Abstract
Biological market theory models the action of natural selection as a marketplace in which animals are viewed as traders with commodities to offer and exchange. Studies of female Old World monkeys have suggested that grooming might be employed as a commodity to be reciprocated or traded for alternative services, yet previous tests of this grooming-trade model in wild adult male chimpanzees have yielded mixed results. Here we provide the strongest test of the model to date for male chimpanzees: we use data drawn from two social groups (communities) of chimpanzees from different populations and give explicit consideration to variation in dominance hierarchy steepness, as such variation results in differing conditions for biological markets. First, analysis of data from published accounts of other chimpanzee communities, together with our own data, showed that hierarchy steepness varied considerably within and across communities and that the number of adult males in a community aged 20–30 years predicted hierarchy steepness. The two communities in which we tested predictions of the grooming-trade model lay at opposite extremes of this distribution. Second, in accord with the grooming-trade model, we found evidence that male chimpanzees trade grooming for agonistic support where hierarchies are steep (despotic) and consequent effective support is a rank-related commodity, but not where hierarchies are shallow (egalitarian). However, we also found that grooming was reciprocated regardless of hierarchy steepness. Our findings also hint at the possibility of agonistic competition, or at least exclusion, in relation to grooming opportunities compromising the free market envisioned by biological market theory. Our results build on previous findings across chimpanzee communities to emphasize the importance of reciprocal grooming exchanges among adult male chimpanzees, which can be understood in a biological markets framework if grooming by or with particular individuals is a valuable commodity.
Keywords: biological market, hierarchy steepness, linear mixed model, M-group, rank, reciprocity, Sonso, trading
Biological market theory (BMT) models the action of natural selection on behavioural strategies as a marketplace in which animals (or at least the strategies they embody) can be viewed as traders with commodities (behavioural interactions) to offer and exchange (Barrett & Henzi, 2001; Noë, 2001, 2006; Noë & Hammerstein, 1994, 1995). The amount or quality of the commodity exchanged is contingent on the classical market forces of supply and demand such that when supply is low, or demand is high, a commodity has greater value (Noë, 2001, 2006; Noë & Hammerstein, 1994, 1995). For example, commodities held by a small proportion of individuals are more valuable than those that can be provided by a larger number of group members (Noë & Hammerstein, 1994, 1995). Consequently, BMT accounts for the presence of asymmetric relationships if some individuals hold a highly attractive commodity for which their partners would compete (Noë, 1990, 2001). Multiple studies have proffered BMT as an explanation for the trade of commodities in social animals, such as the exchange of services in primates (e.g. Barrett, Gaynor, & Henzi, 2002; Barrett & Henzi, 2006; Barrett, Henzi, Weingrill, Lycett, & Hill, 1999; Fruteau, Voelkl, van Damme, & Noë, 2009; Gumert, 2007; Koyama, Caws, & Aureli, 2012; Norscia, Antonacci, & Palagi, 2009), between cleaner fishes and their clients (Bshary, 2001; Bshary & Grutter, 2002; Bshary & Noë, 2003; Bshary & Schäffer, 2002), the exchange of protection for nectar between ants and aphids (Fischer, Hoffmann, & Völkl, 2001; Leimar & Axén, 1993), the nutrient exchange between plants and fungi in mycorrhiza (Hoeksema & Schwartz, 2001; Schwartz & Hoeksema, 1998) and in the trading of grooming for mating between male and female wood mice (Apodemus sylvaticus: Stopka & Macdonald, 1999).
