Abstract
Autistics are presumed to be characterized by cognitive impairment, and their cognitive strengths (e.g., in Block Design performance) are frequently interpreted as low-level by-products of high-level deficits, not as direct manifestations of intelligence. Recent attempts to identify the neuroanatomical and neurofunctional signature of autism have been positioned on this universal, but untested, assumption. We therefore assessed a broad sample of 38 autistic children on the preeminent test of fluid intelligence, Raven’s Progressive Matrices. Their scores were, on average, 30 percentile points, and in some cases more than 70 percentile points, higher than their scores on the Wechsler scales of intelligence. Typically developing control children showed no such discrepancy, and a similar contrast was observed when a sample of autistic adults was compared with a sample of nonautistic adults. We conclude that intelligence has been underestimated in autistics.
Autism is defined by atypical communication, social interaction, interests, and body mannerisms. When Kanner (1943) originally codified the phenomenon of autism, he prognosticated that autistics’1 “excellent memory … and the precise recollection of complex patterns and sequences, bespeak good intelligence” (p. 247). However, more formal measurements in epidemiological studies have placed a substantial percentage of autistics in the range defined as mental retardation (e.g., 40% in Baird et al., 2000; 25% to 64% in Kielinen, Linna, & Moilanen, 2000).
The assumption that autistics are cognitively impaired pervades the popular and scientific literature. Autistics who are considered minimally verbal or nonverbal (i.e., who are severely challenged in their ability to speak fluently) are considered the most cognitively impaired; it is commonplace to refer to such individuals as “low functioning.” And although it has become impolitic to refer to autistics with exceptional abilities as “idiot savants,” superior performance by autistics is frequently considered to be a side effect of abnormal neuroanatomical function, rather than a reflection of genuine human intelligence (Hobson, 2002). We empirically examined these prevalent conceptions to better understand the level and nature of autistic intelligence.
Intelligence tests play a prominent role in autism research and clinical practice. In research, intelligence-test scores serve as variables for matching subject groups, as variables to be covaried (when prior matching was ineffective), and as outcome measures used to test various therapies empirically. In research and clinical practice, autistic intelligence is most commonly measured by performance on Wechsler-based tests of intelligence (Mottron, 2004).
For example, the Wechsler Intelligence Scale for Children (Wechsler, 1991) comprises a dozen subtests. Scores on five of those subtests, which require the examinee to answer orally delivered questions with oral responses, compose a Verbal IQ factor; scores on five other subtests, which require the examinee to answer orally delivered questions with nonoral responses (e.g., arranging cards or blocks), compose a nonverbal or Performance IQ factor. Thus, both Verbal and Performance IQ subtests require competence in understanding language, and Verbal IQ subtests require competence in speaking language. The composite of Verbal and Performance IQ subtests is referred to as Full Scale IQ.
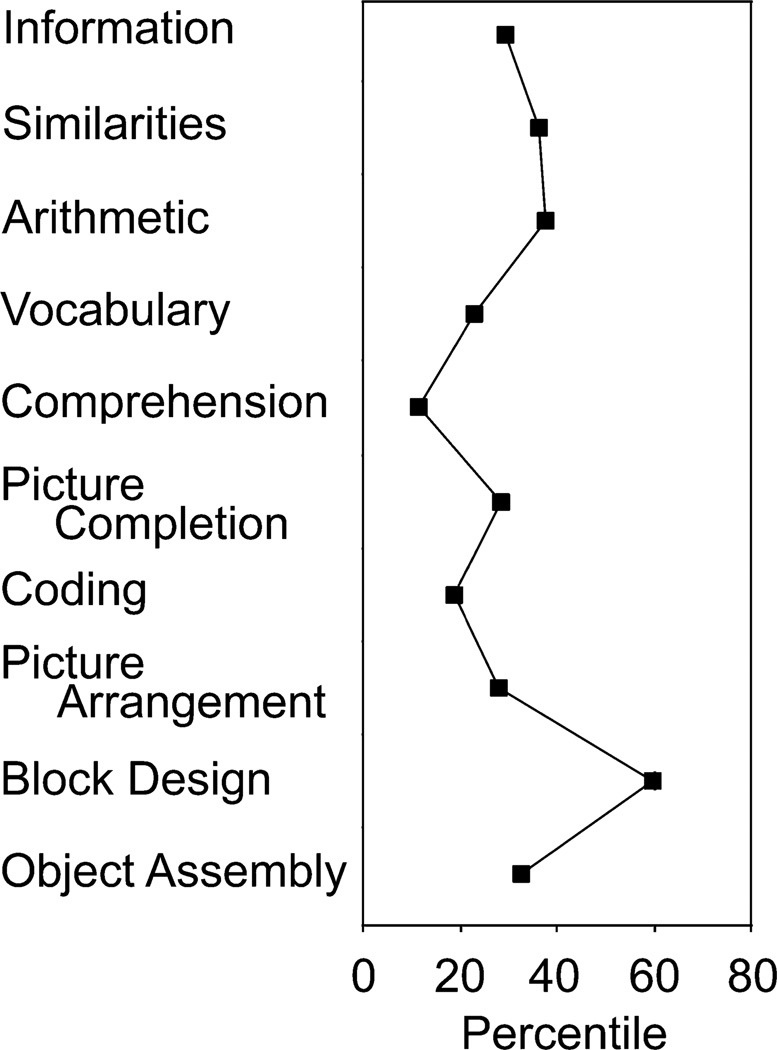
