Abstract
The human brain displays stereotyped and early emerging patterns of cortical asymmetry in health. It is unclear if these asymmetries are highly sensitive to genetic and environmental variation or fundamental features of the brain that can survive severe developmental perturbations. To address this question, we mapped cortical thickness (CT) asymmetry in a group of genetically defined disorders known to impact CT development. Participants included 137 youth with one of five sex-chromosome aneuploidies [SCAs; XXX (n = 28), XXY (n = 58), XYY (n = 26), XXYY (n = 20), and XXXXY (n = 5)], and 169 age-matched typically developing controls (80 female). In controls, we replicated previously reported rightward inferior frontal and leftward lateral parietal CT asymmetry. These opposing frontoparietal CT asymmetries were broadly preserved in all five SCA groups. However, we also detected foci of shifting CT asymmetry with aneuploidy, which fell almost exclusively within regions of significant CT asymmetry in controls. Specifically, X-chromosome aneuploidy accentuated normative rightward inferior frontal asymmetries, while Y-chromosome aneuploidy reversed normative rightward medial prefrontal and lateral temporal asymmetries. These findings indicate that (1) the stereotyped normative pattern of opposing frontoparietal CT asymmetry arises from developmental mechanisms that can withstand gross chromosomal aneuploidy and (2) X and Y chromosomes can exert focal, nonoverlapping and directionally opposed influences on CT asymmetry within cortical regions of significant asymmetry in health. Our study attests to the resilience of developmental mechanisms that support the global patterning of CT asymmetry in humans, and motivates future research into the molecular bases and functional consequences of sex chromosome dosage effects on CT asymmetry.
Keywords: asymmetry, brain, neurodevelopment, sex chromosome
Introduction
Structural asymmetries of the human brain have been described since seminal postmortem dissections in the late 19th century (Broca, 1861; Wernicke, 1874). More recently, the advent of neuroimaging has allowed systematic and spatially fine-grained analyses of human brain asymmetry in vivo. Replicated structural asymmetries in the human brain include: (1) Yakovlevian torque, in which the right frontal and left occipital petalia are more prominent than their contralateral homologs (Kertesz et al., 1990); (2) a leftward volumetric bias in language-related perisylvian cortices (Good et al., 2001; Watkins et al., 2001); and (3) cortical thickness (CT) asymmetry, which is right biased in inferior frontal regions and left biased in parietal regions by early adolescence (Shaw et al., 2009; Zhou et al., 2013).
The biological determinants of human cortical asymmetry remain poorly understood, but several lines of evidence indicate that structural brain asymmetries may be rooted in genetic and/or environmental influences on early brain patterning. For example, the robust structural asymmetry of language-related perisylvian cortices in postnatal human life (Rentería, 2012) is preceded by structural asymmetries that are already apparent in mid-fetal life (Kasprian et al., 2011; Habas et al., 2012), and associated with even earlier asymmetries of perisylvian gene expression at 12 weeks post conception (Sun et al., 2005; Sun and Walsh, 2006). However, few studies have directly assessed the extent to which lateralized cortical development in humans is altered by defined environmental and genetic perturbations. Some aspects of cortical asymmetry appear to be sensitive to environmental influences during postnatal life (Klöppel et al., 2010), but the robustness of human cortical asymmetry to defined genetic perturbations remains largely uncharted. Reports of atypical cortical asymmetry in heritable disorders like schizophrenia (Oertel et al., 2010), autism (Herbert et al., 2002), attention deficit hyperactivity disorder (ADHD; Shaw et al., 2009), and dyslexia (Hier et al., 1978) suggest that the patterning of normative asymmetry may be sensitive to genetically determined disruptions of early brain development. However, these disorders are behaviorally defined (American Psychiatric Association, 2013) and therefore limited in their utility as models for investigating defined biological influences on structural brain asymmetry: specifically, such diagnoses reflect a highly heterogeneous set of underlying genetic and environmental risks that remain unknown in the vast majority of affected individuals.
Here, we test the robustness of human brain asymmetry to defined genetic perturbations by using in vivo neuroimaging to create spatially fine-grained maps of CT asymmetry across five distinct sex-chromosome aneuploidy syndromes [SCAs; XXX, XXY, XYY, XXYY, and XXXXY], and age-matched typically developing controls. Mapping CT across a wide range of SCAs represents a powerful approach to examining genetic influences on human brain asymmetry because (1) normative patterns of CT asymmetry are well described (Shaw et al., 2009; Zhou et al., 2013), (2) SCAs are genetically defined disorders that are known to cause widespread changes in CT (Raznahan et al., 2014), and (3) studying multiple SCAs allows the same gene-dosage change to be studied across different biological context (e.g., a supernumerary X chromosome in both XXX and XXY), which supports stronger inference-making about genetic effects on brain asymmetry.