Nonhuman primates provide good model systems to explore the applicability of BMT, as they show a range of cooperative behavioural exchanges, such as grooming (Henzi & Barrett, 1999; Russell & Phelps, 2013; Schino & Aureli, 2008), agonistic support (Harcourt & de Waal, 1992) and food transfer (Brown, Almond, & Bergen, 2004). Under the framework set by BMT, studies of the social behaviour of female cercopithecoids (Old World monkeys) have suggested that grooming might be used as a commodity to be either reciprocated or traded for alternative services, depending on the goods the individuals can offer in the market (Barrett et al., 2002, 1999; Seyfarth, 1976, 1977, 1980). In addition, a meta-analysis of 14 monkey species found that females have a general tendency to groom high-ranking individuals (Schino, 2001). There is also evidence that monkeys tend to reciprocate grooming time (Carne, Wiper, & Semple, 2011; Frank & Silk, 2009; Leinfelder, de Vries, Deleu, & Nelissen, 2001; Schino & Aureli, 2008; Schino, di Giuseppe, & Visalberghi, 2009; Ventura, Majolo, Koyama, Hardie, & Schino, 2006; Xia et al., 2012; Xia et al., 2013), with less reciprocal grooming in groups with steeper rank relationships (Balasubramaniam et al., 2012), as well as trading grooming for rank-related benefits such as agonistic support (Carne et al., 2011; Hemelrijk, 1994; Schino, di Sorrentino, & Tiddi, 2007; Seyfarth & Cheney, 1984; Silk, 1992), tolerance in feeding contexts (Carne et al., 2011; Richter, Mevis, Malaivijitnond, Schülke, & Ostner, 2009; Tiddi, Aureli, di Sorrentino, Janson, & Schino, 2011) and reduction of aggression (Gumert & Ho, 2008; Ventura et al., 2006; Xia et al., 2012; Xia et al., 2013). This ‘grooming-trade’ model posits that as high-ranking individuals can potentially offer dominance-restricted benefits such as agonistic support (Seyfarth, 1977, 1980) or access to food resources (Barrett et al., 1999), subordinates use grooming as a ‘currency’ with which to ‘buy’ these services. The extent to which grooming is traded for other benefits depends on individuals’ ability (or possibility) to monopolize resources (Barrett et al., 2002, 1999; Henzi et al., 2003). Under the model, when individuals are not able to offer alternative services (commodities), such as when rank relationships are shallow (egalitarian hierarchies: van Schaik, 1989; Vehrencamp, 1983) or access to resources cannot be controlled (e.g. when food is scattered), then grooming is exchanged for itself (i.e. reciprocated: Barrett et al., 1999). In practice, commodities such as agonistic support are likely to be rank-related rather than dominance-restricted as most individuals should be able to offer the commodity, but its quality (e.g. the effectiveness of agonistic support) will depend on the trader’s rank.
Previous tests of the grooming-trade model in wild adult male chimpanzees have yielded mixed results. While there is ample evidence that male chimpanzees reciprocate services such as grooming (Arnold & Whiten, 2003; Boesch & Boesch-Achermann, 2000; Gomes, Mundry, & Boesch, 2009; Mitani, 2006; Newton-Fisher, 1997; Newton-Fisher & Lee, 2011; Watts, 2000a), meat exchange (Gilby, 2006; Mitani, 2006; Mitani & Watts, 2001) and agonistic support (Gomes & Boesch, 2011; Mitani, 2006; Mitani, Merriwether, & Zhang, 2000), evidence that adult male chimpanzees trade grooming to access rank-related services is still scant and inconsistent across communities. In agreement with the grooming-trade model, adult male chimpanzees of the Ngogo community (Kibale National Park, Uganda) tended to direct grooming up the social dominance hierarchy, with most reciprocity occurring between individuals with similar social ranks (Watts, 2000b), while data collected from males of the Sonso community (Budongo Forest Reserve, Uganda) in 1994/1995 revealed that males directed grooming up the hierarchy, but grooming reciprocity was not stronger among males holding similar ranks (Newton-Fisher & Lee, 2011).
By contrast, no relationship was found between rank and the distribution of grooming for chimpanzees of M-group (Mahale National Park, Tanzania), or North-group and South-group in the Taï National Park, Côte D’Ivoire (Boesch & Boesch-Achermann, 2000; Gomes & Boesch, 2011; Gomes et al., 2009; Watts, 2000b). With respect to trading, dominant males in the Ngogo community were more likely than subordinates to provide agonistic support (Watts, 2002) and grooming was traded for agonistic support as well as for meat (Mitani, 2006; Watts, 2002). Conversely, South-group chimpanzees were not found to trade grooming for other commodities, but exchanged agonistic support for meat (Gomes & Boesch, 2011). These findings hint at differences across communities, although the reasons behind them are not yet fully understood. Watts (2000b) argued that group size could partly explain these differences, yet the relationship between grooming distribution and rank in the large Ngogo community is also present in the smaller Sonso community (Newton-Fisher & Lee, 2011), whose size is comparable to M-group where the relationship is absent. A full understanding of the patterns of grooming among male chimpanzees and the extent to which these can be explained by models such as biological market theory therefore requires studies that incorporate data from multiple social groups.