When tested with Wechsler-type intelligence scales (Happé, 1994), autistics usually produce a characteristic profile, as illustrated in Figure 1. A marked deficit on one of the verbal subtests, Comprehension, is usually observed. This subtest reputedly measures social and practical understanding, by including questions such as, “What is the thing to do if you find an envelope in the street that is sealed, addressed, and has a new stamp on it?” and “What is the thing to do when you cut your finger?” The examinee’s oral answers on the Comprehension subtest are scored for their quality by the examiner.
Fig. 1.
Mean subtest scores of the 38 autistic children on the Wechsler Intelligence Scale for Children–Third Edition.
In contrast, autistics typically demonstrate a marked peak on one of the nonverbal subtests, Block Design. On the Block Design subtest, the examinee is shown a two-dimensional red-and-white geometric design, and the task is to reproduce that design by assembling a set of colored blocks. The Block Design subtest is time limited and scored for accuracy.
How should one interpret such peaks and troughs in autistics’ Wechsler subtest scores? One commonsense notion is that the peaks correspond to the intellectual skills that epitomize autistics, the cognitive tasks on which they excel. However, for many years, these peaks were classified as “islets of ability…regarded as something of a myth or else as merely an interesting but theoretically unimportant fact” (Shah & Frith, 1993, p. 1351). Then, in the 1990s, the peaks were imbued with theoretical importance. Exceptional performance on the Block Design subtest, along with exceptionality in rapidly disembedding a target figure from a complex background, drawing “impossible” figures, and perceiving pitch, as well as many savant skills, were all interpreted as a unified deficit: “weak central coherence,” the tendency to focus on details at the expense of configuration (Happé, 1999; Heaton, Hermelin, & Pring, 1998; Shah & Frith, 1983).
We empirically tested this construal of autistics’ intellectual strengths as low-level perceptual penchants resulting from high-level conceptual deficits by administering an intelligence test widely regarded to be a preeminent measure of high-level analytical reasoning, the Raven’s Progressive Matrices (Raven, Raven, & Court, 1998). This test comprises 60 items, divided into five sets of increasing complexity. All items have a similar format: A matrix of geometric designs with one cell of the matrix left blank is presented with six or eight alternatives for the matrix’s completion. Minimal instruction is required for this putatively nonverbal test.
The Raven’s Progressive Matrices has been empirically demonstrated to assay the ability to infer rules, to manage a hierarchy of goals, and to form high-level abstractions (Carpenter, Just, & Shell, 1990). Broadly recognized as a paramount metric of reasoning and problem solving, the Raven’s Progressive Matrices is believed to be a “paradigmatic” measure of fluid intelligence (Mackintosh, 1998, p. 228), and fluid-intelligence tasks are proposed to require coordinated executive function, attentional control, and working memory (Blair, 2006; Kane & Engel, 2002; Newman & Just, 2005). The Raven’s Progressive Matrices occupies psychometric centrality among tests of cognitive ability; in Snow, Kyllonen, and Marshalek’s (1984) classic diagram, which summarizes the intercorrelations among numerous tests of cognitive ability, simple, domain-specific tests lie along the periphery, and Raven’s Progressive Matrices occupies the center, as the most complex and general single test of intelligence.
