Abstract
BACKGROUND
Patients with therapy-related acute promyelocytic leukemia (t-APL) have been commonly exposed to topoisomerase inhibitors and may potentially benefit from induction regimens omitting anthracyclines.
METHODS
Retrospective analysis of the outcomes of 29 patients with t-APL who were either treated with arsenic trioxide (ATO) and all-trans-retinoic acid (ATRA) or with standard ATRA plus anthracycline-based chemotherapy was performed.
RESULTS
Prior therapy included chemotherapy alone, radiation alone, or a combination of the 2 in 19%, 33%, and 47% of patients, respectively. The combination of ATO and ATRA (n = 19) for induction resulted in a similar remission rate compared with ATRA plus chemotherapy (n = 10) (89% vs 70%; P = .35). The median overall survival for the patients treated with ATRA plus ATO was not reached compared with that for patients treated with ATRA plus chemotherapy (161 weeks; P =.79).
CONCLUSIONS
In this cohort of t-APL patients, outcomes with ATO and ATRA appeared to be comparable to anthracycline-containing induction regimens. This combination may be preferable in t-APL patients to avoid any risk of anthracycline-induced toxicities.
Keywords: therapy-related acute promyelocytic leukemia, arsenic trioxide, outcome, all-trans-retinoic acid
Although the majority of patients with acute promyelocytic leukemia (APL) have de novo disease with no clear underlying etiology, an increasing number of cases have been linked to prior exposure to chemotherapy, in particular to topoisomerase II inhibitors.1,2 Recent work has demonstrated specific hot spots in the breakpoint region of the PML and RARA genes, underscoring the causative role of topoisomerase II inhibitors in the etiology of these leukemias.3–5 In addition to topoisomerase II inhibitors, many other classes of cytotoxic agents (such as alkylating agents and nucleoside analogues), as well as radiotherapy alone, have been linked to therapy-related acute myeloid leukemias in general6–13 and therapy-related APL (t-APL) in particular.1 Many of these studies did not specify all the cytogenetic subtypes of the patients with therapy-related acute myeloid leukemias.
Treatment with regimens containing all-trans-retinoic acid (ATRA) has revolutionized outcomes in APL.14 More recently, arsenic trioxide (ATO), either alone or in combination with ATRA, was shown to be effective and less toxic than chemotherapy as frontline therapy for APL.15–17 Previous reports have established outcomes of patients with t-APL,1,2,18 comparing them with de novo APL. However, to the best of our knowledge, none included patients who were treated with ATO as a part of their initial therapy.
The objective of the current study was to characterize clinical parameters and treatment outcomes of 29 patients with t-APL at the study institution, 19 of whom were treated with a frontline ATO-containing regimen.
MATERIALS AND METHODS
Informed consent in accordance with the Declaration of Helsinki and institutional approval were obtained. The database of the Department of Leukemia at The University of Texas M. D. Anderson Cancer Center (MDACC) was searched to identify patients with a diagnosis of APL (total n = 301) from 1980 through 2008. Among these, 35 patients carried a previous diagnosis of a malignancy other than APL, 1 patient had been treated previously for multiple sclerosis (total t-APL; n = 36). Seven patients, treated between 1980 and 1990, had not received ATRA and therefore were excluded from our analysis. Therefore, a total of 29 patients with t-APL, treated between 1992 and 2008, were included in our analysis. Clinical parameters, bone marrow studies, and prior chemotherapy exposure were retrieved by chart review (F.D.) and confirmed by another coauthor (S.P.). Complete response (CR) was defined as normalization of peripheral blood counts and bone marrow findings. Overall survival was measured as the time from diagnosis of APL to the time of death or last follow-up.
Routine workup included bone marrow morphology, cytogenetics, fluorescence in situ hybridization for t(15;17), and reverse transcriptase-polymerase chain reaction (RT-PCR) for both short and long forms of the PML-RARA fusion transcript. Two patients with normal cytogenetics were diagnosed based on bone marrow morphology and RT-PCR.
The chi-square test was used to describe differences of clinical values among groups. Overall survival was calculated by the Kaplan-Meier method and log-rank test.
