Abstract
Malignant B-cells express measurable levels of HLA class II proteins, but often escape immune recognition by CD4+ T cells. Resveratrol (Resv) has been the focus of numerous investigations due to its potential chemopreventive and anti-cancer effects, but it has never been tested in the regulation of immune components in B-cell tumors. Here, we show for the first time that Resv treatment enhances HLA class II-mediated immune detection of B-cell lymphomas by altering immune components and class II presentation in tumor cells. Resv treatment induced an upregulation of both classical and non-classical HLA class II proteins (DR and DM) in B-lymphoma cells. Resv also altered endolysosomal cathepsins (Cat S, B and D) and a thiol reductase (GILT), increasing HLA class II-mediated antigen (Ag) processing in B-cell lymphomas and their subsequent recognition by CD4+ T cells. Mechanistic study demonstrated that Resv treatment activated the recycling class II pathway of Ag presentation through upregulation of Rab 4B protein expression in B-lymphoma cells. These findings suggest that HLA class II-mediated immune recognition of malignant B-cells can be improved by Resv treatment, thus encouraging its potential use in chemoimmunotherapy of B-cell lymphoma.
Keywords: B-cell lymphoma, resveratrol, apoptosis, HLA class II, rab 4b, endocytosis and recycling, Antigen presentation, CD4+ T cell recognition
Introduction
Lymphoma is the most common form of hematological malignancy in the United States [1]. It is the seventh most common cancer in adults and the third most common in children [2–3]. Chemotherapy is currently the primary modality of treatment for this fatal disease. In recent decades conventional chemotherapy has produced a dramatic improvement in lymphoma/leukemia patients’ survival, but patients whose cells exhibit in vitro drug resistance may have a substantially worse prognosis than drug sensitive patients [4–7]. Attempts to improve the survival and diminish the unwanted side effects of cancer treatment largely depend on strategies that reduce tumor resistance to chemotherapy, while restoring anticancer immune responses. Although conventional chemotherapy for lymphoma has advanced greatly in recent years, the majority of B-cell lymphomas in patients remain incurable, and there is a need for novel therapies with less toxicity and more specific targeting of tumor cells. Immunotherapy has become a major interest as a new treatment modality for B-cell lymphoma since the discovery that the lymphoma-associated Ags could be recognized by T cells [8]. B-cell malignancies also appear to be ideal candidates as targets of immunotherapy approaches, since the monoclonal antibody Rituximab has been widely used in treating these tumors, and is also established in widespread clinical practice [9].
In recent years, many dietary compounds have been identified that exhibit chemopreventive properties, and could be exploited in novel drug development [10]. Among them is the hydroxylated stilbene Resveratrol (Resv), a polyphenol found naturally in various plant species, and particularly in grapes [11–12]. Resv has been found to have a wide variety of cellular targets, and has therapeutic and preventive potential against cancers by blocking tumor initiation, promotion, and progression in a number of cell types and cancer models [12–16]. The anti-cancer potential of Resv was observed after application of Resv as single agent or in combination with radiotherapy or conventional chemotherapy [12,17]. It has also been shown that Resv acts by modulating the activity of many different signaling pathways involved in cell cycle regulation and survival [18]. Despite the anti-proliferative and pro-apoptotic features previously shown, it is not known whether Resv is able to induce immune activation in lymphoma cells. Effective tumor immune responses usually involve the stimulation and maintenance of tumor specific CD8+ HLA class I-restricted cytotoxic T cells (CTL) and tumor-specific CD4+ class II-restricted helper T cells [19]. Several groups have shown that B-cell lymphoma especially Burkitt lymphoma (BL) cells can not present Ags by class I molecules [20], which contributes to their escaping immune recognition from tumor-specific CD8+T cells. Most B-cell tumors including BL, express detectable levels of HLA class II molecules, could provide their own HLA class II Ag presentation and be targets for CD4+ T cells.
Unlike HLA class I molecules, which are constitutively expressed by all nucleated cells, HLA class II molecules are conserved to thymic epithelial cells and professional antigen presenting cells (APCs) like; dendritic cells, B cells, macrophages, and some lymphoid malignancies like B-cell lymphoma [21–22]. Antigenic fragments derived from both exogenous and endogenous proteins are taken up by APCs and are presented to the immune system by HLA class II molecules. After association with the invariant chain (Ii) in the endoplasmic reticulum, class II molecules are targeted to the endolysosomal compartment, where it contacts antigenic peptides generated in the endocytic pathway [22]. Upon degradation of the Ii, class II-associated invariant chain peptide (CLIP) remains associated to the class II binding groove. In B cells, CLIP is dissociated with the help of a non-classical class II molecule HLA-DM [23]. The removal of CLIP and the peptide display on class II proteins are thought to be interfered with another non-classical class II molecule, HLA-DO [24]. The HLA class II pathway functions through both classical and non-classical methods of Ag processing, peptide loading and presentation to CD4+ T cells. In the classical pathway, Ags are processed in endolysosomal compartments by acidic cathepsins (e.g., Cat S, B and D) and reductases (e.g., GILT), and the resulting class II-peptide complexes traffic to the cell surface for interaction with CD4+ T cells [25]. Studies in B-cells have reported that early endosomal compartments can serve as a secondary site for peptide loading where recycling class II could play a role in CD4+ T cell stimulation. Whether Resv treatment influences HLA class II Ag processing in the endolysosomal compartments and modulates T cell responses remains unknown. In the non-classical pathway, the regulation of endocytosis and recycling of HLA class II proteins and their ligands is also influenced by the Rab family of small GTPases responsible for intracellular vesicle trafficking [26]. Of these proteins, Rab 4B has been implicated in endosomal recycling and HLA class II-restricted Ag presentation [27]. In this study, we have examined the effects of Resv on the induction of immune activation, and describe the specific mechanisms of Resv-induced class II protein expression in human B-cell lymphoma and their subsequent functions in triggering CD4+ T cells. We found that low doses of Resv upregulate the expression of Rab 4B, resulting in increased HLA class II protein recycling and restoring CD4+ T cell recognition of B-cell lymphomas. While B-cell lymphoma poses a defect in class II-mediated immune recognition, enhancement of endocytic recycling and immune activation by Resv may offer new targets for the development of novel immunotherapeutics for this malignant disease.
Material and methods
Cell lines
Human Burkitt lymphoma cell lines Nalm-6, Ramos and Daudi were maintained in complete RPMI-1640 (Invitrogen) supplemented with 10% fetal bovine serum (FBS) (HyClone), 50 U/ml penicillin and 50 μg/ml streptomycin (Mediatech Inc), and 1% L-glutamine (Mediatech). Nalm-6, Ramos, and Daudi cells were obtained from Dr. Janice Blum (Indiana University, Indianapolis). BL cell line 1G2 was purchased from American Type Culture Collection (ATCC). Nalm-6, Ramos, Daudi, and 1G2 cells were transduced using retroviral vectors for constitutive expression of HLA-DR4 (DRB1-0401) with linked drug selection markers for hygromycin and histidinol resistance [28–30]. Expression of surface HLA-DR4 complexes on cells was confirmed by flow cytometric analysis using the DR4-specific mAb, 359-F10 [28]. The T cell hybridoma line 17.9, which responds to human serum albumin (HSA) residue 64–76K, the T cell hybridoma line 50.84.17, which responds to human HA-flu307-319 peptide, the human IgG κ188-203 specific T cell hybridoma line 2.18a, and κ145-159 specific T cell hybridoma line 1.21 were all obtained from Dr. Janice Blum and maintained in complete RPMI-1640 with 10% FBS, 50 U/ml penicillin, 50 μg/ml streptomycin, and supplemented with 1% L-glutamine and 50 μM β-ME [31–32].