Barrett and colleagues (Barrett et al., 1999; Henzi et al., 2003) proposed that for the grooming-trade model to be valid, it should be shown that (1) high-ranking individuals can offer subordinates one or more commodities, other than grooming, that subordinates cannot or are less likely to offer to dominants (e.g. agonistic support, social tolerance), (2) individuals direct grooming up the hierarchy (i.e. individuals predominantly groom those who are socially dominant to them), (3) dominants receive more grooming than they give and (4) group members adjacent in rank engage in grooming that is more reciprocal than that between individuals holding more distant ranks. Furthermore, these trading patterns should be contingent on hierarchy steepness, decreasing or potentially disappearing in favour of reciprocity of grooming where hierarchies are shallow (Barrett et al., 1999; Stevens, Vervaecke, de Vries, & van Elsacker, 2005). As such, the grooming-trade model is best tested by studying the same group under different conditions, or different groups with different social structures (Balasubramaniam, Berman, Ogawa, & Li, 2011; Barrett et al., 2002, 1999; Jaeggi, Stevens, & van Schaik, 2010; Stevens et al., 2005). Despite this, most investigations have focused on single social groups (Barrett & Henzi, 2001; Kappeler & van Schaik, 2006; Schino & Aureli, 2009).
The ‘grooming-trade’ model has its origins in Seyfarth’s (1977, 1980) classic model of social grooming, in essence a markets-based approach (Barrett & Henzi, 2006). However, Seyfarth’s model differs from BMT in a number of respects (Henzi et al., 2003). In particular, it postulates that the distribution of grooming is constrained by social rank (high-ranking individuals have first choice of partners) whereas BMT assumes this is traded freely according to market forces. Thus, while both predict that grooming should be most reciprocal between individuals close in rank, different mechanisms are assumed: rank-mediated competition under Seyfarth’s model and a lack of alternative, more valuable, commodities under BMT. This difference provides an opportunity to distinguish between the two: Seyfarth’s model predicts that grooming (not just most reciprocity) should be largely restricted to individuals adjacent in rank (Seyfarth, 1977, 1980) whereas a BMT model predicts that grooming should be distributed among all dyads, albeit with reciprocity correlating negatively with rank distance and with high-ranking individuals (or at least, those who hold commodities for which groomers wish to trade) receiving far more grooming than they perform due to outbidding competition (R. Noë, personal communication).
Here we test the grooming-trade model using data from two wild communities of East African chimpanzees, Pan troglodytes schweinfurthii: Sonso (Budongo Forest Reserve, Uganda) and M-group (Mahale Mountains National Park, Tanzania). We test for each of Barrett and colleagues’ (Barrett et al., 1999; Henzi et al., 2003) four conditions, as presented above, namely: (1) the existence of rank-related commodities; (2) grooming directed up the hierarchy; (3) dominants receiving more grooming than subordinates; and (4) closely ranked individuals showing more reciprocity in their grooming (i.e. reciprocity correlating negatively with rank distance). To differentiate between Seyfarth’s (1977) classic model and BMT further, we assess (5) whether total grooming effort is largely restricted to individuals of adjacent rank (as predicted by Seyfarth’s (1977) classic model) or spread across available partners (as predicted by BMT). We consider these explicitly in light of variation in dominance hierarchy steepness, with the expectation that (6) reciprocity in grooming should be stronger where hierarchies are shallower, while under steep hierarchies, grooming should instead be traded for rank-related benefits such as agonistic support and tolerance.
METHODS
Study Sites and Subjects
The Sonso community lives in semi-deciduous tropical forest in the Budongo Forest Reserve, Uganda (Eggeling, 1947; Plumptre, 1996; Reynolds, 2005), and has been studied continuously since 1994 (Newton-Fisher, 1997; Reynolds, 2005). For this study, behavioural data on Sonso chimpanzees were collected between December 2003 and August 2004. During data collection, the community consisted of 63 individuals including eight adult males (≥16 years old) and 21 adult females (≥14 years old). The M-group community lives in the semi-evergreen Kasoje Forest of the Mahale Mountains National Park and has been studied continuously since early 1980s (Nakamura & Nishida, 2012; Nishida, 1990, 2012). Data on M-group chimpanzees were collected for this study between February and November 2011. At the beginning of our observations, this community consisted of 60 individuals, including 10 adult males and 23 adult females (Kaburu & Newton-Fisher, 2013). For both communities, we collected data as part of our broader studies of social behaviour that sampled behaviour of both adult males and adult females. This research complied with the regulations set by the Ethics Committee of the University of Kent, the protocols of both the Budongo Forest Project (now BCFS) and the Mahale Mountains Wildlife Research Center and the legal requirements of Uganda and Tanzania.
