Abstract
Infiltration of tumours by regulatory T cells confers growth and metastatic advantages by inhibiting anti-tumour immunity and by production of RANK ligand, which may directly stimulate metastatic propagation of RANK-expressing cancer cells. Modulation of regulatory T cells can enhance the efficacy of cancer immunotherapy. Strategies include depletion, interference with function, inhibition of tumoural migration and exploitation of T cell plasticity. Problems with these strategies include a lack of specificity, resulting in depletion of anti-tumour effector T cells or global interruption of regulatory T cells, which may predispose to autoimmune diseases. Emerging technologies such as RNA interference and tetramer-based targeting may have the potential to improve selectivity and efficacy.
Keywords: Regulatory T cell, Cancer, Targeted Therapy, Immunotherapy, Tumour Immunology
Introduction
There is renewed optimism that many cancers can be cured or forestalled by immune-based therapies, used either alone or as part of multimodal programmes. This originates from an improved understanding of tumour immune interactions and the availability of gene, cell and ligand-based technologies which promote effector anti-tumour responses. Most tumours develop in the face of normal immune function and anti-tumour responses of varying strength result. A strong immune response against the primary tumour is associated with clearance and induced dormancy of metastatic cancer cells, with a resulting enhanced prognosis. Conversely, global immune deficiencies secondary to disease or therapy are associated with an increased frequency, earlier recurrence, more rapid progression of tumours and poorer prognosis. Responses to chemotherapy and oncolytic virotherapy may in part be immune-determined and there is persuasive evidence that an intact immune system, specifically determined by CD4+ T cells, is required for sustained tumour regression following oncogene inactivation therapies (1).
Adaptive anti-tumour immune responses are durable, tumour antigen-specific and acquired through the integrated intercellular responses of the innate and adaptive immune systems (2) (figure 1, centre). Tumour infiltrating T cells, especially CD8+ cytotoxic T lymphocytes (CTLs) and IFNγ-secreting CD4+ (Th1) cells, are central to effective immune containment. Adaptive immune responses are initiated when cells of the innate immune system (NKT, γδ T, NK and macrophages) are recruited to the tumour microenvironment - the continued process of tumour remodelling results in the shedding of cancer cells and debris with a consequent induction of inflammatory signals. The production of IFN-γ (initially from NK and NKT cells) appears critical as it creates a positive feedback loop by inducing some tumour cell death, the further activation of NK cells and macrophages and the production of chemokines and cytokines which are also tumouricidal and anti-angiogenic. Immature dendritic cells (DCs) are activated following uptake of tumour debris/antigens and migrate to the regional lymph nodes where they present the tumour antigens to naive T cells, which can differentiate into Th1, Th2, Th17 or regulatory T cells (TRegs) depending on the cytokine environment. Th1 cells can license DCs to induce tumour-specific CTLs by cross presentation of antigen on MHC class I. Antigen-specific CD8+ T cells traffic to the tumour where cell-mediated killing of tumour cells is augmented by Th1 and Th17 derived cytokines. However these effector responses can be inhibited by TRegs, induced by or recruited to the growing tumour (3).
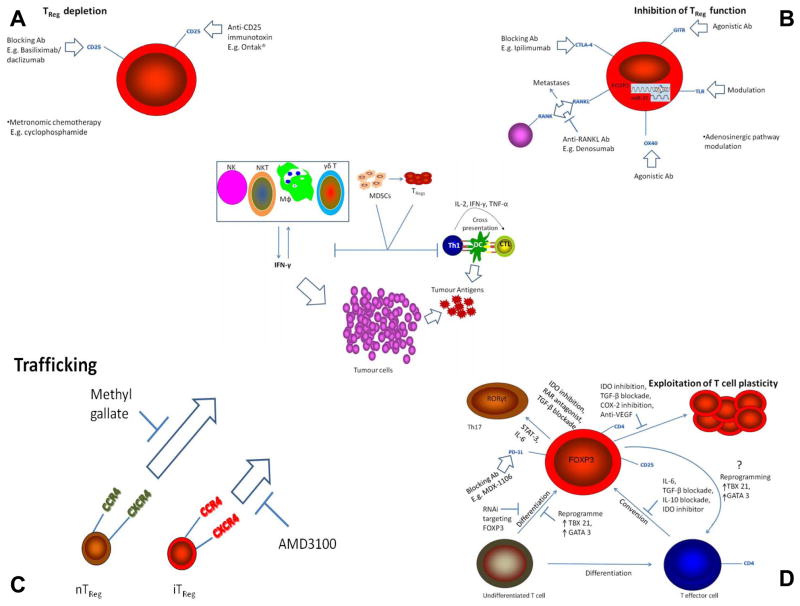
Figure 1.
