Abstract
In a prospective cross-sectional study we quantified HIV viral load within the alveolar macrophage in a cohort of healthy HIV-infected subjects who did not have medical comorbidities or smoke cigarettes to determine if alveolar macrophage proviral DNA was associated with alveolar macrophage phagocytic immune dysfunction. We enrolled 23 subjects who underwent bronchoscopy and bronchoalveolar lavage. Alveolar macrophages were isolated and HIV-1 RNA was quantified in the cells using the Abbott RealTime HIV-1 Assay. Proviral DNA was qualitatively measured using a modified version of the HIV-1 RNA assay. Phagocytosis measured by incubating alveolar macrophages with FITC-labeled Staphylococcus aureus and determining fluorescence with a Zeiss inverted microscope. Phagocytic index was calculated as (% positive cells×mean channel fluorescence)/100. Sixteen subjects had (+) proviral DNA and seven had (−) proviral DNA in their alveolar macrophages. Of all subjects 100% in both groups were on highly active antiretroviral therapy (HAART). The median plasma viral load was 0 in both groups. HIV-1-infected subjects with (+) proviral DNA in their alveolar macrophages had a significantly lower median alveolar macrophage phagocytic index compared to those with (−) proviral DNA in their alveolar macrophages [11.8 (IQR 4.8–39.0) vs. 64.9 (IQR 14.0–166.0), p=0.05]. Alveolar macrophages harbor HIV even in otherwise healthy subjects with undetectable plasma viral loads, representing a potential reservoir for the virus. In addition, HIV viral replication within the macrophage may impair phagocytosis and other immune functions in the lung, leading to an increased risk for lung infection.
Introduction
Despite advances in human immunodeficiency virus-1 (HIV-1) treatment including highly active antiretroviral therapy (HAART) individuals infected with HIV-1 continue to remain susceptible to serious bacterial and viral infections, such as pneumococcus and influenza, which continue to cause significant morbidity and mortality.1–3 A recent study in the Veterans Administration (VA) HIV-1 cohort found that bacterial pneumonia was the most common pulmonary disease, with an incidence of 28.0 per 1,000 person-years [95% confidence interval (CI), 27.2–28.8] compared with 5.8 per 1,000 person-years (95% CI, 5.6–6.0) among HIV-1-uninfected individuals (p=0.001).4 Alveolar macrophage immune dysfunction could contribute to the increased risk of pneumonia in HIV-1-infected individuals, but the exact mechanisms are currently unknown. Alveolar macrophages are the primary innate immune defense cell in the lung and also the most plentiful cells in the lung. They are resistant to apoptosis, thus resulting in a half-life that is approximately 30 days.5,6 There is abundant evidence that HIV-1 infection impairs alveolar macrophage immune functions.7–9 Experimental studies have shown that macrophage phagocytic function by alveolar macrophages was significantly decreased in HIV-1 transgenic animals compared to wild-type animals.7
Eradication of HIV-1 has been difficult to achieve. Evidence for ongoing viral replication and the persistence of latently infected cells, including CD4+ T cells and cells from the monocyte/macrophage lineage, has been shown in HIV-1-infected individuals.10–12 HAART has been ineffective in eliminating these latent reservoirs. Furthermore, studies have shown that despite the lack of detectable replication-competent HIV-1 in patients on HAART, plasma HIV-1 reemerges when HAART is discontinued.13 Studies have also demonstrated the lack of correlation between viral rebound in plasma upon discontinuation of HAART and the size of the pool of latent HIV-1 reservoir prior to discontinuation of therapy, suggesting that the source of rebound HIV-1 viremia may not have come from a pool that is measurable in the circulating blood.14,15
HIV-1 can infect long-lived alveolar macrophages and HIV-1 entry into these cells is preferentially mediated by the CCR5 receptor.16 However, reports comparing the burden of HIV-1 in alveolar macrophages relative to peripheral blood monocytes are conflicting and it is unclear what the exact viral burden is in the lung, as percentages of alveolar macrophages expressing HIV-1 antigens vary considerably.17 Phylogenetic evidence supports compartmentalization of HIV-1 between lungs and peripheral blood, suggesting that alveolar macrophages could be another reservoir for HIV-1.18,19
We hypothesize that the alveolar macrophage is an important reservoir for HIV-1, contributing to cell–cell spread of the virus within the lung and exhibiting abnormal function, which contributes to the increase risk of severe lung infections. Therefore, we sought to quantify HIV-1 viral load within the alveolar macrophage and determine if alveolar macrophage proviral DNA was associated with alveolar macrophage phagocytic immune dysfunction.
