Abstract
Residual HIV viremia, defined by low levels of plasma HIV RNA with enhanced-sensitivity assays, may persist even in the presence of successful antiretroviral therapy, but little is known about its determinants. Our objective was to evaluate the rate and determinants of residual viremia in patients who show stable undetectable plasma HIV-1 RNA with conventional assays. Forty-four multidrug-experienced patients with undetectable levels of HIV RNA for at least 2 years under raltegravir-based regimens were evaluated. An ultrasensitive (2.5 copies/ml) real-time PCR method was used to quantify plasma HIV RNA. After 12 months of salvage treatment, 48.3% of the patients had residual viremia between 2.5 and 37 copies/ml. The proportion of patients with plasma HIV RNA below 2.5 copies/ml decreased from 51.7% at 12 months to 30.8% at 24 months. The presence of residual viremia was not associated with levels of viremia before starting raltegravir. Considering CD4 counts, hepatitis B or C virus (HBV or HCV) coinfection, or other demographic characteristics, for the time interval between HIV diagnosis and initiation of antiretroviral therapy, patients with a longer interval (>1 year) were significant less likely to have RNA levels below 2.5 copies/ml at 12 months compared to patients who started therapy within 1 year of HIV diagnosis (28.6% vs. 73.3%, p=0.027). Half of the patients showing undetectable HIV viremia with conventional assays had low-level viremia with ultrasensitive assays, with no predictive role of viroimmunological status at the start of the regimen. The potential influence of the interval between HIV diagnosis and initiation of treatment should be confirmed in subjects with a known date of seroconversion.
Introduction
Ultrasensitive research-based assays may show residual low-level viremia (LLV) in patients achieving antiretroviral treatment (ART) with no detectable HIV-1 RNA in plasma with common commercial tests.1–5 This persistence could be due to ongoing replication and infection of new cells in sanctuary sites not affected by ART, and to the presence of low-level replication in functional HIV reservoirs of long-lived cells.6–9 These hypotheses have been investigated in many studies, with conflicting results.10–12
Clinical studies have recently shown that neither short-term nor long-term highly active antiretroviral treatment (HAART) eliminates low-level viremia, which may persist in up to 80% of patients with undetectable plasma HIV RNA,9 with no significant decline in longitudinal studies.2,13 Recent studies have also demonstrated that treatment intensification with raltegravir is unable to suppress this persistent residual viremia, with an increase in episomal forms of HIV DNA that indicates maintained active replication.14–19 Ongoing replication is likely to have clinical relevance, because low-level viremia was found to be associated with a significantly increased risk of virological failure, drug resistance, and chronic immune activation.20–22 Identifying predictors of residual viremia is therefore relevant; unfortunately, however, attempts to correlate residual viremia with specific antiretroviral regimens2 or integrated HIV DNA levels22 failed to find significant associations. To investigate this issue, we analyzed residual viremia in a cohort of treatment-experienced patients with long-term HIV infection, who achieved undetectable viral load with raltegravir (RAL)-based salvage therapy. The primary objective of the study was to determine, in the presence of continuous viral suppression under RAL-based regimens, the rate and potential predictors of residual viremia. A second objective was to determine the predictive value of residual viremia on the occurrence of subsequent virological rebound.
Materials and Methods
Study description
The present work is nested in an ongoing (2008–2013) multicenter observational study (ISS-NIA) evaluating new antiretroviral inhibitors (integrase and entry/fusion inhibitors) in long-term treatment-experienced HIV-1+ patients with resistance (n=42) or intolerance (n=2) to nucleoside and nonnucleoside reverse transcriptase inhibitors (NRTI, NNRTI) and to protease inhibitors (PI).23 All patients with resistance had resistance mutations to reverse transcriptase inhibitors, and 37/42 had resistance to protease inhibitors. The study was conducted in compliance with the requirements of the sites' Institutional Review Boards/Ethics Committees, and all patients gave written informed consent.
Inclusion criteria for the present analysis were prescription of RAL-based therapy in a context of salvage regimens, detectable (>50 copies/ml) HIV-1 RNA at the beginning of such regimens, and durable HIV-1 RNA suppression (<50 copies/ml for 24 months).
Sample collection
Blood samples were collected every 6 months concomitantly with routine clinical visits. Plasma samples were separated and then frozen at −80°C until analysis.
HIV RNA quantitation
HIV-1 RNA plasma determinations were performed at month 12 and between month 18 and 24 from the start of the raltegravir-based regimen. When possible, two determinations between months 18 and 24 were performed, and the mean of these two determinations was used.