Ags, peptides and antibodies
Human HSA and IgGκ Ags were purchased from Sigma-Aldrich. The human HSA64-76K (VKLVNEVTEFAKTK), HA-flu307-319 (PKYVKQNTLKLAT), IgGκ188-203 (KHKVYACEVTHQGLSS), IgGκ145-159, (KVQWKVDNALQSGNS) peptides were produced using Fmoc technology and an Applied Biosystems Synthesizer as described [30–31]. Peptide purity (>99%) and sequence were analyzed by reverse phase HPLC purification and mass spectroscopy. Peptides were dissolved in DMSO and stored at −20°C until used. The primary antibodies used were human HLA-DR (L243), cathepsins S, B and D (Santa Cruz Biotechnology Inc), Rab 4B (Santa Cruz), Ii, HLA-DM (Santa Cruz), β-actin (clone AC-15) (Sigma), and HLA-DRβ (XD5) (a gift from Dr. Blum at Indiana University). The secondary antibodies used were horseradish peroxidase conjugated anti-mouse (Pierce), anti-rabbit or anti-goat IgG (Santa Cruz).
MTS assay for Resv cytotoxicity and cell death
A 10 mM stock solution of Resv (> 99 % purity, Cat# R5010 Sigma) in DMSO was prepared and kept at −20°C. For all Resv treatments, DMSO final concentration was ≤ 1%. The Nalm-6.DR4, Ramos.DR4, Daudi.DR4 and 1G2.DR4 cells were seeded at 1 × 105 cells/well in 100 μl of appropriate culture medium in a flat-bottom 96-well plate. Resv was then added to appropriate wells for final concentrations of 0, 50, 100, and 200 μM. Following 24 h of Resv treatment, cell viability was measured using the CellTiter 96 AQueous One Solution Cell Proliferation Assay® (Promega). Cells were also treated with 50 μM of Resv for 6, 12 or 24 h, followed by addition of twenty μl of MTS reagent to all wells. The plate was incubated for 2 h at 37°C, and absorbance was read at 490 nm. Experiments were repeated at least three times and the data were expressed as percent cell death ± SD of triplicate wells.
HLA class II Ag presentation assays
For exogenous presentation, Nalm-6.DR4 and Ramos.DR4 cells (2 × 105 cells/well) were treated with vehicle alone or 50 μM of Resv for 24 h at 37°C in culture media in 96-well plate, followed by the addition of HSA Ag (20 μM), HSA64-76K (10 μM) or HA-Flu307-319 peptide (10 μM) to the appropriate wells for the last 4–6 h of incubation. Cells were then washed and cocultured with the peptide specific T cell hybridomas as described [25,31]. Following incubation, the plates were stored at −80°C and analyzed for IL-2 production. To examine the effect of Resv treatment on the endogenous Ag presentation, Daudi.DR4 (express endogenous IgGκ) treated with vehicle alone or 50 μM of Resv were cocultured with the human IgGκ188-203 and IgGκ145-159 peptide specific T cell hybridomas for 24 h. Following incubation, the plates were stored at −80°C and analyzed for IL-2 production. Cells were also treated with bestatin, colchicines, cytochalasin D, and primaquine (Sigma), followed by the addition of HSA64-76K or κ 145-159 peptide and coculture with the peptide specific T cell hybridoma as described. T cell production of IL-2 was quantified by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (R&D Systems). Anti-IL-2 was purchased from Sigma-Aldrich. All assays were repeated at least three times.
Flow cytometric analysis
Control and Resv (50 μM)-treated Nalm-6.DR4 and Ramos.DR4 cells were stained with mAbs directed against HLA-DR (L243) and HLA-DR4 (359-F10), followed by secondary antibodies labeled with FITC (Santa Cruz) [25]. The B cell line, Frev, which constitutively expresses class II DR4, was also stained with those antibodies and used as controls [33]. Samples were then analyzed on a FACScan using CellQuest software (BD Bioscience, Mountain View, CA). Background fluorescence was evaluated using an irrelevant isotype-matched mAbs NN4 and IN-1 as described [25]. Mean fluorescence intensity (MFI) was used to compare the relative class II protein expression on the cell surface.
Western blot analysis
Nalm-6.DR4 and Ramos.DR4 cells were cultured for 24 h in the presence of 0, 50, and 100 μM of Resv. Following treatment, cells were washed and cell lysate was obtained using a standard lysis buffer (10 mM Trizma base, 150 mm NaCl, 1% Triton-X 100). Equal protein concentrations from designated cell lysates were separated on a 4–12% Bis/Tris NuPage gel (Invitrogen). Proteins were transferred onto a nitrocellulose membrane (Pierce), and probed with antibodies for the expression of caspases (3 and 9), HLA-DR, HLA-DM, Ii, and Rab 4B proteins. The secondary antibodies used were horseradish peroxidase conjugated anti-mouse (Pierce), anti-rabbit, or anti-goat IgG (Santa Cruz). Monoclonal antibody for β-actin was used as a protein loading control. Densitometry was performed using a ChemiDoc XRS station (Bio-Rad) where the protein bands were analyzed using the Quantity One 4.6.3 software (Bio-Rad). Relative protein expression levels were stated as a ratio of specific proteins expressed/β-actin for each sample. Data are representative of at least three separate experiments.
Quantitation of endocytosis and class II protein recycling
Ramos.DR4 cells were treated with vehicle alone or 50 μM of Resv overnight, followed by measurement of endocytosis and recycling of class II molecules as described previously [25]. Briefly, control (vehicle treated) and Resv-treated Ramos.DR4 cells were divided into three groups (samples A, B and C), washed with cold PBS, and cell surface proteins were biotinylated with sulfo-NHS-SS-biotin (Pierce, IL) for 15 min at 4°C. Sample A was immediately harvested, washed with ice cold buffer for subsequent analysis. Sample A, thus represents total cell surface proteins accessible to the biotin-labeling reagent in the absence of endocytosis. For sample B, cells were exposed to a glutathione solution to remove the disulfide-linked biotin from surface proteins, with typically greater than 95% release of this label. Cells in sample C were incubated for 15 min at 37°C to allow endocytosis of biotin-labeled surface molecules, followed by exposure to the glutathione solution to remove any residual biotin-label from proteins remaining on the cell surface. Thus, sample C represents the amount of cell surface protein endocytosed in 15 min. Each set of cells were washed extensively and lysed with PBS + 1% Triton + protease inhibitors. Biotinylated cell surface molecules were then detected by capture ELISA using plates coated with antibodies to HLA class II molecules (37.1) or transferrin receptor (B3/25) followed by the addition of streptavidin-peroxidase and colorimetric detection of bound peroxidase. Background readings were subtracted and the percent endocytosis was calculated by the following formula: % cell surface protein endocytosis = (sample C-sample B) / (sample A-sample B).