Targeting regulatory T cells in cancer. The central schematic depicts the main events involved in mounting an immune response to a tumour. Cells of both the innate and adaptive systems contribute (further details are provided in the text). TRegs offer substantial resistance to this immune assault and thus four different approaches for reducing their immunosuppressive contribution are advanced (figure 1; A, B, C and D); depletion, inhibition of function, blockade of trafficking and modulation of T cell plasticity. Within each approach numerous existing and novel options for therapeutic manipulation are forwarded. Ab – antibody; DC – dendritic cell; IDO - indoleamine 2,3-dioxygenase; MDSCs – myeloid-derived suppressor cells; TReg – regulatory T cell.
Immune evasion is a hallmark of cancer that results from both passive and active tolerising conditions that subvert anti-tumour immune responses (4). Passive tolerisation may result from down-regulation of MHC Class I expression on the tumour cells and/or low antigenicity secondary to immune editing and selective cell growth. Other tolerising mechanisms involve inhibition of immune cells in the tumour domain by depletion of tryptophan by the enzyme 2, 3 indoleamine dioxygenase (IDO). Active tolerisation involves suppression of anti-tumour cell-mediated responses by tumour infiltrating TRegs and myeloid derived suppressor cells (5).
Overall TRegs are considered to be the most powerful inhibitors of anti-tumour immunity and the greatest barrier to successful immunotherapy (6). In the early stages of cancer TRegs are concentrated in the tumour mass, resulting in concomitant immunity, whereby the primary tumour can progress due to local inhibition of effector immune responses, but metastatic cells are eliminated by uninhibited systemic anti-tumour immune responses. In advanced stage disease or for poorly immunogenic cancers there are increased TRegs systemically and absence of concomitant anti-tumour immunity (7). While a correlation between increased TReg number and survival, either negative or positive, remains equivocal, the ratio of TReg to Teffector cells in the tumour mass seems to have greater prognostic significance (8).
There are a number of subtypes of TReg (8), including natural CD4+ TRegs (nTRegs) which originate in the thymus, express CD25, FOXP3, CTLA-4, LAG3 and GITR and suppress innate and adaptive immune cells. Induced CD4+ TRegs (iTRegs) control immune responses to tissue antigens, including tumour antigens and include CD4+ nTReg-like, Tr1 and Th3 cells that suppress through production of IL-10 and TGF-β. The iTRegs develop in the periphery following engagement of the TCR of naive T cells and under the influence of innate IL-10 and TGF-β. Their cell-surface markers are often indistinguishable from those of nTRegs and they differ principally in their mechanism of suppression. Although less well characterised, there are also populations of natural and induced CD8+ TRegs.
While the field is still in its infancy, evidence is emerging that inhibition of TRegs may help in tumour containment, especially when combined with appropriate immunotherapies that activate effector T cells. Systemic TReg depletion in patients induced regression of melanoma metastases (9) and in mice when combined with immunogene stimulation of intratumoural immune effector cells resulted in cure of 90% of animals who had large and weakly immunogenic sarcomas (10). The clinical objective will be to provide sustained reduction of TReg function, particularly in the tumour environment, allowing enhancement of anti-tumour effector functions and with minimal risk of developing systemic autoimmune diseases.
Current approaches to TReg modulation
Regulatory T cell depletion (figure 1 A)
Depletion strategies are not T cell subset-specific but have a selective advantage when the TReg accumulation provides functional dominance in the tumour environment. TReg depletion strategies have focused on monoclonal antibodies or ligand-directed toxins targeted to a TReg cell surface receptor such as CD25. Daclizumab and basiliximab are anti-CD25 antibodies which invoke cell death by cytokine deprivation (IL-2) and also by triggering ADCC or CDC. Results from an ongoing clinical trial have shown that Daclizumab reduces TRegs and thereby enhances cytotoxic T lymphocyte responses to tumour antigen induced by vaccination (11).