The standard assay method for HIV RNA plasma viral load quantitation was a real time PCR (VERSANT HIV-1 RNA 1.0 kPCR assay, Siemens Healthcare Diagnostics, Berkeley, CA), with a detection limit of 37 copies/ml (1.57 log/ml). To quantify the residual viremia in patients under virological suppression, some modifications were introduced to convert the above assay into an “ultrasensitive” assay. Such modifications included the following: the calibration curve extended toward lower HIV-1 RNA concentrations, the initial plasma sample volume increased 3-fold, an increase in the number of amplification cycles from 40 to 50, and the adoption of an “open-mode” software for quantification. The new calibration curve was composed of two points, using new calibrators (Siemens Healthcare Diagnostics, Berkeley, CA) of 397 copies/ml and 66 copies/ml, respectively. Two replicates of each new calibrator were included in each analytical session, together with negative, low-positive, and high-positive controls. To further increase the sensitivity of the modified protocol, 3×concentrated input samples were prepared by ultracentrifugation of 2.0 ml of plasma at 23,500×g for 2 h at 4°C. The excess plasma supernatant was discarded and the pellet was resuspended in 0.65 ml for extraction and quantification. The new calibrators were treated as plasma samples. Amplification and quantification were performed with the VERSANT kPCR Molecular System, Siemens, equipped with “open-mode” software. The modified method was highly specific and sensitive, with a detection limit of 2.5 HIV-1 RNA copies/ml.
HIV RNA resistance and definition of virological failure
The genotypic susceptibility score (GSS) was calculated using the Stanford HIV Resistance Database24 as previously decribed.25 After a 24-month period of viral suppression (HIV RNA <50 copies/ml) all patients continuing therapy were monitored for a median period of 18 additional months. Virological blip was defined by an isolated occurrence of HIV-1 RNA levels >50 copies/ml and virological failure by two or more consecutive determinations of HIV-1 RNA levels >200 copies/ml.26
Statistical analysis
The Shapiro–Wilk test was performed to verify whether the variables fit a normal distribution. Continuous variables were compared using the Mann–Whitney or Wilcoxon test. Categorical variables were compared using the χ2 test or, when appropriate, the Fisher exact test. For correlation analyses, the Spearman test was used. p values<0.05 were considered statistically significant. All the analyses were performed using SPSS software, Version 20.0 (IBM, Somers, NY).
Results
Patients characteristics
Forty-four patients were evaluated; their general characteristics are reported in Table 1. Their median age was 46 years (IQR: 43–50), with a long history of HIV infection (median time from HIV diagnosis: 17.5 years). Sexual (63.6%) and intravenous (IV) drug use (36.4%) were the two reported transmission routes. Hepatitis C virus (HCV) coinfection was relatively common (38.6%). At study entry, the median levels of HIV RNA and CD4 cells were 4.07 log copies/ml and 288/mm3, respectively, and 38.6% of patients were in stage C of the CDC classification.
Table 1.
Patient Characteristics
| Characteristic | Number |
|---|---|
| Patients | 44 |
| Age (years) | 46 (43–50) |
| Male (n, %) | 20 (74.1) |
| CDC (A/B/C, %) | 40.9/20.5/38.6 |
| HBV (n, %) | 4 (9.1) |
| HCV (n, %) | 17 (38.6) |
| Route of infection (n, %) | |
| Sexual | 28 (63.3) |
| Drug use | 16 (36.4) |
| Years from HIV diagnosis | 17.5 (13.3–21.0) |
| Years of antiretroviral therapy | 14.0 (11.0–16.8) |
| Patients with resistance (n, %) | 23 (92.0) |
| Reasons to start salvage therapy (n, %) | |
| Immunovirological failure | 40 (90.9) |
| Other reasons | 4 (9.1) |
| CD4 nadir (cells/mm3) | 95 (31–238) |
| CD4 (cells/mm3) | 289 (156–428) |
| HIV-1 RNA (log copies/ml) | 4.07 (3.53–4.70) |
Values are expressed as median, interquartile range, and percentage.
HBV, HBV, hepatitis B and C virus.