RESULTS
Patients
Twenty-nine patients who had received prior chemotherapy and/or radiotherapy developed t-APL between 1992 and 2008. Table 1 shows the patient characteristics. The median age of the patients was 54 years (range, 35–81 years) and 14 (48%) were female. The median white blood cell count (WBC) at the time of presentation was 1.6 × 1000/µL (range, 0.6–162.5× 1000/µL). As a comparison, the median age at presentation in 265 patients with de novo APL treated at our institution in the same time interval was 42 years (range, 13–80 years; P <.001), and 49% were female (P value was not significant) with a median WBC of 3.5 ×1000/µL (range, 0.2–195 × 1000/µL; P value not significant.).
Table 1.
Patient Characteristicsa
| Gender | Age, Years |
Year of Diagnosis |
WBC, ×1000/ µL |
Cytogenetics | RT-PCR Isoform |
Primary Malignancy |
Prior Chemotherapy |
Prior XRT |
Frontline Regimen for APL |
Response to Induction |
Status | Remission Duration, Weeks |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATRA ± Idarubicin ± GO | ||||||||||||
| Man | 37 | 1994 | 17.2 | 46,XY,t(15;17),del(16) | NA | Testis | A+CIS+BLEO | No | IDA+ATRA | Fail | Dead | 0 |
| Man | 60 | 1999 | 2 | 46,XY,t(15;17) | Short | NHL | CHOP+FND | Yes | IDA+ATRA | CR | Dead | 57 |
| Man | 63 | 1992 | 11 | 46,XY, t(15;17) | Neither | Prostate | None | Yes | IDA+ATRA | CR | Died in CR | 552 |
| Woman | 46 | 1992 | 1.2 | 46XX,t(15;17) | Long | Cervix | None | Yes | IDA+ATRA | CR | Alive | 304 |
| Woman | 59 | 1996 | 0.6 | 46,XX,t(15;17), 46,XX,t(2;4) | Neither | NHL | CHOP+ESHAP | Yes | IDA+ATRA | CR | Alive | 500 |
| Man | 75 | 1996 | 3 | 46,XY,del(7),t(15;17) | Short | Prostate | None | Yes | IDA+ATRA | Fail | Dead | 0 |
| Man | 65 | 1999 | 0.7 | 46,XY,t(15;17) | Long | HNSCC | CIS | Yes | IDA+ATRA | CR | Dead | 118 |
| Man | 35 | 2001 | 0.8 | 46,XY,t(15;17) | Long | NHL | CHOP | Yes | GO+ATRA | CR | Alive | 255 |
| Woman | 54 | 2001 | 1 | 45,XX,t(15;17),−21 | Short | Breast | A+C+TAX | Yes | GO+ATRA | CR | Died in CR | 172 |
| Woman | 48 | 2001 | 30.6 | 47,XX,+8,t(15;17) | Neither | Breast | None | Yes | GO+IDA+ATRA | Fail | Dead | 0 |
| ATO + ATRA ± GO | ||||||||||||
| Woman | 45 | 2003 | 9.9 | 46,XX,t(15;17) | Short | Breast | Capecitabine | Yes | ATO+ATRA | CRp | Died in CR | 14 |
| Man | 47 | 2006 | 1.6 | 46,XY,t(15;17) | Long | Prostate | A | Yes | ATO+ATRA | CR | Died in CR | 21 |
| Woman | 63 | 2002 | 12.7 | 46,XX,t(8;14),t(15;17), der(18),t(8;18) | Short | Breast | Not recorded for breast cancer | Yes | ATO+ATRA+GO | CR | Alive | 277 |
| Arthritis | MTX for arthritis | |||||||||||
| Man | 72 | 2004 | 1.2 | 46,XY,inv(2),t(15;17) | Long | NHL | CHOP+RITUX+F | No | ATO+ATRA | CR | Died in CR | 71 |
| Man | 60 | 2005 | 0.9 | 46,XY,t(15;17) | Long | HNSCC | None | Yes | ATO+ATRA | CR | Alive | 104 |
| Man | 81 | 2004 | 1.5 | CG not done (FISH 60.5%) | Short | Stomach | 5-FU | Yes | ATO+ATRA | CR | Alive | 167 |
| Woman | 56 | 2004 | 11.5 | 47,XX,t(15;17),+mar | Short | Breast | Not recorded | Yes | ATO+ATRA+GO | Fail | Dead | 0 |
| Woman | 46 | 2004 | 0.8 | 46,XX,del(12),t(15;17) | Long | SLL | Flu+FND+RITUX | No | ATO+ATRA | CR | Alive | 120 |
| Woman | 65 | 2006 | 1.4 | 47,XX,+8,t(15;17) | Short | Breast | None | Yes | ATO+ATRA | CR | Alive | 65 |
| Man | 66 | 2005 | 162.5 | 46,XY,inv(9),t(15;17) | Short | Prostate | None | Yes | ATO+ATRA+GO | Fail | Dead | 0 |
