements of the HIV-1 reservoir were performed during the ASCT procedure, only one focused specifically on patients who remained on therapy with HIV-RNA below the threshold.13 However, this study lacked information on reservoir size in peripheral blood before transplantation and in autografts. Aiming to obtain information suggesting functional cure strategies including the concept of purging HIV-1 from the autograft, we assessed autograft HIV-DNA load, its association with the peripheral blood HIV-1 reservoir before transplantation, and its value in predicting peripheral HIV-1 reservoir size and stability under cART during longitudinal follow-up up to 1 year after infusion. Herein, we retrospectively evaluated data on the HIV-1 reservoir from patients belonging to one of the largest and well-characterized monoinstitutional cohorts of cART-treated HIV-positive lymphoma patients who underwent ASCT.14
Materials and Methods
Patients and study design
Patients for this retrospective study were elected among all HIV-positive subjects with relapse/refractory non-Hodgkin lymphoma (NHL) and Hodgkin lymphoma (HL) after first line conventional chemotherapy (CT), submitted to salvage therapy with high-dose chemotherapy (HDC) plus ASCT in 2002–2007, as previously described.14 The selection criteria were (1) to have a follow-up of at least 6 months after transplantation, namely, until recovery of at least baseline CD4 T cell counts was attained14 and (2) to have recorded HIV-RNA assessments and clinical information highlighting cART adherence during the transplant procedure in order to evaluate the impact of antiretroviral and ASCT treatment on the HIV-1 reservoir.
Thirteen patients were eligible. Based on cART interruption after autograft infusion, patients were stratified into two groups: continuously treated and interrupted cART patients. They had undergone HIV-DNA load and CD4 T cell percentage assessments in all the available samples of apheretic products (n=13) and of peripheral blood at baseline [i.e., before starting debulking chemotherapy (DCT); n=12] and at months 1 (n=9), 3 (n=12), 6 (n=11), and 12 (n=11) after transplantation. A slightly smaller number of patients had undergone CD4 memory T cell percentage evaluation in autograft products (n=12) and in peripheral blood (at baseline, n=11; at months 1, 3, 6, and 12 after transplantation, n=9, 12, 11, and 11, respectively). The Ethics Committee of the CRO National Cancer Institute Aviano approved this study. A written informed consent was obtained from the patients in accordance with the Declaration of Helsinki. All the patients' information was deidentified prior to analysis.
Salvage procedure and samples collection
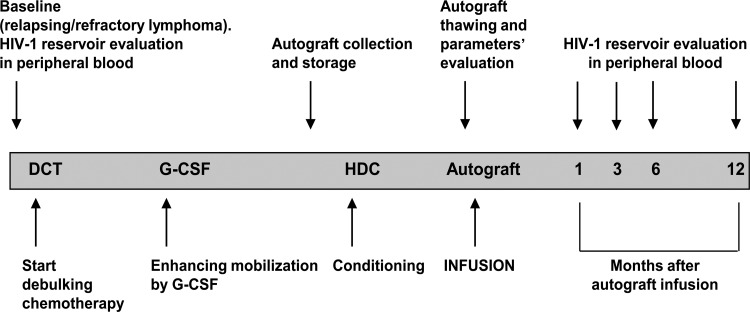
Figure 1 provides an outline of the salvage procedure and the timing of the evaluated parameters. In brief, conventional second-line DCT for all NHL patients consisted of ESHAP (etoposide, cytarabine, cisplatinum, and methylprednisone) or DHAOx schemes (dexamethasone, high-dose cytarabine, and oxaliplatinum). Rituximab was administered before each cycle to all patients with CD20-positive NHL. DCT in HL relapsed patients consisted of the MINE regimen (mesna, iphosphamide, mitoxantrone, etoposide, and prednisolone). Stem cell mobilization was enhanced through a challenge with 10 μg/kg/day of granulocyte colony-stimulating factor (G-CSF) after the fourth CT cycle, until a stable CD34+ cell count of at least 10 cells/μl was achieved. CD34+ stem cell-enriched autografts were harvested by leukapheresis using a COM.TEC cell separator (Fresenius Hemocare, Germany) and frozen with a double bag storage procedure.22 Two leukapheresis products within 48 h were harvested in 6 out of 13 patients.
FIG. 1.
Outline of autologous stem cell transplantation (ASCT) procedure and timing of parameter evaluation. DCT, debulking chemotherapy; G-CSF, granulocyte colony-stimulating factor; HDC, high dose chemotherapy.
The second phase of the treatment consisted of a conditioning regimen with HDC BEAM (carmustine, etoposide, cytarabine, and melphalan). Two days after the end of HDC, the cryopreserved autograft was thawed, evaluated for cellular viability by the ISHAGE protocol,23 and infused, while an aliquot was used for genomic DNA extraction by the phenol/chloroform technique.
Whole blood samples were collected by venipuncture in ethylenediaminetetraacetic acid; peripheral blood mononuclear cells (PBMCs) were then isolated by density gradient centrifugation over Ficoll-Hypaque and cryopreserved at −80°C until genomic DNA extraction by the phenol/chloroform technique.
Immunological parameters
Peripheral blood percentage values and absolute counts of lymphocyte subsets were evaluated by a single platform whole blood lysing technique, Flow-Count fluorospheres and EPICS XL flow cytometer (Beckman-Coulter), as previously described.24 In brief, staining was performed by monoclonal antibody (MoAb) combinations CD45-FITC/CD4-PE/CD8-ECD/CD3-PC5 and CD45-FITC/CD56-PE/CD19-ECD/CD3-PC5 (Beckman-Coulter). The proportion of CD4+ T cells in the thawed autografts was evaluated by lymphocyte gating on the basis of scatter properties and CD45-FITC reactivity (2D1, BD Biosciences) and by MoAb CD4-PC5 (MT310, DAKO). The proportions of CD4+ T cells expressing memory phenotype in peripheral blood and thawed autografts were studied in detail using the MoAb combinations CD4-FITC/CD45R0-PE (MT310/UCHL1, DAKO).