Drug regimens and resistance profile
All patients were drug experienced, with a median past treatment time of 14 years. More than half of the patients (26, 59.1%) had their first antiretroviral regimen within a year of HIV diagnosis, with the remaining 18 (40.9%) without antiretroviral therapy for a median of 6 years (IQR: 3.75–8.25) after HIV diagnosis. Salvage regimens for the 44 patients studied included 29 different drug combinations, indicating a high level of treatment individualization. The global GSS score and number of full active drugs are reported in Table 2. During the 2 years on the study with undetectable RNA, only eight patients changed or interrupted one or more drugs, always maintaining RAL. Among these changes, two were due to adverse events and six to treatment simplification.
Table 2.
Profile of Drug Regimens and Resistance in Patients
| Number of patients starting ARV within 1 year of HIV diagnosis (n, %) | 26 (59.1) |
| Number of drugs in the regimen (RAL included) | 4.5 (4.0–6.0) |
| Global GSS (median, IQR) | 3.0 (2.0–3.5) |
| Number of full active drugs (median, IQR) | 2.0 (2.0–3.0) |
| Number | % | |
|---|---|---|
| 3TC, ABC, ATV, RTV, RAL | 1 | 2.3 |
| 3TC, ABC, SQV, RAL | 1 | 2.3 |
| 3TC, ATV, RTV, RAL | 2 | 4.5 |
| 3TC, DRV, RTV, MVC, RAL | 1 | 2.3 |
| 3TC, DRV, RTV, RAL | 1 | 2.3 |
| 3TC, MVC, RAL | 1 | 2.3 |
| 3TC, TDF, FTC, ATV, RTV, RAL | 1 | 2.3 |
| AZT, 3TC, ABC, ETV, DRV, RTV, T20, RAL | 1 | 2.3 |
| AZT, 3TC, ABC, ETV DRV, RTV, RAL | 1 | 2.3 |
| AZT, 3TC, DRV, RTV, RAL | 1 | 2.3 |
| AZT, 3TC, DRV, RTV, T20, RAL | 1 | 2.3 |
| AZT, DRV, RTV, RAL | 1 | 2.3 |
| TDF, DRV, RTV, RAL | 2 | 4.5 |
| TDF, FTC, ATV, RAL | 1 | 2.3 |
| TDF, FTC, DRV, RTV, ETV, RAL | 1 | 2.3 |
| TDF, FTC, DRV, RTV, RAL | 7 | 15.9 |
| TDF, FTC, ETV, DRV, RTV, RAL | 5 | 11.4 |
| TDF, FTC, T20, MVC, RAL | 1 | 2.3 |
| FTC, DRV, RTV, RAL | 1 | 2.3 |
| d4T, TDF, DRV, RTV, T20, RAL | 1 | 2.3 |
| NVP, RAL | 1 | 2.3 |
| ETV, DRV, RTV, RAL | 1 | 2.3 |
| ETV, T20, MVC, RAL | 2 | 4.5 |
| LPV, RTV, RAL | 1 | 2.3 |
| fAPV, MVC, RAL | 1 | 2.3 |
| ATV, MVC, RAL | 1 | 2.3 |
| ATV, RTV, RAL | 1 | 2.3 |
| DRV, RTV, ETV, RAL | 3 | 6.8 |
| DRV, RTV, RAL | 1 | 2.3 |
Values are expressed as proportions or as median and interquartile range (IQR).
GSS, genotypic susceptibility score; 3TC, lamivudine; ABC, abacavir; ATV, atazanavir; RTV, ritonavir; RAL, raltegravir; SQV, saquinavir; DRV, darunavir; MVC, maraviroc; TDF, tenofovir; FTC, emtracitabine; ETV, etravirine; T20, fuzeon; d4T, stavudine; NVP, nevirapine; LPV, lopinavir; fAMP, fosamprenavir.
Immunovirological outcome during 24 months of therapy
All patients achieved undetectable HIV RNA within 9 months of RAL-based therapy, and remained below 50 HIV-1 RNA copies/ml until the end of the observation period (no less than 24 months). CD4 cell counts increased significantly during the first year of RAL-based therapy (median increase: 115, IQR: 53–210 cells/mm3, p<0.0001) remaining stable at month 24 (median increase from baseline: 124, IQR: 44–283 cells/mm3, p=0.502).
