Abstract
Significant upregulation of the integrin αvβ6 has been described as a prognostic indicator in several cancers, making it an attractive target for tumor imaging. This study compares variants of a PEGylated αvβ6-targeting peptide, bearing either an [18F]fluorobenzoyl prosthetic group ([18F]FBA-PEG-A20FMDV2) or different [64Cu]copper chelators (DOTA-PEG-A20FMDV2, CB-TE2A-PEG-A20FMDV2). The compounds were evaluated in vitro by enzyme-linked immunosorbent assay (against the integrin αvβ6 and the closely related integrin αvβ3) and by cell labeling (αvβ6-positive DX3puroβ6/αvβ6-negative DX3puro) and in vivo using micro-positron emission tomography in a mouse model bearing paired DX3puroβ6/Dx3puro xenografts. In vitro, all three compounds showed excellent αvβ6-specific binding (50% inhibitory concentration [IC50](αvβ6) = 3 to 6 nmol/L; IC50(αvβ3) > 10 µmol/L). In vivo, they displayed comparable, preferential uptake for the αvβ6-expressing xenograft over the αvβ6-negative control (> 4:1 ratio at 4 hours postinjection). Whereas [64Cu]Cu-DOTA-PEG-A20FMDV2 resulted in increased levels of radioactivity in the liver, [64Cu]Cu-CB-TE2A-PEG-A20FMDV2 did not. Significantly, both 64Cu-labeled tracers showed unexpectedly high and persistent levels of radioactivity in the kidneys (> 40% injected dose/g at 4 and 12 hours postinjection). The findings underscore the potential influence of the prosthetic group on targeted in vivo imaging of clinically relevant markers such as αvβ6. Despite identical targeting peptide moiety and largely equal in vitro behavior, both 64Cu-labeled tracers displayed inferior pharmacokinetics, making them in their present form less suitable candidates than the 18F-labeled tracer for in vivo imaging of αvβ6.
Integrins are a family of heterodimeric cell surface receptors involved in cell–cell and cell–extracellular matrix interactions.1,2 Expression of the epithelium-specific integrin αvβ6 is generally low or undetectable in healthy adult tissue.3 Significant upregulation of αvβ6 has been linked to several particularly challenging cancers, such as pancreatic ductal adenocarcinoma, where nearly uniform widespread and high expression levels were found in the patient population.4 More recently, αvβ6 expression has been described as a prognostic indicator for lung, colon, cervical, and gastric cancer.5–8 Thus, selective in vivo imaging of αvβ6 could provide a useful tool for cancer detection and monitoring of therapy. Positron emission tomography (PET) is particularly attractive for localization of tumors and metastases in the clinical setting, with exquisite sensitivity and no depth limitation.9–11 We previously demonstrated in a mouse model bearing αvβ6-expressing DX3puroβ6 xenografts the feasibility of αvβ6-selective PET using an 18F-labeled peptide, 4-[18F]fluorobenzoic acid ([18F]FBA)-A20FMDV2.12 In the present study, we show that incorporation of a small, monodisperse polyethylene glycol (PEG) unit did not influence the high binding affinity and specificity for αvβ6 (enzyme-linked immunosorbent assay [ELISA]) while greatly improving retention in αvβ6-expressing xenografts (PET and biodistribution studies). However, the relatively rapid radioactive decay of fluorine-18 (half-life T1/2[18F] = 110 minutes) effectively limits the useful PET window to approximately 4 to 6 hours (approximately 2 to 3 half-lives). For that reason, we decided to evaluate tracers containing copper-64, another popular PET radioisotope with a nearly seven times longer half-life (T1/2[64Cu] = 12.7 hours).13 Monocyclic 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA)14 and cross-bridged 4,11-bis(carboxymethyl)-1,4,8,11-tetraazabicyclo[6.6.2] hexadecane (CB-TE2A)15,16 were chosen as chelators for [64Cu]Cu2+. DOTA is a readily available, very widely used monocyclic chelator for a variety of radiometals. However, it has been shown to be prone to demetallation in vivo, resulting in increased levels of radioactivity in the liver caused by strong binding of the copper to liver proteins (transchelation).13,17 Given that cross-bridged (ie, bicyclic) CB-TE2A forms very stable complexes with [64Cu]Cu2+, it has been suggested as a potential alternative to DOTA and other 64Cu chelators,16,18–20 even though it requires elevated temperatures for [64Cu]Cu2+ incorporation (approximately 90°C).
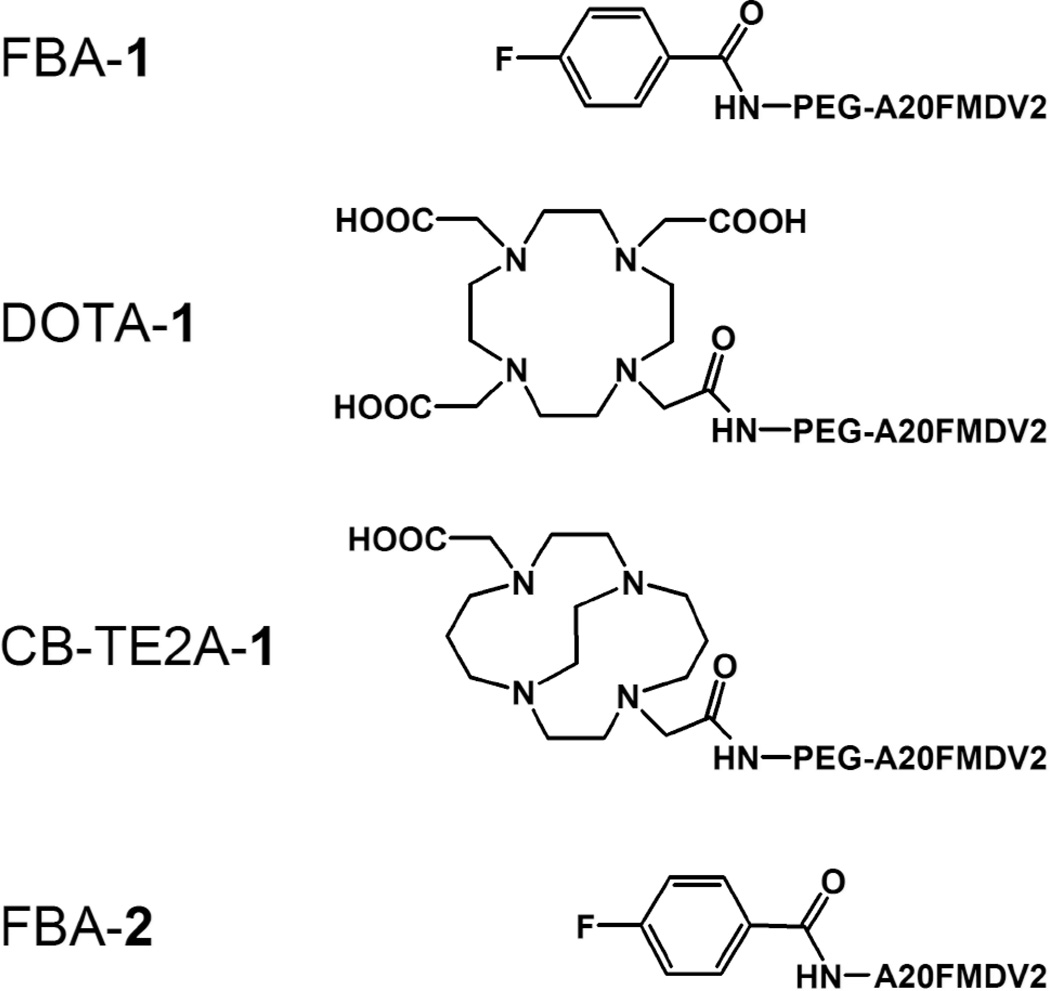
Here we describe a comparison of three PEGylated tracers, one bearing fluorine-18 ([18F]FBA-PEG-A20FMDV2, [18F]FBA-1) and two different 64Cu tracers ([64Cu]Cu-DOTA-1 and [64Cu]Cu-CB-TE2A-1; Figure 1, Table 1). The binding characteristics of FBA-1, DOTA-1, and CB-TE2A-1, as well as the unPEGylated reference compound FBA-A20FMDV2, (FBA-2) toward αvβ6 were first evaluated in vitro (1) using purified integrin αvβ6 (ELISA with FBA-1, FBA-2, DOTA-1, CB-TE2A-1) and (2) using αvβ6-expressing DX3puroβ6 and αvβ6-negative control DX3puro cell lines (cell binding assay with radiolabeled compounds). The in vivo pharmacokinetic behavior of the radiolabeled compounds was compared by PET and biodistribution studies in a mouse model bearing paired DX3puroβ6 and DX3puro xenografts.