Virological parameters
HIV-DNA load was assessed in each sample of DNA by quantitative real-time PCR (qPCR) with primers and probe detecting a 121-bp DNA fragment in the long terminal repeat of the HIV-1 genome as previously described.25 In brief, qPCR amplification was performed in a 25 μl volume containing 2× TaqMan Universal Master Mixture, 37.5 pmol of each primer, 3.75 pmol of the fluorogenic probe,26 and 5 μl of sample. The ACH-2 cell line, which stably harbors one integrated copy of the HIV-1 genome, was used to generate the standard curve. The intra- and interassay coefficient variation (CV) of standard quantity from 5,000 to 2 copies per reaction was always within 30%. Ten-fold and, for quantification of reactions containing HIV-DNA below 5 copies per well, 2-fold serial dilutions of the standard curve were run in triplicate along with the samples in each plate experiment.
Analytic validation assays of the standard curve from five to one HIV-DNA copies per reaction, in a background of 1×105 cell equivalents of genomic DNA, showed that quantification of two and one copies of target HIV-DNA per reaction occurred in 85% and 67% of the cases, respectively. PBMCs and autograft DNA samples were run in duplicate or quadruplicate using a range of 2.1–46.3×104 cell equivalents per qPCR reaction. Mean intraassay CV of the sample quantity was 28.2 (range 3.1–67.7). Samples with an HIV-DNA quantity between one and two copies per reaction (with CVs higher than 60%) were repeated in other sessions on more than three replicates of the same DNA extract and/or after reextraction of the sample.
The lack of inhibitory substances was evaluated by coamplification of an additional one or two replicates seeded with 300 copies of ACH-2 HIV-1 DNA. Normalization of the HIV-DNA copies per 106 PBMCs in each peripheral blood DNA sample was obtained by qPCR on the human β-globin gene as previously reported.27 Since thawed autografts contained a considerable and not removable granulocyte proportion (range 10.5–67.1%), HIV-1 DNA qPCR data in this compartment were normalized per 106 autograft mononuclear cells (AMCs) using qPCR for the β-globin gene and the lymphocytes and monocytes percent estimated by hemocytometer.
When two leukapheresis products were harvested and infused, a weighted mean of the two HIV-DNA concentrations was reported. Normalization of HIV-DNA per 106 CD4 and CD4 memory T cells was attained by using CD4+ and CD4+CD45R0+ T cell percentages assessed in peripheral blood or in autograft. Data were collected using an ABI Prism 7900 HT Sequence Detector and analyzed using Sequence Detection System Software (2.1 v., Applera, Italy). For statistical analysis, a value of two HIV-DNA copies/106 PBMCs was given to qPCR reactions persistently showing a detected HIV-DNA quantity below the limit of the assessed sensitivity. HIV-RNA levels were tested using the HIV-RNA b-DNA assay (3.0 v., Siemens, Berkeley, CA) with a detection limit of 50 copies/ml.
Statistical analysis
Univariate linear regression analyses were computed to evaluate associations between covariates. To increase the power of regression analyses between autograft and peripheral blood HIV-1 reservoir after transplantation, we combined HIV-reservoir determinations from 12 patients at month 3 after autograft infusion with the HIV-1 reservoir determination at month 1 for one continuously treated cART patient. Moreover, we added patients having HIV-1 reservoir assessments at month 12 after transplantation (n=11) to patients showing HIV-1 reservoir determination at month 6 (n=1, continuously treated cART patient; n=1, interrupted cART patient). The Wilcoxon rank sum test for continuous variables and Fisher exact test for proportions were used to evaluate differences between groups. The Wilcoxon signed-rank test was used to evaluate differences between paired covariates. Statistical analyses were performed using STATA (version 7) and MedCalc (version 13.1.2) softwares. Results were considered statistically significant at a two-tailed p value ≤0.05.
Results
Patients
As shown in Table 1, at baseline, the majority of patients had a long history of HIV-1 infection. Nine of them were treated with protease inhibitor (PI)-based cART regimens and all received nucleoside reverse transcriptase inhibitors (NRTIs). The minimum time of cART treatment was 8 months. Most of the patients showed HIV-RNA levels <50 copies/ml. Previous CT consisted of one to three doxorubicin-based intensive regimens and patients' immunodeficiency was severe. We found a wide variation within patients in CD34+ stem cells mobilization efficiency (range harvested CD34+, 2.1–20.0×106 cells/kg). The CD34+ cell concentration in leukapheresis was significantly correlated with CD4+ T cell numbers at baseline (r=0.60, p=0.037) with a nearly significant reduction in patients treated with PI-based cART [median harvested CD34+ in patients treated with PI-based cART vs. NNRTI-based cART, 882 cells/μl (range, 500–7,031 cells/μl) vs. 3,000 cells/μl (range 1,618–11,148); p=0.09].
Table 1.