Materials and Methods
Study design and protocol
All participants signed an Emory University Institutional Review Board approved consent to participate in the study. The research study purpose, procedures, risks and benefits, and alternative treatment options were described in detail and discussed with each subject. The subject was given an opportunity to read the informed consent form and ask questions. The subject also verbalized understanding of the study and all related visits and procedures, verbalized understanding of the HIPAA authorization, and signed and received a copy of the informed consent form. No study procedures were performed prior to obtaining informed consent. We performed a cross-sectional study of HIV-1-infected subjects without other medical problems within the Grady Health System in Atlanta, GA. Inclusion criteria included all subjects with HIV-1 infection. Exclusion criteria included active liver disease (known cirrhosis and/or total bilirubin >2.0 mg/dl), heart disease [ejection fraction <50%, history of acute myocardial infarction, New York Heart Association (NYHA) II–IV cardiac symptoms, severe valvular dysfunction], current renal disease (dialysis dependent or creatinine >2.0 mg/dl), current lung disease [spirometry revealing a forced vital capacity (FVC) or forced expiratory volume in 1 s (FEV1) <80% of predicted], diabetes, current pregnancy, malnutrition (body mass index <17), current tobacco use, and age <25 years.
The study coordinator entered the date, patient's name, medical record number, and study ID number in the patient enrollment log. Data were initially collected using our established case report forms and then entered into a HIPAA-compliant, secure database. All subjects completed a preenrollment evaluation (visit 1) that included (1) a complete history and physical examination, (2) routine blood chemistries (basic chemistry, liver function tests, complete blood count, coagulation parameters, hemoglobin A1C), (3) urine pregnancy test (qualitative β-HCG) for women of child-bearing potential, (4) urine dipstick for cotinine, (5) spirometry (FEV1, FVC), (6) Short Michigan Alcohol Screening Test (SMAST) and Alcohol Use Disorders Identification Test (AUDIT) alcohol use questionnaires, and (7) body mass index (BMI). Demographic data were collected. Subjects with exclusions identified at the time of the preenrollment evaluation were excluded from further participation.
After completing the preenrollment evaluation to confirm eligibility, subjects presented to the Grady Memorial Hospital Clinical Interaction Site after an overnight fast. Enrolled volunteers had the following interventions on visit 2: (1) CD4+ count and viral load for HIV-1-infected subjects only and (2) flexible fiberoptic bronchoscopy with standardized bronchoalveolar lavage (BAL) performed using standard conscious sedation techniques. BAL was performed by installing 30-ml aliquots of 0.9% nonbacteriostatic normal saline solution, followed by withdrawal with low-pressure hand suction until a total of 180 ml had been administered. Subjects were contacted by phone 24 h after completing the study to ensure patient safety. A Data Safety Monitoring Representative was appointed to review each patient's enrollment procedures and bronchoscopy report on a monthly basis.
Alveolar macrophage phagocytosis
BAL fluid was centrifuged at 1,200 rpm for 7 min for recovery of cell pellet. Manual cell counts were performed with a hemocytometer and differentials were obtained from 300 consecutive cells after Diff-Quik staining (Andwin Scientific, Addison, IL). The cell pellet was resuspended in 10 ml of 1:1 Dulbecco's modified Eagle's medium/Ham's F-12 solution containing 2% fetal bovine serum (FBS), l-glutamine, 15 mmol/liter HEPES, penicillin (10,000 U), streptomycin (10,000 mg/ml), amphotericin (25 mg/ml), and gentamicin (4 μg/ml). Alveolar macrophages (100,000 cells) were added to glass-chamber slides containing 100 μl of medium and 20 μl of phosphate-buffered saline (PBS). Alveolar macrophages were incubated at 37°C with 10% CO2 for 15 h, after which 10×105 particles of pH-sensitive pHrodo fluorescein isothiocyanate-conjugated inactivated Staphylococcus aureus were added (10:1 ratio of S. aureus/alveolar macrophages) to the cultures and incubated for 2 h. Cells were washed, fixed with 4% paraformaldehyde, and stored at 4°C until analysis. Fluorescence was determined by quantitative computer analysis of images taken with a Zeiss inverted microscope using 10 fields per experimental condition with the same pinhole, detector gain, and amplifier offset. The basis of this assay is that S. aureus is internalized and the pHrodo component fluoresces once it reaches the lower pH (pH=4) present in the phagolysosome. Macrophages with any internalized bacteria were considered positive for phagocytosis. Phagocytosis was quantified by phagocytic index, which was calculated as the percentage of cells positive for phagocytosis multiplied by the mean relative fluorescence units of S. aureus per cell.