Ultrasensitive analysis of HIV RNA
To develop a modified ultrasensitive kPCR Versant assay for specificity and sensitivity, in vitro spiking experiments were conducted to determine internal validation. Samples of known HIV-1 viral load copy number (1.25, 2.5, 5, 10, 20, and 40 copies/ml) were tested, and four replicates of each sample were included in each analytical session. The new calibration curve was extended toward lower HIV-1 RNA concentrations, using new calibrators (Siemens Healthcare Diagnostics, Berkeley, CA) of 397 copies/ml and 66 copies/ml, respectively. For assay validation, a total of 10 consecutive runs were performed, and slope, intercept, r2 of the linear regression equation, and cycle threshold (Ct) of the new calibration curve were considered. The slopes of the standard curves (range, −2.912 to −3.330) were nearly identical to the expected slope (–3.3) for 100% efficiency of PCR amplification. The modified method showed a good correlation between expected and observed results (Pearson test: r2=0.9968).
Ultrasensitive plasma viremia at 12 and at 18–24 months from the beginning of RAL-based therapy
At 12 months, 51.7% of patients had viremia below 2.5 copies/ml with the ultrasensitive assay, and the median HIV RNA level for the remaining patients was 5.13 copies/ml (IQR: 3.28–11.62). At the second time point (months 18–24), only 30.8% of patients maintained HIV RNA levels below 2.5 copies/ml, and median HIV RNA level was 7.26 copies/ml (IQR: 4.09–11.05) (Fig. 1).
FIG. 1.
Percentage of patients with detectable (gray) and undetectable (black) residual viremia (detection limit: 2.5 HIV RNA copies/ml) at 12 and 18–24 months following the beginning of raltegravir-based therapy.
Factors influencing residual viremia and immunovirological response
Demographic (age, sex, risk factor for HIV) and clinical virological variables (CDC clinical stage, HBV coinfection, years of antiretroviral therapy, GSS score, and numbers of drugs in regimens) did not influence levels of residual viremia. Levels of pre-RAL-based therapy viremia were not associated with the levels of residual viremia (p=0.231). HCV coinfection was strictly associated with IV drug use route of infection (p<0.001), with a mild correlation with residual viremia levels at 12 and at 18–24 months (p=0.103 and p=0.050, respectively). The presence of NRTI in regimens was associated with a better control of residual viremia (p=0.014) only at 12 months, and no other associations were observed with other HIV drug classes.
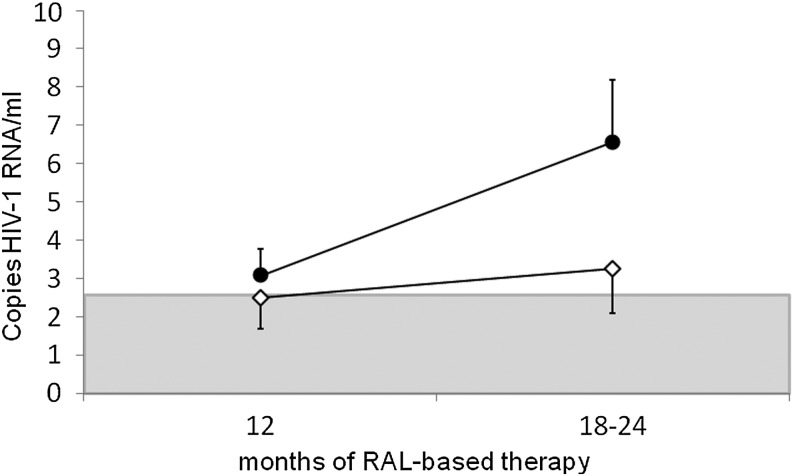
Levels of residual viremia were positively associated with the time between the time of HIV diagnosis and the start of initial antiretroviral therapy (r=0.331, p=0.073). Figure 2 shows the reported trend in residual viremia levels. Patients who started therapy within 1 year of HIV diagnosis achieved and maintained HIV RNA levels below 2.5 copies/ml easily compared to patients who had no antiretroviral therapy for more than 1 year after HIV diagnosis (76.9% vs. 31.3%, p=0.025). Importantly, despite different intervals between HIV diagnosis and start of treatment, there were no differences in terms of CD4 cell count at the start of the first treatment between patients (p=0.745).
FIG. 2.
Trend in residual viremia levels (median and standard errors) during 12–24 months of HIV RNA suppressive raltegravir-based therapy. The detection threshold (<2.5 copies/ml) is shown in gray. White rhombus: patients starting their first antiretroviral therapy within 1 year of HIV diagnosis. Black circle: patients without antiretroviral therapy more than 1 year (median time: 6 years) after HIV diagnosis.