Materials and Methods
Participants included 137 youth with non-mosaic SCA (28 XXX, 58 XXY, 26 XYY, 20 XXYY, and 5 XXXXY), and 169 age-matched karyotypically normal controls (80 XX and 89 XY). Participant characteristics are detailed in Table 1. Participant recruitment was as previously described (Lee et al., 2012). To be included in the study, SCA participants must have had a non-mosaic X/Y aneuploidy confirmed by karyotype and no acquired head injury or condition that would result in gross brain abnormalities. Typically developing participants were all singletons recruited from the United States and were enrolled in an ongoing longitudinal study of typical brain development (Raznahan et al., 2011). Inclusionary criteria for healthy participants included never having required special education services, taken psychiatric medications, received mental health treatment, or having had any medical condition impacting the nervous system.
Table 1.
Participant characteristics
| Characteristic | Group |
||||||
|---|---|---|---|---|---|---|---|
| XX | XY | XXX | XXY | XYY | XXYY | XXXXY | |
| Sample size age (years) | 80 | 89 | 28 | 58 | 26 | 20 | 5 |
| Mean | 12.8 | 12.8 | 12.3 | 12.8 | 12.4 | 14.1 | 12.9 |
| SD | 5.07 | 4.61 | 5.68 | 4.93 | 4.91 | 5.45 | 4.82 |
| Range | 5–25 | 5–25 | 5–24 | 5–25 | 5–23 | 5–22 | 7–17 |
| IQ* | |||||||
| Full-scale mean (SD) | 115 (14.1) | 116 (14.6) | 93 (13.5) | 97 (17.8) | 91 (14.6) | 87 (12.3) | 56 (7.2) |
| Verbal mean (SD) | 115 (14.8) | 113 (15.8) | 93 (14.1) | 95 (17.5) | 89 (14.1) | 81 (11.9) | 61 (9.8) |
| Performance mean (SD) | 111 (12.3) | 114 (14.3) | 94 (13.7) | 99 (17.8) | 95 (17.4) | 95 (11.5) | 56 (2.6) |
| Handedness | |||||||
| Right | 69 | 80 | 22 | 46 | 21 | 18 | 3 |
| Mixed | 5 | 6 | 4 | 7 | 1 | 1 | 1 |
| Left | 6 | 3 | 2 | 5 | 4 | 1 | 1 |
| SES* | |||||||
| Mean (SD) | 47 | 48 | 41 | 55 | 59 | 46 | 69 |
SES, socioeconomic status.
* p < 0.01 for omnibus test of significant variation across groups.
T1-weighted structural MRI images with contiguous 1.5 mm axial slices and 2.0 mm coronal slices were obtained on a 1.5 T General Electric Signa scanner, using a 3D spoiled gradient-recalled echo sequence. Native structural MRI scans were then submitted to the CIVET pipeline for automated morphometric analysis (Ad-Dab'bagh et al., 2006) to derive measures of cortical gray matter thickness at 40,962 vertices across the cortical sheet of each cerebral hemisphere. For every scan, CT asymmetry indices were calculated at each left–right pair of homologous vertices using a standard formula: (Left − Right)/[0.5*(Left + Right)]. This resulted in 40,962 estimates of CT asymmetry across the cortical sheet for each individual. All scans included in analyses passed rigorous quality assessment and control of CIVET output for evidence of motion artifact, errors in skull removal, and definition of cortical surfaces. This process involved visual inspection of preprocessed and postprocessed data, randomized by karyotype by two trained raters who were blind to karyotype.