Denileukin diftitox (Ontak®) is a fusion protein of human IL-2 and the enzymatically active and membrane-translocating domains of diphtheria toxin. After binding to CD25 and internalisation, release of the toxin is cytocidal. Clinical data on the use of Ontak® for alternative indications has led to its application for CD25 targeting of TRegs and the emergence of similar CD25-targeted immunotoxins LMB-2 and RFT5-SMPT-dgA. With one exception, Ontak® depleted TReg numbers, albeit transiently, with TReg nadirs persisting for less than 3 weeks (11). The TReg elimination was mirrored by a concomitant increase in the prevalence of IFN-γ+CD3+ T-cells in the blood and de novo appearance of melanoma antigen-specific CD8+ T cells (9). However the clinical benefits were modest. Regression of melanoma metastases in five out of sixteen patients represents the most promising outcome (9). Consistency of response is an issue as two patients who developed antigen-specific T cells failed to show any tumour regression and another study in melanoma patients failed to yield a single objective clinical response (11).
Ontak® is the subject of numerous clinical trials but to date fails to realise its clinical promise. Since CD25 is also expressed on activated Teffector cells, Ontak® may also restrain protective anti-tumour immune responses. Ontak® transiently depleted various T subsets including tumour antigen-specific CD8+ T cells (9). Its indiscriminate effects on CD4+CD25− cells are difficult to rationalise.
Low-dose oral metronomic cyclophosphamide induced a profound, selective reduction in TRegs and restored T and NK cell function in advanced cancer patients (12). This invoked temporary disease stabilisation in a number of patients without clinical improvement. The mechanism underpinning its selective toxicity towards TRegs is unexplained. Metronomic cyclophosphamide also has anti-angiogenic and direct cytotoxic effects, which contribute to tumour stabilisation or shrinkage.
Depleting TRegs may have further consequences aside from an unintended treatment-mediated elimination of activated Teffector cells (13). Their depletion leads to an increase in tumour-mediated Teffector to TReg conversion with a diminution in anti-tumour immune responses. This does not seem to occur with the other TReg modulation approaches.
Suppression of TReg function (figure 1 B)
Similar to CD25, CTLA-4 is not exclusively expressed on TRegs – it is also found on activated CD4+ and CD8+ T cells (8). CTLA-4 inhibits antigen priming of Teffectors by competing with CD28 for the costimulation of CD80/CD86 on APCs. Furthermore, it induces IDO in DCs (14). The consequent depletion of tryptophan and production of tryptophan metabolites, such as kynurenines and picolinic acid, inhibit Teffector proliferation and function. The anti-CTLA-4 antibodies ipilimumab (MDX-010) and tremelimumab (CP-675206) are currently undergoing clinical evaluation. Ipilimumab, as monotherapy or in combination with peptide vaccination improved survival in patients with previously treated metastatic melanoma (15). Tremelimumab promotes anti-tumour responses but recently these have been shown to result from Teffector activation rather than TReg modulation (16). This may also be true of Ipilimumab as its clinical mode of action has yet to be fully defined and could be ascribed to direct effects on either TRegs, Teffectors or a combination.
The glucocorticoid-induced TNF receptor (GITR) is constitutively expressed on TRegs but also at lower levels on activated Teffectors. Intratumoural injection of an agonistic antibody to GITR (DTA-1) invoked potent anti-tumour immunity and eradicated established tumours in mice (17). The exact mechanism by which this approach achieves its effects is controversial. One study showed that the benefit of DTA-1 was TReg-mediated, facilitated by their selective modulation (18). However a more recent study suggested Teffector costimulation as the predominant outcome (19). Regardless of mechanism of action, GITR approaches have yet to recapitulate these promising findings in humans. Receptor activator of nuclear factor-kB (RANK) ligand (RANKL) expression on TRegs engages the RANK receptor on cancer cells and promotes metastases (20). Inhibitors of RANK signalling, such as the anti-RANKL antibody denosumab, already used against osteoclastic-mediated bone resorption, may block direct TReg-induced metastases of certain cancers.