The patients' virological and clinical status was still monitored beyond the first 24 months with undetectable (<50 copies/ml) HIV RNA levels under RAL-based therapy, with other 18 month data available for most of the patients (39/44). All patients continued on RAL-based regimens; one died during follow-up of hepatic failure following liver transplantation and four were lost to follow-up. Overall, 64.1% of patients always remained below 50 copies/ml, independent of levels of residual viremia. Virological rebound (two consecutive detectable viral load measurements above 200 copies) was observed in two patients.
Discussion
The aim of the present study was to explore residual viremia during successful salvage therapy in multidrug-experienced patients receiving RAL-based salvage treatments. In this study we establish a new protocol for the quantification of residual viremia. With the introduction of some changes to the standard procedure of commercially available diagnostic assays, we developed a procedure showing high reproducibility and sensitivity that can be used as a valid tool to explore the limits of conventional assays.
Among study participants on viral suppression for at least 2 years, ultrasensitive assays showed detectable levels of viral replication in 48.3% of patients after 1 year of therapy. Interestingly, although the usual clinical monitoring (performed each 3 months) confirmed the maintenance of HIV RNA levels below 50 copies during the entire study, a generalized increase in the number of patients with more than 2.5 copies/ml over time was observed. This finding may indicate a progressive loss of efficacy of salvage regimens, and is also consistent with some results from drug intensification studies, in which the introduction of potent drugs, such as RAL, lowered but did not abrogate residual viremia, which plateaued at approximately 3–10 copies/ml.2,9,14,18,19,27 Persistence and levels of residual viremia in our patients with multiple prior treatment failures appeared to be similar to those described in studies investigating strategies for viral eradication14,16,18,19,28,29 among patients with no previous virological failure and stable undetectable viremia, suggesting that factors other than past duration of antiretroviral treatment and previous treatment failures impact residual viremia.
No association was found between levels of residual viremia and age, gender, route of transmission, years under ART, and CDC status. Although our results might suggest a role of HCV coinfection in favoring the presence of residual viremia, adherence issues in this particular group of patients, composed primarily of former drug users, should also be considered.30 The potential role of HCV coinfection in reducing the response to antiretroviral treatment is consistent with findings from clinical trials in which lower efficacy or an attenuated response to HAART in subjects with HCV coinfection has been observed, possibly caused by poorer tolerance, lower adherence, or the development of adverse events leading to treatment discontinuation.31–33
We did not find associations of residual viremia with regimen complexity, GSS score, and number of fully active drugs. Other studies also found no specific influence of regimen composition on residual viremia.2,9 In our population, NRTI use showed some association with increased lowering of residual HIV-1 RNA. Given the long-term background of past treatment history, that suggests only a minor role of NRTI in terms of activity; however, this association should be considered cautiously.
Looking for possible factors explaining the persistence of residual viremia, we found a potential influence of the time interval between HIV diagnosis and initiation of antiretroviral therapy on levels of residual viremia: among patients equally suppressed for viral load, patients who had a longer interval between HIV diagnosis and start of the first ART had higher levels of residual viremia and were less likely to have HIV-1 RNA levels below 2.5 copies/ml, compared to patients who started antiretroviral treatment within 1 year of HIV diagnosis.
It could be hypothesized that patients who remained without any ART for a longer period may have a larger latent viral reservoir of infected cells established before therapy.2,9 Consistent with this view, it has been reported that the initiation of ART shortly after primary infection may facilitate decay HIV DNA in CD4 in blood.34–36 Early ART treatment has long-term benefits; sustained immunovirological control has been observed in patients receiving ART during primary HIV infection, and has been associated with a significant reduction of the HIV DNA reservoir.37–39
However, in evaluating the role of time before starting antiretroviral treatment, the main limitation of our study is that it does not include information on the time of seroconversion and we used the time of diagnosis as a marker of time of infection. This may not be considered entirely accurate, since the time of HIV diagnosis may include early diagnosis as well as later diagnosis during the course of HIV infection. The population was, however, well balanced for several factors, including age, route of infection, drug resistance patterns, and immunovirological status. Most importantly, despite different intervals between HIV diagnosis and the start of treatment, there were no differences in terms of CD4 cell count at the start of treatment between patients.
Although there is consensus regarding the initiation of ART in asymptomatic patients based on CD4 cell count, the optimal level at which ART is best initiated remains unclear. According to recent published studies, approximately 77% of persons diagnosed with HIV are linked to care within 3–4 months of diagnosis,40,41 and immediate ART for all individuals found to be HIV positive (test and treat strategy) has been proposed as a potential improvement for individual health outcomes and as a strategy to eliminate HIV.42,43 In this context, our study should be considered as exploratory, with a formal confirmation possible only through studies on subjects with known time of seroconversion.