Descriptions of the cognitive processes required to solve Raven’s Progressive Matrices and to perform fluid-intelligence tasks read like compendia of the cognitive processes that autistics are assumed to lack. For example, whereas autistics are expected to perform adequately on simple tests of executive function and working memory, they are expected to lack the cognitive abilities required to perform well on more complex assays of cognition (Minshew, Webb, Williams, & Dawson, 2006). Autistics are assumed to excel at tests of rote memory or low-level pattern matching, but to be disproportionately challenged by tests of high-level integration or abstraction (Courchesne & Pierce, 2005; Just, Cherkassky, Keller, & Minshew, 2004). Indeed, it has been specifically predicted that autistics should be disproportionately impaired in fluid reasoning (Blair, 2006; Pennington & Ozonoff, 1996), but this prediction has never been submitted to empirical scrutiny. Our goal was to directly examine these claims.
METHOD
Subjects
Autistic Children
This group comprised 38 autistic children (35 males, 3 females) between 7 and 16 years of age (M = 10.39, SD = 2.69). They were diagnosed at the Pervasive Developmental Disorders Specialized Clinic at Rivière-des-Prairies Hospital, Montreal, Canada. All met diagnostic criteria for autistic disorder, rather than any of the other diagnostic categories of the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (e.g., pervasive developmental disorder not otherwise specified or Asperger’s disorder), according to two gold-standard research-diagnostic instruments (the Autism Diagnostic Interview–Revised, by Lord, Rutter, & Le Couteur, 1994, and the Autism Diagnosis Observation Schedule–General, by Lord, Rutter, DiLavore, & Risi, 1999) and experienced clinicians. After the diagnostic evaluation, data from all patients were entered in a Digimed© Database, with their prior informed consent.
Data from all consecutive cases who met criteria for autism on both diagnostic instruments and who had completed both the French-Canadian version of the Wechsler Intelligence Scale for Children–Third Edition (WISC-III; Wechsler, 1991) and Raven’s Progressive Matrices (Raven et al., 1998) were retrieved from the database. From this sample, autistic children who had a known, diagnosable genetic condition or additional neurological condition were excluded. The autistic subjects selected in this way represented primary, or idiopathic, autism, that is, autism without a known and possibly confounding etiology.
Nonautistic Control Children
This group comprised 24 typically developing, nonautistic children (19 males, 5 females) between 6 and 16 years of age (M = 11.0, SD = 3.28). They were recruited via advertisements placed in a local newspaper. A semistructured interview allowed the exclusion of subjects with a history of psychiatric treatment, learning disabilities, or neurological disorders, or a familial history of psychiatric or neurological disorders.
Autistic Adults
This group comprised 13 autistic adults (11 males, 2 females) between 16 and 43 years of age (M = 25.38, SD = 8.86). These adults were also diagnosed at the Pervasive Developmental Disorders Specialized Clinic. Diagnosis was made by experienced clinicians using the Autism Diagnostic Interview–Revised (Lord et al., 1994) and the Autism Diagnosis Observation Schedule (Lord et al., 1999). The same inclusion and exclusion criteria that were applied to the autistic children were applied to the autistic adults.
Nonautistic Control Adults
This group comprised 19 typical adults (all males) between 19 and 32 years of age (M = 22.37, SD = 4.57). They were recruited and screened in the same way as the nonautistic children.
Materials
Wechsler Scales
The WISC-III was administered to both groups of children, and the Wechsler Adult Intelligence Scale–Third Edition (WAIS-III; Wechsler, 1997) was administered to both groups of adults. Both the WISC-III and the WAIS-III were scored with Canadian norms.
Raven’s Progressive Matrices
The standard version of the Raven’s Progressive Matrices was administered to all subjects, with no time limit. Norms for North American children were taken from the test’s manual (Raven et al., 1998), and norms for the adults came from Burke (1985).