Figure 1.
Structures of the compounds evaluated.
Table 1.
IC50 Values of Nonradioactive Compounds for Integrins αvβ6 and αvβ3 Determined by ELISA
| IC50 | |||
|---|---|---|---|
| Compound | αvβ6 (nmol/L) | αvβ6 (µmol/L) | |
| FBA-PEG-A20FMDV2 | FBA-1 | 6 ± 4 | > 10 |
| DOTA-PEG-A20FMDV2 | DOTA-1 | 3 ± 2 | > 10 |
| CB-TE2A-PEG-A20FMDV2 | CB-TE2A-1 | 4 ± 2 | > 10 |
| FBA-A20FMDV2* | FBA-2 | 3 ± 1 | > 10 |
| A20FMDV2* | 2 | 3 ± 1 | > 10 |
ELISA = enzyme-linked immunosorbent assay; IC50 = 50% inhibitory concentration.
Experiments were performed in triplicate, and values are expressed as mean ± SD (where appropriate).
Data from Hausner SH et al.12
Materials and Methods
General Information
Solvents and chemicals were purchased from Aldrich (Milwaukee, WI) unless stated otherwise. 9-Fluorenylmethoxycarbonyl (Fmoc)-protected amino acids and coupling reagents were purchased from NovaBiochem (San Diego, CA) or GL Biochem (Shanghai, China). Monodisperse Fmoc-amino-PEG-carboxylic acid and Rink Amide NovaGel HL resin (0.63 mmol/g) were obtained from NovaBiochem (San Diego, CA). Sep-Pak SPE cartridges were obtained from Waters (Milford, MA), and 18F Trap & Release Columns were purchased from ORTG Inc. (Oakdale, TN). Mass spectrometry analysis was performed using an ABI 4700 matrix-assisted laser desorption ionization time of flight/time of flight (MALDI TOF/TOF) spectrometer (Applied Biosystems, Foster City, CA). The DX3puro and DX3puroβ6 cell lines, recombinant soluble αvβ6 (rsαvβ6), and mouse monoclonal anti-αv-antibody (clone P2W7) were generated in the laboratory of J.F.M. and have been described previously.12 Cells were cultured at 37°C and 8% CO2 in Dulbecco’s Modified Eagle’s Medium (DMEM; Mediatech, Manassas, VA) containing 10% heat-inactivated fetal calf serum. Recombinant soluble αvβ3 was a gift from Genentech (San Francisco, CA). IgG1-κ (clone MOPC 21) and ExtrAvidin-peroxidase conjugate were purchased from Sigma (St. Louis, MO). AlexaFluor488-conjugated goat anti-mouse IgG was obtained from Molecular Probes (Invitrogen, Carlsbad, CA). MaxiSorp 96-well microtiter plates were used for ELISA (Nunc, Rochester, NY). 3,3'-5,5'-Tetramethylbenzidine (TMB) substrate was purchased from Dako (Carpinteria, CA).
[18F]Fluoride was produced from the 18O(p,n)18F nuclear reaction on [18O]H2O purchased from Marshall Isotopes Ltd. (Tel Aviv, Israel) using an RDS 111 negative ion cyclotron (CTI, Knoxville, TN). [18F]FBA was prepared on a TRACERlab FX F-N (GE Healthcare, Chalfont St Giles, UK). Preparation and in vivo evaluation of FBA-2 and [18F]FBA-2 have been described previously.21 [64Cu]CuCl2 was purchased from Trace Life Sciences (Denton, TX). CB-TE2A (bistrifluoroacetyl salt) was prepared according to published procedures.18,22
Thin-layer chromatography (TLC) was used to monitor the 64Cu-radiolabeling reactions. TLC was performed on silica gel plates (Whatman, Florham Park, NJ) using 10% (wt/v) aqueous ammonium acetate/methanol (1:1 v/v) as eluent.
Reverse-phase high-pressure liquid chromatography (HPLC) was used to purify and analyze the compounds: solvent A, 0.05% trifluoroacetic acid (TFA) in water (v/v); solvent B, acetonitrile. HPLC systems were equipped with both an ultraviolet (UV) absorbance detector (UV 220 nm) and a radioactivity detector (photomultiplier tube [PMT]). The detectors were connected in series, resulting in a slight difference in retention times observed for radioactive compounds and their corresponding cold standards:
Analytical HPLC system A: Phenomenex Jupiter 4 µ Proteo 90 Å column (250 × 4.6 mm, 4 µm), solvent B isocratic 9% for 2 minutes, then linear gradient to 81% over 30 minutes, flow rate 1.5 mL/min.
Semipreparative HPLC system B: Phenomenex Jupiter 10 µ Proteo 90 Å (250 × 10 mm, 10 µm), solvent B isocratic 9% for 2 minutes, then linear gradient to 81% over 30 minutes, flow rate 3 mL/min.
Isocratic HPLC system C: Phenomenex Jupiter 10 µ C18 300 Å (250 × 10 mm, 10 µm), isocratic 35% acetonitrile–0.05% TFA in water (v/v), flow rate 3 mL/min.
Cell-labeling solutions and biodistribution samples were analyzed on a Wizard 1470 gamma counter (Perkin-Elmer, Waltham, MA). MicroPET scans were acquired on a Focus120 microPET scanner (Siemens Medical Solutions USA, Malvern, PA), and data were processed with the accompanying ASIpro software.