Statistical analysis
Data from each experimental group were subjected to statistical analysis. Differences between experimental groups were analyzed for statistical significance using Student’s t-test. Values of P ≤ 0.05 were considered significant.
Results
Resv treatment restores HLA class II-restricted Ag processing, presentation, and CD4+ T cell recognition of B-cell lymphomas
Ag presentation by HLA class II molecules plays an important role in inducing anti-tumor immunity and tumor clearance. Resv is a natural polyphenolic antioxidant and has been shown to exhibit cardio-protective as well as anti-inflammatory and anti-tumor effects on various types of cancers [34–37]. However, its effect on HLA class II Ag presentation and immune recognition of B-cell lymphomas remains unknown. B-cell lymphomas each express measurable levels of surface HLA class II molecules. However, in order to gain a more direct comparison of class II-mediated Ag presentation between these cells following Resv treatment, we expressed a common HLA class II allele in several B-cell lymphoma cell lines. B-cell lymphoma cell lines Nalm-6, Ramos, and Daudi were retrovirally transduced to express the DR4 allele, HLA DRB1*0401. Flow cytometric analysis showed that all 3 cell lines were successfully transfected, constitutively expressing the common DR4 allele [Figure 1(A)]. Following DR4 transfection, Nalm-6.DR4 and Ramos.DR4 cells were treated with vehicle alone (DMSO) or different concentrations of Resv (0, 50, 100 and 200 μM) for 24 h. Data obtained from MTS assay showed that the 24 h Resv treatment induced dose-dependent killing in Nalm-6.DR4 and Ramos.DR4 lymphoma cells [Supplemental Figure 1(A)]. The concentration of Resv inhibiting cell growth by 50% (IC50) ranged from about 55.9 to 125.5 μM. Thus, a low concentration of 50 μM of Resv was selected and tested for cell (Nalm-6.DR4) viability at various time points (0, 6, 12 and 24 h) using MTS assay [Supplemental Figure 1(B)]. These results suggest that a low concentration of Resv (50 μM) after 24 h can also induce lymphoma cell death. To determine whether caspases played a role in Resv induced cell death, we tested cell viability in the presence or absence of Resv and the pan-caspase inhibitor Z-VAD-FMK, which irreversibly binds to the catalytic site of caspases. Increased cell viability was observed when Z-VAD-FMK was added to the Resv-treated cells [Supplemental Figure 1(C) and 1(D)], suggesting that active caspases played a key role in inducing apoptosis by Resv.
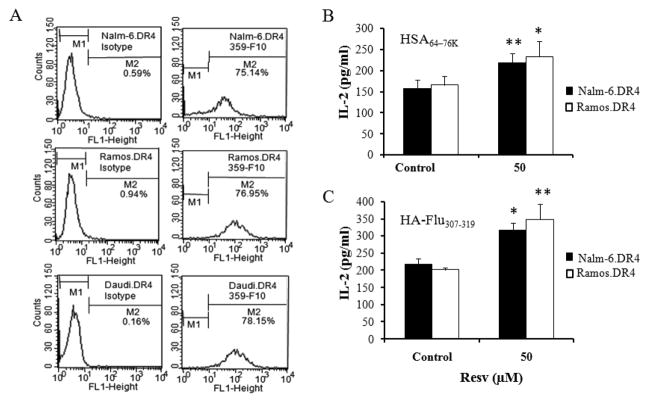
Figure 1.
Resv treatment restores CD4+ T cell recognition of B-lymphoma. (A) B-lymphoma cell lines Nalm-6, Ramos and Daudi were retrovirally transduced with a class II allele HLA-DR4 (DR4B*0401). Cells treated with vehicle alone or Resv were then stained with the HLA-DR4-specific antibody (359-F10) to confirm cell surface DR4 expression. An isotype-matched irrelevant antibody, NN4, was used as a specificity control as described in the method section. Data shown are representative of three independent experiments with similar patterns. (B–C) HLA class II Ag presentation and CD4+ T cell recognition of B-lymphoma cells were carried out on cells seeded in 96-well plates and treated with vehicle alone (Control) or Resv (50 μM) for 24 h, followed by addition of 10 μM of human HSA64-76K or HA-flu307-319 peptide for another 4 h. Thereafter, cells were washed, and cocultured with the peptide specific T cells (17.9 for HSA64-76K and 50.84.17 for HA-flu307-319) as described. The production of IL-2 was measured by ELISA and expressed as mean IL-2 in pg/ml ± SD of triplicate wells. Significant differences were calculated by student’s t-test; * indicates p <0.05, ** indicates p <0.01.
To investigate whether Resv treatment alters HLA class II-restricted Ag processing and presentation, Nalm-6.DR4 and Ramos.DR4 cells were treated with vehicle alone or 50 μM of Resv, followed by the addition of HSA64-76K or HA-flu307-319 peptide for 4 h as described in the methods. After treatment, cells were washed and co-cultured with the peptide specific T cell hybridomas for 24 h, and the production of IL-2 was quantitated by ELISA. Data showed that Resv treatment enhanced the presentation of HSA64-76K and HA-flu307-319 peptides by both B-lymphoma cell lines Nalm-6.DR4 and Ramos.DR4 [Figure 1(B) and 1(C)]. These data suggest that Resv treatment enhances HLA class II-restricted peptide presentation by B-cell lymphomas to CD4+T cells.