Other limitations of our study are represented by its observational nature and by the limited availability of some clinical data (such as pre-ART viral load, nadir CD4), which reduces the strength of the conclusions. This lack of information on historical patient data is, however, a consequence of the particular characteristics of the cohort, mostly represented by patients with long-term infection and with a complex clinical history.
Nonetheless, we provided information on rate of residual viremia in patients with viral suppression at conventional levels. Residual viremia is increasingly considered as a potential predictor of treatment failure, and as such our results contribute information on the expected rate of such occurrence. The current clinical significance of residual viremia, especially between 1 and 10 copies/ml, is controversial; in exploring a potential association between residual viremia and virological outcome, our results could be consistent with the hypothesis that detectable residual HIV-1 RNA might increase the risk of suboptimal virological control, as observed by others.20,44 Most of our patients maintained undetectable HIV-1 RNA through the entire study period, with an occasional occurrence of virological blips, but no correlations were recorded, probably due to the low frequency of failure. As for the immunological correlates of residual viremia, we did not find differences in CD4 cell recovery between the two groups with and without residual viremia, suggesting that residual viremia (lower than 10 copies/ml) has a limited impact on CD4 cell recovery. The persistence of residual viremia has been associated with higher levels of microbial translocation45,46 and with a reduced CD4 cell recovery,47 while other studies reported only a limited impact on systemic immune activation48,49; overall, further studies based on more sensible markers of immune activation should be performed.
In conclusion, in this exploratory study, almost half of the patients achieving undetectable viral load with current assays showed with the use of more sensitive assays residual viremia between 2.5 and 37 copies/ml, with no clear association with viroimmunological status. Even if a longer interval between HIV diagnosis and the start of treatment was associated with the presence of residual viremia, the hypothesis that prompt initiation of ART upon HIV diagnosis produces a better control of residual viremia needs confirmation in studies with a known date of seroconversion.
Acknowledgments
We thank Mrs. Roberta Amici for her valuable technical help, Dr. Marina Franco for editorial assistance, Mrs. Stefania Donnini for secretarial help, and Stefano Lucattini for computer support. We thank Patrizia Cocco and Ferdinando Costa for technical support and all the patients participating in this study. This work was supported by the National Program on Research on AIDS 2009–2010. Ethical approval was given by the Istituto Superiore di Sanità Ethics Committee (05/12/2007), no. CE/ISS07-181.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Havlir DV, Koelsch KK, Strain MC, et al. : Predictors of residual viremia in HIV-infected patients successfully treated with efavirenz and lamivudine plus either tenofovir or stavudine. J Infect Dis 2005;19:1164–1168 [DOI] [PubMed] [Google Scholar]
- 2.Palmer S, Maldarelli F, Wiegand A, et al. : Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci USA 2008;105:3879–3884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dornadula G, Zhang H, VanUitert B, et al. : Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. JAMA 1999;282:1627–1632 [DOI] [PubMed] [Google Scholar]
- 4.Pinzone MR, Di Rosa M, Cacopardo B, et al. : HIV RNA suppression and immune restoration: Can we do better? Clin Dev Immunol 2012;2012:515962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmer S: Advances in detection and monitoring of plasma viremia in HIV-infected individuals receiving antiretroviral therapy. Curr Opin HIV AIDS 2013;8:87–92 [DOI] [PubMed] [Google Scholar]
- 6.Finzi D, Blankson J, Siliciano JD, et al. : Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med 1999;5:512–517 [DOI] [PubMed] [Google Scholar]
- 7.Siliciano JD, Kajdas J, Finzi D, et al. : Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med 2003;9:727–728 [DOI] [PubMed] [Google Scholar]
- 8.Grossman Z, Polis M, Feinberg MB, et al. : Ongoing HIV dissemination during HAART. Nat Med 1999;5:1099–1104 [DOI] [PubMed] [Google Scholar]