| Woman | 42 | 2005 | 24.7 | 46,XX,t(15;17) | Long | Breast | A+C+TAX | No | ATO+ATRA+GO | CR | Alive | 107 |
| MDS/AML | Ara-C+IDA | |||||||||||
| Man | 47 | 2005 | 0.6 | 46,XY,del(9),inv(9),t(15;17) | Short | Seminoma | None | Yes | ATO+ATRA | CR | Alive | 93 |
| Woman | 36 | 2006 | 1.5 | 46,XX | Short | MS | FND | No | ATO+ATRA | CR | Alive | 73 |
| Woman | 53 | 2008 | 65.3 | 46,XX | Short | Breast | 5-FU+E+C+TAX | Yes | ATO+ATRA+GO | CR | Alive | 54 |
| Woman | 52 | 2008 | 46.3 | 46,XX,t(15;17) | Long | Breast | A+C+DOC+TAX | Yes | ATO+ATRA+GO | CR | Alive | 32 |
| Man | 46 | 2007 | 68.5 | 46,XY,t(15;17) | Short | Testis | BEP | No | ATO+ATRA+GO | CR | Alive | 0 |
| Man | 77 | 2008 | 0.7 | 46,XY,add(8),t(15;17) | Long | Prostate | None | Yes | ATO+ATRA | CR | Alive | 0 |
| Woman | 53 | 2001 | 2.6 | 47,XX,t(15;17),del(20),+mar | Short | Cervix | None | Yes | ATO+ATRA+GO | CR | Dead | 69 |
| Man | 60 | 1999 | 0.5 | 46,XY,t(15;17) | Long | MFH | A+I | Yes | ATO+ATRA | CR | Dead | 34 |
WBC indicates white blood cell count; RT-PCR, reverse transcriptase-polymerase chain reaction; XRT, radiotherapy; APL, acute promyelocytic leukemia; ATRA, all-trans-retinoic acid; GO, gemtuzumab ozogamicin; t, translocation; del, deletion; NA, not applicable; A: Adriamycin (doxorubicin); CIS, cisplatin; BLEO, bleomycin; IDA, idarubicin; NHL, non-Hodgkin lymphoma; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; FND, fludarabine, mitoxantrone, and dexamethasone; CR, complete response; ESHAP, etoposide, methylprednisolone, high-dose cytarabine, and cisplatin; HNSCC, head and neck squamous cell carcinoma; C, cyclophosphamide; TAX, paclitaxel; ATO, arsenic trioxide; CRp, pathologic complete response; MTX, methotrexate; RITUX, rituximab; F, FND (mitoxantrone); CG, cytogenetics; FISH, fluorescence in situ hybridization; 5-FU, 5-fluorouracil; SLL, small lymphocytic lymphoma; Flu, fludarabine; MDS/AML, myelodysplastic syndrome/acute myelogenous leukemia; Ara-C, cytarabine; MS, multiple sclerosis; E, epirubicin; DOC, docetaxel; BEP, bleomycin, etoposide, and cisplatin; MFH, malignant fibrous histiocytoma; I, ifosfamide.
Two patients with negative cytogenetics were diagnosed before RT-PCR was introduced into clinical practice, and therefore the diagnosis of t-APL was based on morphology only.
Prior Malignancies
The most common primary cancer was breast cancer (9 of 29 patients), followed by prostate cancer (5 of 29 patients), and lymphoma (4 of 29 patients). Six patients had been previously treated with chemotherapy only, 10 with radiotherapy only, and 13 had received both treatment modalities. Thirteen of 29 patients had prior exposure to topoisomerase II inhibitors (10 patients with anthracyclines, 1 with etoposide, and 3 with mitoxantrone; 2 patients had received 2 different topoisomerase II inhibitors). The exact prior chemotherapy was not documented for 1 patient with breast cancer.
The median interval from primary disorder to the diagnosis of t-APL was 3.5 years (range, 1–19 years). The incidence of t-APL has increased with advancing decades (9% of all patients with APL diagnosed between 1992 and 1999 vs 16% of patients diagnosed between 2000 and 2008; P = .027). This trend confirms previous reports,1,19 possibly explained by the more widespread use of topoisomerase II inhibitors, mainly for the treatment of breast cancer.