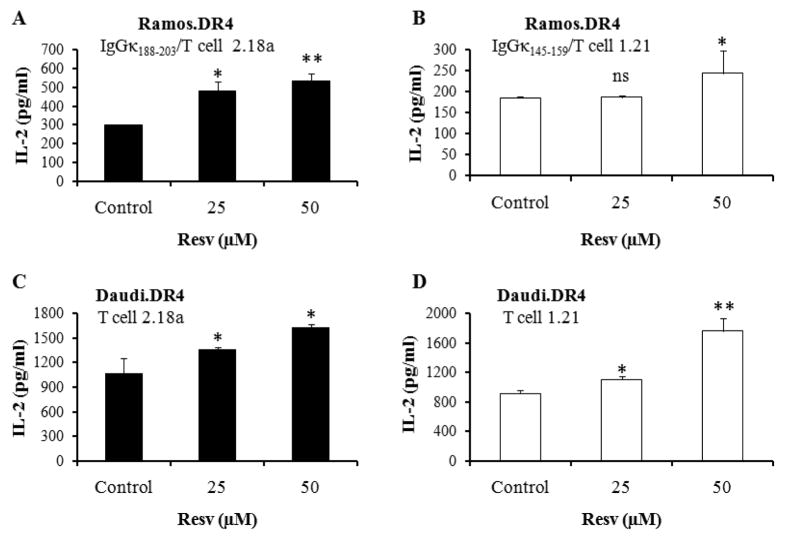
To investigate whether Resv treatment also enhances whole Ag processing and HLA class II-mediated presentation of processed epitopes to CD4+ T cells, Ramos.DR4 cells were pretreated with Resv and incubated with whole IgGκ Ag as described in the methods. Functional Ag presentation assay showed that Resv treatment significantly enhanced the presentation of IgGκ188-203 and IgGκ145-159 epitopes to CD4+ T cells by Ramos.DR4 cells [Figure 2(A) and 2(B)]. To examine whether endogenous presentation of κ epitopes is also altered by Resv treatment, we used Daudi lymphoma cells which naturally express endogenous IgGκ Ag. Daudi.DR4 cells treated with Resv were cocultured with the IgGκ188-203 or IgGκ145-159 epitope specific T cell hybridoma for 24 h. T cell production of IL-2 was measured, and data showed that Resv treatment significantly enhanced endogenous presentation of HLA-DR4-restricted IgGκ epitopes by Daudi cells to CD4+ T cells [Figure 2(C) and 2(D)]. These functional data suggest that Resv treatment enhances HLA class II Ag presentation and CD4+ T cell recognition of B-cell lymphoma.
Figure 2.
Resv treatment enhances HLA class II-restricted Ag processing, presentation, and CD4+ T cell cognition of B-lymphoma cells. (A, B) Briefly, Resv- or vehicle-treated Ramos.DR4 cells were incubated with the whole Ag IgGκ for 24 h. (C, D) Another B-lymphoma cell line Daudi.DR4, which endogenously expresses IgGκ, was also treated with vehicle alone (control) or Resv (25 μM and 50 μM) for 24 h as described in the methods. Both cell lines were washed, and co-cultured with the IgGκ188-203 or IgGκ145-159 epitope specific T cell hybridomas (2.18a for κ188-203 and 1.21 for κ145-159) for 24 h as described. Epitope presentation was measured by T cell production of IL-2 (pg/ml) which was quantitated by ELISA. Data are representative of at least three independent experiments. Significant differences were calculated by student’s t-test; * = p <0.05, ** = p <0.01, ns (not significant).
Resv treatment upregulates intracellular HLA class II molecules, cathepsins and reductases but does not alter surface DR expression in B-cell lymphomas
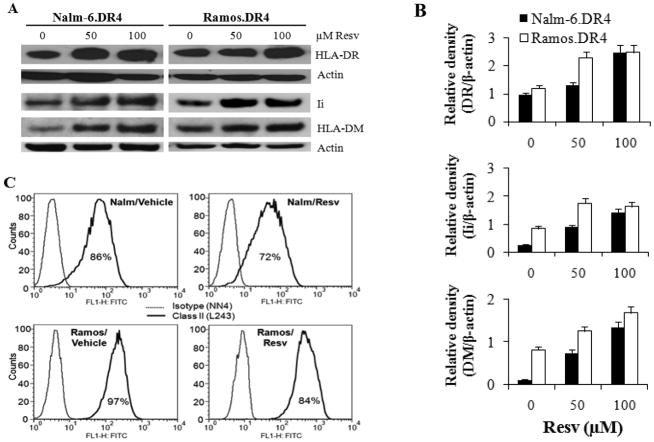
To examine whether Resv treatment altered class II proteins and the components of the class II pathway for immune restoration, we first analyzed intracellular HLA class II protein expression levels in a number of B-lymphoma cell lines treated with either Resv or a vehicle control. Western blot analysis showed that treatment with various concentrations (50–100 μM) of Resv caused significant upregulation of HLA class II molecules in Nalm-6.DR4 and Ramos.DR4 lymphoma cells [Figure 3(A)]. The major components of the class II pathway, such as Ii and HLA-DM molecules were also significantly elevated in Nalm-6.DR4 and Ramos.DR4 cells when treated with 50 μM of resveratrol [Figure 3(A)]. Densitometric analyses of protein bands confirmed that Resv treatment significantly increased class II DR, DM, and Ii expression in Nalm-6 and Ramos cells [Figure 3(B)]. This is an interesting finding because Resv-induced upregulation of HLA-DM molecules may influence epitope loading in the endolysosomal compartments, enhancing Ag presentation by the classical class II pathway. However, the intracellular pool of HLA class II molecules does not often correlate with the amount of cell surface class II proteins expressed by APC or tumor cells. To determine if Resv treatment had any effect on surface DR protein expression, flow cytometry was used on both Nalm-6.DR4 and Ramos.DR4 cell lines. Data showed that Resv treatment did not significantly alter surface DR expression (MFI: vehicle 62.9 vs. Resv 50.3) in Nalm-6 cells [Figure 3(C)]. However, Resv treatment upregulated surface DR expression (MFI: vehicle 189.2 vs. Resv 413.6) in Ramos cells, which were not statistically significant as this treatment also increased isotype control values (MFI: vehicle 10.6 vs. Resv 193) as compared with those of the vehicle group [Figure 3(C)]. Both constitutive and inducible expression of HLA class II gene requires class II transactivator (CIITA), which is the master regulator of HLA class II molecules. Thus, we sought to analyze the role of Resv in CIITA as well as DRα and DRβ gene expression by semi-quantitative PCR (Supplemental Figure 2). Data showed that the CIITA gene expression in lymphoma cells was not significantly changed, implicating a secondary mechanism responsible for the observed increases in CD4+ T cell activation.
Figure 3.
Resv treatment induces upregulation of intracellular HLA class II proteins in B-cell lymphoma. (A) Western blot analysis of whole cell lysates from Nalm-6.DR4 and Ramos.DR4 cell lines treated with vehicle alone (control) or Resv (50 μM or 100 μM) for 24 h. Cell lysates were probed with antibodies for HLA-DR, Ii, and HLA-DM molecules. (B) Relative density of HLA-DR, Ii, and HLA-DM protein bands expressed in Nalm-6.DR4 and Ramos.DR4 cells treated with Resv. Quantitative data were obtained from densitometric analysis of the protein bands detected in Fig. 3A. β-actin was used as a reference band to normalize the original protein loading and to quantitate the expression of HLA-DR, Ii, and HLA-DM proteins in both control and Resv treated lymphoma cells. (C) Flow cytometric analysis of cell surface class II molecules on Nalm-6.DR4 and Ramos.DR4 cells treated with vehicle alone or Resv. Data are representative of at least three separate experiments.