Solid-Phase Peptide Synthesis
Peptides were synthesized on Rink Amide NovaGel HL resin. Manual synthesis was performed using a threefold excess of amino acids and O-(7-azabenzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate (HATU)/N,N-diisopropylethylamine (DIPEA) activation with a 2-hour coupling time, followed by 30 minutes of Fmoc deprotection with 20% piperidine in N,N-dimethylformamide (DMF). Fmoc-PEG28-COOH (formula weight = 1,544.8) was coupled in an overall ratio of 1.65:1 (Fmoc-PEG28-COOH/peptide on resin), split into two 18-hour coupling cycles (1.1:1 and 0.55:1 ratios, respectively).
Nonradioactive FBA or DOTA tris(t-Bu ester) was coupled to the N-terminus of the peptide on solid phase using a threefold excess of the acid and HATU/DIEA activation for 1 hour. CB-TE2A was preactivated18 with N,N'-diisopropylcarbodiimide (DIC) in the presence of DIEA for 30 minutes at room temperature before being coupled to the peptide on solid phase.
The progress of the coupling reactions was monitored by the ninhydrin-based Kaiser test23 or the picrylsulfonic acid test.24 Side-chain protecting groups were removed and the peptides were cleaved off the solid support using TFA/1,2-ethanedithiol/triisopropyl-silane/water 94:2.5:1:2.5 (v/v/v/v). The crude peptides, dissolved in water, were washed three times with diethyl ether before purification on semipreparative HPLC (system B) and characterization using mass spectrometry.
Radiochemical Synthesis of [18F]FBA-1
[18F]FBA was prepared according to a published procedure and coupled to the N-terminally deprotected peptide on solid phase as previously described.12,21,25 Briefly, the [18F]FBA in DMF (50 µL) was withdrawn into a 1 mL fritted syringe containing the peptide resin (5 mg) swollen in DMF, followed by HATU (5 mg) in DMF (30 µL) and DIEA (10 µL) in DMF (30 µL). The reaction mixture was shaken at room temperature for 30 minutes. The solvent was removed, and the resin was washed with DMF (3 × 0.5 mL) and methanol (3 × 0.5 mL). The TFA cleavage mixture (0.7 mL; TFA/triisopropylsilane/water 95:2.5:2.5 v/v/v) was drawn into the syringe, and the reaction mixture was incubated at 30°C for 20 minutes. The solution was collected. Following evaporation of the cleavage solvents, the product was purified by semipreparative HPLC (system C). The fractions containing the product were collected, diluted with water to a total volume of 20 mL, and passed through a C18 Sep-Pak cartridge. The product, trapped on the cartridge, was washed with water (5 mL) and eluted with 1% acetic acid in ethanol (v/v). The solvent was removed by a stream of nitrogen and the product was reconstituted in phosphate-buffered saline (PBS), followed by pH adjustment. The specific activity was determined by comparison of the radiotracer with the nonradioactive standard (HPLC, system A).
Radiochemical Synthesis of [64Cu]Cu-DOTA-1
Aqueous ammonium acetate (1 M, pH 8.5, 7.5 µL) and a solution of DOTA-1 (22 nmol) in water (10 µL) were added to a solution of [64Cu]CuCl2 (4.8 mCi) in 0.04 M aqueous hydrochloric acid (67 µL). The pH was adjusted to 7 to 8 with aqueous ammonium acetate (1 M). The reaction mixture was incubated at room temperature for 1 hour. Ethylenediaminetetraacetic acid (EDTA) (disodium salt, 4 µmol) in aqueous ammonium acetate (0.25 M, pH 7.5, 400 µL) was added to scavenge any remaining free [64Cu]Cu2+. [64Cu]Cu-DOTA-1 was purified by C18 solid-phase extraction (ORTG Inc.) and eluted in ethanol (400 µL). The solution was concentrated to a small volume (approximately 50 µL) using a vacuum-centrifuge and then formulated in PBS. The radiotracer was evaluated for radiochemical purity (TLC, HPLC) and specific activity (HPLC).
Radiochemical Synthesis of [64Cu]Cu-CB-TE2A-1
The synthesis was similar to that of [64Cu]Cu-DOTA-1 except the reaction solution was buffered to pH 7.5 to 8.5 and incubated at 90 to 95°C. Formulation and evaluation (TLC, HPLC) were performed as with [64Cu]Cu-DOTA-1.
In Vitro Experiments
IC50 values were determined as previously described12 using competitive ELISA of the nonradioactive compounds FBA-1, DOTA-1, CB-TE2A-1, FBA-2, and the parent peptide A20FMDV2 (2) against biotinylated natural ligands, either biotinylated fibronectin (αvβ6 assays) or vitronectin (αvβ3 assays), in triplicate on microtiter plates. For cell binding experiments, 0.2 µCi aliquots of the radiotracer in 50 µL serum-free DMEM (pH 7.2) was added to the cell suspension (3.75 × 106 cells in 50 µL serum-free DMEM) and incubated at room temperature for 1 hour. The assay tubes, pretreated with bovine serum albumin (5% wt/v in PBS) to block nonspecific binding, were regularly agitated to prevent settling of the cells. Following gentle centrifugation, the supernatant was removed and the cells were washed with 0.5 mL serum-free DMEM. The supernatants were combined, and the cells were resuspended in 0.6 mL serum-free DMEM. To determine the fraction of radioactivity bound to cells, levels of radioactivity in the cell suspension and in the supernatants were measured in a gamma counter. For each radiotracer, experiments with DX3puroβ6 and DX3puro cell lines were carried out simultaneously (n ≥ 4/cell line).
In Vivo Experiments
All animal experiments were conducted under a protocol approved by the University of California, Davis, Animal Use and Care Committee. Male athymic mice (nu/nu; Charles River Laboratories, Wilmington, MA) were inoculated subcutaneously on opposite flanks in the shoulder region with 3 × 106 DX3puro or DX3puroβ6 cells in 100 µL serum-free DMEM. Cell lines were analyzed by flow cytometry before injection to confirm levels of integrin expression. Food and water were available ad libitum. Imaging was conducted 2 to 4 weeks after injection once tumors had reached a diameter of approximately 0.5 cm.
MicroPET Imaging
The radiotracer (approximately 100–250 µCi) in isotonic solution (150–200 µL) was injected intravenously into the tail vein via a catheter in mice (n = 3/tracer) anesthetized with 3% isoflurane. The animals were placed in a feet-first, prone position on the scanner bed and maintained under 1.5 to 2.0% isoflurane. The body temperature was measured by a rectal probe and maintained with a heating pad, supplemented by a heating lamp. For [18F]FBA-1, dynamic 4 × 15-minute scans were acquired starting 15 minutes after injection, followed by single-frame 15-minute scans at 2 and 4 hours after injection. For [64Cu]Cu-DOTA-1 and [64Cu]Cu-CB-TE2A-1, single-frame 15-minute scans were acquired 4 hours after injection. Full body image reconstructions were obtained using a maximum a posteriori algorithm (ASIpro software).