HIV-1 RNA and DNA tests
The Abbott RealTime HIV-1 Assay (Abbott Molecular Inc., Des Plaines, IL) is a real-time reverse transcription polymerase chain reaction (RT-PCR) assay for the quantitation of HIV-1 RNA on the automated m2000 System over the range of 40 to 10,000,000 copies/ml. The assay is standardized against a viral standard from the Virology Quality Assurance laboratory of the AIDS Clinical Trial Group and against the WHO 1st International standard for HIV-1 RNA. An internal control is introduced into specimens at the beginning of the sample preparation that is an RNA sequence unrelated to the HIV-1 target (pumpkin plant hydroxypyruvate reductase gene). An input of 1 million alveolar macrophages suspended in 100 μl PBS was used in the HIV-1 RNA test, the elution volume is 50 μl, all of which is used for amplification.
The Abbott RealTime HIV-1 Proviral DNA research use only assay is a qualitative modification of the above mentioned HIV-1 RNA viral load assay. The modifications allow amplification of HIV-1 proviral DNA only by removing the reverse transcription stage of the amplification protocol, modifying the nucleic acid extraction procedure from and RNA extraction to a DNA extraction, and adding a DNA internal control. The HIV-1 Proviral DNA test is qualitative and can detect an input of 153 copies of HIV-1 DNA. An input of 600,000 alveolar macrophages suspended in 100 μl of PBS was used in the HIV-1 DNA test; the elution volume is 50 μl, all of which is used for amplification.
Statistical analyses
Univariate comparisons between HIV-1 subjects with and without proviral DNA were calculated and evaluated for a significance level of 0.05 using a chi-squared test for categorical variables and a two-sample t-test for continuous variables. The data were log-transformed or a Wilcoxon rank-sum test was used when the data were not normally distributed.
Results
Baseline characteristics of HIV-1-infected subjects
A total of 23 HIV-1 subjects were enrolled. Table 1 shows the demographic characteristics of HIV-1-infected subjects enrolled in the study divided into two groups—those who had HIV-1 proviral DNA present in their alveolar macrophages compared to those who did not. We had 16 HIV-1-infected subjects with (+) proviral DNA in their alveolar macrophages and 7 HIV-1-infected subjects with (−) proviral DNA in their alveolar macrophages. There were no significant differences in median age, gender, or race. The majority of subjects in both groups were African American. Of the subjects, 100% in both groups were on HAART. HAART regimens varied and are listed in Table 2. HIV-1-infected subjects with (+) proviral DNA trended toward a lower median CD4 count compared to HIV-1-infected subjects with (−) proviral DNA [349.5 (IQR 248.5–452.3) vs. 560 (390–730), p=0.06]. Approximately 71.4% of subjects with (+) proviral DNA had an undetectable plasma viral load vs. 100% of subjects with (−) proviral DNA in their alveolar macrophages. However, the median plasma viral load was 0 in both groups.
Table 1.
Characteristics of HIV-1-Infected Subjects (n=24) Enrolled in the Study
| Variables | (+) Proviral DNA | (−) Proviral DNA | p |
|---|---|---|---|
| N | 16 | 7 | |
| Median age (IQR) | 49.5 (44–54.3) | 50 (47–53) | 0.84 |
| Gender (% male) | 43.8 | 57.1 | 0.55 |
| Race | 0.53 | ||
| White (n, %) | 1 (6.3) | 1 (14.3) | |
| Black (n, %) | 15 (93.7) | 6 (85.7) | |
| On HAART (n, %) | 16 (100) | 7 (100) | |
| Median CD4 (IQR) | 349.5 (248.5–452.3) | 560 (390–730) | 0.06 |
| % Undetectable plasma viral load | 71.4% | 100% | 0.12 |
| Median plasma viral load copies/ml (IQR) | 0 (0–27.3) | 0 (0–0) | 0.18 |
HAART, highly active antiretroviral therapy.
Table 2.