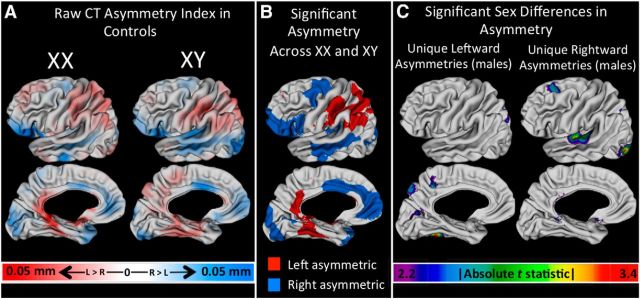
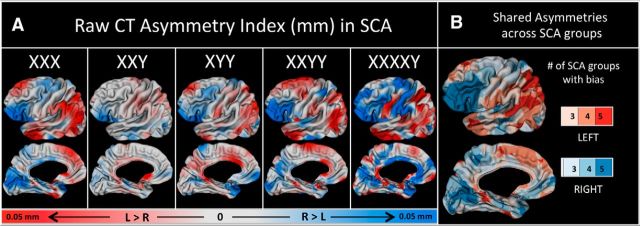
To provide a context for SCA findings, we first characterized CT asymmetry in controls by (1) mapping mean CT asymmetry across the cortical sheet in each sex, (2) identifying cortical regions of significant leftward and rightward CT asymmetry that are shared across typically developing males and females, and (3) identifying cortical regions of sexually dimorphic CT asymmetry (Fig. 1). These analyses were conducted after first testing for, and failing to find, evidence of interactive effects of age and gonadal sex (i.e., statistical significance of an age*Sex term) on CT asymmetry. Having established this normative context, we derived vertex-wise maps of mean CT asymmetry in each SCA group (Fig. 2A). Qualitative similarities in CT asymmetries across all SCA groups were visualized by creating a conjunction map that classified vertices according to the number of karyotype groups that showed a leftward or rightward CT bias (Fig. 2B).
Figure 1.
CT asymmetry in controls. A, Maps of mean CT asymmetry in female and male controls. B, Vertices of statistically significant leftward (red) and rightward (blue) CT asymmetries that are indistinguishable between males and females. C, Regions of statistically significant sexual dimorphism in CT asymmetry.
Figure 2.
CT asymmetries in SCA groups. A, Maps of mean CT asymmetry in each SCA group. B, A conjunction map showing regions where three or more SCA groups share the same lateralized bias in CT. Color intensity codes greater consistency across groups.
Next, we localized statistically significant changes in CT asymmetry with SCA, using the following linear model at each vertex across all 306 participants in our study:
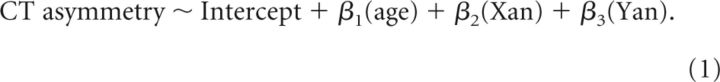 |
In this model, the intercept term estimates CT asymmetry in the absence of X and Y aneuploidy (i.e., across all controls) at mean age, and Xan and Yan terms estimate changes in CT with X- and Y-chromosome aneuploidy, respectively (Fig. 3A). Age was modeled as a main effect after testing for and ruling out interactive effects between age and sex-chromosome dosage (i.e., significance of age*Xan and age*Yan terms) on CT asymmetry. We also ran separate analyses within subsets of the karyotypes represented in our sample to test for potential interactions between sex-chromosome dosage and gonadal sex (using XX, XY, XXY, XYY, and XXYY karyotypes), as well as interactions between X- and Y-chromosome aneuploidy (using XY, XXY, XYY, XXYY, and XXXXY karyotypes).
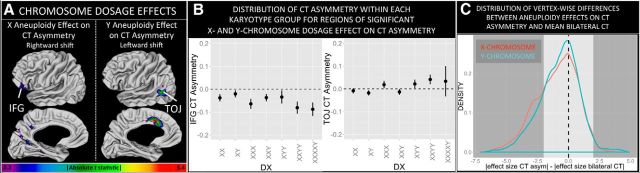
Figure 3.
X- and Y-chromosome dosage effects on CT asymmetry. A, Vertices showing a statistically significant relationship between CT asymmetry and X- and Y-chromosome aneuploidy. B, Plots of mean CT asymmetry (±95% confidence intervals) per karyotype group for representative foci of significant X- and Y-chromosome aneuploidy effect on CT asymmetry. C, Density plots demonstrating that X- and Y-chromosome aneuploidy effects on CT asymmetry across the cortical sheet tend to be less than aneuploidy effects on mean bilateral CT.
To quantify the relative stability of CT asymmetry to SCA, we (1) used the predictor variables in Equation1 to estimate scaled βcoefficients (equivalent to effect size estimates) for the influence of X- and Y-chromosome aneuploidy on both CT asymmetry and mean bilateral CT at every vertex, and then (2) calculated the following difference metric at every vertex: absolute aneuploidy effect size on CT asymmetry − absolute aneuploidy effect size on mean bilateral CT. The distribution of these 40,0962 difference scores for X- and Y-chromosome effects (Fig. 3C) quantifies the relative stability of CT asymmetry versus mean bilateral CT to SCA, with negative values indicating greater relative stability of CT asymmetry.
Vertex-wise asymmetry indices, and t values for βcoefficients of interest, were visualized by projection onto a left-hemisphere template. All t statistic maps were displayed after thresholding for multiple comparisons using False Discovery Rate correction with q (the expected proportion of false positives) set at 0.05 (Genovese et al., 2002).