Targeting FOXP3, the essential transcription factor of TRegs, by RNA interference (RNAi) could also modulate their function. Lentiviral-mediated delivery of miR-31 (a negative regulator of FOXP3) to TRegs abolished their suppressor capability (21). Translation to clinical application is challenging, as miR-31 would need to be delivered specifically to TRegs because FOXP3 is also transiently expressed on activated human Teffectors. FOXP3 is also expressed (both mRNA and protein) in numerous cancer cell lines (22) but the effects of its down-regulation are unknown and could even be counterproductive.
Further options for disrupting TReg function include Toll-like receptor (TLR) modulation, OX40 stimulation or interference with the adenosinergic pathway. Exposure of TRegs to the TLR8 ligand, poli-G10 abolished their suppressive influence on CD8+ T cells, leading to improved anti-tumour immunity (23). More recently a synthetic TLR1/TLR2 agonist, an analogue of bacterial lipoprotein, mediated a dose-dependent tumour regression and a long-lasting protective response against tumour rechallenge through a reciprocal downregulation of TRegs and upregulation of CTL function (24). These findings suggest that TLR signaling is a worthwhile pursuit but caution is advised as TLR agonists can promote regulatory as well as effector responses (25). Stimulation of OX40 (a co-stimulatory member of the TNF receptor family) inhibits the suppressive function of TRegs in vitro (by downregulation of FOXP3) and abolishes protection against graft-versus-host disease in mice (13). The paradoxical stimulatory effects on Teffectors make it an enticing target for cancer immunotherapy. Another potential target on TRegs is ectonucleotidase activity which facilitates local generation of adenosine which has immunosuppressive capability. Ectoenzyme inhibitors such as ARL67156 and other modulators of the adenosinergic pathway, such as inhibitors of the A2A adenosine receptor, have been shown to block TReg-induced immunosuppression (26).
Disrupting Tumoural Homing of Regulatory T Cells (figure 1 C)
Chemokine-chemokine receptor and integrin-integrin ligand interactions attract TRegs to the tumour, a phenomenon first observed for the CCL22-CCR4 interaction in ovarian cancer (27). Importantly CCL22 expression was not confined to tumour cells but also included bystander cells such as tumour-associated macrophages. Further chemokines/integrins have been implicated in the selective recruitment and retention of TRegs at tumour sites including CXCR4, CD103 and CCR2 (8). Because chemoattraction is ubiquitous in the immune system efforts to block TReg recruitment to the tumour mass may be limited by the concurrent effects on Teffectors. Nevertheless, disruption of CCR5/CCL5 signalling blocks TReg migration to tumours and inhibits pancreatic tumour growth in mice (28). Methyl gallate has also recently been shown to inhibit infiltration of TRegs into tumours resulting in reduced tumour growth and prolonged survival rates (29).
Immuno-stimulatory therapies may inadvertently promote tumoural homing of TRegs. Therapy with IL-2 can enhance CCR4 expression on TRegs, which stimulates their migration to the tumour mass and an upregulation of CXCR4, the receptor for CXCL12, a chemokine linked to development of organ-specific metastases (8). These findings endorse a more prudent use of IL-2 or perhaps its use in combination with agents such as AMD-3100 which antagonise the CXCR4-CXCL12 interaction..
Exploiting T cell plasticity (figure 1 D)
The origins of iTRegs within the tumour microenvironment are diverse as varying degrees of plasticity exist within the helper CD4+ T cell population (TRegs, Th1, Th2, Th17, Tfh) (30); Pre-differentiated TRegs may migrate under the influence of chemokines (27), TRegs may arise from de novo generation via differentiation and expansion or may derive from conversion of CD4+CD25− T cells. The plasticity inherent in each of these processes is a potentially exploitable therapeutic niche.