Biodistribution
The radiotracer (approximately 15–30 µCi for the 1-, 2-, and 4-hour time points; 100 µCi for the 12-hour time point) in isotonic solution (150–200 µL) was injected intravenously into the tail vein via a catheter in mice anesthetized with 3% isoflurane. Following a conscious uptake period, the mice were anesthetized (4% isoflurane), sacrificed, and dissected (n = 3/tracer). Organs were rapidly collected, and radioactivity was measured in a gamma counter. Calibrated, decay-corrected radioactivity concentrations are expressed as the percentage of injected dose per gram of tissue (% ID/g).
Statistical Analysis
Data are reported as mean ± SD. Two-tailed Student t-tests were performed to evaluate statistical significance, where p < .05 was considered statistically significant.
Results
Nonradioactive Peptides
The compounds were prepared as described and obtained in high purity. The analytic data for the peptides are as follows: FBA-1, HPLC (system A, UV 220 nm) retention time (Rt) = 17.0 minutes; mass spectrometry (MS) (MALDI) m/z = 3,588.2280 [M + H]+, calculated (C159H285FN33O57) 3,588.0401; DOTA-1, HPLC (system A, UV 220 nm) Rt = 15.3 minutes; MS (MALDI) m/z = 3,851.9736 [M + H]+, calculatd (C168H308N37O63) 3,852.2035; CB-TE2A-1, HPLC (system A, UV 220 nm) Rt = 15.3 minutes; MS (MALDI) m/z = 3,790.0168 [M + H]+, calculated (C168H310N37O59) 3,790.2395.
Radiotracer Preparation
[18F]FBA-1 was prepared by solid-phase radiolabeling with a decay-corrected radiochemical yield of 5.0 ± 1.8% (n = 3). The overall synthesis time was 138 ± 12 minutes. HPLC analysis (system A, PMT, Rt = 17.4 minutes) indicated a radiochemical purity of > 99% and a specific activity of > 1 Ci/µmol.
The 64Cu tracers were radiolabeled with [64Cu]Cu2+ in solution by incubation for 1 hour either at room temperature (DOTA-1) or 90 to 95°C (CB-TE2A-1), with an overall synthesis time of approximately 2.5 hours. The radiochemical yield for [64Cu]Cu-DOTA-1 was > 99%, with a specific activity of 0.48 Ci/µmol. For [64Cu]Cu-CB-TE2A-1, the radiochemical yield was > 99%, with a specific activity of 0.52 Ci/µmol. TLC analysis of [64Cu]Cu-DOTA-1 and [64Cu]Cu-CB-TE2A-1 showed retention factor (Rf) = 0.4 for both compounds. (Free [64Cu]Cu2+ and [64Cu]Cu2+ chelated with EDTA had Rf = 0.0 and Rf = 0.7, respectively.) No degradation of the peptide was observed for CB-TE2A-1 during radiolabeling at the elevated temperatures; analysis by TLC and HPLC (system A, PMT; [64Cu]Cu-DOTA-1: Rt = 15.8 minutes; [64Cu]Cu-CB-TE2A-1: Rt = 15.7 minutes) indicated radiochemical purities of ≥ 99% for both tracers.
In Vitro Evaluation: ELISA
Assays were performed to evaluate the ability of non-radioactive FBA-1, DOTA-1, and CB-TE2A-1 to inhibit binding of biotinylated natural ligands (fibronectin or vitronectin) to the immobilized integrin αvβ6 or the closely related αvβ3. For comparison, the unPEGylated FBA-2 and the parent peptide A20FMDV2 (2) were included as well. Binding values are listed in Table 1. For all compounds, the data show IC50 values in the low nanomolar range for integrin αvβ6, together with no significant binding (IC50 > 10 µmol/L) for integrin αvβ3.
In Vitro Evaluation: Cell Labeling
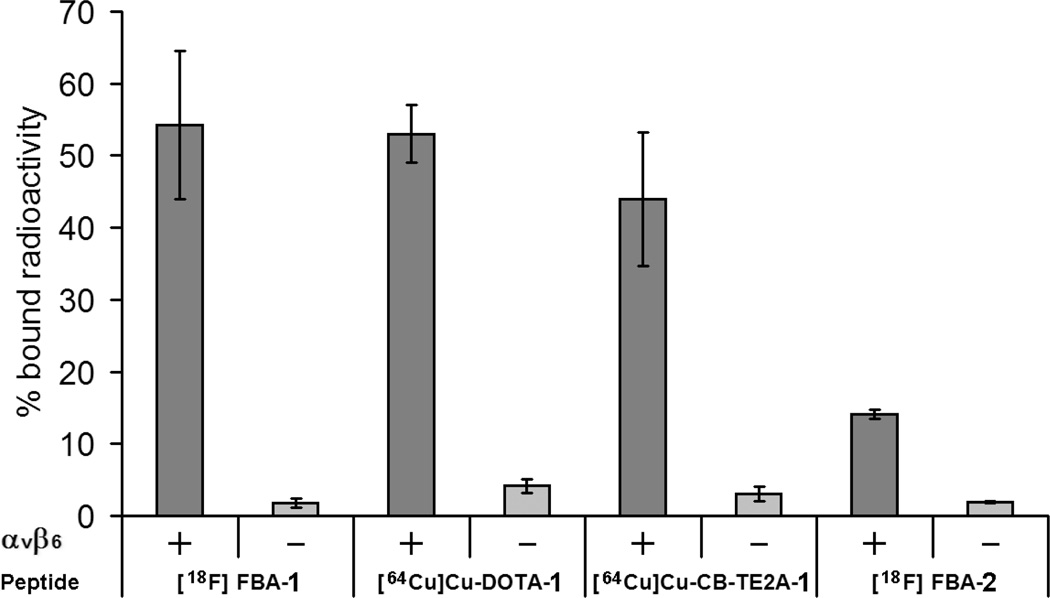
The radiolabeled compounds were evaluated in cell binding assays (Figure 2). The cell line DX3puroβ6, stably transduced to express αvβ6, and the paired control line DX3puro, lacking expression of αvβ6, were analyzed as positive and negative cell lines, respectively.12 Both lines expressed equal levels of integrin αvβ3 and other integrins. For all tracers, less than 5% of radioactivity bound to the αvβ6-negative DX3puro cell line. When the tracers were incubated with αvβ6-positive DX3puroβ6 cells, binding levels of 14% were observed for the unPEGylated FBA-2, whereas the PEGylated tracers [18F]FBA-1, [64Cu]Cu-DOTA-1, and [64Cu]Cu-CB-TE2A-1 showed binding levels between 44 and 54%. The ratio of binding to DX3puroβ6 versus DX3puro was 7.4:1 for FBA-2; it increased to 30.1:1, 12.6:1, and 14.7:1 for [18F]FBA-1, [64Cu]Cu-DOTA-1, and [64Cu]Cu-CB-TE2A-1, respectively.
Figure 2.