Results
Our analyses of CT asymmetry within controls replicated previously reported patterns of CT asymmetry in typical development by revealing a significant rightward CT asymmetry in the inferior frontal region and leftward CT asymmetry in the lateral parietal cortex across both sexes (Luders et al., 2006; Shaw et al., 2009; Zhou et al., 2013; Fig. 1A,B). We also identified foci of sexually dimorphic CT asymmetry, where significant CT asymmetry was present in males, but not females (Fig. 1C). These male-specific asymmetries were leftward in the precuneus, calcarine sulcus, and fusiform gyrus, versus rightward in the superior frontal gyrus, Heschl's sulcus/planum temporale, and lateral occipital regions.
The normative pattern of opposing inferior frontal (rightward) and lateral parietal (leftward) CT asymmetry that we detected in controls was also evident in maps of mean CT asymmetry within each of the five SCA groups studied (Fig. 2A). A conjunction map of CT asymmetry across all five SCA groups confirms the consistency of this inferior frontal versus lateral parietal distinction (Fig. 2B).
Analysis of sex-chromosome dosage effects on CT asymmetry at each vertex detected four cortical regions where CT asymmetry was significantly altered by sex-chromosome aneuploidy (Fig. 3). Three of these four regions lay within areas of significant CT asymmetry in controls. Specifically, X-chromosome aneuploidy significantly accentuated the normative rightward inferior frontal gyrus (IFG) CT asymmetry and produced a rightward medial occipitoparietal CT asymmetry. In contrast, Y-chromosome aneuploidy was associated with a leftward CT bias that fully inverted the typical pattern of rightward anterior cingulate and lateral temporo-occipital CT asymmetry. Notably, none of these foci of X- and Y-chromosome dosage effects on CT asymmetry overlapped with regions of sexually dimorphic CT asymmetry in health (Fig. 1C).
We next compared sex chromosome dosage effects on CT asymmetry versus mean bilateral CT across all vertices in the cortical sheet. For both X- and Y-chromosomes, where aneuploidy exerted a disparate effect on CT asymmetry versus mean bilateral CT, CT asymmetry was usually the lesser impacted of the two neuroanatomical phenotypes.
Selected supplemental analyses were performed to test for potential modifiers of the above results including interactions between gonadal sex and X-chromosome aneuploidy, interactions between X- and Y-chromosome aneuploidy, handedness, and general cognitive ability. Interaction analyses indicated that the X-chromosome effects on IFG and medial occipitoparietal CT asymmetry were not altered by gonadal sex. However, outside these regions we identified male-specific effects of X-chromosome aneuploidy on CT asymmetry within the posterior cingulate (leftward bias) and female-specific effects within medial prefrontal and anterior temporal cortex (leftward bias). We found no significant interactive effects of X- and Y-chromosome aneuploidy on CT asymmetry. Our findings were not modified by exclusion of the few nondextral participants in our study. We also performed supplemental analyses to test if X- and Y-dosage effects on CT asymmetry could be detected above and beyond potential relationships between IQ variation across aneuploidy groups and CT asymmetry. Re-running Equation 1 with IQ included as a covariate did not alter the findings reported in Figure 3 or detect statistically significant relationships between IQ and CT asymmetry controlling for X- and Y-chromosome dosage.
Discussion
Our study provides several insights into the basic and clinical science of structural asymmetry within the human brain. First, we replicate previous reports of rightward inferior frontal and leftward lateral parietal CT asymmetry in typically developing controls (Luders et al., 2006; Shaw et al., 2009; Koelkebeck et al., 2014), which are shared by both males and females (Luders et al., 2006; Hamilton et al., 2007). However, we also identify discrete regions of sexually dimorphic cortical asymmetry where CT is significantly asymmetric in males, but not females. This finding adds to prior reports of greater structural asymmetry in males versus females at the group level, although this literature remains mixed (Rentería, 2012). Important next steps would be confirming that the sex differences we report in CT asymmetry can be replicated in large independent cohorts (e.g., Satterthwaite et al., 2014), and examining how these patterns might relate to recently reported sex differences in cognitive profile (Gur et al., 2012) and interhemispheric connectivity (Ingalhalikar et al., 2014).