Molecular Studies
Cytogenetics data were available for 28 of 29 patients (Table 1). Cytogenetic abnormalities in addition to t(15;17) occurred in 13 of 29 patients (45%) and most frequently involved chromosome 8 (4 of 29 patients; 14%). The presence of additional cytogenetic abnormalities was not associated with a worse outcome. Among 25 patients with available RT-PCR data, detection of the short PML-RARA isoform (14 of 25 patients; 56%) was associated with a trend toward shorter survival compared with the long isoform (11 of 25 patients; 44%) (161 weeks vs 344 weeks; P = .29).
Prognostic Factors
A WBC >10,000/µL was associated with fewer CRs and worse survival. The CR rate in 10 patients with a WBC >10,000/µL was 60% (6 of 10 patients), with a median survival of 5 weeks (range, 0–282 weeks) versus 95% (18 of 19 patients) and a median survival of 117 weeks (range, 4–650 weeks) for 19 patients with a WBC <10,000/µL. Four of 10 patients with a WBC >10,000/µL failed to achieve a CR, and all had died within 2 weeks of the initiation of induction therapy for t-APL.
Response to Therapy and Survival
The detailed doses and schedules for the drugs used for t-APL induction regimens are shown in Table 2. Postremission therapy was varied depending on the regimen, and the details have been published previously.16,20,21 The combination of ATO and ATRA (n = 19) for induction resulted in a CR rate comparable to that of ATRA plus chemotherapy (n = 10) (89% vs 70%; P = .35). The median overall survival for the patients treated with ATRA plus ATO was not reached compared with that for patients treated with ATRA plus chemotherapy (161 weeks; P=.79) (Fig. 1). The difference in the median survival was not significantly different between the 2 groups and likely was a result of a shorter follow-up interval in the ATRA plus ATO group. The median follow-up for all patients (both alive and dead) in the ATRA plus chemotherapy cohort was 51 months (range, 1–117 months), whereas the median follow-up time for all patients in the ATRA plus ATO cohort was 18 months (range, 1–65 months). The median ages for patients treated with ATRA plus ATO and ATRA plus chemotherapy were 53 years and 56.5 years, respectively (P value not significant). The percentage of patients with a presenting WBC >10,000/µL was 37% (7 of 19 patients) in patients treated with ATRA plus ATO versus 30% (3 of 10 patients) in patients treated with ATRA plus chemotherapy.
Table 2.
Drug Doses and Schedules in t-APL Induction Regimensa
| Drug | Dose | Schedule | Route |
|---|---|---|---|
| ATRA + ATO ± GO16 | |||
| ATRA | (45 mg/m2) | Starting on D 1, once daily | PO |
| ATO | (0.15 mg/kg) | Starting D 1 or D 10 once daily until CR | iv |
| GO | (9 mg/m2) | D 1 for WBC >10,000/µL | iv |
| ATRA + IDA ± GO21 | |||
| ATRA | (45 mg/m2) | Starting D 1 once daily | PO |
| IDA | (12 mg/m2) | D 1–4 | iv |
| GO | (9 mg/m2) | D1 | iv |
| ATRA + GO20 | |||
| ATRA | (45 mg/m2) | Starting D 1 once daily | PO |
| GO | (9 mg/m2) | D1 | iv |
t-APL indicates therapy-related acute promyelocytic leukemia; ATRA, all-trans-retinoic acid; ATO, arsenic trioxide; GO, gemtuzumab ozogamicin; PO, orally; CR, complete response; iv, intravenously; WBC, white blood cell count; IDA, idarubicin.
GO was added to the regimen for patients with a WBC >10,000/µL.