Characteristics of Patients Included in the Study
| Patient ID | Age in years (sex) | Lymphoma histology | Months since first HIV infection report until baseline | Kind of cART | Months on cART until baseline | Baseline HIV-RNA (copies/ml) | Baseline CD4 (cells/μl) | Harvested CD34+(cells × 106/Kg) | HIV-DNA in autograft (copies/106 AMCs) | Days of cART interruption early after ASCT | Max HIV-RNA level early after ASCT (copies/ml) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 36 (M) | NHL-DLBCL | 23 | 1NNRTI+2NRTI | 8 | <50 | 189 | 6.4 | 112 | 0 | <50 |
| 2 | 51 (M) | HL-NS | 53 | 1PI+2NRTI | 46 | <50 | 191 | 2.1 | 112 | 0 | <50 |
| 3 | 42 (M) | NHL-DLBCL | 25 | 1NNRTI+2NRTI | 24 | <50 | 211 | 20.0 | 422 | 0 | 103a |
| 4 | 34 (M) | NHL-DLBCL | 68 | 1PI+2NRTI | 39 | <50 | 212 | 10.0 | 22 | 0 | <50 |
| 5b | 46 (M) | HL | 250 | 1NNRTI+2NRTI | 105 | <50 | 630 | 6.2 | 679 | 0 | <50 |
| 6 | 47 (M) | NHL-DLBCL | 199 | 1NNRTI+2NRTI | 46 | <50 | 150 | 5.0 | 330 | 0 | <50 |
| 7 | 40 (M) | HL-NS | 174 | 1PI+2NRTI | 62 | <50 | 172 | 4.1 | 13 | 27 | >500,000 |
| 8 | 39 (M) | NHL-DLBCL | 12 | 1PI+2NRTI | 10 | 204 | 172 | 4.3 | 706 | 15 | >500,000 |
| 9 c | 44 (M) | NHL-DLBCL | 241 | 1PI+2NRTI | 73 | 1,272 | 293 | 3.9 | 181 | 4 | >500,000 |
| 10 | 67 (M) | NHL-DLBCL | 27 | 1PI+2NRTI | 21 | <50 | 13 | 2.4 | 215 | 41 | >500,000 |
| 11 | 42 (M) | NHL-DLBCL | 247 | 1PI+1NNRTI+1NRTI | 86 | NA | NA | 3.4 | 68 | 77 | 6,615 |
| 12b | 52 (M) | NHL-IB | 249 | 1PI+2NRTI | 124 | <50 | 369 | 10.0 | 334 | 27 | 3,800 |
| 13 | 48 (F) | NHL-DLBCL | 241 | 1PI+2NRTI | 118 | 56 | 45 | 3.8 | 609 | 7 | 180,735 |
| Overall | |||||||||||
| median (range) | 44 (34–67) | 174 (12–250) | 46 (8–124) | <50 (<50–1,272) | 190 (13–630) | 4.30 (2.1–20.0) | 215 (13–706) | 4 (0–77) | 3,800 (<50–>500,000) | ||
| CcART | |||||||||||
| median (range) | 44 (34–51) | 61 (23–250) | 43 (8–105) | <50 (<50–<50) | 201 (150–630) | 6.3 (2.1–20.0) | 221 (22–679) | 0 (0–0) | <50 (<50–103) | ||
| IcART | |||||||||||
| median (range) | 44 (39–67) | 241 (12–249) | 73 (10–124) | 53 (<50–1,272) | 172 (13–369) | 3.9 (2.4–10.0) | 215 (13–706) | 27 (4–77) | >500,000 (3,800–>500,000) | ||
| pd CcART vs. IcART | 0.48 | 0.56 | 0.47 | 0.021 | 0.25 | 0.06 | 0.34 | 0.13 | 0.89 | 0.002 | 0.002 |
At month 1 after ASCT.
Follow-up until month 6.
Patient on suboptimal cART due to low adherence at baseline.
Wilcoxon rank-sum or Fisher exact test when applicable.
cART, combination antiretroviral therapy; M, male; F, female; NHL, non-Hodgkin lymphoma; DLBCL, diffuse large B-cell lymphoma; HL, Hodgkin lymphoma; NS, nodular sclerosis; IB, immunoblastic; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; NA, not available; CcART, patients on continuous cART; IcART, patients who interrupted cART; ASCT, autologous stem cell transplantation; AMCs, autograft mononuclear cells.
cART was maintained throughout the DCT/mobilization procedure and HIV-RNA load was undetectable during DCT and at autograft collection time. After autograft infusion, patients 1–6 received continuous cART and patients 7–13 received interrupted cART within month 0.5–1 for a median of 27 days (range 4–77 days). All continuously treated cART patients showed undetectable HIV-RNA levels until month 6 or 12 after transplantation, except for one who exhibited a transient increase to 103 copies/ml at month 1. Conversely, median HIV-RNA levels sharply increased at month 0.5 after transplantation in interrupted cART patients. Reintroduction of the same cART produced a 0.5–3 log10 copies/ml reduction in HIV-RNA levels in about 4 weeks. HIV-RNA was always detectable during follow-up in one patient due to low adherence.
At baseline, no statistically significant difference was noticed between continuously treated and interrupted cART patients, except for the antiretroviral treatment that was PI based in the latter subjects (p=0.02). No statistically significant difference between continuously treated and interrupted cART patients emerged in harvested CD34+ cell number [median harvested CD34+ in continuously treated vs. interrupted, 6.3×106 cells/kg (range 2.1–7.5×106 cells/kg) vs. 3.9×106 cells/kg (range 3.1–7.5×106 cells/kg); p=0.13] or in leukapheresis number (1/6 continuously treated and 5/7 interrupted patients harvested two autografts; p=0.10).