Procedure
For the autistic children and adults, the two tests (Wechsler scales and Raven’s Progressive Matrices) were routinely included in the diagnostic evaluation at the Pervasive Developmental Disorders Specialized Clinic. Both tests were administered individually by neuropsychologists unaware of the study and hypotheses. The nonautistic, control children and adults were tested by neuropsychologists under conditions similar to those for autistic subjects and received compensation.
RESULTS
Autistic and Nonautistic Children
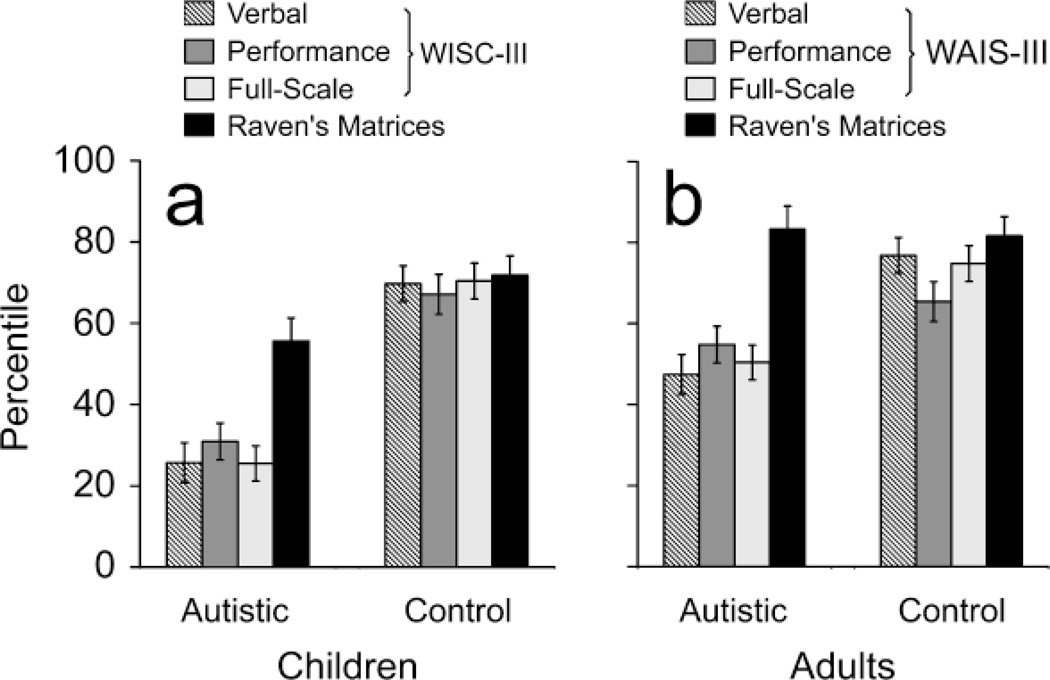
The autistic children’s WISC-III subtest scores exhibited the prototypical autistic profile (see Fig. 1). Their WISC-III factor scores (see Fig. 2a) were at the 26th percentile (SD = 30.17) for Verbal IQ, the 31st percentile (SD = 27.47) for Performance IQ, and the 26th percentile (SD = 26.58) for Full Scale IQ, each falling in the range of low average. In contrast, the autistic children’s scores on the Raven’s Progressive Matrices were at the 56th percentile (SD = 35.11), indicating an average level of performance. Indeed, analyses of variance (ANOVAs) indicated that the autistic children’s Raven’s Matrices scores were significantly higher than their WISC-III Full Scale, Verbal, and Performance scores (two-tailed, all preps = .996, Cohen’s ds = 0.78–0.97).
Fig. 2.
Performance of the (a) autistic and nonautistic children and (b) autistic and nonautistic adults on the Wechsler scales and Raven’s Progressive Matrices. Error bars represent 2 SEMs. WAIS-III = Wechsler Adult Intelligence Scale–Third Edition; WISC-III = Wechsler Intelligence Scale for Children–Third Edition.