As previously mentioned, intracellular proteases especially acidic cathepsins are responsible for the processing of Ags and class II molecules within endolysosomal compartments of B-cells. We investigated if Resv treatment enhances cathepsin processing and generation of active catalytic subunits, potentially explaining the increases in Ag presentation in B-lymphoma cells and activation of CD4+ T cells. Western blot analysis of the B-cell lymphoma cell lines Nalm-6.DR4 and Ramos.DR4 showed increased expression of active cathepsins S, B, and D [Figure 4(A)] following treatment with low concentration of Resv (50 μM). Densitometric analysis confirmed that several active forms of cathepsins were significantly upregulated by Resv treatment [Figure 4(B)]. These active forms of cathepsins could be involved in both Ag processing (Cat D and B), and class II/Ii processing (Cat S), which could further influence immune recognition of B-cell lymphomas. Western blot analysis also showed Resv treatment slightly upregulated GILT expression in B-cell lymphomas Nalm-6.DR4 and Ramos.DR4 [Figure 4]. Previous study has implicated GILT in many roles in enzymatic processing, including the reduction of oxidized and cysteinylated proteins and peptides, the enhancement of acidic cathepsin activity, and in upregulating HLA-DM protein expression [33]. Taken together these results suggest, Resv treatment increases intracellular pool of HLA class II proteins, enhances Ag processing by increased active cathepsins and reductases within endolysosomal compartments of B-lymphoma cells, potentially contributing to increased activation of CD4+ T cells.
Figure 4.
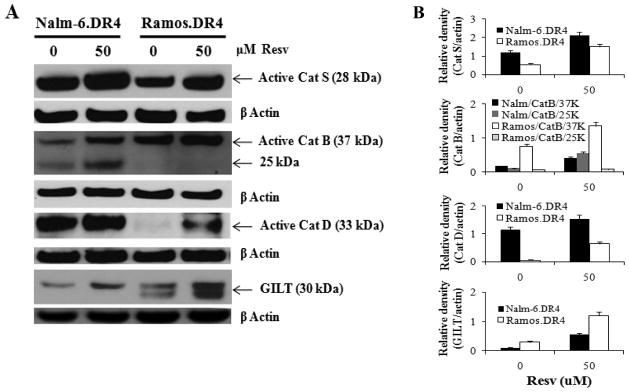
Resv treatment modulates endolysosomal cathepsins and GILT in B-cell lymphoma. (A) Nalm-6.DR4 and Ramos.DR4 cells were treated with vehicle alone or Resv (50 μM) for 24 h. Cells were washed and subjected to western blotting for cathepsins S, B, D, and GILT proteins. β-actin was used as a loading control. Data are representative of at least three separate experiments. (B) Densitometric analysis of the protein bands detected in Fig. 4A. β-actin was used as a reference to quantitate the relative expression of proteins in both control and Resv-treated lymphoma cells.
Resv treatment elevates Rab 4B protein expression and enhances endocytic recycling of HLA class II molecules in B-lymphoma cells
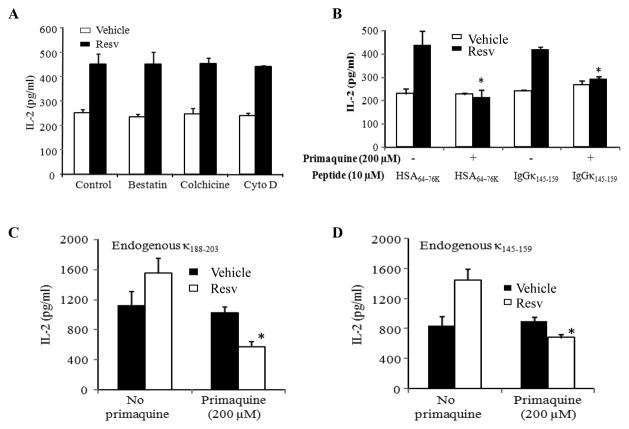
The presence of increased intracellular class II expression observed in Figure 3, suggets that endocytic recycling of class II could be a potential secondary mechanism responsible for increased CD4+ T cell activation following Resv treatment. To determine if this increase in intracellular pool of class II proteins is the result of enhanced cellular endocytosis and recycling, experiments were performed utilizing various inhibitors that interfere with endocytosis and the recycling process. Ramos.DR4 cells were pretreated with vehicle alone or Resv, and incubated in the presence of either bestatin (a competitive protease inhibitor), cytochalasin D (microfilament inhibitor), colchicine (microtubule inhibitor), cytochalasin D (actin polymerization inhibitor) and primaquine (inhibitor of endocytic recycling) for 30 min, followed by the addition of HSA64-76K or IgGκ145-159 peptide (10 μM) for 4 h. Cells were then washed, fixed with 1% paraformaldehyde, and cocultured with the peptide specific T cells for 24 h. The production of IL-2 was quantitated by ELISA as described [25,33]. Blocking intracellular microtubule formation, peptidases or microfilament activity responsible for vesicle trafficking did not alter CD4+ T cell activation [Figure 5(A)]. However, the endocytic recycling inhibitor primaquine significantly reduced Resv treated B-cell lymphoma activation of CD4+ T cells [Figure 5(B)]. Treatment of cells with primaquine also reduced endogenous presentation of kappa epitopes by Resv treated Daudi lymphoma cells [Figure 5(C) and 5(D)]. These results suggest that Resv treatment may enhance endocytic recycling of HLA class II proteins in the early endosomal compartments resulting in increased class II presentation and immune activation in B-cell lymphomas.
Figure 5.
Resv treatment enhances the recycling pathway for Ag presentation by HLA class II molecules in B-cell lymphoma. (A) Ramos.DR4 cells were pretreated with vehicle alone or Resv (50 μM) for overnight and incubated with either bestatin (60 μM), colchicine (40 μM) or cytochalasin D (40 μM) for 30 min, followed by the addition of HSA64-76K peptide (10 μM) for 4 h. (B) Ramos.DR4 cells pretreated with vehicle alone or Resv were also incubated with primaquine (200 μM) for 30 min, followed by the addition of HSA64-76K or κ145-159 peptide (10 μM) for 4 h. Cells were then washed, fixed with paraformaldehyde (1%) and cocultured with the peptide specific T cell hybridoma as described in the methods. (C and D) Daudi.DR4 cells which express endogenous Ig kappa Ag were also pretreated with vehicle alone or Resv (50 μM), followed by the addition of primaquine for overnight, washed and cocultured with the κ188-203 (C) and κ145-159 (D) peptide specific T cell hybridoma. T cell production of IL-2 was measured by ELISA. Data is a representative of at least three separate experiments. * indicates p <0.01.
Previous studies have shown endocytic recycling is a highly regulated system under the control of the small GTPase Rab family of proteins [26,38]. In B cells, Rab 4B has been identified as the key protein responsible for HLA class II endocytic recycling [31,39–40]. Thus, studies next examined the mechanism responsible for Resv-induced endocytic recycling through western blot analysis for Rab 4B protein expression. Ramos.DR4 cells were treated with 50 μM of Resv and subjected to western blot analysis as outlined in the methods section. Results showed that Resv treatment enhanced Rab 4B proteins (31/29 kDa) expression in Ramos.DR4 cells [Figure 6(A)], potentially resulting in increased endocytic recycling. To directly measure this effect, Ramos.DR4 cells were treated with either vehicle alone or Resv (50 μM) and monitored for endocytosis and recycling of HLA class II and transferrin receptor (TfR) through the use of biotin labeling on the cell surface, as described in methods. Results indicated that Resv treatment enhanced class II endocytosis and recycling versus vehicle alone (22.1% vs. 7.9%) in Ramos.DR4 cells, and slightly increased TfR (HLA class I and clathrin dependent endocytosis) recycling as well (11.8% vs. 6.1%) [Figure 6(B)]. These results confirm Resv treatment enhances HLA class II endocytic recycling in B-cell lymphomas resulting in increased Ag presentation and CD4+ T cell activation.