Second, we find that the normative pattern of rightward inferior frontal and leftward lateral parietal CT asymmetry is robust to the gross changes in chromosome and gene dosage that accompany SCA. The qualitative replicability of this pattern across five different SCAs is striking, and we were also able to quantitatively demonstrate the robustness of CT asymmetry to SCA relative to mean bilateral CT. These findings suggest that the mechanisms regulating CT differences between the left and right hemisphere are dissociable from, and more resilient to, SCA (and perhaps other genetic perturbations) than the mechanisms that shape nonlateralized variation in CT. This relative resilience of CT asymmetry supports the notion that structural asymmetry of the cortical sheet represents a prenatally embedded effect of genetic patterning on cortical organization (Sun and Walsh, 2006). While some studies of asymmetric gene expression within early human cortical development have been inconsistent (Pletikos et al., 2014), others have found differentially expressed genes between the two hemisphere in utero within cortical regions destined for later structural asymmetry. A study by Sun and et al., 2005 verified 27 differentially expressed genes between the left and right planum temporale, of which LMO4, critical for cortical development in mice, was consistently more expressed in the right perisylvian region. It is important to note that hypothesizing a strong role for genetic factors in the early patterning of structural brain asymmetries does not necessarily require that structural brain asymmetry traits show high heritability in twin studies: lateralized traits that show little variation between individuals (e.g., a left-sided heart), or arise through the influence of genes with little allelic variation at the population level, will not show high heritability in twin studies (Eyler et al., 2014).
Third, our results indicate that the few cortical foci where CT asymmetry is impacted by SCA lie almost exclusively within regions that are significantly asymmetric in healthy controls. This convergence suggests that while the mechanisms supporting emergence of normative cortical asymmetry are relatively robust in global terms, they may confer local vulnerability to asymmetric effects of disease on the developing brain. In support of this hypothesis, reports of aberrant CT asymmetry in clinical groups, such as 22q11 Deletion Syndrome (Bearden et al., 2007) and ADHD (Shaw et al., 2009), both highlight the IFG as an area where significant normative asymmetries are focally disrupted. However, further studies across a wider range of neurodevelopmental disorders will be needed to more robustly test this hypothesis.
Fourth, we find no overlap between X- and Y-chromosome aneuploidy effects on CT asymmetry. Also, while greater X-chromosome dosage accentuates normative asymmetries, greater Y-chromosome dosage reverses normative asymmetries. These disjunctions between the effects of X- and Y-chromosome dosage are in keeping with the fact that X and Y chromosomes differ drastically in size and gene content (Skaletsky et al., 2003), and suggests that sex-chromosome effects on CT asymmetry may reflect the action of X and Y chromosome-specific genes. This inference implicates X- and Y-chromosome genes outside the small distal pseudo-autosomal regions of sequence homology and obligate recombination between sex chromosomes (Otto et al., 2011). Furthermore, our observed X-chromosome effects are most likely to be accounted for by ∼15% of X-chromosome genes that “escape” the process of X inactivation (Nguyen and Disteche, 2006). X inactivation operates in karyotypically normal females to silence 85% of the genes borne by one of the two X chromosomes in each female cell, and is thought to help equate effective X-chromosome gene dosage between males and females. The X-chromosome genes that escape this inactivation have been hypothesized to underlie several phenotypic consequences of X aneuploidy syndromes (Vawter et al., 2007). A valuable next step would be to test for asymmetric expression of these candidate X-linked genes in the IFG cortex using publically available atlases of gene expression during human brain development (Miller et al., 2014).
Finally, the focal sex-chromosome dosage effects we observe on CT asymmetry may inform mechanistic models for normative sex differences in CT asymmetry. We found no spatial overlap between the effects of sex and sex-chromosome dosage on CT asymmetry. This indicates that differences in X- and Y-chromosome dosage between typically developing males and females either (1) do not contribute to normative sex differences in CT asymmetry or (2) are compensated for by other biological sex differences, such as gonadal hormones (De Vries, 2005). There is some evidence for lateralized sex-steroid effects on cortical organization in animal models (Stewart and Kolb, 1988), and such effects have been hypothesized to operate in humans as well (Patwardhan et al., 2000).
Our study does not address the functional correlates of X- and Y-dosage effects on CT asymmetry, and this will be an important area for future work. However, several unresolved issues complicate analysis of the relationship between structural asymmetry and behavior in clinical groups. First, very little is known regarding normative inter-relationships between structural asymmetry, functional lateralization, and behavior in typical development (Greve et al., 2013) and there is a pressing need to systematically characterize such structure—function relationships across a wider range of cognitive domains, anatomical phenotypes, and developmental stages in health. Second, some clinical groups show changes in structural asymmetry within cortical regions, such as the IFG, which have distinct functional specializations in left (semantic language processing; Dapretto and Bookheimer, 1999) versus right (response inhibition; Hampshire et al., 2010) hemispheres, making it harder to assess the functional valence of a given shift in structural asymmetry. Finally, establishing a correlation between altered asymmetry and altered behavior within a clinical cohort still leaves open several causal scenarios that are hard to disambiguate in humans (Bishop, 2013).