αvβ6-Dependent binding of radiolabeled tracers to DX3puroβ6 cells (αvβ6 positive, +) and DX3puro (αvβ6 negative, −). Both cell lines express comparable levels of αvβ3 and other integrins.12 Data are expressed as % bound radioactivity ± SD.
In Vivo Evaluation
The in vivo evaluation of [18F]FBA-1, [64Cu]Cu-DOTA-1, and [64Cu]Cu-CB-TE2A-1 by PET and biodistribution studies was performed using our previously described mouse model12 bearing paired human xenografts of DX3puroβ6 (αvβ6 positive) and DX3puro (αvβ6 negative) (Figure 3 and Figure 4).
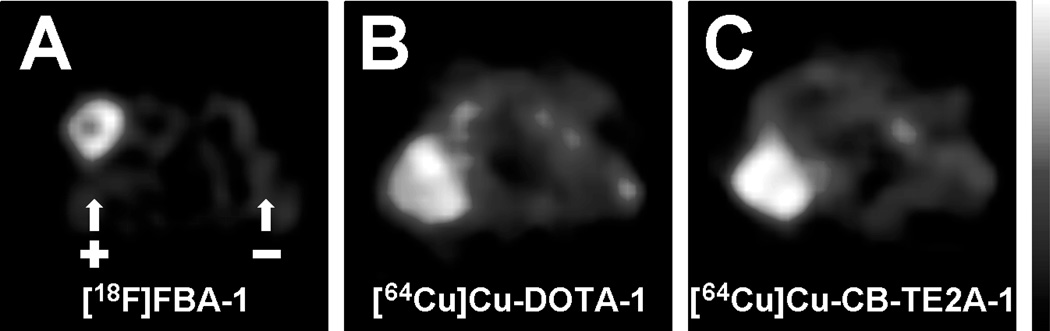
Figure 3.
In vivo microPET imaging in a mouse model (male nu/nu nude mice bearing paired DX3puroβ6 (αvβ6 positive, +) and DX3puro (αvβ6 negative, −) xenografts; n = 3/tracer per experiment). Depicted are representative maximum a posteriori (MAP) transaxial cross sections through the plane of the tumors obtained with [18F]FBA-1 (A), [64Cu]Cu-DOTA-1 (B), and [64Cu]Cu-CB-TE2A-1 (C) 4 hours after injection.
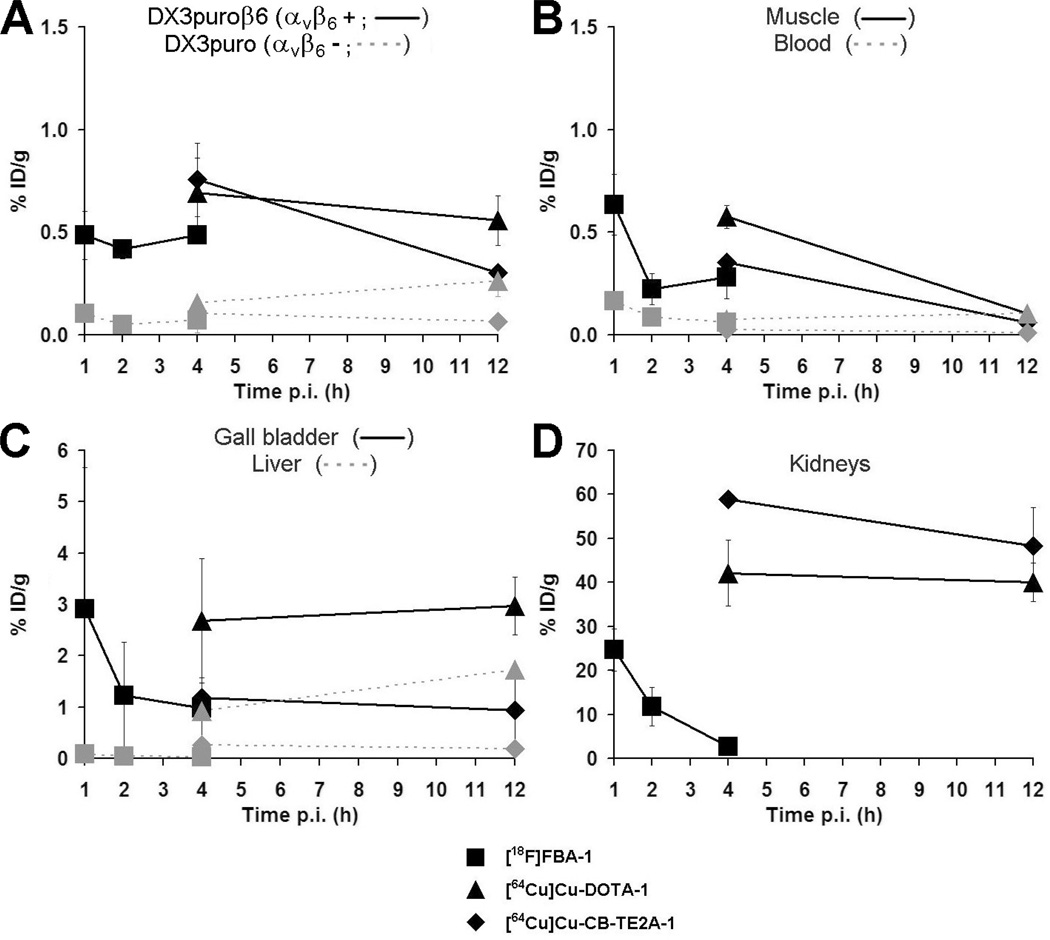
Figure 4.
Biodistribution data for tumors (A) and select organs (B: muscle, blood; C: gall bladder, liver; D: kidneys) obtained 1, 2, and 4 hours postinjection ([18F]FBA-1) or 4 and 12 hours postinjection ([64Cu]Cu-DOTA-1 and [64Cu]Cu-CB-TE2A-1) in the DX3puroβ6/DX3puro mouse model (n = 3/tracer per experiment per time point). Data are expressed as decay corrected % ID/g (points, % ID/g; bars, SD, smaller than the size of the symbol for some data points).
Initial microPET scans obtained 15 to 75 minutes (4 × 15-minute frames), 2 hours, and 4 hours after injection with [18F]FBA-1 (n = 3 animals) showed selective uptake in the αvβ6-positive over the αvβ6-negative xenograft (see Figure 3A), along with renal clearance as the major route of elimination from the body (not shown). The later time points indicated efficient clearance from the kidneys. MicroPET scans for [64Cu]Cu-DOTA-1 and [64Cu]Cu-CB-TE2A-1 obtained 4 hours after injection (see Figure 3, B and C; n = 3 animals per tracer) also showed preferential uptake in the αvβ6-positive xenograft. However, very high levels of activity were also observed with both [64Cu]Cu tracers in the kidneys. These observations prompted a more detailed evaluation by biodistribution studies: Data obtained 1, 2, and 4 hours postinjection for [18F]FBA-1 (n = 3 animals/time point) revealed that uptake in the αvβ6-positive DX3puroβ6 xenograft remained steady at approximately 0.5% ID/g throughout (see Figure 4A), with αvβ6-positive/αvβ6-negative xenograft ratios > 4.5:1 (p < .006). Values for blood, muscle, and liver were low and showed good clearance over the duration evaluated (see Figure 4, B and C). Clearance from the kidneys (see Figure 4D) and excretion in the urine were rapid (kidneys: 1 hour, 24.7 ± 4.8% ID/g; 4 hours, 2.7 ± 0.8% ID/g; urine: 1 hour, 231 ± 21% ID/g; 4 hours, 103 ± 110% ID/g).