- 9.Maldarelli F, Palmer S, King MS, et al. : ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog 2007;3:e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Günthard HF, Frost SD, Leigh-Brown AJ, et al. : Evolution of envelope sequences of human immunodeficiency virus type 1 in cellular reservoirs in the setting of potent antiviral therapy. J Virol 1999;73:9404–9412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharkey ME, Teo I, Greenough T, et al. : Persistence of episomal HIV-1 infection intermediates in patients on highly active anti-retroviral therapy. Nat Med 2000;6:76–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petitjean G, Al Tabaa Y, Tuaillon E, et al. : Unintegrated HIV-1 provides an inducible and functional reservoir in untreated and highly active antiretroviral therapy-treated patients. Retrovirology 2007;4:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng L, Bosch RJ, Chan ES, et al. : Predictors of residual viraemia in patients on long-term suppressive antiretroviral therapy. Antivir Ther 2013;18:39–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gandhi RT, Zheng L, Bosch RJ, et al. : The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: A randomized controlled trial. PLoS Med 2010;7(8):e1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatano H, Hayes TL, Dahl V, et al. : A randomized, controlled trial of raltegravir intensification in antiretroviral-treated, HIV-infected patients with a suboptimal CD4+ T cell response. J Infect Dis 2011;203:960–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMahon D, Jones J, Wiegand A, et al. : Short-course raltegravir intensification does not reduce persistent low-level viremia in patients with HIV-1 suppression during receipt of combination antiretroviral therapy. Clin Infect Dis 2010;50:912–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buzón MJ, Massanella M, Llibre JM, et al. : HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med 2010;16:460–465 [DOI] [PubMed] [Google Scholar]
- 18.Dinoso JB, Kim SY, Wiegand AM, et al. : Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc Natl Acad Sci USA 2009;106:9403–9408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vallejo A, Gutierrez C, Hernandez-Novoa B, et al. : The effect of intensification with raltegravir on the HIV-1 reservoir of latently infected memory CD4 T cells in suppressed patients. AIDS 2012;26:1885–1894 [DOI] [PubMed] [Google Scholar]
- 20.Maggiolo F, Callegaro A, Cologni G, et al. : Ultrasensitive assessment of residual low-level HIV viremia in HAART-treated patients and risk of virological failure. J Acquir Immune Defic Syndr 2012;60:473–482 [DOI] [PubMed] [Google Scholar]
- 21.Corbeau P. and Reynes J: Immune reconstitution under antiretroviral therapy: The new challenge in HIV-1 infection. Blood 2011;117:5582–5590 [DOI] [PubMed] [Google Scholar]
- 22.Cohen C: Low-level viremia in HIV-1 infection: Consequences and implications for switching to a new regimen. HIV Clin Trials 2009;10:116–124 [DOI] [PubMed] [Google Scholar]
- 23.Bucciardini R, D'Ettorre G, Baroncelli S, et al. : Virological failure at one year in triple-class experienced patients switching to raltegravir-based regimens is not predicted by baseline factors. Int J STD AIDS 2012;23:459–463 [DOI] [PubMed] [Google Scholar]
- 24.Stanford University HIV Drug Resistance Database, Stanford University: http://hivdb.stanford.edu/ Accessed January5, 2014
- 25.Rhee SY, Fessel WJ, Liu TF, et al. : Predictive value of HIV-1 genotypic resistance test interpretation algorithms. J Infect Dis 2009;200:453–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panel on Antiretroviral Guidelines for Adults and Adolescents: Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available at http://aidsinfo.nih.gov/ContentFiles/Adultand AdolescentGL.pdf Accessed January10, 2014
- 27.Di Mascio M, Dornadula G, Zhang H, et al. : In a subset of subjects on highly active antiretroviral therapy, human immunodeficiency virus type 1 RNA in plasma decays from 50 to <5 copies per milliliter, with a half-life of 6 months. J Virol 2003;77:2271–2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramratnam B, Ribeiro R, He T, et al. : Intensification of antiretroviral therapy accelerates the decay of the HIV-1 latent reservoir and decreases, but does not eliminate, ongoing virus replication. J Acquir Immune Defic Syndr 2004;35:33–37 [DOI] [PubMed] [Google Scholar]
- 29.Llibre JM, Buzón MJ, Massanella M, et al. : Treatment intensification with raltegravir in subjects with sustained HIV-1 viraemia suppression: A randomized 48-week study. Antivir Ther 2012;17:355–364 [DOI] [PubMed] [Google Scholar]