Our findings should be considered in the context of two additional limitations. First, we modeled brain asymmetry using cross-sectional data within a wide age-range developmental window when CT is known to undergo significant developmental changes (Shaw et al., 2008; Raznahan et al., 2011). However, our focus on relatively rare patient groups necessarily makes it difficult to gather longitudinal data of large cross-sectional samples within a narrow age range. Also, our groups were age matched and we controlled for the linear age effects on CT, which predominate in this age range (Shaw et al., 2008). Finally, we tested for, but found no evidence of age-by-group interactions for asymmetry. Nevertheless, age-related variations in CT asymmetry have been reported (Shaw et al., 2009), and future replication of our study using a longitudinal approach will be of value. Second, we focus here on CT because it is the surface-based metric that has been most extensively charted by multiple independent groups with respect to both normative asymmetry (Shaw et al., 2009; Zhou et al., 2013) and alterations in SCA (Lepage et al., 2013; Raznahan et al., 2014). Further research will be required to determine whether asymmetries of other cortical properties such as surface area (Koelkebeck et al., 2014) show the global resilience and focal sensitivities to SCA that we describe for CT.
Notwithstanding these caveats, this current study is the first to chart patterns of cortical asymmetry across multiple genetically defined disorders known to impact cortical development. Our approach provides some of the strongest evidence to date that global patterns of CT asymmetry represent a deep-seated aspect of cortical patterning in humans, which can withstand severe karyotypic abnormalities. Our study also suggests that sex chromosomes may exert regionally specific effects in brain asymmetry, which motivates future studies to investigate molecular determinants and functional consequences of these effects.
Footnotes
This work was funded by the National Institutes of Health Intramural Research Program. We thank the participants and families who took part in this study. We are also grateful to Dr Stephen J. Gotts of the NIH IRP Laboratory of Brain and Cognition for his advice on data modeling methods.
The authors declare no competing financial interests.
References
- Ad-Dab'bagh Y, Lyttelton O, Muehlboeck JS, Lepage C, Einarson D, Mok K, Ivanov O, Vincent RD, Lerch J, Fombonne E, Evans AC. The CIVET image-processing environment: a fully automated comprehensive pipeline for anatomical neuroimaging research. Proceedings of the 12th Annual Meeting of the Organization for Human Brain Mapping; Florence, Italy. 2006. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Ed 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Bearden CE, van Erp TG, Dutton RA, Tran H, Zimmermann L, Sun D, Geaga JA, Simon TJ, Glahn DC, Cannon TD, Emanuel BS, Toga AW, Thompson PM. Mapping cortical thickness in children with 22q11.2 deletions. Cereb Cortex. 2007;17:1889–1898. doi: 10.1093/cercor/bhl097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DV. Cerebral Asymmetry and language development: cause, correlate, or consequence? Science. 2013;340:1230531. doi: 10.1126/science.1230531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broca MP. Remarques sur le siège de la faculté du langage articulé, suivies d'une observation d'aphémie (perte de la parole) Bulletin de la Société Anatomique. 1861;6:330–357. [Google Scholar]
- Dapretto M, Bookheimer SY. Form and content: dissociating syntax and semantics in sentence comprehension. Neuron. 1999;24:427–432. doi: 10.1016/S0896-6273(00)80855-7. [DOI] [PubMed] [Google Scholar]
- De Vries GJ. Sex steroids and sex chromosomes at odds? Endocrinology. 2005;146:3277–3279. doi: 10.1210/en.2005-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyler LT, Vuoksimaa E, Panizzon MS, Fennema-Notestine C, Neale MC, Chen CH, Jak A, Franz CE, Lyons MJ, Thompson WK, Spoon KM, Fischl B, Dale AM, Kremen WS. Conceptual and data-based investigation of genetic influences and brain asymmetry: a twin study of multiple structural phenotypes. J Cogn Neurosci. 2014;26:1100–1117. doi: 10.1162/jocn_a_00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Greve DN, Van der Haegen L, Cai Q, Stufflebeam S, Sabuncu MR, Fischl B, Brysbaert M. A surface-based analysis of language lateralization and cortical asymmetry. J Cogn Neurosci. 