Figure 6.
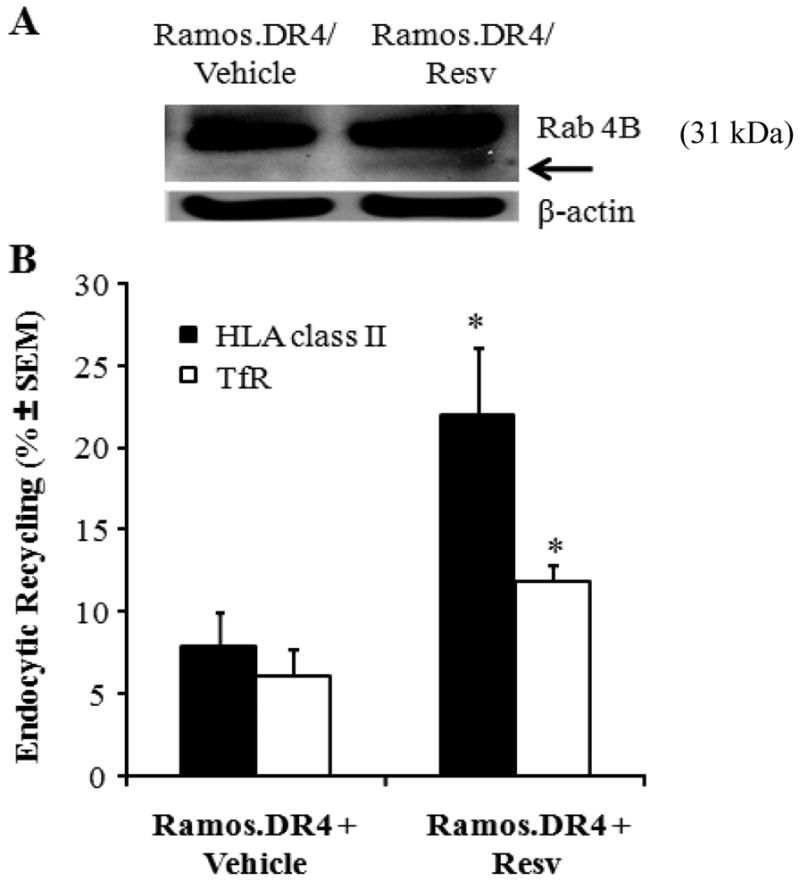
Resv treatment influences Rab 4B protein expression and HLA class II recycling in B-cell lymphoma. (A) Western blot analysis showing an upregulation and cleavage of Rab 4B proteins (arrow) following Resv treatment of Ramos.DR4 lymphoma cells. Cells were treated with vehicle alone or 50 μM of Resv for 24 h, and subjected to western blotting for Rab 4B molecules. Actin was used as a loading control. (B) Ramos.DR4 cells treated with vehicle alone or Resv were also washed in cold PBS, and biotinylated with sulfo-NHS-SS-biotin and divided into three groups. Cells in group I were kept on ice while cell in group II were warmed (37°C for 15 min) to allow internalization of these biotin-labeled surface molecules, followed by cleavage of residual biotin from cell surface proteins with a glutathione solution as described in the methods. The biotin-labeled endocytosed class II proteins were then detected in a capture ELISA, and the percent endocytosis was calculated as described. Data are representative of triplicate wells, and is expressed as percent class II endocytosis/recycling ± SEM.
Discussion
The effectiveness of anti-cancer chemotherapeutics mainly depends on their ability to induce apoptotic cell death. Unfortunately, they also have the drawback of exhibiting high levels of treatment-associated toxicities, which are even more pronounced in HIV-positive and elderly patients [41]. In this regard, the search for natural molecules with limited bystander toxicity is of paramount importance in treating lymphoma and leukemia. Resv and its analogs have received a great deal of attention recently because of their well-known anti-proliferative and anti-tumor activities [42–43]. Particularly relevant functions are its demonstrated antitumor properties through inhibition of cell proliferation, alteration of intracellular signaling pathways, and induction of apoptosis [13,44–45]. Here, we have shown that in addition to the induction of cytotoxicity in a variety of malignancies, low doses of Resv treatment can enhance immune recognition of B-cell lymphoma via the HLA class II pathway.
While chemotherapy treatment has made a major impact on tumor control and remission particularly in hematological malignancies, it often fails to prevent disease recurrence. Surprisingly, interactions between cancer chemotherapy and the immune surveillance of cancer have been given less attention, with some exceptions [46]. It is important that while gaining the advantage of tumor control with cytotoxic chemotherapy, restoring the immune system could reinstate much more effective treatment preventing disease recurrence. We and others have shown that both malignant and nonmalignant B-cells express detectable levels of HLA class II proteins, yet, malignant B-cells often escape immune recognition via the class II pathway [24,47]. Thus, the induction of effective HLA class II Ag presentation in B-cell lymphoma by Resv could aid in the immune surveillance of B-cell malignancies during cytotoxic drug therapy, improving overall treatment efficacy. Our present investigation suggests that in addition to apoptosis induction, Resv induces immune activation in B-cell lymphoma. We have shown that Resv treatment increases the presentation of HLA class II-restricted antigenic peptides in B-cell lymphoma. Both exogenous and endogenous pathways of Ag presentation by B-cell lymphoma are enhanced by Resv. Mechanistic study indicated that Resv treatment upregulated intracellular class II and DM molecules while inducing apoptotic cell death. Interestingly, endolysosomal active cathepsins (Cat S, Cat B, and D) and thiol-reductase (GILT) proteins were also increased following Resv treatment of lymphoma cells. Our previous study demonstrated that GILT colocalizes with acidic cathepsins and enhances cathepsin activity, increases HLA-DM expression, and processes oxidized or cysteinylated proteins and peptides [33]. These functions taken together suggest a heighten HLA class II processing state which could be responsible for some enhanced Ag processing and presentation within Resv treated B-cell lymphomas. However, intracellular expression of these proteins does not always correlate with increased surface expression of HLA class II or increased activation of CD4+ T cells. Our study indicated no significant changes in surface class II protein expression in Resv-treated B-lymphoma cells. These data suggest that an increased level of intracellular DR expression by Resv might not be responsible for the increased activation of T cells via the classical class II presentation pathway; rather a secondary route of Ag presentation could be activated by Resv treatment resulting in enhanced T cell activation.