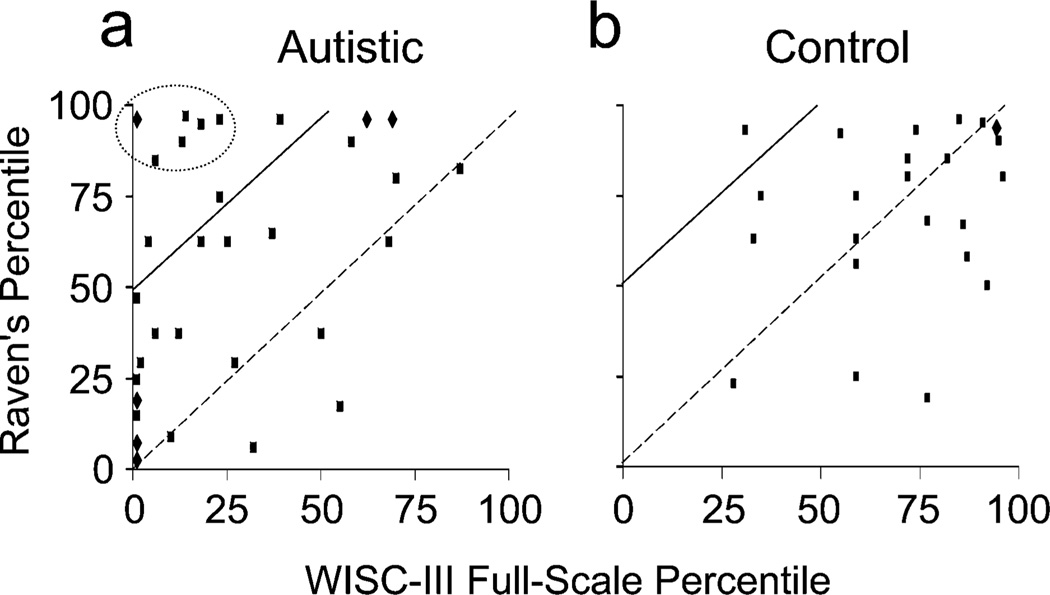
Discrepancies between the autistic children’s WISC-III Full Scale IQs and their Raven’s Matrices scores occurred throughout the entire WISC-III range, as illustrated in Figure 3a. For example, no autistic child scored in the “high intelligence” range on the WISC-III, whereas a third of the autistic children scored at or above the 90th percentile on the Raven’s Matrices. Only a minority of the autistic children scored in the “average intelligence” range or higher on the WISC-III, whereas the majority scored at or above the 50th percentile on the Raven’s Matrices. Whereas a third of the autistic children would be called “low functioning” (i.e., in the range of mental retardation) according to the WISC-III, only 5% would be so judged according to the Raven’s Matrices.
Fig. 3.
Relation between Full Scale IQ on the Wechsler Intelligence Scale for Children–Third Edition (WISC-III) and Raven’s Progressive Matrices scores in (a) autistic children and (b) control children. Data points to the left of the dashed diagonal lines represent subjects whose Raven’s Matrices scores were greater than their WISC-III scores; data points to the left of the solid diagonal lines represent subjects whose Raven’s Matrices scores were 50 percentile points greater than their WISC-III scores. In (a), a circle surrounds the data points for 7 autistic children whose Raven’s Matrices scores exceeded their WISC-III scores by more than 70 percentile points. Diamonds represent identical data points from 2 subjects.
The control children’s WISC-III factor scores were at the 70th percentile (SD = 21.35) for Verbal IQ, the 67th percentile (SD = 23.79) for Performance IQ, and the 70th percentile (SD = 21.77) for Full Scale IQ (see Fig. 2a). Similarly, the control children’s Raven’s Progressive Matrices scores were at the 72nd percentile (SD = 23.69). In striking contrast to the autistic children, the nonautistic control children did not show a significant difference between their Raven’s Matrices scores and their WISC-III Full Scale, Verbal Scale, or Performance Scale scores (ANOVA, all preps < .53, ds = 0.06–0.2). Thus, the magnitude of the difference between the Raven’s Progressive Matrices scores and WISC-III scores differed significantly between the autistic and nonautistic children, F(1, 60) = 12.89, prep = .986, d = 0.94. In fact, for nearly half the nonautistic children, the WISC-III Full Scale and Raven’s Progressive Matrices scores differed by fewer than 10 percentile points (see Fig. 3b). For only 1 nonautistic control child was the discrepancy between the WISC-III Full Scale and Raven’s Progressive Matrices scores greater than 50 percentile points.