- 30.Li JZ, Gallien S, Ribaudo H, et al. : Incomplete adherence to antiretroviral therapy is associated with higher levels of residual HIV-1 viremia. AIDS 2014;28:181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pulido F, Hill A, van Delft Y, et al. : Impact of hepatitis C co-infection on response to antiretroviral treatment. AIDS Rev 2012;14:124–131 [PubMed] [Google Scholar]
- 32.Hua L, Andersen JW, Daar ES, et al. : Hepatitis C virus/HIV coinfection and responses to initial antiretroviral treatment. AIDS 2013;27:2725–2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Motta D, Brianese N, Focà E, et al. : Virological effectiveness and CD4+ T-cell increase over early and late courses in HIV infected patients on antiretroviral therapy: Focus on HCV and anchor class received. AIDS Res Ther 2012;1:9–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chun TW, Justement JS, Moir S, et al. : Decay of the HIV reservoir in patients receiving antiretroviral therapy for extended periods: Implications for eradication of virus. J Infect Dis 2007;195:1762–1764 [DOI] [PubMed] [Google Scholar]
- 35.Strain MC, Little SJ, Daar ES, et al. : Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. J Infect Dis 2005;191:1410–1418 [DOI] [PubMed] [Google Scholar]
- 36.Chun TW, Murray D, Justement JS, et al. : Relationship between residual plasma viremia and the size of HIV proviral DNA reservoirs in infected individuals receiving effective antiretroviral therapy. J Infect Dis 2011;204:135–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hocqueloux L, Prazuck T, Avettand-Fenoel V, et al. : Long-term immunovirologic control following antiretroviral therapy interruption in patients treated at the time of primary HIV-1 infection. AIDS 2010;24:1598–1601 [DOI] [PubMed] [Google Scholar]
- 38.Hocqueloux L, Avettand-Fènoël V, Jacquot S, et al. : Long-term antiretroviral therapy initiated during primary HIV-1 infection is key to achieving both low HIV reservoirs and normal T cell counts. J Antimicrob Chemother 2013;68:1169–1178 [DOI] [PubMed] [Google Scholar]
- 39.Sáez-Cirión A, Bacchus C, Hocqueloux L, et al. : Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy. ANRS VISCONTI Study. PLoS Pathog 2013;9:e1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marks G, Gardner LI, Craw J, et al. : Entry and retention in medical care among HIV-diagnosed persons: A meta-analysis. AIDS 2010;24:2665–2678 [DOI] [PubMed] [Google Scholar]
- 41.Jennessa SM, Myersbc JE, Neaigusd A, et al. : Delayed entry into HIV medical care after HIV diagnosis: Risk factors and research methods. AIDS Care 2012; 24(10):1240–1248 [DOI] [PubMed] [Google Scholar]
- 42.Siegfried N, Uthman OA, and Rutherford GW: Optimal time for initiation of antiretroviral therapy in asymptomatic, HIV-infected, treatment-naive adults. Cochrane Database Syst Rev 2010;3:CD008272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kretzschmar ME, van der Loeff MF, Birrell PJ, et al. : Prospects of elimination of HIV with test-and-treat strategy. Proc Natl Acad Sci USA 2013;110:15538–15543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Widdrington J, Payne B, Medhi M, et al. : The significance of very low-level viraemia detected by sensitive viral load assays in HIV infected patients on HAART. J Infect 2011;62:87–92 [DOI] [PubMed] [Google Scholar]
- 45.Baroncelli S. Galluzzo C, Pirillo MF, et al. : Microbial translocation is associated with residual viral replication in HAART treated HIV+ subjects with<50 copies/ml HIV-1 RNA. J Clin Virol 2009;46:367–370 [DOI] [PubMed] [Google Scholar]
- 46.d'Ettorre G, Paiardini M, Zaffiri L, et al. : HIV persistence in the gut mucosa of HIV-infected subjects undergoing antiretroviral therapy correlates with immune activation and increased levels of LPS. Curr HIV Res 2011;9:148–153 [DOI] [PubMed] [Google Scholar]
- 47.Gianotti N, Galli L, Racca S, et al. : Residual viraemia does not influence 1 year virological rebound in HIV-infected patients with HIV RNA persistently below 50 copies/mL. J Antimicrob Chemother 2012;67:213–217 [DOI] [PubMed] [Google Scholar]
- 48.Eastburn A, Scherzer R, Zolopa AR, et al. : Association of low level viremia with inflammation and mortality in HIV-infected adults. PLoS One 2011;6:e26320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Charpentier C, Landman R, Laouénan C, et al. : Persistent low-level HIV-1 RNA between 20 and 50 copies/mL in antiretroviral-treated patients: Associated factors and virological outcome. J Antimicrob Chemother 2012;67:2231–2235 [DOI] [PubMed] [Google Scholar]