IL-6 is central to T cell plasticity (30). It helps to convert FOXP3+ TRegs into IL-17 secreting T cells (Th17). It potently abolishes conversion of conventional T cells into iTRegs and in its absence no other cytokine can substitute for this inhibition. Thus, IL-6 merits further investigation as a therapeutic for cancer. TGF-β acts at the axis between TReg and Th17 differentiation, enhancing the function of FOXP3 and inhibiting the function of RORγt, their essential transcription factors respectively. TGF-β-induced FOXP3 expression is inhibited by proinflammatory cytokines (IL-6 and IL-21 for example) in a Stat-3-dependent manner. Thus Stat-3 may also represent a therapeutic option – indeed forced expression of Stat3 augmented IL-17 production, most likely through increased RORγt expression (30). Re-directing differentiation towards a Th17 phenotype might also be achieved by direct introduction of RORγt, as this has been shown to induce IL-17 expression upon transduction of naive CD4+ T cells (30). Conversely, selective methylation at the FOXP3 locus would likely hinder differentiation along a suppressor pathway. Aside from the epigenetic level, targeting FOXP3 at the mRNA and protein levels would also be worthwhile. Other approaches include antagonists for retinoic acid receptors which facilitate differentiation into Th17 cells over TRegs (30). TReg differentiation can be redirected towards lineages other than Th17. Specific inactivation of the transcription factor interferon regulatory factor 4 (IRF-4) elevates Th2 cytokine production while IL-4-driven growth factor independent 1 (Gfi-1) facilitates optimal Th2 differentiation (30).
Blocking TReg proliferation is an obvious goal. This can be achieved by either direct inhibition of TGF-β, inhibition of IDO directly with 1-methyl-D-tryptophan or indirectly by CTLA-4 blockade. Aside from directly stimulating TReg expansion, COX-2-derived PGE2 facilitates tolerogenic APC-led TReg recruitment and is itself a functional instrument of TRegs in certain tumours (8). Thus, use of COX-2 inhibitors like celecoxib may be justified. Alternatively, bevacizumab or blockade of PD1-L on TRegs with MDX-1106 (Phase II) may halt TReg proliferation.
Inhibiting the peripheral conversion of CD4+CD25− T cells into CD4+CD25+ TRegs may be a useful therapeutic approach. The TGF-β-blocking antibody, 1D11 abolished this conversion and reduced tumour burden in mice. Subsequently other TGF-β-modulators including antibodies, soluble TGF-β receptors and the antisense oligonucleotide AP-12009 have reached Phase I/II clinical trials. However systemic TGF-β-blockade may carry the risk of developing autoimmune disorders. Furthermore, under subimmunogenic conditions T cell conversion can occur in the absence of TGF-β; IL-10 and IDO have also been shown to promote induction of TRegs (8).
Novel approaches to TReg modulation
The multitude of strategies discussed in this review deliver only marginal efficacy. While some strategies have lacked potency the majority flounder on specificity. This dearth of specificity is understandable given the intersecting differentiation pathways shared by all cells of the T cell lineage. Selective approaches to TReg modulation are warranted. Simple depletion of TRegs may be naive and the benefit short-lived, while inhibiting their migration to the tumour ignores the in situ generation of these cells. Thus strategies focused on negating TReg function or reprogramming their functional phenotype would seem more meritorious.
A unique cell surface marker which facilitates selective targeting of TRegs has yet to be uncovered. Thus targeting CD25 or CTLA-4 has been encumbered by a concomitant effect on Teffectors. Introducing a second layer of specificity, so called dual specificity, to receptor targeting would likely be synergistic. This is a strategy under investigation in our laboratory whereby a relatively TReg-specific gene therapy approach is coupled to ligand selectivity.
A global TReg modulation is undesirable as it may increase susceptibility to autoimmunity. Tumour-TRegs could be targeted via their antigen-specific T cell receptors (TCRs); antigen-specific TRegs engaged melanoma-expressed LAGE1 and ARTC1 (31) and in colorectal cancer patients CEA, telomerase, HER2/neu and MUC-1 reactive TRegs were detected in the peripheral blood (32). On a practical level this could be achieved by harnessing tetramer technology; Saporin-coupled MHC class I tetramers specifically ablated IGRP-autoreactive T cells and delayed diabetes in NOD mice (33). Identification of CD4+ TRegs specific for a given tumour antigen would facilitate their targeting with MHC class II tetramers by similar means.