Highly Active Antiretroviral Therapy Regimens of the HIV-1-Infected Subjects Enrolled in the Study
| Subject ID | Proviral DNA detected in alveolar macrophage (+/−) | HAART regimen |
|---|---|---|
| 1 | + | Emtricitabine/tenofovir/efavirenz |
| 2 | + | Raltegravir+emtricitabine/tenofovir |
| 3 | − | Emtricitabine/tenofovir/efavirenz |
| 4 | − | Raltegravir+emtricitabine/tenofovir+lopinavir/ritonavir |
| 5 | + | Raltegravir+lamivudine/zidovudine |
| 6 | + | Emtricitabine/tenofovir/efavirenz |
| 7 | − | Emtricitabine/tenofovir/efavirenz |
| 8 | + | Efavirenz+abacavir |
| 9 | + | Emtricitabine/tenofovir+darunavir+ritonavir |
| 10 | + | Emtricitabine/tenofovir/efavirenz |
| 11 | + | Ritonavir |
| 12 | + | Lopinavir/ritonavir+abacavir |
| 13 | + | Atazanavir+ritonavir+abacavir/lamivudine |
| 14 | + | Raltegravir+tenofovir/emtricitabine |
| 15 | − | Tenofovir/emtricitabine |
| 16 | + | Emtricitabine/tenofovir/efavirenz |
| 17 | + | Emtricitabine/tenofovir/efavirenz |
| 18 | − | Emtricitabine/tenofovir/efavirenz |
| 19 | + | Raltegravir+emtricitabine/tenofovir |
| 20 | − | Raltegravir+tenofovir/emtricitabine+etravirine |
| 21 | − | Emtricitabine/tenofovir/efavirenz |
| 22 | + | Emtricitabine/tenofovir/efavirenz |
| 23 | + | Atazanavir+ritonavir |
HIV-1 Proviral DNA and RNA are detectable and quantifiable in alveolar macrophages
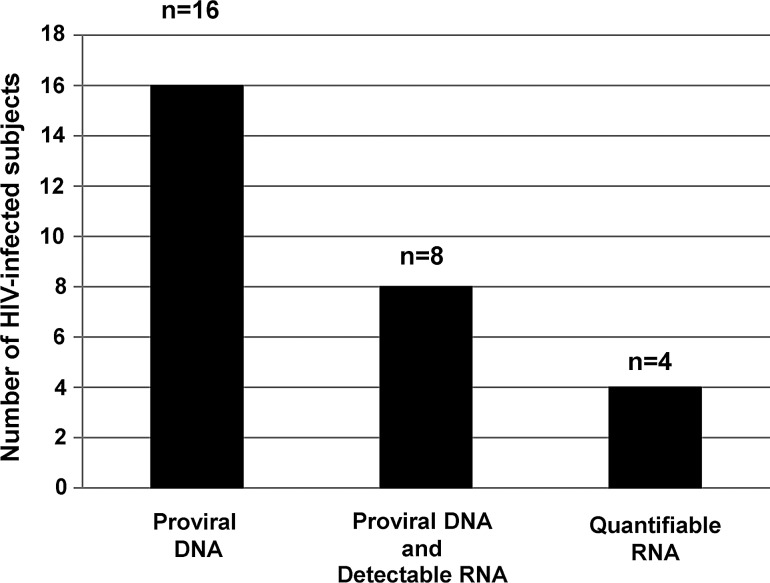
Sixteen HIV-1-infected subjects had (+) proviral DNA present in their alveolar macrophages (Fig. 1). Eight of these subjects had (+) proviral DNA and (+) HIV-1 RNA. Of these eight subjects, four had quantifiable HIV-1 RNA in their alveolar macrophages. Table 3 details the four subjects who, in addition to detectable proviral DNA, had quantifiable HIV-1 RNA in their alveolar macrophages. Subject 1 had the highest HIV-1 RNA count in the plasma and the second highest count within the alveolar macrophage. However, Subjects 2–4 had quantifiable HIV-1 RNA within their alveolar macrophages, whereas their plasma HIV-1 RNA levels were undetectable.
FIG. 1.
HIV proviral DNA and RNA are detectable and quantifiable in alveolar macrophages. Alveolar macrophages were isolated from otherwise healthy HIV-infected subjects. The Abbott RealTime HIV-1 Assay was used to measure both HIV DNA and RNA.
Table 3.