While the HLA class II pathway may operate through the classical and non-classical pathways of Ag presentation, our data suggest that Resv treatment favors endocytic recycling of class II and immune activation in B-cell lymphoma. We have previously shown that the HSA64-76K peptide requires endocytic recycling while the IgGκ145-159 peptide is presented via the classical class II pathway for T cell activation [30–32]. In the present study, we found that recycling class II, but not peptide recycling may have played a role in CD4+ T cell recognition of Resv-treated B-lymphoma cells. To further understand this mechanism, the small GTPase Rab 4B which can direct endocytic recycling of HLA class II proteins was analyzed. Results showed that Resv treatment upregulated Rab 4B proteins, supporting the notion that endocytic recycling is enhanced by Resv. However, it was necessary to determine if Resv treatment was inducing generalized increases in endocytosis or if the reaction was specific for HLA class II presentation. Experiments using surface biotinylation definitively showed that Resv treatment significantly enhanced recycling of HLA class II proteins while slightly increased those of the HLA class I associated transferrin receptor (TfR) complex. Thus, Resv may increase the immune surveillance of B-cell lymphomas through the recycling class II pathway while simultaneously reducing tumor burden through antiproliferative and apoptotic properties.
In summary, low doses of Resv enhance HLA class II Ag presentation and CD4+ T cell recognition of B-cell lymphoma. This immunostimulatory role is caused in part by enhanced DR/DM protein expression as well as an increased processing of cathepsins and reductases due to Resv treatment. However, Resv does not increase cell surface HLA class II expression suggesting a secondary mechanism of T cell activation. This study also found that Resv treatment increases Rab 4B proteins in B-cell lymphoma, which may be involved in enhanced HLA class II endocytic recycling and peptide presentation. Considering that Resv is not a potent cytotoxic compound when compared with other chemotherapeutics, this natural drug offers promise of a new combination therapy for improving the ability of chemotherapy based treatments to destroy cancer cells, while restoring immune defense to prevent cancer recurrence.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01 CA129560 and R01 CA129560-S1) to A. Haque. The research presented in this article was also supported in part by the Flow Cytometry Shared Resource as part of the Hollings Cancer Center at the Medical University of South Carolina which is funded by a Cancer Center Support Grant P30 CA138313. We also thank Dr. Janice Blum (Indiana University) for providing us with the B-lymphoma lines, Drs. L. Xiang, J. Norris, O. Moussa, C. Johnson, and A. Das (Medical University of South Carolina) for antibodies and reagents.
Abbreviations
- Resv
resveratrol
- HLA
human leukocyte antigen
- IgGκ
immunoglobulin G kappa
- HSA
human serum albumin
- HA-Flu
human influenza hemagglutinin
- Rab-4B
Ras-related protein
Footnotes
Conflicts of Interest
The authors have no financial conflicts of interest.
References
- 1.Society AC. Cancer Facts & Figures 2010. Atlanta: American Cancer Society; 2010. [Google Scholar]
- 2.Jaglowski SM, Linden E, Termuhlen AM, Flynn JM. Lymphoma in adolescents and young adults. Semin Oncol. 2009;36(5):381–418. doi: 10.1053/j.seminoncol.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Ward EM, Thun MJ, Hannan LM, Jemal A. Interpreting cancer trends. Ann N Y Acad Sci. 2006;1076:29–53. doi: 10.1196/annals.1371.048. [DOI] [PubMed] [Google Scholar]
- 4.Zelenetz AD. Lymphoid malignancies: a decade and a half of dramatic improvements in outcome. Clin Lymphoma Myeloma. 2008;8 (Suppl 4):S126–127. doi: 10.3816/CLM.2008.s.007. [DOI] [PubMed] [Google Scholar]
- 5.Cabanillas F. Front-line management of diffuse large B cell lymphoma. Curr Opin Oncol. 2010;22(6):642–645. doi: 10.1097/CCO.0b013e32833ed848. [DOI] [PubMed] [Google Scholar]
- 6.Friedberg JW. Treatment of follicular non-Hodgkin’s lymphoma: the old and the new. Semin Hematol. 2008;45(3 Suppl 2):S2–6. doi: 10.1053/j.seminhematol.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Igney FH, Krammer PH. Immune escape of tumors: apoptosis resistance and tumor counterattack. J Leukoc Biol. 2002;71(6):907–920. [PubMed] [Google Scholar]
- 8.Passoni L, Scardino A, Bertazzoli C, et al. ALK as a novel lymphoma-associated tumor antigen: identification of 2 HLA-A2. 1-restricted CD8+ T-cell epitopes. Blood. 2002;99(6):2100–2106. doi: 10.1182/blood.v99.6.2100. [DOI] [PubMed] [Google Scholar]
- 9.Cartron G, Trappe RU, Solal-Celigny P, Hallek M. Interindividual variability of response to rituximab: from biological origins to individualized therapies. Clin Cancer Res. 2011;17(1):19–30. doi: 10.1158/1078-0432.CCR-10-1292. [DOI] [PubMed] [Google Scholar]
- 10.Lucas DM, Still PC, Perez LB, Grever MR, Kinghorn AD. Potential of plant-derived natural products in the treatment of leukemia and lymphoma. Curr Drug Targets. 2010;11(7):812–822. doi: 10.2174/138945010791320809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jang M, Cai L, Udeani GO, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275(5297):218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 12.Saiko P, Szakmary A, Jaeger W, Szekeres T. Resveratrol and its analogs: defense against cancer, coronary disease and neurodegenerative maladies or just a fad? Mutat Res. 2008;658(1–2):68–94. doi: 10.1016/j.mrrev.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Athar M, Back JH, Kopelovich L, Bickers DR, Kim AL. Multiple molecular targets of resveratrol: Anti-carcinogenic mechanisms. Arch Biochem Biophys. 2009;486(2):95–102. doi: 10.1016/j.abb.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Athar M, Back JH, Tang X, et al. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007;224(3):274–283. doi: 10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delmas D, Lancon A, Colin D, Jannin B, Latruffe N. Resveratrol as a chemopreventive agent: a promising molecule for fighting cancer. Curr Drug Targets. 2006;7(4):423–442. doi: 10.2174/138945006776359331. [DOI] [PubMed] [Google Scholar]