While such agents would be specific for a given subset of TRegs they would also target other CD4+ helper cells expressing the same antigen specificity – CD8+ cells would be unaffected. To circumvent this issue the effector component attached to the tetramer could be modified to confer another level of specificity. It could be miR-31 as 100% of target cells internalise the tetrameric complexes (33). Although the consequence of FOXP3 knockdown in non-TRegs is unknown TCR engagement in these cells may simply lead to activation – further augmenting the immune effector response.
Alternatively one could target tumour-TRegs indirectly by modulating dendritic cell activation. This could be achieved by blockade of DC p38 MAPK, COX-2 or PI3K which inhibits innate production of TGF-β and IL-10 and thereby suppresses induction of TRegs. Such strategies enhance the efficacy of TLR agonists or HSPs as immunotherapeutics or adjuvants for DC vaccines and permit an un-restrained development of protective Th1 and Th17 cells (25).
Conclusion
TReg inhibition in the cancer environment would permit an anti-tumour immune effector competency with containment or elimination of disease. Such responses would be tumour specific and durable and should be effective against systemic disease, particularly micrometastases. There is clinical potential for TReg inhibitory strategies as part of multimodal programmes or combined with targeted therapies or local immunogene stimulation of anti-tumour immune effector cells. The objective should be to selectively modulate TRegs within the tumour microenvironment rather than their global depletion in order to minimize the risk of autoimmune manifestations. Strategies targeting TReg function or differentiation seem currently to be the best option as they are less susceptible to compensatory mechanisms. Emerging technologies such as tetramer or RNA interference approaches should improve specificity and efficacy and thus favour the preferential inhibition of TRegs within the tumour environment.
Acknowledgments
Funding Sources: William L Byrne is funded by the Health Research Board in Ireland, PhD Scholars programme in Cancer Biology. Kingston HG Mills is funded by Science Foundation Ireland (PI award 06/IN.1/B87). James A Lederer is funded by an NIH grant (R01 GM035633-23). Gerald C O’Sullivan is funded by Science Foundation Ireland Research Frontiers Programme (08-RFP-NSC-1655).
References
- 1.Rakhra K, Bachireddy P, Zabuawala T, Zeiser R, Xu L, Kopelman A, et al. CD4(+) T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation. Cancer Cell. 2010;18:485–98. doi: 10.1016/j.ccr.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2010;29:235–71. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 3.Steer HJ, Lake RA, Nowak AK, Robinson BW. Harnessing the immune response to treat cancer. Oncogene. 2010;29:6301–13. doi: 10.1038/onc.2010.437. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–74. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 6.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 7.Turk MJ, Guevara-Patino JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200:771–82. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mougiakakos D, Choudhury A, Lladser A, Kiessling R, Johansson CC. Regulatory T cells in cancer. Adv Cancer Res. 2010;107:57–117. doi: 10.1016/S0065-230X(10)07003-X. [DOI] [PubMed] [Google Scholar]
- 9.Rasku MA, Clem AL, Telang S, Taft B, Gettings K, Gragg H, et al. Transient T cell depletion causes regression of melanoma metastases. J Transl Med. 2008;6:12. doi: 10.1186/1479-5876-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whelan MC, Casey G, MacConmara M, Lederer JA, Soden D, Collins JK, et al. Effective immunotherapy of weakly immunogenic solid tumours using a combined immunogene therapy and regulatory T-cell inactivation. Cancer Gene Ther. 2010;17:501–11. doi: 10.1038/cgt.2010.8. [DOI] [PubMed] [Google Scholar]
- 11.Rech AJ, Vonderheide RH. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann N Y Acad Sci. 2009;1174:99–106. doi: 10.1111/j.1749-6632.2009.04939.x. [DOI] [PubMed] [Google Scholar]
- 12.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–8. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colombo MP, Piconese S. Regulatory-T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat Rev Cancer. 2007;7:880–7. doi: 10.1038/nrc2250. [DOI] [PubMed] [Google Scholar]