HIV-DNA reservoir
HIV-DNA levels in autograft were higher than in peripheral blood at baseline in the patients before cART interruption [median, 215 copies/106 AMCs (range 13–706 copies/106 AMCs) vs. 82 copies/106 PBMCs (range 13–479 copies/106 PBMCs); p=0.03]. Although not statistically significant, increased autograft HIV-DNA levels were also observed after adjustment of HIV-1 reservoir per CD4 [median, 886 copies/106 CD4 (range 90–7,385 copies/106 CD4) vs. 685 copies/106 CD4 (range 45–2,818 copies/106 CD4); p=0.15]. Since the HIV-1 reservoir resides for the most part in memory T cell subsets, we also normalized HIV-DNA per million CD4+CD45R0+ memory T cells. The findings obtained resemble those attained from the HIV reservoir expressed per million PBMCs [median, 1,535 copies/106 CD4R0 (range 146–16,411 copies/106 CD4R0) vs. 860 copies/106 CD4R0 (range 66–3,023 copies/106 CD4R0); p=0.017].
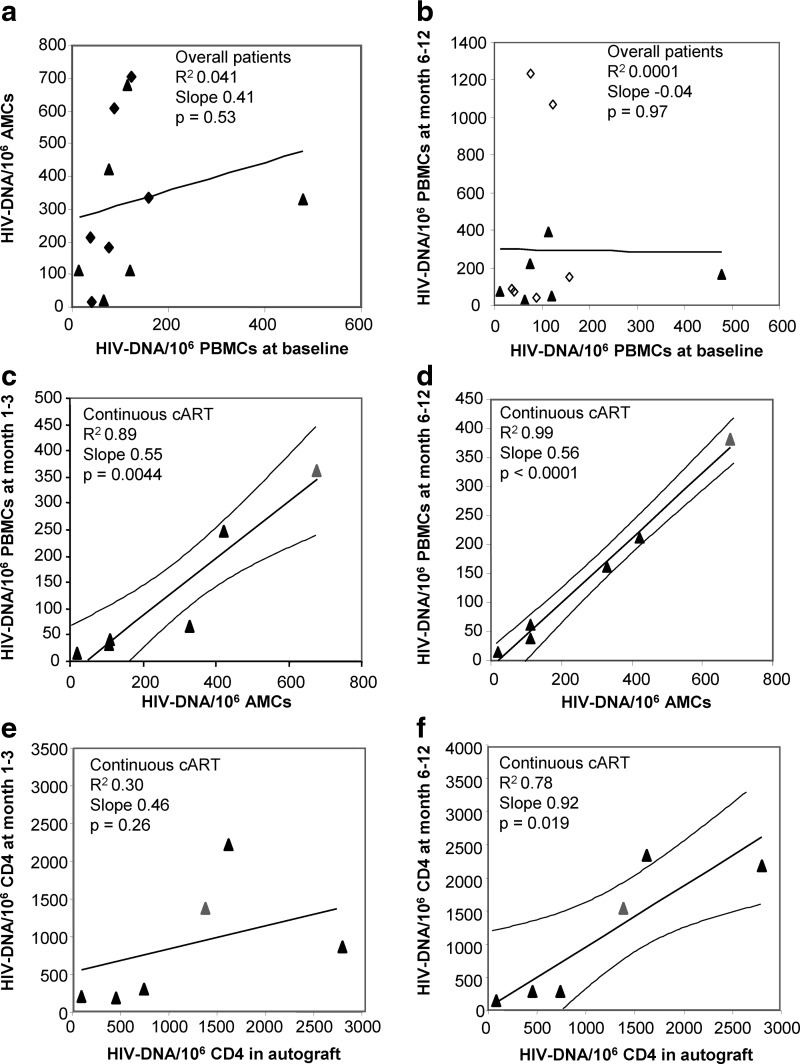
No association emerged between HIV-DNA levels in peripheral blood at baseline and in autograft (n=12) (R2=0.04, p=0.53; Fig. 3a). Similar associations were confirmed by normalizing the HIV-1 reservoir per CD4 or CD4R0 memory T cells (baseline vs. autograft: HIV-DNA/106 CD4 in n=12 patients, R2=0.1, p=0.34; HIV-DNA/106 CD4R0 in n=10 patients, R2=0.23, p=0.16).
FIG. 3.
(a) Linear regression between reservoir size in the autograft vs. reservoir size in peripheral blood at baseline (before HDC) in overall patients: R2=0.04, slope=0.41, p (slope≠0)=0.53; (b) linear regression between reservoir size in peripheral blood at month 6 or 12 vs. reservoir size in peripheral blood at baseline (before HDC) in overall patients: R2=0.0001, slope=–0.04, p (slope≠0)=0.97; (c) linear regression and 95% CI (curved lines) between reservoir size in peripheral blood at month 1 or 3 vs. reservoir size in autograft, continuously cART-treated patients: R2=0.89, slope=0.55, p (slope≠0)=0.0044; interrupted cART patients: R2=0.008, slope=0.03, p (slope≠0)=0.85; (d) linear regression and 95% CI between reservoir size in peripheral blood at month 6 or 12 vs. reservoir size in autograft, continuously cART-treated patients: R2=0.99, slope=0.56, p (slope≠0)<0.0001; interrupted cART patients: R2=0.09, slope=0.61, p (slope≠0)=0.51. (e) linear regression between reservoir size per million CD4 in peripheral blood at month 1 or 3 vs. reservoir size per million CD4 in autograft, continuously cART-treated patients: R2=0.30, slope=0.46, p (slope≠0)=0.26; interrupted cART patients: R2=0.004, slope=0.02, p (slope≠0)=0.91; (f) linear regression and 95% CI between reservoir size per million CD4 in peripheral blood at month 6 or 12 vs. reservoir size per million CD4 in autograft, continuously cART-treated patients: R2=0.78, slope=0.92, p (slope≠0)=0.019; interrupted cART patients: R2=0.004, slope=–0.15, p (slope≠0)=0.89. HDC, high dose chemotherapy; cART, combination antiretroviral therapy; CI, confidence intervals; AMCs, autograft mononuclear cells; PBMCs, peripheral blood mononuclear cells. black triangles, continuously cART-treated patients; black diamonds, interrupted cART patients before interruption; open diamonds, interrupted cART patients after interruption; gray triangle, continuously cART-treated patients at month 1 (c–e) or month 6 (d–f).