Autistic and Nonautistic Adults
Similar results were observed when the autistic and nonautistic adults’ scores on the Raven’s Progressive Matrices and WAIS-III were compared (see Fig. 2b). The autistic adults’ Raven’s Progressive Matrices scores (M = 83.30 percentile, SD = 19.26) were, on average, more than 30 percentile points higher than their WAIS-III scores (M = 50.38 percentile, SD = 30.57; prep= .986, d = 1.29). In contrast, the nonautistic adults’ Raven’s Progressive Matrices scores (M = 81.64 percentile, SD = 16.78) and WAIS-III scores (M = 74.80 percentile, SD = 16.57) did not differ significantly (prep = .852, d = 0.41). As found for the children, the magnitude of the difference between the Raven’s Progressive Matrices and WISC-III scores differed significantly between the autistic and nonautistic adults, F(1, 30) = 13.19, prep = .986, d = 1.31.
DISCUSSION
In addition to addressing the level of autistic intelligence, these data address the nature of autistic intelligence. These data challenge the assumption that autistic intelligence is only simple, low-level, perceptual expertise, which enables autistics to solve only tasks based on rote memory or the manipulation of geometric cubes, such as the Block Design task. Although autistics can be described as possessing enhanced perceptual functioning (Mottron, Dawson, Soulières, Hubert, & Burack, 2006), their performance on the Block Design subtest is correlated with their performance on the other Wechsler subtests (e.g., for the autistic children in the current study, r = .65, prep = .986). In addition, when autistics perform a series of Block Design tasks, altered so as to be optimally solved either through perception of local details or through configural processing, they display more versatility and better performance than nonautistics (Caron, Mottron, Berthiaume, & Dawson, 2006). Furthermore, in the current study, the relative difficulty of the 60 Raven’s Progressive Matrices items was highly correlated between the autistic and nonautistic children, r(58) = .96, suggesting that the test measured the same construct in the two groups.
We have shown that autistics are not disproportionately impaired on a test of fluid intelligence, as many current theories of autism predict they should be. Instead of being limited to isolated Wechsler subtests assumed to measure only low-level rote memory and perception, autistic intelligence is manifested on the most complex single test of general intelligence in the literature. Although autistics no doubt deploy atypical cognitive processes in performing many tasks, we strongly caution against declaring these processes dysfunctional or assuming that autistics’ peaks and troughs on Wechsler scales “flout the premise of … general intelligence” (Scheuffgen, Happé, Anderson, & Frith, 2000, pp. 83–84).
Acknowledgments
We thank É. Ménard, C. Berthiaume, P. Jelenic, G. Martel, M.-J. Dubuc, and W.K. Dawson for contributing to the project, and D.M. Goldsmith for providing motivation. This work was supported by a grant from the Canadian Institutes of Health Research to L.M.
Footnotes
See Sinclair (1999) to appreciate our respectful use of the term “autistic” rather than “person with autism.”