Subjects with Quantifiable HIV-1 RNA in Their Alveolar Macrophages
| Subjects | Alveolar macrophage HIV RNA (copies/million cells) | Plasma HIV RNA (copies/ml) |
|---|---|---|
| 1 | 182 | 27,542 |
| 2 | 588 | Undetectable |
| 3 | 70 | Undetectable |
| 4 | 48 | Undetectable |
Proviral DNA within alveolar macrophages is associated with impaired bacterial phagocytosis
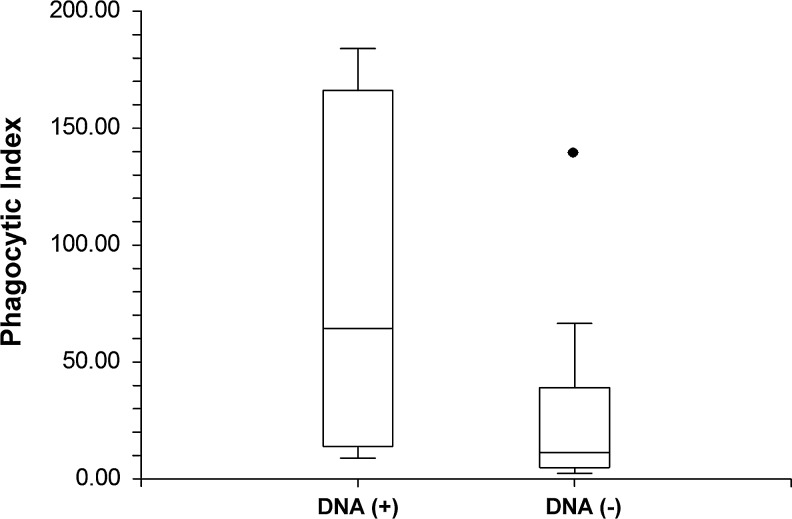
Twelve out of 16 HIV-1-infected subjects with (+) proviral DNA and six out of seven HIV-1-infected subjects with (−) proviral DNA had alveolar macrophage phagocytosis data. HIV-1-infected subjects with (+) proviral DNA in their alveolar macrophages had a significantly lower median alveolar macrophage phagocytic index compared to those with (−) proviral DNA in their alveolar macrophages [11.8 (IQR 4.8–39.0 vs. 64.9 (IQR 14.0–166.0), p=0.05] (Fig. 2).
FIG. 2.
Proviral DNA within alveolar macrophages is associated with decreased bacterial phagocytosis. Alveolar macrophages were isolated from otherwise healthy HIV-infected subjects and incubated with FITC-labeled S. aureus. Phagocytosis was determined by measuring fluorescence with a Zeiss inverted microscope. The phagocytic index was calculated as (% positive cells×mean channel fluorescence)/100. The Abbott RealTime HIV-1 Assay was used to measure HIV proviral DNA.
Discussion
The results from this study showed that HIV-1 was both detectable and quantifiable within alveolar macrophages even in otherwise healthy, nonsmoking, HIV-1-infected individuals with plasma viral suppression. HIV-1 DNA was present in approximately 70% of this “healthy” cohort, while both HIV-1 DNA and RNA were detectable in 34.8% and HIV-1 RNA was quantifiable in about 17.4% of all subjects. In addition, the presence of proviral DNA within alveolar macrophages was associated with impaired alveolar macrophage bacterial phagocytic capacity, even in this population with no history of lung infections or lung disease. The alveolar macrophage may, therefore, represent a potential reservoir for the virus. This is the first study demonstrating that (1) HIV-1 DNA and RNA are present within alveolar macrophages in otherwise healthy HIV-1-infected subjects with systemic viral suppression and (2) that the presence of HIV-1 DNA within the alveolar macrophages was associated with impaired lung immune function, as reflected by impairment in alveolar macrophage phagocytic capacity.
This study adds to the accumulating evidence supporting the hypothesis that the lung may be a reservoir for HIV-1. Certain studies have shown a greater HIV-1 burden in unfractionated BAL fluid and undifferentiated BAL cells compared to the periphery.20–22 In one of the largest studies, Clarke et al. compared HIV-1 DNA copy number within lung cells from BAL specimens to peripheral blood cells from 78 HIV-1-infected individuals not on HAART.20 They determined that the mean HIV-1 DNA copy number was greater in BAL inflammatory cells compared to peripheral blood in approximately 56% of subjects. Furthermore, in both BAL and peripheral blood, a higher level of HIV-1 DNA was detected in the monocyte/macrophage lineage compared to leukocytes. In a subgroup of individuals receiving azidothymidine (AZT) therapy, the authors found a reduced mean HIV-1 proviral DNA copy number in each subpopulation of cells.20 However, in contrast to this current study, all of these individuals were undergoing bronchoscopy for pulmonary symptoms and approximately 38% of the HIV-1-infected individuals proceeded to AIDS with a diagnosis of Pneumocystis carinii (now known as Pneumocystis jiroveci) pneumonia (PCP). Although this evidence suggests that the lung is a significant focus of HIV-1 infection and may be important in the pathogenesis of disease, there is significant heterogeneity in the current literature including the presence of lung infections, other lung diseases, and tobacco use in the different study populations.