2013;25:1477–1492. doi: 10.1162/jocn_a_00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, Loughead J, Connolly JJ, Qiu H, Mentch FD, Abou-Sleiman PM, Hakonarson H, Gur RE. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychology. 2012;26:251–265. doi: 10.1037/a0026712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas PA, Scott JA, Roosta A, Rajagopalan V, Kim K, Rousseau F, Barkovich AJ, Glenn OA, Studholme C. Early folding patterns and asymmetries of the normal human brain detected from in utero MRI. Cereb Cortex. 2012;22:13–25. doi: 10.1093/cercor/bhr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton LS, Narr KL, Luders E, Szeszko PR, Thompson PM, Bilder RM, Toga AW. Asymmetries of cortical thickness: effects of handedness, sex, and schizophrenia. Neuroreport. 2007;18:1427–1431. doi: 10.1097/WNR.0b013e3282e9a5a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. 2010;50:1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert MR, Harris GJ, Adrien KT, Ziegler DA, Makris N, Kennedy DN, Lange NT, Chabris CF, Bakardjiev A, Hodgson J, Takeoka M, Tager-Flusberg H, Caviness VS., Jr Abnormal asymmetry in language association cortex in autism. Ann Neurol. 2002;52:588–596. doi: 10.1002/ana.10349. [DOI] [PubMed] [Google Scholar]
- Hier DB, LeMay M, Rosenberger PB, Perlo VP. Developmental dyslexia: evidence for a subgroup with a reversal of cerebral asymmetry. Arch Neurol. 1978;35:90–92. doi: 10.1001/archneur.1978.00500260028005. [DOI] [PubMed] [Google Scholar]
- Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, Hakonarson H, Gur RE, Gur RC, Verma R. Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci U S A. 2014;111:823–828. doi: 10.1073/pnas.1316909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprian G, Langs G, Brugger PC, Bittner M, Weber M, Arantes M, Prayer D. The prenatal origin of hemispheric asymmetry: an in utero neuroimaging study. Cereb Cortex. 2011;21:1076–1083. doi: 10.1093/cercor/bhq179. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Polk M, Black SE, Howell J. Sex, handedness, and the morphometry of cerebral asymmetries on magnetic resonance imaging. Brain Res. 1990;530:40–48. doi: 10.1016/0006-8993(90)90655-U. [DOI] [PubMed] [Google Scholar]
- Klöppel S, Mangin JF, Vongerichten A, Frackowiak RS, Siebner HR. Nurture versus nature: long-term impact of forced right-handedness on structure of pericentral cortex and basal ganglia. J Neurosci. 2010;30:3271–3275. doi: 10.1523/JNEUROSCI.4394-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelkebeck K, Miyata J, Kubota M, Kohl W, Son S, Fukuyama H, Sawamoto N, Takahashi H, Murai T. The contribution of cortical thickness and surface area to gray matter asymmetries in the healthy human brain. Hum Brain Mapp. 2014;35:6011–6022. doi: 10.1002/hbm.22601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NR, Wallace GL, Adeyemi EI, Lopez KC, Blumenthal JD, Clasen LS, Giedd JN. Dosage effects of X and Y chromosomes on language and social functioning in children with supernumerary sex chromosome aneuploidies: implications for idiopathic language impairment and autism spectrum disorders. J Child Psychol Psychiatry. 2012;53:1072–1081. doi: 10.1111/j.1469-7610.2012.02573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage JF, Hong DS, Mazaika PK, Raman M, Sheau K, Marzelli MJ, Hallmayer J, Reiss AL. Genomic imprinting effects of the X chromosome on brain morphology. J Neurosci. 2013;33:8567–8574. doi: 10.1523/JNEUROSCI.5810-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, Rex DE, Jancke L, Toga AW. Hemispheric asymmetries in cortical thickness. Cereb Cortex. 2006;16:1232–1238. doi: 10.1093/cercor/bhj064. [DOI] [PubMed] [Google Scholar]
- Miller JA, Ding SL, Sunkin SM, Smith KA, Ng L, Szafer A, Ebbert A, Riley ZL, Royall JJ, Aiona K, Arnold JM, Bennet C, Bertagnolli D, Brouner K, Butler S, Caldejon S, Carey A, Cuhaciyan C, Dalley RA, Dee N, et al. Transcriptional landscape of the prenatal human brain. Nature. 2014;508:199–206. doi: 10.1038/nature13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DK, Disteche CM. Dosage compensation of the active X chromosome in mammals. Nat Genet. 2006;38:47–53. doi: 10.1038/ng1705. [DOI] [PubMed] [Google Scholar]