- 14.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176:6752–61. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 15.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan S, Burt DJ, Ralph C, Thistlethwaite FC, Hawkins RE, Elkord E. Tremelimumab (anti-CTLA4) mediates immune responses mainly by direct activation of T effector cells rather than by affecting T regulatory cells. Clin Immunol. 2010;138:85–96. doi: 10.1016/j.clim.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Ko K, Yamazaki S, Nakamura K, Nishioka T, Hirota K, Yamaguchi T, et al. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J Exp Med. 2005;202:885–91. doi: 10.1084/jem.20050940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coe D, Begom S, Addey C, White M, Dyson J, Chai JG. Depletion of regulatory T cells by anti-GITR mAb as a novel mechanism for cancer immunotherapy. Cancer Immunol Immunother. 2010;59:1367–77. doi: 10.1007/s00262-010-0866-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cote AL, Zhang P, O’Sullivan JA, Jacobs VL, Clemis CR, Sakaguchi S, et al. Stimulation of the glucocorticoid-induced TNF receptor family-related receptor on CD8 T cells induces protective and high-avidity T cell responses to tumor-specific antigens. J Immunol. 2010;186:275–83. doi: 10.4049/jimmunol.1001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM, et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011;470:548–53. doi: 10.1038/nature09707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amendola M, Passerini L, Pucci F, Gentner B, Bacchetta R, Naldini L. Regulated and multiple miRNA and siRNA delivery into primary cells by a lentiviral platform. Mol Ther. 2009;17:1039–52. doi: 10.1038/mt.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karanikas V, Speletas M, Zamanakou M, Kalala F, Loules G, Kerenidi T, et al. Foxp3 expression in human cancer cells. J Transl Med. 2008;6:19. doi: 10.1186/1479-5876-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, et al. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–4. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Luo F, Cai Y, Liu N, Wang L, Xu D, et al. TLR1/TLR2 agonist induces tumor regression by reciprocal modulation of effector and regulatory T cells. J Immunol. 2011;186:1963–9. doi: 10.4049/jimmunol.1002320. [DOI] [PubMed] [Google Scholar]
- 25.Conroy H, Marshall NA, Mills KH. TLR ligand suppression or enhancement of Treg cells? A double-edged sword in immunity to tumours. Oncogene. 2008;27:168–80. doi: 10.1038/sj.onc.1210910. [DOI] [PubMed] [Google Scholar]
- 26.Mandapathil M, Hilldorfer B, Szczepanski MJ, Czystowska M, Szajnik M, Ren J, et al. Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. J Biol Chem. 2010;285:7176–86. doi: 10.1074/jbc.M109.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 28.Tan MC, Goedegebuure PS, Belt BA, Flaherty B, Sankpal N, Gillanders WE, et al. Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J Immunol. 2009;182:1746–55. doi: 10.4049/jimmunol.182.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee H, Kwon Y, Lee JH, Kim J, Shin MK, Kim SH, et al. Methyl gallate exhibits potent antitumor activities by inhibiting tumor infiltration of CD4+CD25+ regulatory T cells. J Immunol. 2010;185:6698–705. doi: 10.4049/jimmunol.1001373. [DOI] [PubMed] [Google Scholar]
- 30.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–55. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Wang HY, Peng G, Guo Z, Shevach EM, Wang RF. Recognition of a new ARTC1 peptide ligand uniquely expressed in tumor cells by antigen-specific CD4+ regulatory T cells. J Immunol. 2005;174:2661–70. doi: 10.4049/jimmunol.174.5.2661. [DOI] [PubMed] [Google Scholar]
- 32.Bonertz A, Weitz J, Pietsch DH, Rahbari NN, Schlude C, Ge Y, et al. Antigen-specific Tregs control T cell responses against a limited repertoire of tumor antigens in patients with colorectal carcinoma. J Clin Invest. 2009;119:3311–21. doi: 10.1172/JCI39608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vincent BG, Young EF, Buntzman AS, Stevens R, Kepler TB, Tisch RM, et al. Toxin-coupled MHC class I tetramers can specifically ablate autoreactive CD8+ T cells and delay diabetes in nonobese diabetic mice. J Immunol. 2010;184:4196–204. doi: 10.4049/jimmunol.0903931. [DOI] [PMC free article] [PubMed] [Google Scholar]