We also found that HIV-1 replication in alveolar macrophages was compartmentalized compared to the serum in eight subjects in whom HIV-1 RNA and DNA were detected. In these subjects, three had undetectable plasma viral loads. Furthermore, three out of four subjects with quantifiable HIV-1 RNA had undetectable viral loads in the periphery. These data suggest that HIV-1 replication in the lungs occurs separately from the blood and is not just from simple diffusion from the periphery. Koziel et al. examined the relationship of viral burden in cell-free BAL fluid to that of serum in asymptomatic HIV-1-infected individuals and found that 16% of asymptomatic subjects had evidence of HIV-1 RNA in cell-free BAL fluid.21 However, in contrast to this current study, all seven asymptomatic subjects with HIV-1 RNA in BAL fluid had detectable HIV-1 RNA in their serum and six of these seven subjects were not taking antiretroviral medications. Furthermore, unlike this current study, all seven were active tobacco users.
In addition, in this study of otherwise healthy HIV-1-infected subjects, we found that the presence of HIV-1 DNA within alveolar macrophages significantly impaired alveolar macrophage phagocytic capacity. Experimental data show us that HIV-1 infection can affect alveolar macrophage immune functions,7–9 including the finding that abnormal activation impaired oxidative burst and cytokine secretion.23–25 In humans, similar macrophage dysfunctions are seen26,27; however, the data with respect to phagocytic functions are conflicting.28–31 Gordon et al. found that alveolar macrophages from healthy, nonsmoking HIV-1-infected adults showed normal internalization of opsonized pneumococci,29 whereas others have shown that HIV-1-infected adults have impaired macrophage phagocytic capacity.30,32,33 Most recently, Jambo et al. found that HIV-1 present in small alveolar macrophages from asymptomatic HIV-1-infected HAART-naive individuals was associated with impaired phagocytic capacity and proteolytic activity.32 However, prior to the current study, the presence of intracellular HIV-1 DNA or RNA has not been associated with impaired alveolar macrophage phagocytic function.
The effect of HAART on lung viral load is still being studied. In a prospective, longitudinal study, Twigg et al. determined that HAART was associated with a significant decline in plasma viral load, but the decline in lung viral load, both in the acellular and cellular compartments, was more rapid even after just 4 weeks of HAART, which was sustained for 6 months.22 These results suggest that the lung seemed to respond to HAART just as well as the vascular compartment. These subjects all had CD4 counts ≥500 cells/μl and a plasma HIV-1 RNA load of ≥5,000 copies/ml. The finding of 34.8% of our asymptomatic HIV-1-infected adults with HIV-1 RNA within their alveolar macrophages, despite HAART, suggests that HIV-1 may be compartmentalized to an area in the lung where HAART is not as effective. At this time, it is still unclear what the pharmacokinetics of HAART in the lung are and whether HAART can restore lung immune function. Currently, CD4 counts and viral loads are used as prognostic markers in this population. However, our study revealed that even otherwise healthy HIV-1-infected individuals have persistent HIV-1 within alveolar macrophages that is associated with macrophage immune dysfunction, despite systemic viral suppression and higher CD4 counts. If this is corroborated in larger clinical trials, then clinicians will need new diagnostic and prognostic biomarkers of susceptibility to lung infection in this vulnerable population.
There are a number of limitations of this study. This is an observational cross-sectional study and so is subject to bias when comparing the two groups. However, confounding was minimized by restricting the population to a select group of individuals who were otherwise healthy, nonsmokers, and without any major medical problems. HAART regimens were self-reported by the study subjects. Although we confirmed regimens from subjects who were followed in the clinic, there were a number of subjects who received their care at other hospitals and we were not able to confirm their medications. In addition, we are currently unable to confirm the duration of HAART. Furthermore, although HIV-1 RNA and DNA were measured in the alveolar macrophages, we did not determine whether the HIV-1 within these alveolar macrophages was replication competent. Although we are unable to assess this on the current samples, we intend to address this important question in the future.
In conclusion, HIV-1 viral infection of alveolar macrophages may impair macrophage phagocytosis and other immune functions, leading to an increased risk for lung infections. At this time, there are a number of unanswered questions that are critical to understanding the effects of HIV-1 infection in the lung. It is unknown what the clinical impact of persistent viral replication in the lung is: Does it, in fact, lead to an increase risk of pulmonary infections? Is impairment of macrophage function implicated in the pathogenesis of other lung diseases, such as obstructive lung disease? Furthermore, if even otherwise healthy HIV-1-infected individuals on HAART with systemic viral suppression have HIV-1 present in the lung, and this presence is associated with impaired immune function, then clinicians need better diagnostics to determine which individuals are at risk for lung infections. Finally, supplemental therapies to HAART may be necessary to target the alveolar macrophage reservoir and improve lung immune function.