HDC induced a radical decrease in absolute white blood cell counts (WBCs), with the nadir reached approximately 1 week after the end of the conditioning period [median, 50 WBC/μl (range 10–130 WBC/μl), at median 6 days after HDC (range 5–8 days)] and 98.6% median reduction from levels before conditioning (range 97.0–99.7%).
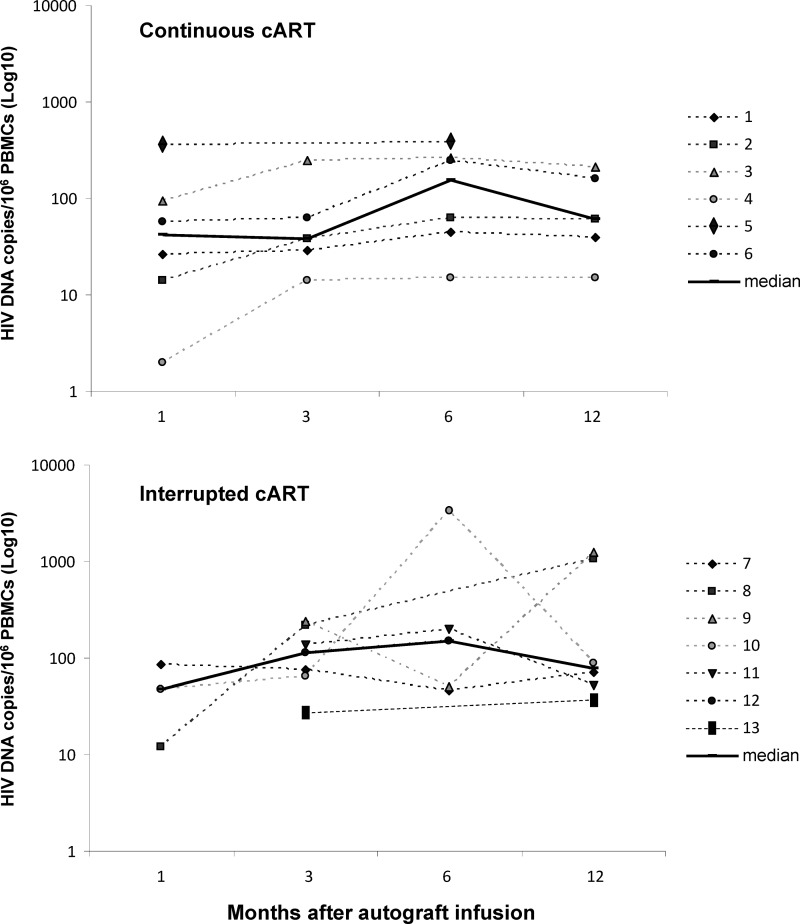
After autograft infusion, the median peripheral blood HIV-1 reservoir in continuously treated and interrupted cART patients reached relatively stable levels between months 6 and 12 follow-up (median HIV-DNA levels at months 3, 6, and 12 in continuous and interrupted cART patients: 38, 156, and 61 copies/106 PBMCs and 114, 148, and 79 copies/106 PBMCs, respectively) (Fig. 2). Peripheral blood HIV-DNA levels at plateau were not associated with HIV-DNA values at baseline either in overall patients (R2=0.0001, p=0.97) (Fig. 3b) or in patients stratified by cART interruption (interrupting cART patients: R2=0.04, p=0.70; continuously cART treated patients: R2=0.02, p=0.78). In addition, peripheral HIV-DNA levels at baseline were not associated with peripheral HIV-DNA values in the initial months after autograft infusion in both interrupting (between month 1 and 6, range R2=0.10–0.70, p>0.37) and continuously cART-treated patients (between month 1 and 6, range R2=0.0–0.12, p>0.51). Conversely, only in the last subgroup was a stable significant linear association between reservoir size in autograft and in peripheral blood observed from month 1 to 12 after transplantation (month 1: R2=0.84, p=0.01; month 3: R2=0.72, p=0.07; month 6: R2=0.97, p=0.0004; month 12: R2=0.99, p=0.0005). Regression analyses of pooled data were reported in Fig. 3c and d.
FIG. 2.
HIV-1 reservoir posttransplant dynamics in continuously (top) and interrupted combined antiretroviral therapy (cART)-treated patients (bottom).
Adjustments of HIV-1 reservoir per 106 CD4 and CD4R0 yielded analogous associations. A statistically significant association between autograft and posttransplantation peripheral HIV-1 reservoir was particularly evidenced at plateau in continuous cART-treated subjects (Fig. 3f). It is worth noting that in the last subgroup, associations between HIV-1 reservoir per million CD4R0 memory T cells in autograft and in posttransplant peripheral blood were highly significant (at months 1–3, R2=0.94, p=0.007; at months 6–12, R2=0.97, p=0.003).
Discussion
ASCT is a well-tolerated salvage therapy for HIV-positive relapse/refractoring-lymphoma patients.28 In this monocentric study focusing on cART-treated patients submitted to this procedure, we evaluated the importance of autograft HIV-1 reservoir size in determining the peripheral HIV-1 reservoir, ultimately aiming to obtain information in support of the concept that purging HIV-1 from the autograft could represent a strategy contributing to functionally curing HIV-1 infection.