- Oertel V, Knochel C, Rotarska-Jagiela A, Schönmeyer R, Lindner M, van de Ven V, Haenschel C, Uhlhaas P, Maurer K, Linden DE. Reduced laterality as a trait marker of schizophrenia–evidence from structural and functional neuroimaging. J Neurosci. 2010;30:2289–2299. doi: 10.1523/JNEUROSCI.4575-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto SP, Pannell JR, Peichel CL, Ashman TL, Charlesworth D, Chippindale AK, Delph LF, Guerrero RF, Scarpino SV, McAllister BF. About PAR: the distinct evolutionary dynamics of the pseudoautosomal region. Trends Genet. 2011;27:358–367. doi: 10.1016/j.tig.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Patwardhan AJ, Eliez S, Bender B, Linden MG, Reiss AL. Brain morphology in Klinefelter syndrome: extra X chromosome and testosterone supplementation. Neurology. 2000;54:2218–2223. doi: 10.1212/WNL.54.12.2218. [DOI] [PubMed] [Google Scholar]
- Pletikos M, Sousa AM, Sedmak G, Meyer KA, Zhu Y, Cheng F, Li M, Kawasawa YI, Sestan N. Temporal specification and bilaterality of human neocortical topographic gene expression. Neuron. 2014;81:321–332. doi: 10.1016/j.neuron.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Lerch JP, Lee N, Greenstein D, Wallace GL, Stockman M, Clasen L, Shaw PW, Giedd JN. Patterns of coordinated anatomical change in human cortical development: a longitudinal neuroimaging study of maturational coupling. Neuron. 2011;72:873–884. doi: 10.1016/j.neuron.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Lee NR, Greenstein D, Wallace GL, Blumenthal JD, Clasen LS, Giedd JN. Globally divergent but locally convergent X- and Y-chromosome influences on cortical development. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu174. doi: 10.1093/cercor/bhu174. Advance online publication. Retrieved Aug. 21, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentería ME. Cerebral asymmetry: a quantitative, multifactorial, and plastic brain phenotype. Twin Res Hum Genet. 2012;15:401–413. doi: 10.1017/thg.2012.13. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Ruparel K, Loughead J, Prabhakaran K, Calkins ME, Hopson R, Jackson C, Keefe J, Riley M, Mentch FD, Sleiman P, Verma R, Davatzikos C, Hakonarson H, Gur RC, Gur RE. Neuroimaging of the Philadelphia neurodevelopmental cohort. Neuroimage. 2014;86:544–553. doi: 10.1016/j.neuroimage.2013.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Lalonde F, Lepage C, Rabin C, Eckstrand K, Sharp W, Greenstein D, Evans A, Giedd JN, Rapoport J. Development of cortical asymmetry in typically developing children and its disruption in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2009;66:888–896. doi: 10.1001/archgenpsychiatry.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaletsky H, Kuroda-Kawaguchi T, Minx PJ, Cordum HS, Hillier L, Brown LG, Repping S, Pyntikova T, Ali J, Bieri T, Chinwalla A, Delehaunty A, Delehaunty K, Du H, Fewell G, Fulton L, Fulton R, Graves T, Hou SF, Latrielle P, et al. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature. 2003;423:825–837. doi: 10.1038/nature01722. [DOI] [PubMed] [Google Scholar]
- Stewart J, Kolb B. The effects of neonatal gonadectomy and prenatal stress on cortical thickness and asymmetry in rats. Behav Neural Biol. 1988;49:344–360. doi: 10.1016/S0163-1047(88)90354-8. [DOI] [PubMed] [Google Scholar]
- Sun T, Walsh CA. Molecular approaches to brain asymmetry and handedness. Nat Rev Neurosci. 2006;7:655–662. doi: 10.1038/nrn1930. [DOI] [PubMed] [Google Scholar]
- Sun T, Patoine C, Abu-Khalil A, Visvader J, Sum E, Cherry TJ, Orkin SH, Geschwind DH, Walsh CA. Early asymmetry of gene transcription in embryonic human left and right cerebral cortex. Science. 2005;308:1794–1798. doi: 10.1126/science.1110324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vawter MP, Harvey PD, DeLisi LE. Dysregulation of X-linked gene expression in Klinefelter's syndrome and association with verbal cognition. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:728–734. doi: 10.1002/ajmg.b.30454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, Paus T, Lerch JP, Zijdenbos A, Collins DL, Neelin P, Taylor J, Worsley KJ, Evans AC. Structural asymmetries in the human brain: a voxel-based statistical analysis of 142 MRI scans. Cereb Cortex. 2001;11:868–877. doi: 10.1093/cercor/11.9.868. [DOI] [PubMed] [Google Scholar]
- Wernicke C. Der aphasische Symptomenkomplex: eine psychologische Studie auf anatomischer Basis. Breslau, Poland: Cohn and Welgert; 1874. [Google Scholar]
- Zhou D, Lebel C, Evans A, Beaulieu C. Cortical thickness asymmetry from childhood to older adulthood. Neuroimage. 2013;83:66–74. doi: 10.1016/j.neuroimage.2013.06.073. [DOI] [PubMed] [Google Scholar]