Acknowledgments
The authors would like to thank a number of people for contributing to this article: (1) Joel Andrews, RN, BSN for all his help with recruitment and enrollment of study subjects and IRB regulations; (2) Annette Esper, MD, MSc and Greg Martin, MD, MSc for their help with bronchoscopies; (3) Jessica Ingersoll, MS, MB (ASCP) and Deborah Abdul-Ali, MT (ASCP) for technical assistance with specimen processing and HIV DNA and RNA testing; (4) Janine Ward, BS and Frank Harris, BS for technical assistance with specimen processing and analyzing alveolar macrophage phagocytic function and (5) Abbott Molecular for providing reagents for the study. In addition, the corresponding author, Dr. Cribbs, would like to thank her co-authors for the following contributions: (1) study design and recruitment (D.G., J.L., A.C., L.B.); (2) analysis and interpretation of HIV-1 RNA and DNA detection (D.G., J.L., A.C., L.B.); (3) analysis and interpretation of alveolar macrophage phagocytic immune function (D.G., J.L., L.B.); and (4) manuscript preparation (D.G., J.L., A.C., L.B.).
This study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (KL2 TR000454 and UL1 TR000454) and Emory Center for AIDS Research (P30 AI050409).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.El-Sadr WM, Lundgren JD, Neaton JD, et al. : CD4+count-guided interruption of antiretroviral treatment. N Engl J Med 2006;355(22):2283–2296 [DOI] [PubMed] [Google Scholar]
- 2.Gordin FM, Roediger MP, Girard PM, et al. : Pneumonia in HIV-infected persons: Increased risk with cigarette smoking and treatment interruption. Am J Respir Crit Care Med 2008;178(6):630–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jordano Q, Falco V, Almirante B, et al. : Invasive pneumococcal disease in patients infected with HIV: Still a threat in the era of highly active antiretroviral therapy. Clin Infect Dis 2004;38(11):1623–1628 [DOI] [PubMed] [Google Scholar]
- 4.Crothers K, Huang L, Goulet JL, et al. : HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med 2011;183(3):388–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy J, Summer R, Wilson AA, Kotton DN, and Fine A: The prolonged life-span of alveolar macrophages. Am J Respir Cell Mol Biol 2008;38(4):380–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan J. and Sattentau QJ: The HIV-1-containing macrophage compartment: A perfect cellular niche? Trends Microbiol 2013;21(8):405–412 [DOI] [PubMed] [Google Scholar]
- 7.Joshi PC, Raynor R, Fan X, and Guidot DM: HIV-1-transgene expression in rats decreases alveolar macrophage zinc levels and phagocytosis. Am J Respir Cell Mol Biol 2008;39(2):218–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joshi PC. and Guidot DM: HIV-1 transgene expression in rats induces differential expression of tumor necrosis factor alpha and zinc transporters in the liver and the lung. AIDS Res Ther 2011;8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pugliese A, Vidotto V, Beltramo T, and Torre D: Phagocytic activity in human immunodeficiency virus type 1 infection. Clin Diagn Lab Immunol 2005;12(8):889–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furtado MR, Callaway DS, Phair JP, et al. : Persistence of HIV-1 transcription in peripheral-blood mononuclear cells in patients receiving potent antiretroviral therapy. N Engl J Med 1999;340(21):1614–1622 [DOI] [PubMed] [Google Scholar]
- 11.Wong JK, Hezareh M, Gunthard HF, et al. : Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 1997;278(5341):1291–1295 [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Ramratnam B, Tenner-Racz K, et al. : Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N Engl J Med 1999;340(21):1605–1613 [DOI] [PubMed] [Google Scholar]
- 13.Chun TW, Davey RT, Jr, Engel D, Lane HC, and Fauci AS: Re-emergence of HIV after stopping therapy. Nature 1999;401(6756):874–875 [DOI] [PubMed] [Google Scholar]
- 14.Chun TW, Davey RT, Jr, Ostrowski M, et al. : Relationship between pre-existing viral reservoirs and the re-emergence of plasma viremia after discontinuation of highly active anti-retroviral therapy. Nat Med 2000;6(7):757–761 [DOI] [PubMed] [Google Scholar]