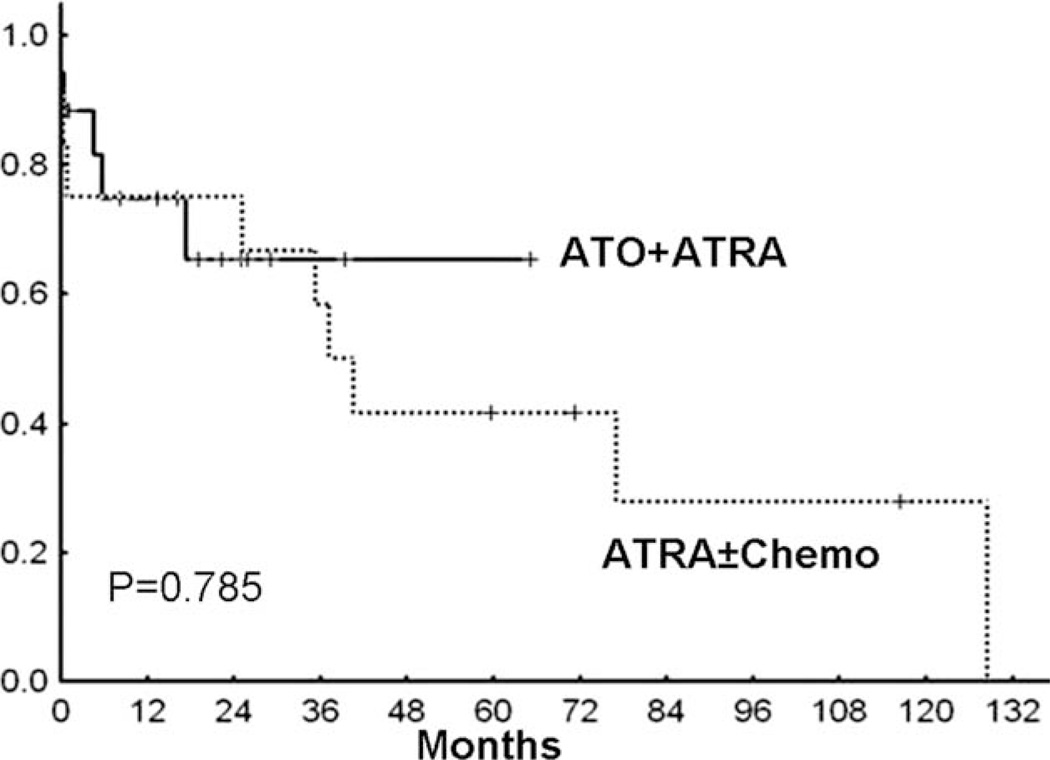
Figure 1.
Overall survival in patients with therapy-related acute promyelocytic leukemia (t-APL) who were treated with arsenic trioxide (ATO) plus all-trans-retinoic acid (ATRA) as a frontline regimen compared with patients treated with ATRA plus anthracycline-containing regimens is shown. The bold line indicates ATO plus ATRA (n = 19); dotted line, ATRA plus chemotherapy (Chemo) (n = 10). The median survival was not reached in the group of patients treated with ATO plus ATRA but was 161 weeks in the group of patients treated with ATRA plus chemotherapy (P = .79).
At the time of last analysis (December 2009), 7 of 19 patients in the ATRA plus ATO group and 7 of 10 patients in the ATRA plus chemotherapy group had died. The cause of death was due to leukemia in 4 of 7 patients in the ATRA plus ATO group and 5 of 7 patients in the ATRA plus chemotherapy group. There was no difference in early mortality noted between the 2 cohorts, because in each group, 2 patients died within 4 weeks of the initiation of induction therapy for t-APL.
We also compared the outcome of the patients with t-APL with a cohort of patients with de novo APL who were treated at our institution during the same time interval. As expected, the de novo APL cohort had a significantly younger age at the time of diagnosis (median age, 42 years; range, 13–80 years [P < .0001 vs t-APL]). Otherwise, clinical parameters and outcomes were similar to t-APL patients: the median presenting WBC was 3500/µL (range, 200–195,000 µL [P = .106 vs t-APL]) and the 3-year survival rate was 65% (P = .175 vs t-APL). In the de novo APL cohort, 80 of 85 patients (94%) who were treated with ATRA plus ATO (P = .099 vs t-APL) and 26 of 110 patients (76%) treated with ATRA plus chemotherapy achieved a CR.