Uptake values of [64Cu]Cu-DOTA-1 and [64Cu]Cu-CB-TE2A-1 (4 and 12 hours post injection, n = 3 animals per time point per tracer) in the αvβ6-positive xenograft were in the same range as seen for [18F]FBA-1 (see Figure 4A; DX3puroβ6/DX3puro ratio 4 hours post injection: [64Cu]Cu-DOTA-1, 4.4:1, p = .006; [64Cu]Cu-CB-TE2A-1, 7.3:1, p = .003). However, the biodistribution studies also confirmed the unexpectedly high and persistent levels (40 to 60% ID/g; see Figure 4D) of radioactivity in the kidneys for the two 64Cu tracers. Clearance from blood and muscle was good for both compounds and comparable to the values seen for the 18F tracer (see Figure 4B). Whereas [64Cu]Cu-CB-TE2A-1 showed low levels of radioactivity in the liver and gallbladder (see Figure 4C; 4 hours: 0.25 ± 0.02% ID/g and 1.2 ± 0.3% ID/g, respectively, again similar to those of the 18F tracer), [64Cu]Cu-DOTA-1 showed higher and, for the liver, increasing levels of radioactivity (liver: 4 hours: 0.93 ± 0.18% ID/g, 12 hours: 1.7 ± 0.1% ID/g, p = .002; gallbladder: 4 hours: 2.7 ± 1.2% ID/g, 12 hours: 3.0 ± 0.6% ID/g, p = not significant).
Discussion
The peptide A20FMDV2 has been recognized as a high-affinity, high-specificity ligand for the integrin αvβ612,26 and provides an attractive platform for the development of targeted in vivo imaging tracers. For the present study, solid-phase peptide synthesis and radiolabeling of the 18F and 64Cu tracers followed robust protocols,15,21,25 yielding the radiotracers in high radiochemical purity (≥ 99%).
Given that modifications of a peptide such as the N-terminal addition of the prosthetic group and/or PEGylation can potentially influence the targeted binding behavior, the affinities and selectivities for αvβ6 of FBA-1, DOTA-1, and CB-TE2A-1 were first compared with A20FMDV2 (2)12 and unPEGylated FBA-2 (see Figure 1). The results obtained by competitive ELISA (see Table 1) demonstrated that neither the introduction of the PEG moiety nor the choice of prosthetic group had any significant deleterious effect on binding to purified αvβ6. All compounds selectively bound to integrin αvβ6 with the same high affinity as the parent A20FMDV2 and exhibited > 1,600-fold selectivity over the closely related integrin αvβ3. These findings were corroborated by subsequent cell binding studies with the radiotracers using the melanoma cell line DX3puroβ6, stably transfected to express αvβ6, and its paired control line DX3puro (see Figure 2): 44 to 54% of [18F]FBA-1, [64Cu]Cu-DOTA-1, and [64Cu]Cu-CB-TE2A-1 bound to DX3puroβ6 within 1 hour, whereas less than 5% bound to DX3puro under identical conditions. Notably, both cell lines expressed comparable levels of integrins αvβ3 and other integrins,12 underscoring the high selectivity of the A20FMDV2 peptide sequence for αvβ6. Preliminary studies with the 18F tracers indicated some internalization into cells (not shown). PEGylation is known to positively affect the characteristics of biologically important drug or diagnostic molecules.27,28 In this cell binding assay, the presence of a small PEG spacer between the prosthetic group and the targeting peptide was also found to have a significant, positive effect on binding to αvβ6, whereas an additional change of the prosthetic group at the end of the PEG spacer had only a slight effect: only 14% of the unPEGylated [18F]FBA-2 bound to DX3puroβ6, compared with the 54% for its PEGylated derivative, [18F]FBA-1, and 53% and 44% for [64Cu]Cu-DOTA-1 and [64Cu]Cu-CB-TE2A-1, respectively. These results highlight the significant differences between αvβ6 captured on microtiter plates and αvβ6 expressed on a cell surface. They underscore the importance of considering the biologic environment early in the development of targeted imaging tracers.
In preliminary studies, [18F]FBA-1 had shown the greatest promise in vivo, combining generally good clearing from nontarget tissues with excellent retention in αvβ6-expressing xenografts. Besides improved tumor retention, introduction of the PEG moiety also resulted in elevated levels of radioactivity in the kidneys, especially at the early time points, but renal clearing remained efficient (see Figure 4D): values dropped from 24.7 ± 4.8% ID/g at 1 hour post injection to 2.7 ± 0.8% ID/g at 4 hours postinjection (compared with 3.6 ± 1.4% ID/g and 0.15 ± 0.09% ID/g for [18F]FBA-2 at the same time points).12 [64Cu]Cu-DOTA-1 and [64Cu]Cu-CB-TE2A-1 were prepared to evaluate the pharmacokinetics of PEGylated A20FMDV2-based radiotracers over longer periods of time. For all three tracers, selective uptake in the αvβ6-expressing DX3puroβ6 xenograft was observed by microPET in the mouse model (see Figure 3). In images obtained 4 hours postinjection [18F]FBA-1 appeared to show slightly better DX3puroβ6 to background contrast compared with [64Cu]Cu-DOTA-1 and [64Cu]Cu-CB-TE2A-1. However, most notably, the 4-hour data for both 64Cu tracers also revealed high levels of radioactivity in the kidneys. Biodistribution studies subsequently corroborated these observations: uptake in the kidneys was high and persistent (4 and 12 hours: > 40% ID/g), in stark contrast to the good clearing behavior observed for [18F]FBA-1. The rapid clearing of [18F]FBA-1 indicates that the metal chelates, rather than the PEG moiety, are likely key reasons for the retention. Other studies have suggested several possible reasons for uptake and trapping of radiopharmaceuticals in the kidneys.13,29 It is generally understood that glomerular filtration and reabsorption in the proximal tubules (endocytosis and lysosomal metabolism) play key roles in the retention and that different mechanisms may be involved to various degrees for different radiotracers. To reduce trapping of the radiotracer or its metabolites in the tubular cells and thus reduce the radiation dose to the kidney, either the rate of endocytosis needs to be low or exocytosis and subsequent excretion in the urine need to be efficient.29,30 In some instances, reducing the positive charge of metal-chelating radiotracers resulted in significantly improved clearance behavior, whereas the correlation was less clear in other studies.29,31,32 When charge effects were evaluated specifically for 64Cu-labeled macrocyclic complexes, increased positive formal charges were also found to result in increased kidney accumulation, whereas the related negatively charged or neutral complexes showed better renal clearance.33 Compared with the high values seen for our 64Cu tracers, in these studies, kidney uptake of [64Cu]Cu-DOTA was comparatively modest, reaching only 1.26 ± 0.14% ID/organ (2 hours post injection; rat) and dropping to 0.54 ± 0.08% ID/organ 24 hours after injection.