First, this study showed that the HIV-1 reservoir size in autograft was higher than the HIV-1 reservoir size in peripheral blood at baseline. It is possible that HIV-1 infection compartmentalization might be disrupted by the G-CSF effect on mononuclear cell mobilization from multiple niches characterized by differential levels of infection, as already demonstrated in T cell trafficking within peripheral blood in response to this mobilizing agent.29
In addition, HIV-1 reservoir sizes in autograft and in peripheral blood at baseline were not associated, suggesting an influence of the DCT/mobilization phase in changing the autograft HIV-1 reservoir. This was also emphasized by the absence of a relationship between peripheral blood HIV-DNA levels at baseline and after infusion not only in interrupting cART patients, where this was expected given the rapid rebound of viremia in this subgroup, but also in continuously cART-treated patients. In fact, this suggests that autograft infusion might have contributed to perturbing a relationship that, otherwise, would be maintained in well-suppressed patients.
Second, patients not interrupting cART maintained a stable highly statistically significant linear association between autograft and peripheral blood HIV-1 reservoir size since month 1 after transplantation. To the best of our knowledge, this finding was never reported in the literature. Studies measuring the HIV-1 reservoir in selected lymphocyte populations reported that the proliferation process driven by the complex cytokinic network and/or by antigens within the lymphopenic environment30,31 could lead to the differential expansion of subsets32 and stimulate virus replication in productively and latently infected CD4+ T cells.33,34 Our results indicated that a homeostatic and/or antigenic milieu during posttransplant lymphopenia exerted its proliferative pressure on lymphomonocytes and HIV-1 replication without apparent consequences for the proportion of the HIV-1 reservoir in each patient until plateau. This occurred due to the continuous action of cART in efficiently preventing virus dissemination to susceptible cells.
Our observations resemble the association found between viral reporter gene expression in the autograft and in the reconstituted immune system at set point (6 months) described in autologous HSCT of nonhuman primates following total body irradiation.35 Six to twelve months is also the lag time needed to reach baseline CD4+ T cell count levels in an analogous cohort of HIV-1-positive patients.14 Of note, our previous findings showed a correlation between CD4+ and CD8+ T cell counts in autograft and in peripheral blood until month 6 or 12 after transplantation, indicating a remarkable role of the infused T cell subsets and their expansion in immune reconstitution.14 It is known that the recovery of immune function occurs rapidly in CD34+ unselected if compared to selected autologous transplantation; although the pattern of TCRVB expression is very similar before and after conditioning plus autograft infusion, the expansion of oligoclones within 100 days after infusion suggests the proliferation of mature postthymic cells.36
Although straightforward and direct proof ascertaining the origin of the posttransplant HIV-1 reservoir has not been reported in human studies and further investigations are needed to establish the relative contribution of the autograft to immune system recovery, collectively these observations suggest that, in preconditioned HIV-1 hosts, the reconstituted immune system could stem primarily from the autograft, rather than from homeostatic proliferation of the immune cells surviving the conditioning regimen.
Possible weaknesses of this study are its retrospective nature and the small number of patients, which might, for instance, have masked the presence of associations not found in our group of patients due to the low power of the analysis. In addition, this study is grounded on HIV-1 reservoir assessments in PBMCs, which could be an approximate marker of the HIV-1 reservoir. However, we have demonstrated that similar associations emerged also expressing the HIV-1 reservoir per CD4 T cells, in particular per CD4 memory T cells. The loss of strength in the associations that consider HIV-1 reservoir inferred per million CD4 (Fig. 3e) could be related to statistical noise due to the low sample size as well as to systematic errors introduced by the difference in staining and gating strategy between peripheral blood and autograft samples, or distinct dynamics of monocytes and CD4 T cell subset recoveries during transplantation.37–39
Despite these limitations, our results point out the importance of autograft HIV-DNA levels in determining the HIV-1 reservoir after transplantation. Consequently, clearing autografts from HIV-1, during or after the DCT/mobilization phase, could have a positive impact on the subsequent peripheral HIV-DNA load. Theoretically, >99% replacement of the immune system from a fully purged autograft would reduce the reservoir size, in cART settings, of more than 2 logs. Such a strategy would make it possible to reach a threshold below which the reservoir could be eradicated or kept under control possibly in combination with other immune-based approaches, e.g., through enhancement of HIV-1-specific boosting of the reconstituted immune system prior to interruption of antiretroviral therapy.40,41
In line with other findings, our results showed that the greatest efficiency of CD34+ cell mobilization was associated with high CD4+ T cell absolute counts at baseline and suggested that cART might influence progenitors' availability, confirming an interdependence between immune status, cART treatment, and hematopoiesis in these patients.42–45 It is conceivable that patients preserving a quite functional bone marrow and immune system during HIV-1 infection could fully benefit from HIV-1 purging interventions, because they exhibit lower defects in the quantity and quality of CD34+ and effector T cells with presumably low HIV-1 infection load. In these subjects, risks associated with delayed lymphocyte recovery and infection-related mortality after transplantation might be managed by increasing the dose of infused purified CD34+ progenitor cells and by infection surveillance and prevention.46,47
In conclusion, this study provides evidence that autograft HIV-1 reservoir size influences posttransplant peripheral HIV-1 reservoir size in patients on effective cART. The latter prevents substantial reseeding of the infused HIV-1 reservoir to the reconstituting immune system. Since the DCT/mobilization phase within the ASCT procedure could change the quantity and/or the quality of the autograft HIV-1 reservoir, additional studies on larger patient numbers are needed to assess the autograft composition in HIV-1-infected subsets predicting subsequent HIV-1 cell-associated load. To this end, the ASCT procedure could be a useful platform to subsequently design strategies reducing the HIV-1 reservoir.