DISCUSSION
The long-term effects of anthracycline-containing chemotherapy include myelodysplastic syndromes (MDS) and cardiac dysfunction. Therefore, it is plausible that front-line treatment of patients with t-APL previously exposed to anthracyclines, with regimens that omit chemotherapy, particularly topoisomerase II inhibitors, might be beneficial. To our knowledge, this is the first study examining the outcome of patients with t-APL who were treated with ATRA and ATO. In our experience, ATO and ATRA appear to produce results comparable to anthracycline-containing regimens. Although not statistically significant due to the relatively small number of patients, the combination of ATO plus ATRA was found to be associated with a similar response rate and overall survival compared with ATRA plus chemotherapy. In addition to the risk of MDS and cardiotoxicity, there are patient-related reasons why such a regimen would be desirable in patients with t-APL. Pulsoni et al reported a significantly higher proportion of t-APL patients with poor performance status (Eastern Cooperative Oncology Group score >2) when compared with de novo APL patients.19 Furthermore, their higher median age makes these patients more susceptible to chemotherapy-induced adverse events and organ toxicities. The higher median age as well as worse performance status in t-APL patients compared with de novo APL patients might have contributed to the inferior outcomes in our cohort of t-APL patients compared with de novo APL patients treated during the same time interval. In addition, consistent with the referral pattern to a major cancer center, it is possible that there was a bias toward poorer prognosis patients treated at our center compared with patients treated at other centers.
There are several limitations to the current study. The small number of patients diagnosed with t-APL during the time span included in the study, the lack of randomization, and the retrospective nature of the analysis make the results susceptible to uncontrolled biases that might influence the validity of our conclusions. However, the most important prognostic factor (WBC >10,000/µL) remained valid. It would be ideal to determine the exact causes of death of the patients on study. However, the majority of patients died outside of our institution with no detailed record of the cause of death available, making it difficult to determine the influence of the toxicities of the 2 strategies on the long-term outcome. Finally, supportive care has improved over the past 3 decades, possibly accounting for differences in survival among our patients. Nevertheless, a higher percentage of patients treated with ATRA and ATO achieved a CR, an endpoint that should not be as significantly influenced by supportive care.
In summary, the current study presents our single-institution experience of what to the best of our knowledge is the largest series of patients with t-APL treated with a frontline regimen with no chemotherapy reported to date. Although we cannot draw definitive conclusions because of the limited sample size, the combination of ATO plus ATRA appears not to be inferior and therefore might be preferable in patients with t-APL to reduce the possible risk of anthracycline-induced cardiomyopathy or MDS. Although ideally, given the low incidence of t-APL, prospective randomized trials are required to confirm our observation, it will be a difficult task to accrue enough patients for such a trial. Thus, although lacking controlled randomized trials data, we might need to resort to observational studies such as this one, despite its limitations, to gain insight into possible treatment options for patients with t-APL.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Dr. Ravandi has been a member of advisory boards and has received honoraria from Cephalon.
REFERENCES
- 1.Beaumont M, Sanz M, Carli PM, et al. Therapy-related acute promyelocytic leukemia. J Clin Oncol. 2003;21:2123–2137. doi: 10.1200/JCO.2003.09.072. [DOI] [PubMed] [Google Scholar]
- 2.Jantunen E, Heinonen K, Mahlamaki E, et al. Secondary acute promyelocytic leukemia: an increasingly common entity. Leuk Lymphoma. 2007;48:190–191. doi: 10.1080/10428190600961736. [DOI] [PubMed] [Google Scholar]