The calculated charges for [18F]FBA-1, [64Cu]Cu-DOTA-1, and [64Cu]Cu-CB-TE2A-1 are +2, +1, and +3, respectively. Even if slightly different charges are assumed under physiologic conditions, the relative order should remain unchanged as the tracers differ only in the prosthetic group. Thus, if kidney retention were chiefly charge related, uptake values for [18F]FBA-1 should be comparable to and fall between those of the two 64Cu tracers. Clearly, this assumption in not supported by our observations. Similar, seemingly contradictory results have been reported for charge modifications of some octreotide radiotracers.13,29 Anderson and colleagues showed that radiometal chelate–conjugated peptides that bind to cell surface receptors such as somatostatin are taken up intracellularly into lysosomes, and then the compounds are metabolized to radiometal chelate–N-terminal amino acids.34,35 These metal chelate–based metabolites are retained in the kidneys, whereas halogenated metabolites are likely effluxed from the kidney cells. Interestingly, PEGylation has been suggested as a general strategy to improve the pharmacokinetics of radiopharmaceuticals and thereby reduce renal retention.14,27,29
Despite the unfavorable renal pharmacokinetics encountered for [64Cu]Cu-DOTA-1 and [64Cu]Cu-CB-TE2A-1, some noteworthy differences were observed for other organs. Most notably, the CB-TE2A tracer was superior to the DOTA tracer with respect to liver uptake (see Figure 4). Corroborating our initial assumption, only [64Cu]Cu-DOTA-1 showed elevated and increasing levels of radioactivity in the liver. Even though this study was not extended beyond the 12-hour time point, other reports support the assumption that this trend, caused by in vivo transchelation of [64Cu]Cu2+, would have continued.36 Thus, despite the limitations observed here for the 64Cu chelators in conjunction with A20FMDV2, CB-TE2A has shown encouraging results in other studies,16,18,20 making it a valuable alternative to other 64Cu chelators for peptide radiotracers.
Conclusion
Peptide radiotracers based on the A20FMDV2 sequence hold promise for targeted detection of the integrin αvβ6, a cell surface receptor implicated in many aggressive cancers. Three PEGylated A20FMDV2 tracers, [18F]FBA-1, [64Cu]Cu-DOTA-1, and [64Cu]Cu-CB-TE2A-1, were compared. In vitro, all three compounds showed excellent selectivity for αvβ6. ELISA data were equivalent to those of the parent peptide A20FMDV2. Cell binding assays showed 13- to 30-fold preferential binding to αvβ6-expressing cells over the αvβ6-negative control, confirming maintained specificity αvβ6 in biologic systems. In vivo, all three tracers also showed similar, preferential uptake for the αvβ6-expressing xenograft over the αvβ6-negative control. The use of 64Cu tracers allowed studies over an extended period of time. Elevated levels of radioactivity in the liver for [64Cu]Cu-DOTA-1 indicated some transchelation. By contrast, [64Cu]Cu-CB-TE2A-1 did not show any loss of [64Cu]Cu2+. Significantly, both 64Cu-labeled tracers, but not [18F]FBA-1, showed unexpectedly high and persistent levels of radioactivity in the kidneys, making them in their present form less suitable candidates than [18F]FBA-1 for in vivo imaging of αvβ6. However, a potential application of the 64Cu-labeled compounds in their current form, particularly [64Cu]Cu-CB-TE2A-1, could be to image αvβ6-expressing neoplasms that are not obstructed by the kidney radioactivity.
Acknowledgments
We thank J. Choi (mass spectrometry) and the staff of the University of California, Davis, Center for Molecular and Genomic Imaging (imaging support).
Financial disclosure of authors: National Institutes of Health funding is acknowledged: grants R21 CA107792 and R01 CA093375.
Footnotes
Financial disclosure of reviewers: None reported.
References
- 1.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 2.Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 3.Thomas GJ, Nystrom ML, Marshall JF. αvβ6 integrin in wound healing and cancer of the oral cavity. J Oral Pathol Med. 2006;35:1–10. doi: 10.1111/j.1600-0714.2005.00374.x. [DOI] [PubMed] [Google Scholar]
- 4.Sipos B, Hahn D, Carceller A, et al. Immunohistochemical screening for β6-integrin subunit expression in adenocarcinomas using a novel monoclonal antibody reveals strong up-regulation in pancreatic ductal adenocarcinomas in vivo and in vitro. Histopathology. 2004;45:226–236. doi: 10.1111/j.1365-2559.2004.01919.x. [DOI] [PubMed] [Google Scholar]
- 5.Elayadi AN, Samli KN, Prudkin L, et al. A peptide selected by biopanning identifies the integrin αvβ6 as a prognostic biomarker for nonsmall cell lung cancer. Cancer Res. 2007;67:5889–5895. doi: 10.1158/0008-5472.CAN-07-0245. [DOI] [PubMed] [Google Scholar]
- 6.Bates RC, Bellovin DI, Brown C, et al. Transcriptional activation of integrin β6 during the epithelial-mesenchymal transition defines a novel prognostic indicator of aggressive colon carcinoma. J Clin Invest. 2005;115:339–347. doi: 10.1172/JCI23183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hazelbag S, Kenter GG, Gorter A, et al. Overexpression of the αvβ6 integrin in cervical squamous cell carcinoma is a prognostic factor for decreased survival. J Pathol. 2007;212:316–324. doi: 10.1002/path.2168. [DOI] [PubMed] [Google Scholar]
- 8.Zhang ZY, Xu KS, Wang JS, et al. Integrin αvβ6 acts as a prognostic indicator in gastric carcinoma. Clin Oncol. 2008;20:61–66. doi: 10.1016/j.clon.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Cherry SR. The 2006 Henry N. Wagner Lecture: Of mice and men (and positrons)—advances in PET imaging technology. J Nucl Med. 2006;47:1735–1745. [PubMed] [Google Scholar]
- 10.Alavi A, Kung JW, Zhuang HM. Implications of PET based molecular imaging on the current and future practice of medicine. Semin Nucl Med. 2004;34:56–69. doi: 10.1053/j.semnuclmed.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 11.McLarty K, Reilly RM. Molecular imaging as a tool for personalized and targeted anticancer therapy. Clin Pharmacol Ther. 2007;81:420–424. doi: 10.1038/sj.clpt.6100096. [DOI] [PubMed] [Google Scholar]