Acknowledgments
We thank Luigina Mei for editorial assistance. This work was supported by grants from “Ministero del lavoro, della salute e delle politiche sociali” (grant 40H73). The content of this article has been presented as an oral communication to the VI Italian Conference on AIDS and Retroviruses, Rome, May 25–27, 2014.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.High KP, Brennan-Ing M, Clifford DB, et al. : HIV and aging: State of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr 2012;60(Suppl 1):S1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apostolova N, Blas-García A, and Esplugues JV: Mitochondrial toxicity in HAART: An overview of in vitro evidence. Curr Pharm Des 2011;17:2130–2144 [DOI] [PubMed] [Google Scholar]
- 3.Deeks SG: HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med 2011;62:141–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International AIDS Society Scientific Working Group on HIV Cure, Deeks SG, Autran B, Berkhout B, et al. : Towards an HIV cure: A global scientific strategy. Nat Rev Immunol 2012;12:607–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hütter G, Nowak D, Mossner M, et al. : Long-term control of HIV by CCR5 delta32/delta32 stem-cell transplantation. N Engl J Med 2009;360:692–698 [DOI] [PubMed] [Google Scholar]
- 6.Deeks SG. and Walker BD: Human immunodeficiency virus controllers: Mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity 2007;27:406–416 [DOI] [PubMed] [Google Scholar]
- 7.Van Gulck E, Bracke L, Heyndrickx L, et al. : Immune and viral correlates of “secondary viral control” after treatment interruption in chronically HIV-1 infected patients. PLoS One 2012;7:e37792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendoza D, Johnson SA, Peterson BA, et al. : Comprehensive analysis of unique cases with extraordinary control over HIV replication. Blood 2012;119:4645–4655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitchen SG. and Zack JA: Stem cell-based approaches to treating HIV infection. Curr Opin HIV AIDS 2011;6:68–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woolfrey AE, Malhotra U, Harrington RD, et al. : Generation of HIV-1-specific CD8+ cell responses following allogeneic hematopoietic cell transplantation. Blood 2008;112:3484–3487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henrich TJ, Hu Z, Li JZ, et al. : Long-term reduction in peripheral blood HIV type 1 reservoirs following reduced-intensity conditioning allogeneic stem cell transplantation. J Infect Dis 2013;207:1694–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henrich T, Hanhauser E, Sirignano M, et al. . :In depth investigation of peripheral and gut HIV-1 reservoirs, HIV-specific cellular immunity, and host microchimerism following allogeneic hematopoietic stem cell transplantation [Abstract WELBA05]. In Program and Abstract book from the International AIDS Society 7th Conference on HIV Pathogenesis and Prevention, June30–July3, 2013, Kuala Lumpur, Malaysia [Google Scholar]
- 13.Cillo AR, Krishnan A, Mitsuyasu RT, et al. : Plasma viremia and cellular HIV-1 DNA persist despite autologous hematopoietic stem cell transplantation for HIV-related lymphoma. J Acquir Immune Defic Syndr 2013;63:438–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simonelli C, Zanussi S, Pratesi C, et al. : Immune recovery after autologous stem cell transplantation is not different for HIV-infected versus HIV-uninfected patients with relapsed or refractory lymphoma. Clin Infect Dis 2010;50:1672–1679 [DOI] [PubMed] [Google Scholar]
- 15.Resino S, Pérez A, Seoane E, et al. : Short communication: Immune reconstitution after autologous peripheral blood stem cell transplantation in HIV-infected patients: Might be better than expected? AIDS Res Hum Retroviruses 2007;23:543–548 [DOI] [PubMed] [Google Scholar]
- 16.Mavigner M, Watkins B, Lawson B, et al. : Persistence of virus reservoirs in ART-treated SHIV-infected rhesus macaques after autologous hematopoietic stem cell transplant. PLoS Pathog 2014;10:e1004406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiGiusto DL, Krishnan A, Li L, et al. : RNA-based gene therapy for HIV with lentiviral vector-modified CD34(+) cells in patients undergoing transplantation for AIDS-related lymphoma. Sci Transl Med 2010;2:36ra43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tebas P, Stein D, Tang WW, et al. : Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med 2014;370:901–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pace M. and O'Doherty U: Hematopoietic stem cells and HIV Infection. J Infect Dis 2013;207:1790–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Josefsson L, Eriksson S, Sinclair E, et al. : Hematopoietic precursor cells isolated from patients on long-term suppressive HIV therapy did not contain HIV-1 DNA. J Infect Dis 2012;206:28–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durand CM, Ghiaur G, Siliciano JD, et al. : HIV-1 DNA is detected in bone marrow populations containing CD4+ T cells but is not found in purified CD34+ hematopoietic progenitor cells in most patients on antiretroviral therapy. J Infect Dis 2012;205:1014–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbruzzese L, Michieli M, Rupolo M, et al. : A new freezing and storage procedure improves safety and viability of haematopoietic stem cells and neutrophil engraftment: A single institution experience. Vox Sang 2010;98:172–180 [DOI] [PubMed] [Google Scholar]
- 23.Keeney M, Chin-Yee I, Weir K, et al. : Single platform flow cytometric absolute CD34+ cell counts based on the ISHAGE guidelines. International Society of Hematotherapy and Graft Engineering. Cytometry 1998;34:61–70 [PubMed] [Google Scholar]