Data Collection and Analysis
Data on grooming and agonistic interactions were collected using all-occurrence sampling (Altmann, 1974) within focal parties (i.e. all occurrences of these interactions that occurred in a party that contained a nominal focal animal, where party is defined as a subgroup produced by the fluid fission–fusion social system). We used 15 min interval instantaneous scan sampling to record the identities of the individuals present in the same party as the focal animal (i.e. association). Parties were followed for as long as possible from first encounter until nesting; focal animals were identified to allow unbiased decisions on which animals to observe when parties fissioned. If contact with chimpanzees was lost due to terrain and/or chimpanzee movement patterns, we searched for and observed the next party encountered that contained one of the predetermined focal animals. We recorded data on association, grooming and agonistic interactions, hunting and agonistic support through audio narration, by pen and paper, or, for some social interactions, on videotape. We recorded a total of 1109.5 h of observation of the Sonso community over 159 days/follows (median observation per day = 7 h); we conducted 141 focal follows of the M-group chimpanzees for a total of 800.9 h of observation (median observation per day = 6.3 h). Individual Sonso adult males were under observation for a mean ± SD duration of 358.8 ± 74.5 h (median observation: 356.7 h). Individual M-group adult male chimpanzees were under observation for a mean ± SD duration of 49.7 ± 5.4 h (median observation: 49.3 h). Except where stated, all analyses are of these two data sets. During data collection, the alpha male of M-group was killed during an intracommunity gang attack (Kaburu, Inoue, & Newton-Fisher, 2013). A period of social instability followed, during which aggression rates were significantly elevated, male rank was unstable and grooming bouts were significantly shorter when compared to the stable period before (Kaburu et al., 2013; Kaburu & Newton-Fisher, 2013). For the purposes of our analyses, we divided the data from M-group into two periods, ‘stable’ or ‘unstable’ (following our previous practice: Kaburu & Newton-Fisher, 2013), depending on whether they were collected before or after the killing of the alpha male, respectively.
Grooming was defined as the visual examination, searching and manipulation of the skin and hair with one or both hands, with the aid occasionally of the lower lip to part the hair (Yerkes, 1933). During grooming interactions, start and end time were recorded, as well as the identities of groomer and receiver. A grooming bout was considered to have ended when neither individual groomed for at least 30 s (Kaburu & Newton-Fisher, 2013; Newton-Fisher & Lee, 2011). Except where stated otherwise, all our analyses of grooming use durations (grooming effort: Newton-Fisher & Lee, 2011), rather than bout frequency. We follow Barrett et al. (1999) in defining reciprocity as the exchange of the same behaviour. We further consider reciprocity as quantifiable (Mitani, 2009; Newton-Fisher & Lee, 2011; Nishida, 1988; Silk, Seyfarth, & Cheney, 1999) such that a dyad’s degree of reciprocity is a measure of the extent to which the members of that dyad match one another’s investment in terms of either effort or participation in bouts. In contrast to cercopithecine monkeys (Barrett et al., 1999), many grooming bouts in chimpanzees include the simultaneous exchange of grooming in addition to unidirectional grooming in which an individual is either grooming or receiving, sometimes taking these roles alternately (Goodall, 1986; Kaburu & Newton-Fisher, 2013). We consider here only the total amount of grooming that was directed towards a partner and do not distinguish between simultaneous and unidirectional modes of delivery.
For these analyses, we restricted agonistic interactions to physical (contact) aggression, chases and directed charging displays (i.e. where these displays had a clear target, as chimpanzees will often perform undirected charging displays, which we excluded); we recorded time of occurrence, the identities of aggressor and victim and type of aggression. We determined winners of agonistic interactions from outcomes and the behaviour of the target of the aggression. Agonistic support was identified when two males either engaged together in charging displays against a group of individuals, attacked a common victim at the same time, or when one male intervened in favour of a second during an ongoing conflict (Goodall, 1986; Nishida & Hosaka, 1996; Nishida, Kano, Goodall, McGrew, & Nakamura, 1999): in addition to data on aggression, we recorded the identities and roles of the participants. As the possibility for individual A to support partner B depended on the number of times B was involved in agonistic interactions, rates of agonistic support were calculated by dividing the number of times A supported B by the total number of agonistic interactions involving B, excluding those between A and B. We assessed tolerance using rates of aggressive interactions received, calculated by dividing the number of times individual A attacked individual B by the total number of attacks given by individual A.