- 12.Hausner SH, DiCara D, Marik J, et al. Use of a peptide derived from foot-and-mouth disease virus for the non-invasive imaging of human cancer: generation and evaluation of 4-[18F]fluorobenzoyl A20FMDV2 for in vivo imaging of integrin αvβ6 expression with positron emission tomography. Cancer Res. 2007;67:7833–7840. doi: 10.1158/0008-5472.CAN-07-1026. [DOI] [PubMed] [Google Scholar]
- 13.Smith SV. Molecular imaging with copper-64. J Inorg Biochem. 2004;98:1874–1901. doi: 10.1016/j.jinorgbio.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 14.De Leon-Rodriguez LM, Kovacs Z. The synthesis and chelation chemistry of DOTA-peptide conjugates. Bioconjug Chem. 2008;19:391–402. doi: 10.1021/bc700328s. [DOI] [PubMed] [Google Scholar]
- 15.Wadas TJ, Anderson CJ. Radiolabeling of TETA- and CB-TE2A-conjugated peptides with copper-64. Nat Protoc. 2006;1:3062–3068. doi: 10.1038/nprot.2006.431. [DOI] [PubMed] [Google Scholar]
- 16.Sprague JE, Peng Y, Fiamengo AL, et al. Synthesis, characterization and in vivo studies of Cu(II)-64-labeled cross-bridged tetraazamacrocycle-amide complexes as models of peptide conjugate imaging agents. J Med Chem. 2007;50:2527–2535. doi: 10.1021/jm070204r. [DOI] [PubMed] [Google Scholar]
- 17.Boswell CA, Sun XK, Niu WJ, et al. Comparative in vivo stability of copper-64-labeled cross-bridged and conventional tetraazamacrocyclic complexes. J Med Chem. 2004;47:1465–1474. doi: 10.1021/jm030383m. [DOI] [PubMed] [Google Scholar]
- 18.Sprague JE, Peng YJ, Sun XK, et al. Preparation and biological evaluation of copper-64-labeled Tyr3-octreotate using a cross-bridged macrocyclic chelator. Clin Cancer Res. 2004;10:8674–8682. doi: 10.1158/1078-0432.CCR-04-1084. [DOI] [PubMed] [Google Scholar]
- 19.Boswell CA, Regino CAS, Baidoo KE, et al. Synthesis of a cross-bridged cyclam derivative for peptide conjugation and 64Cu radiolabeling. Bioconjugate Chem. 2008;19:1476–1484. doi: 10.1021/bc800039e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sprague JE, Kitaura H, Zou W, et al. Noninvasive imaging of osteoclasts in parathyroid hormone-induced osteolysis using a 64Cu-labeled RGD peptide. J Nucl Med. 2007;48:311–318. [PMC free article] [PubMed] [Google Scholar]
- 21.Hausner SH, Marik J, Gagnon MKJ, et al. In vivo positron emission tomography (PET) imaging with an αvβ6 specific peptide radiolabeled using 18F-“click” chemistry: evaluation and comparison with the corresponding 4-[18F]fluorobenzoyl- and 2-[18F]fluoropropionyl-peptides. J Med Chem. 2008;51:5901–5904. doi: 10.1021/jm800608s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong EH, Weisman GR, Hill DC, et al. Synthesis and characterization of cross-bridged cyclams and pendant-armed derivatives and structural studies of their copper(II) complexes. J Am Chem Soc. 2000;122:10561–10572. [Google Scholar]
- 23.Kaiser E, Colescott RL, Bossinger CD, et al. Color test for detection of free terminal amino groups in solid-phase synthesis of peptides. Anal Biochem. 1970;34:595–598. doi: 10.1016/0003-2697(70)90146-6. [DOI] [PubMed] [Google Scholar]
- 24.Hancock WS, Battersby JE. New micro-test for detection of incomplete coupling reactions in solid-phase peptide-synthesis using 2,4,6-trinitrobenzene-sulphonic acid. Anal Biochem. 1976;71:260–264. doi: 10.1016/0003-2697(76)90034-8. [DOI] [PubMed] [Google Scholar]
- 25.Sutcliffe-Goulden JL, O’Doherty MJ, Marsden PK, et al. Rapid solid phase synthesis and biodistribution of 18F-labelled linear peptides. Eur J Nucl Med Mol Imaging. 2002;29:754–759. doi: 10.1007/s00259-001-0756-3. [DOI] [PubMed] [Google Scholar]
- 26.DiCara D, Rapisarda C, Sutcliffe JL, et al. Structure-function analysis of Arg-Gly-Asp helix motifs in αvβ6 integrin ligands. J Biol Chem. 2007;282:9657–9665. doi: 10.1074/jbc.M610461200. [DOI] [PubMed] [Google Scholar]
- 27.Harris JM, Chess RB. Effect of PEGylation on pharmaceuticals. Nat Rev Drug Discovery. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 28.Greenwald RB, Choe YH, McGuire J, et al. Effective drug delivery by PEGylated drug conjugates. Adv Drug Deliv Rev. 2003;55:217–250. doi: 10.1016/s0169-409x(02)00180-1. [DOI] [PubMed] [Google Scholar]
- 29.Akizawa H, Uehara T, Arano Y. Renal uptake and metabolism of radiopharmaceuticals derived from peptides and proteins. Adv Drug Deliv Rev. 2008;60:1319–1328. doi: 10.1016/j.addr.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Boerman OC, Oyen WJG, Corstens FHM. Between the Scylla and Charybdis of peptide radionuclide therapy: hitting the tumor and saving the kidney. Eur J Nucl Med. 2001;28:1447–1449. doi: 10.1007/s002590100597. [DOI] [PubMed] [Google Scholar]
- 31.Akizawa H, Arano Y, Mifune M, et al. Effect of molecular charges on renal uptake of 111In-DTPA-conjugated peptides. Nucl Med Biol. 2001;28:761–768. doi: 10.1016/s0969-8051(01)00241-4. [DOI] [PubMed] [Google Scholar]
- 32.Froidevaux S, Calame-Christe M, Tanner H, et al. Melanoma targeting with DOTA-α-melanocyte-stimulating hormone analogs: structural parameters affecting tumor uptake and kidney uptake. J Nucl Med. 2005;46:887–895. [PubMed] [Google Scholar]
- 33.Jones-Wilson TM, Deal KA, Anderson CJ, et al. The in vivo behavior of copper-64-labeled azamacrocyclic complexes. Nucl Med Biol. 1998;25:523–530. doi: 10.1016/s0969-8051(98)00017-1. [DOI] [PubMed] [Google Scholar]
- 34.Duncan JR, Stephenson MT, Wu HP, et al. Indium-111-diethylenetriaminepentaacetic acid octreotide is delivered in vivo to pancreatic, tumor cell, renal, and hepatocyte lysosomes. Cancer Res. 1997;57:659–671. [PubMed] [Google Scholar]
- 35.Bass LA, Lanahan MV, Duncan JR, et al. Identification of the soluble in vivo metabolites of indium-111-diethylenetriaminepentaacetic acid-D-Phe1-octreotide. Bioconjug Chem. 1998;9:192–200. doi: 10.1021/bc970158h. [DOI] [PubMed] [Google Scholar]
- 36.Wadas TJ, Wong EH, Weisman GR, et al. Copper chelation chemistry and its role in copper radiopharmaceuticals. Curr Pharm Des. 2007;13:3–16. doi: 10.2174/138161207779313768. [DOI] [PubMed] [Google Scholar]