- 24.Zanussi S, Vaccher E, Caffau C, et al. : Interferon-gamma secretion and perforin expression are impaired in CD8+ T lymphocytes from patients with undifferentiated carcinoma of nasopharyngeal type. Cancer Immunol Immunother 2003;52:28–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bortolin MT, Zanussi S, Talamini R, et al. : Predictive value of HIV type 1 DNA levels on overall survival in HIV-related lymphoma patients treated with high-dose chemotherapy (HDC) plus autologous stem cell transplantation (ASCT). AIDS Res Hum Retroviruses 2010;26:245–251 [DOI] [PubMed] [Google Scholar]
- 26.Viard JP, Burgard M, Hubert JB, et al. : Impact of 5 years of maximally successful highly active antiretroviral therapy on CD4 cell count and HIV-1 DNA level. AIDS 2004;18:45–49 [DOI] [PubMed] [Google Scholar]
- 27.Bortolin MT, Pratesi C, Dolcetti R, et al. : Clinical value of Epstein–Barr virus DNA levels in peripheral blood samples of Italian patients with undifferentiated carcinoma of nasopharyngeal type. Cancer Lett 2006;233:247–254 [DOI] [PubMed] [Google Scholar]
- 28.Michieli M, Mazzucato M, Tirelli U, and De Paoli P: Stem cell transplantation for lymphoma patients with HIV infection. Cell Transplant 2011;20:351–370 [DOI] [PubMed] [Google Scholar]
- 29.Savkovic B, Macpherson JL, Zaunders J, et al. : T-lymphocyte perturbation following large-scale apheresis and hematopoietic stem cell transplantation in HIV-infected individuals. Clin Immunol 2012;144:159–171 [DOI] [PubMed] [Google Scholar]
- 30.Mackall C, Fry T, Gress R, et al. : Background to hematopoietic cell transplantation, including post transplant immune recovery. Bone Marrow Transplant 2009;44:457–462 [DOI] [PubMed] [Google Scholar]
- 31.Surh CD. and Sprent J: Homeostasis of naive and memory T cells. Immunity 2008;29:848–862 [DOI] [PubMed] [Google Scholar]
- 32.Sasson SC, Zaunders JJ, Seddiki N, et al. : Progressive activation of CD127+132− recent thymic emigrants into terminally differentiated CD127−132+T-cells in HIV-1 infection. PLoS One 2012;7:e31148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaunders JJ, Ip S, Munier ML, et al. : Infection of CD127+ (interleukin-7 receptor+) CD4+ cells and overexpression of CTLA-4 are linked to loss of antigen-specific CD4 T cells during primary human immunodeficiency virus type 1 infection. J Virol 2006;80:10162–10172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vandergeeten C, Fromentin R, DaFonseca S, et al. : Interleukin-7 promotes HIV persistence during antiretroviral therapy. Blood 2013;121:4321–4329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uchida N, Bonifacino A, Krouse AE, et al. : Accelerated lymphocyte reconstitution and long-term recovery after transplantation of lentiviral-transduced rhesus CD34+ cells mobilized by G-CSF and plerixafor. Exp Hematol 2011;39:795–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benicchi T, Ghidini C, Re A, et al. : T-cell immune reconstitution after hematopoietic stem cell transplantation for HIV-associated lymphoma. Transplantation 2005;80:673–682 [DOI] [PubMed] [Google Scholar]
- 37.Talmadge JE, Reed E, Ino K, et al. : Rapid immunologic reconstitution following transplantation with mobilized peripheral blood stem cells as compared to bone marrow. Bone Marrow Transplant 1997;19:161–172 [DOI] [PubMed] [Google Scholar]
- 38.Krause SW, Rothe G, Gnad M, et al. : Blood leukocyte subsets and cytokine profile after autologous peripheral blood stem cell transplantation. Ann Hematol 2003;82:628–636 [DOI] [PubMed] [Google Scholar]
- 39.Chomont N, El-Far M, Ancuta P, et al. : HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 2009;15:893–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shan L, Deng K, Shroff NS, et al. : Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity 2012;36:491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiss RA: Immunotherapy for HIV infection. N Engl J Med 2014;370:379–380 [DOI] [PubMed] [Google Scholar]
- 42.Re A, Cattaneo C, Skert C, et al. : Stem cell mobilization in HIV seropositive patients with lymphoma. Haematologica 2013;98:1762–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacEneaney OJ, Connick E, and DeSouza CA: Effects of HIV-1 gp120 and protease inhibitors on apoptotic susceptibility of CD34+ hematopoietic progenitor cells. J Acquir Immune Defic Syndr 2011;56:e49–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Isgrò A, Leti W, De Santis W, et al. : Altered clonogenic capability and stromal cell function characterize bone marrow of HIV-infected subjects with low CD4+ T cell counts despite viral suppression during HAART. Clin Infect Dis 2008;46:1902–1910 [DOI] [PubMed] [Google Scholar]
- 45.Gómez-Garre D, Estrada V, Ortega-Hernández A, et al. : Association of HIV-infection and antiretroviral therapy with levels of endothelial progenitor cells and subclinical atherosclerosis. J Acquir Immune Defic Syndr 2012;61:545–551 [DOI] [PubMed] [Google Scholar]
- 46.Witzens-Harig M, Heilmann C, Hensel M, et al. : Long-term follow-up of patients with non-Hodgkin lymphoma following myeloablative therapy and autologous transplantation of CD34+-selected peripheral blood progenitor cells. Stem Cells 2007;25:228–235 [DOI] [PubMed] [Google Scholar]
- 47.Schwinger W, Weber-Mzell D, Zois B, et al. : Immune reconstitution after purified autologous and allogeneic blood stem cell transplantation compared with unmanipulated bone marrow transplantation in children. Br J Haematol 2006;135:76–84 [DOI] [PubMed] [Google Scholar]