- 16.Signorelli P, Ghidoni R. Resveratrol as an anticancer nutrient: molecular basis, open questions and promises. J Nutr Biochem. 2005;16(8):449–466. doi: 10.1016/j.jnutbio.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Mertens-Talcott SU, Percival SS. Ellagic acid and quercetin interact synergistically with resveratrol in the induction of apoptosis and cause transient cell cycle arrest in human leukemia cells. Cancer Lett. 2005;218(2):141–151. doi: 10.1016/j.canlet.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Cecchinato V, Chiaramonte R, Nizzardo M, et al. Resveratrol-induced apoptosis in human T-cell acute lymphoblastic leukaemia MOLT-4 cells. Biochem Pharmacol. 2007;74(11):1568–1574. doi: 10.1016/j.bcp.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411(6835):380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 20.Khanna R, Burrows SR, Thomson SA, et al. Class I processing-defective Burkitt’s lymphoma cells are recognized efficiently by CD4+ EBV-specific CTLs. J Immunol. 1997;158(8):3619–3625. [PubMed] [Google Scholar]
- 21.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 22.Cresswell P. Assembly, transport, and function of MHC class II molecules. Annu Rev Immunol. 1994;12:259–293. doi: 10.1146/annurev.iy.12.040194.001355. [DOI] [PubMed] [Google Scholar]
- 23.Kropshofer H, Vogt AB, Thery C, et al. A role for HLA-DO as a co-chaperone of HLA-DM in peptide loading of MHC class II molecules. EMBO J. 1998;17(11):2971–2981. doi: 10.1093/emboj/17.11.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amria S, Cameron C, Stuart R, Haque A. Defects in HLA class II antigen presentation in B-cell lymphomas. Leuk Lymphoma. 2008;49(2):353–355. doi: 10.1080/10428190701814305. [DOI] [PubMed] [Google Scholar]
- 25.Haque A, Hajiaghamohseni LM, Li P, Toomy K, Blum JS. Invariant chain modulates HLA class II protein recycling and peptide presentation in nonprofessional antigen presenting cells. Cell Immunol. 2007;249(1):20–29. doi: 10.1016/j.cellimm.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10(9):597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazzarino DA, Blier P, Mellman I. The monomeric guanosine triphosphatase rab4 controls an essential step on the pathway of receptor-mediated antigen processing in B cells. J Exp Med. 1998;188(10):1769–1774. doi: 10.1084/jem.188.10.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiraiwa A, Yamanaka K, Kwok WW, et al. Structural requirements for recognition of the HLA-Dw14 class II epitope: a key HLA determinant associated with rheumatoid arthritis. Proc Natl Acad Sci U S A. 1990;87(20):8051–8055. doi: 10.1073/pnas.87.20.8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovats S, Nepom GT, Coleman M, Nepom B, Kwok WW, Blum JS. Deficient antigen-presenting cell function in multiple genetic complementation groups of type II bare lymphocyte syndrome. J Clin Invest. 1995;96(1):217–223. doi: 10.1172/JCI118023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haque MA, Hawes JW, Blum JS. Cysteinylation of MHC class II ligands: peptide endocytosis and reduction within APC influences T cell recognition. J Immunol. 2001;166(7):4543–4551. doi: 10.4049/jimmunol.166.7.4543. [DOI] [PubMed] [Google Scholar]
- 31.Pathak SS, Blum JS. Endocytic recycling is required for the presentation of an exogenous peptide via MHC class II molecules. Traffic. 2000;1(7):561–569. doi: 10.1034/j.1600-0854.2000.010706.x. [DOI] [PubMed] [Google Scholar]
- 32.Amria S, Hajiaghamohseni LM, Harbeson C, et al. HLA-DM negatively regulates HLA-DR4-restricted collagen pathogenic peptide presentation and T cell recognition. Eur J Immunol. 2008;38(7):1961–1970. doi: 10.1002/eji.200738100. [DOI] [PubMed] [Google Scholar]
- 33.Goldstein OG, Hajiaghamohseni LM, Amria S, Sundaram K, Reddy SV, Haque A. Gamma-IFN-inducible-lysosomal thiol reductase modulates acidic proteases and HLA class II antigen processing in melanoma. Cancer Immunol Immunother. 2008;57(10):1461–1470. doi: 10.1007/s00262-008-0483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Das DK, Maulik N. Resveratrol in cardioprotection: a therapeutic promise of alternative medicine. Mol Interv. 2006;6(1):36–47. doi: 10.1124/mi.6.1.7. [DOI] [PubMed] [Google Scholar]
- 35.Wu JM, Wang ZR, Hsieh TC, Bruder JL, Zou JG, Huang YZ. Mechanism of cardioprotection by resveratrol, a phenolic antioxidant present in red wine (Review) Int J Mol Med. 2001;8(1):3–17. doi: 10.3892/ijmm.8.1.3. [DOI] [PubMed] [Google Scholar]
- 36.Harikumar KB, Kunnumakkara AB, Sethi G, et al. Resveratrol, a multitargeted agent, can enhance antitumor activity of gemcitabine in vitro and in orthotopic mouse model of human pancreatic cancer. Int J Cancer. 2010;127(2):257–268. doi: 10.1002/ijc.25041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harikumar KB, Aggarwal BB. Resveratrol: a multitargeted agent for age-associated chronic diseases. Cell Cycle. 2008;7(8):1020–1035. doi: 10.4161/cc.7.8.5740. [DOI] [PubMed] [Google Scholar]
- 38.Krawczyk M, Leimgruber E, Seguin-Estevez Q, Dunand-Sauthier I, Barras E, Reith W. Expression of RAB4B, a protein governing endocytic recycling, is co-regulated with MHC class II genes. Nucleic Acids Res. 2007;35(2):595–605. doi: 10.1093/nar/gkl980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinet V, Vergelli M, Martin R, Bakke O, Long EO. Antigen presentation mediated by recycling of surface HLA-DR molecules. Nature. 1995;375(6532):603–606. doi: 10.1038/375603a0. [DOI] [PubMed] [Google Scholar]
- 40.Lindner R, Unanue ER. Distinct antigen MHC class II complexes generated by separate processing pathways. EMBO J. 1996;15(24):6910–6920. [PMC free article] [PubMed] [Google Scholar]
- 41.Aldoss IT, Weisenburger DD, Fu K, et al. Adult Burkitt lymphoma: advances in diagnosis and treatment. Oncology (Williston Park) 2008;22(13):1508–1517. [PubMed] [Google Scholar]
- 42.Marques FZ, Markus MA, Morris BJ. Resveratrol: cellular actions of a potent natural chemical that confers a diversity of health benefits. Int J Biochem Cell Biol. 2009;41(11):2125–2128. doi: 10.1016/j.biocel.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 43.Pervaiz S, Holme AL. Resveratrol: its biologic targets and functional activity. Antioxid Redox Signal. 2009;11(11):2851–2897. doi: 10.1089/ars.2008.2412. [DOI] [PubMed] [Google Scholar]
- 44.Mukherjee S, Dudley JI, Das DK. Dose-dependency of resveratrol in providing health benefits. Dose Response. 2010;8(4):478–500. doi: 10.2203/dose-response.09-015.Mukherjee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pandey PR, Okuda H, Watabe M, et al. Resveratrol suppresses growth of cancer stem-like cells by inhibiting fatty acid synthase. Breast Cancer Res Treat. 2010 doi: 10.1007/s10549-010-1300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prestwich RJ, Errington F, Hatfield P, et al. The immune system--is it relevant to cancer development, progression and treatment? Clin Oncol (R Coll Radiol) 2008;20(2):101–112. doi: 10.1016/j.clon.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 47.God JM, Haque A. Burkitt lymphoma: pathogenesis and immune evasion. J Oncol. 2010;2010 doi: 10.1155/2010/516047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.