- 3.Hasan SK, Mays AN, Ottone T, et al. Molecular analysis of t(15;17) genomic breakpoints in secondary acute promyelocytic leukemia arising after treatment of multiple sclerosis. Blood. 2008;112:3383–3390. doi: 10.1182/blood-2007-10-115600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mays AN, Osheroff N, Xiao Y, et al. Evidence for direct involvement of epirubicin in the formation of chromosomal translocations in t(15;17) therapy-related acute promyelocytic leukemia. Blood. 2010;115:326–330. doi: 10.1182/blood-2009-07-235051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mistry AR, Felix CA, Whitmarsh RJ, et al. DNA topoisomerase II in therapy-related acute promyelocytic leukemia. N Engl J Med. 2005;352:1529–1538. doi: 10.1056/NEJMoa042715. [DOI] [PubMed] [Google Scholar]
- 6.Greene MH, Harris EL, Gershenson DM, et al. Melphalan may be a more potent leukemogen than cyclophosphamide. Ann Intern Med. 1986;105:360–367. doi: 10.7326/0003-4819-105-3-360. [DOI] [PubMed] [Google Scholar]
- 7.Coso D, Costello R, Cohen-Valensi R, et al. Acute myeloid leukemia and myelodysplasia in patients with chronic lymphocytic leukemia receiving fludarabine as initial therapy. Ann Oncol. 1999;10:362–363. doi: 10.1023/a:1008397226387. [DOI] [PubMed] [Google Scholar]
- 8.McLaughlin P, Estey E, Glassman A, et al. Myelodysplasia and acute myeloid leukemia following therapy for indolent lymphoma with fludarabine, mitoxantrone, and dexamethasone (FND) plus rituximab and interferon alpha. Blood. 2005;105:4573–4575. doi: 10.1182/blood-2004-08-3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olney HJ, Mitelman F, Johansson B, Mrozek K, Berger R, Rowley JD. Unique balanced chromosome abnormalities in treatment-related myelodysplastic syndromes and acute myeloid leukemia: report from an international workshop. Genes Chromosomes Cancer. 2002;33:413–423. doi: 10.1002/gcc.10045. [DOI] [PubMed] [Google Scholar]
- 10.Morrison VA, Rai KR, Peterson BL, et al. Therapy-related myeloid leukemias are observed in patients with chronic lymphocytic leukemia after treatment with fludarabine and chlorambucil: results of an intergroup study, cancer and leukemia group B 9011. J Clin Oncol. 2002;20:3878–3884. doi: 10.1200/JCO.2002.08.128. [DOI] [PubMed] [Google Scholar]
- 11.Martin-Salces M, Canales MA, de Paz R, Hernandez-Navarro F. Treatment-related acute myeloid leukemia with 11q23 translocation following treatment with fludarabine, cyclophosphamide and rituximab. Leuk Res. 2008;32:199–200. doi: 10.1016/j.leukres.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 12.Curtis RE, Boice JD, Jr, Stovall M, et al. Risk of leukemia after chemotherapy and radiation treatment for breast cancer. N Engl J Med. 1992;326:1745–1751. doi: 10.1056/NEJM199206253262605. [DOI] [PubMed] [Google Scholar]
- 13.Godley LA, Larson RA. Therapy-related myeloid leukemia. Semin Oncol. 2008;35:418–429. doi: 10.1053/j.seminoncol.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tallman MS, Andersen JW, Schiffer CA, et al. All-trans-retinoic acid in acute promyelocytic leukemia. N Engl J Med. 1997;337:1021–1028. doi: 10.1056/NEJM199710093371501. [DOI] [PubMed] [Google Scholar]
- 15.Mathews V, George B, Lakshmi KM, et al. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: durable remissions with minimal toxicity. Blood. 2006;107:2627–2632. doi: 10.1182/blood-2005-08-3532. [DOI] [PubMed] [Google Scholar]
- 16.Ravandi F, Estey E, Jones D, et al. Effective treatment of acute promyelocytic leukemia with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab ozogamicin. J Clin Oncol. 2009;27:504–510. doi: 10.1200/JCO.2008.18.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alimoghaddam K, Shariftabrizi A, Tavangar SM, et al. Anti-leukemic and anti-angiogenesis efficacy of arsenic trioxide in new cases of acute promyelocytic leukemia. Leuk Lymphoma. 2006;47:81–88. doi: 10.1080/10428190500300373. [DOI] [PubMed] [Google Scholar]
- 18.Andersen MK, Larson RA, Mauritzson N, Schnittger S, Jhanwar SC, Pedersen-Bjergaard J. Balanced chromosome abnormalities inv(16) and t(15;17) in therapy-related myelo-dysplastic syndromes and acute leukemia: report from an international workshop. Genes Chromosomes Cancer. 2002;33:395–400. doi: 10.1002/gcc.10043. [DOI] [PubMed] [Google Scholar]
- 19.Pulsoni A, Pagano L, Lo Coco F, et al. Clinicobiological features and outcome of acute promyelocytic leukemia occurring as a second tumor: the GIMEMA experience. Blood. 2002;100:1972–1976. doi: 10.1182/blood-2001-12-0312. [DOI] [PubMed] [Google Scholar]
- 20.Estey EH, Giles FJ, Beran M, et al. Experience with gemtuzumab ozogamycin (“Mylotarg”) and all-trans retinoic acid in untreated acute promyelocytic leukemia. Blood. 2002;99:4222–4224. doi: 10.1182/blood-2001-12-0174. [DOI] [PubMed] [Google Scholar]
- 21.Estey E, Thall PF, Pierce S, Kantarjian H, Keating M. Treatment of newly diagnosed acute promyelocytic leukemia without cytarabine. J Clin Oncol. 1997;15:483–490. doi: 10.1200/JCO.1997.15.2.483. [DOI] [PubMed] [Google Scholar]