- 15.Davey RT, Jr., Bhat N, Yoder C, et al. : HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci USA 1999;96(26):15109–15114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park IW, Koziel H, Hatch W, et al. : CD4 receptor-dependent entry of human immunodeficiency virus type-1 env-pseudotypes into CCR5-, CCR3-, and CXCR4-expressing human alveolar macrophages is preferentially mediated by the CCR5 coreceptor. Am J Respir Cell Mol Biol 1999;20(5):864–871 [DOI] [PubMed] [Google Scholar]
- 17.Agostini C, Trentin L, Zambello R, and Semenzato G: HIV-1 and the lung. Infectivity, pathogenic mechanisms, and cellular immune responses taking place in the lower respiratory tract. Am Rev Respir Dis 1993;147(4):1038–1049 [DOI] [PubMed] [Google Scholar]
- 18.Alimohammadi A, Coker R, Miller R, et al. : Genotypic variants of HIV-1 from peripheral blood and lungs of AIDS patients. AIDS 1997;11(6):831–832 [PubMed] [Google Scholar]
- 19.Itescu S, Simonelli PF, Winchester RJ, and Ginsberg HS: Human immunodeficiency virus type 1 strains in the lungs of infected individuals evolve independently from those in peripheral blood and are highly conserved in the C-terminal region of the envelope V3 loop. Proc Natl Acad Sci USA 1994;91(24):11378–11382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clarke JR, Gates AJ, Coker RJ, et al. : HIV-1 proviral DNA copy number in peripheral blood leucocytes and bronchoalveolar lavage cells of AIDS patients. Clin Exp Immunol 1994;96(2):182–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koziel H, Kim S, Reardon C, et al. : Enhanced in vivo human immunodeficiency virus-1 replication in the lungs of human immunodeficiency virus-infected persons with Pneumocystis carinii pneumonia. Am J Respir Crit Care Med 1999;160(6):2048–2055 [DOI] [PubMed] [Google Scholar]
- 22.Twigg HL, Soliman DM, Day RB, et al. : Lymphocytic alveolitis, bronchoalveolar lavage viral load, and outcome in human immunodeficiency virus infection. Am J Respir Crit Care Med 1999;159(5 Pt 1):1439–1444 [DOI] [PubMed] [Google Scholar]
- 23.Denis M. and Ghadirian E: Alveolar macrophages from subjects infected with HIV-1 express macrophage inflammatory protein-1 alpha (MIP-1 alpha): Contribution to the CD8+alveolitis. Clin Exp Immunol 1994;96(2):187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans MR. and Wansbrough-Jones MH: Alveolar macrophage activation in HIV infection. J Infect 1996;33(2):91–94 [DOI] [PubMed] [Google Scholar]
- 25.Koziel H, O'Riordan D, Warner A, and Rose RM: Alveolar macrophage interaction with Pneumocystis carinii. Immunol Ser 1994;60:417–436 [PubMed] [Google Scholar]
- 26.Buhl R, Jaffe HA, Holroyd KJ, et al. : Activation of alveolar macrophages in asymptomatic HIV-infected individuals. J Immunol 1993;150(3):1019–1028 [PubMed] [Google Scholar]
- 27.Day RB, Wang Y, Knox KS, et al. : Alveolar macrophages from HIV-infected subjects are resistant to Mycobacterium tuberculosis in vitro. Am J Respir Cell Mol Biol 2004;30(3):403–410 [DOI] [PubMed] [Google Scholar]
- 28.Elssner A, Carter JE, Yunger TM, and Wewers MD: HIV-1 infection does not impair human alveolar macrophage phagocytic function unless combined with cigarette smoking. Chest 2004;125(3):1071–1076 [DOI] [PubMed] [Google Scholar]
- 29.Gordon SB, Jagoe RT, Jarman ER, et al. : The alveolar microenvironment of patients infected with human immunodeficiency virus does not modify alveolar macrophage interactions with Streptococcus pneumoniae. Clin Vaccine Immunol 2013;20(6):882–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koziel H, Eichbaum Q, Kruskal BA, et al. : Reduced binding and phagocytosis of Pneumocystis carinii by alveolar macrophages from persons infected with HIV-1 correlates with mannose receptor downregulation. J Clin Invest 1998;102(7):1332–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Musher DM, Watson DA, Nickeson D, et al. : The effect of HIV infection on phagocytosis and killing of Staphylococcus aureus by human pulmonary alveolar macrophages. Am J Med Sci 1990;299(3):158–163 [DOI] [PubMed] [Google Scholar]
- 32.Jambo KC, Banda DH, Kankwatira AM, et al. : Small alveolar macrophages are infected preferentially by HIV and exhibit impaired phagocytic function. Mucosal Immunol 2014;7(5):1116–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reardon CC, Kim SJ, Wagner RP, Koziel H, and Kornfeld H: Phagocytosis and growth inhibition of Cryptococcus neoformans by human alveolar macrophages: Effects of HIV-1 infection. AIDS 1996;10(6):613–618 [DOI] [PubMed] [Google Scholar]