REFERENCES
- Baird G, Charman T, Baron-Cohen S, Cox A, Swettenham J, Wheelwright S, Drew A. A screening instrument for autism at 18 months of age: A 6-year follow-up study. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:694–702. doi: 10.1097/00004583-200006000-00007. [DOI] [PubMed] [Google Scholar]
- Blair C. How similar are fluid cognition and general intelligence? A developmental neuroscience perspective on fluid cognition as an aspect of human cognitive ability. Behavioral and Brain Sciences. 2006;29:109–125. doi: 10.1017/S0140525X06009034. [DOI] [PubMed] [Google Scholar]
- Burke HR. Raven’s Progressive Matrices (1938): More on norms, reliability, and validity. Journal of Clinical Psychology. 1985;41:231–235. [Google Scholar]
- Caron M-J, Mottron L, Berthiaume C, Dawson M. Cognitive mechanisms, specificity and neural underpinnings of visuospatial peaks in autism. Brain. 2006;129:1789–1802. doi: 10.1093/brain/awl072. [DOI] [PubMed] [Google Scholar]
- Carpenter PA, Just MA, Shell P. What one intelligence test measures: A theoretical account of the processing in the Raven Progressive Matrices Test. Psychological Review. 1990;97:404–431. [PubMed] [Google Scholar]
- Courchesne A, Pierce K. Why the frontal cortex in autism might be talking only to itself: Local over-connectivity but long-distance disconnection. Current Opinion in Neurobiology. 2005;15:225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Happé F. Wechsler IQ profile and theory of mind in autism: A research note. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1994;35:1461–1471. doi: 10.1111/j.1469-7610.1994.tb01287.x. [DOI] [PubMed] [Google Scholar]
- Happé F. Autism: Cognitive deficit or cognitive style? Trends in Cognitive Sciences. 1999;3:216–222. doi: 10.1016/s1364-6613(99)01318-2. [DOI] [PubMed] [Google Scholar]
- Heaton P, Hermelin B, Pring L. Autism and pitch processing: A precursor for savant musical ability? Music Perception. 1998;15:291–305. [Google Scholar]
- Hobson P. The cradle of thought: Exploring the origins of thinking. New York: Oxford University Press; 2002. [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: Evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engel RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual-differences perspective. Psychonomic Bulletin & Review. 2002;9:637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. The Nervous Child. 1943;2:217–250. [Google Scholar]
- Kielinen M, Linna SL, Moilanen I. Autism in Northern Finland. European Child and Adolescent Psychiatry. 2000;9:162–167. doi: 10.1007/s007870070039. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule. Los Angeles: Western Psychological Services; 1999. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnosis Interview–Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. IQ and human intelligence. New York: Oxford University Press; 1998. [Google Scholar]
- Minshew N, Webb SJ, Williams DL, Dawson G. Neuropsychology and neurophysiology of autism spectrum disorders. In: Moldin SO, Rubenstein JLR, editors. Understanding autism. Boca Raton, FL: Taylor & Francis; 2006. pp. 379–415. [Google Scholar]
- Mottron L. Matching strategies in cognitive research with individuals with high-functioning autism: Current practices, instrument biases, and recommendations. Journal of Autism and Developmental Disorders. 2004;34:19–27. doi: 10.1023/b:jadd.0000018070.88380.83. [DOI] [PubMed] [Google Scholar]
- Mottron L, Dawson M, Soulières I, Hubert B, Burack J. Enhanced perceptual functioning in autism: An update, and eight principles of autistic perception. Journal of Autism and Developmental Disorders. 2006;36:27–43. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- Newman SD, Just MA. The neural bases of intelligence: A perspective based on functional neuroimaging. In: Sternberg RJ, Pretz JE, editors. Cognition and intelligence. Cambridge, England: Cambridge University Press; 2005. pp. 88–103. [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1996;37:51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Raven J, Raven JC, Court JH. Raven manual: Section 3. Standard progressive matrices. Oxford, England: Oxford Psychologists Press; 1998. [Google Scholar]
- Scheuffgen K, Happé F, Anderson M, Frith U. High “intelligence,” low “IQ”? Speed of processing and measured IQ in children with autism. Development and Psychopathology. 2000;12:83–90. doi: 10.1017/s095457940000105x. [DOI] [PubMed] [Google Scholar]
- Shah A, Frith U. An islet of ability in autistic children: A research note. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1983;24:613–620. doi: 10.1111/j.1469-7610.1983.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Shah A, Frith U. Why do autistic individuals show superior performance on the Block Design task? Journal of Child Psychology and Psychiatry and Allied Disciplines. 1993;34:1351–1364. doi: 10.1111/j.1469-7610.1993.tb02095.x. [DOI] [PubMed] [Google Scholar]
- Sinclair J. Why I dislike “person first” language. [Retrieved October 2006];1999 from http://web.syr.edu/~jisincla/person_first.htm. [Google Scholar]
- Snow RE, Kyllonen PC, Marshalek B. The topography of ability and learning correlations. In: Sternberg RJ, editor. Advances in the psychology of human intelligence. Vol. 2. Hillsdale, NJ: Erlbaum; 1984. pp. 47–103. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children–third edition: Canadian (WISC-III) Toronto, Ontario, Canada: Psychological Corp.; 1991. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale–third edition: Canadian. Toronto, Ontario, Canada: Psychological Corp.; 1997. [Google Scholar]