Dyadic association was calculated by dividing the number of 15 min interval scan samples in which two males were observed within the same party by the total number of scan samples, as previous work has suggested that 15 min is a good interval to consider data independent both for Sonso (Newton-Fisher, 1999a) and M-group (Kutsukake, 2003) chimpanzees. Finally, after a successful hunt, the identity of the individuals who shared the meat was recorded (following Nishida et al., 1999).
Grooming Reciprocity
Degree of grooming reciprocity was calculated through the reciprocity index RI (Mitani, 2009; Newton-Fisher & Lee, 2011; Nishida, 1988):
in which gAb is the grooming that individual A directed towards B, gBa is the grooming that B directed towards A and gAb + gBa is the total grooming exchanged between A and B. This index can range between 0 (no reciprocity) and 1 (complete reciprocity).
To determine whether male chimpanzees reciprocated grooming and/or traded it for other commodities we used linear mixed model (LMM) analysis to test the relationship between a dependent variable and multiple independent (or fixed) factors while controlling for the repeated sampling of the same individuals (Millar & Anderson, 2004; Pinheiro & Bates, 2000; Zuur, Ieno, Walker, Saveliev, & Smith, 2009). We used the ‘lme’ function in the ‘nlme’ package (Zuur et al., 2009) in R 2.14 (R Development Core Team, 2012). LMM models were fitted with a Gaussian error structure with restricted maximum likelihood. We checked that the assumptions of homogeneity and normality of residuals of the models were respected (Zuur et al., 2009). Grooming durations were log transformed to achieve normality for the Sonso data and for the M-group data during the period of social stability. For the data collected during the period of social instability in M-group, this transformation failed to normalize the data because of an excessive number of zeros: LMM analysis requires a normally distributed dependent variable (Pinheiro & Bates, 2000), so we instead removed the zeros from the dependent variable (following Gomes & Boesch, 2011) for this analysis. To assess the effect size of a variable, we removed that variable to create a reduced model and compared this to the complete model (i.e. the model containing all the variables) through a likelihood ratio test (LRT: Pinheiro & Bates, 2000) using the R function ‘anova’.
Male Rank
We determined male dominance ranks across each of our study periods (Sonso: 2003/2004; M-group: 2011) by calculating Eloratings derived from directed aggressive interactions (Albers & de Vries, 2001) using the R function ‘elo.sequence’ (Neumann et al., 2011). This method allowed us to track rank over time and thereby determine dominance ranks of interacting males on any day for which grooming or other social interactions were recorded. We confirmed ranks were consistent with the direction of pant-grunt vocalizations (performed by subordinates towards dominants: Bygott, 1979; Goodall, 1986). Following convention, ordinal rankings of males assigned a value of 1 to the highest-ranked individual (the alpha male), with numerically larger values indicating lower-ranked individuals.
Dominance Hierarchy Steepness
We also used our data on aggressive interactions to determine the steepness of male dominance hierarchies for both Sonso 2003/2004, and M-group 2011 using the R package ‘steepness’ (Leiva & de Vries, 2011). This package calculates David’s scores (DS: David, 1988) from dyadic agonistic interactions:
where w represents the number of times an individual, A, wins, l is the number of times A loses, w2 is the number of times individuals defeated by A win, and l2 is the number of times the individuals that won against A lose. ‘Steepness’ normalizes the DS values and determines the slope of the best-fit line of these against dominance rank to generate an index of hierarchy steepness that varies from 0 (flat, or egalitarian) to 1 (steep, or despotic). It also conducts a permutation test (9999 replications) to determine whether a detected degree of steepness is significantly different from random (de Vries, Stevens, & Vervaecke, 2006). To determine whether hierarchy steepness varied within these communities, we also calculated steepness values for the Sonso community in 1995 (Newton-Fisher, 1997, 2004, n.d.) and, using published interaction matrices, for M-group in 1985/1986 (Hayaki, Huffman, & Nishida, 1989) and 1992 (Nishida, 2012; Nishida & Hosaka, 1996). To place these dominance steepness values in cross-community context, we used published data to calculate values for the Kanyawara (Kibale National Park, Uganda) community in 1998 (Muller & Wrangham, 2004) and North group (Taï National Park, Cote D’Ivoire) in 1993 (Boesch & Boesch-Achermann, 2000).
Tests of the Grooming-trade Model
Are there rank-related commodities?
Agonistic support
Although any individual in a community can potentially offer agonistic support, requests for support are typically directed to the strongest (i.e. highest-ranking) individuals (Harcourt & deWaal, 1992). Among chimpanzees, agonistic support is known to play a role in increasing or maintaining social dominance rank (Gilby et al., 2013; Goodall, 1986; Nishida, 1983; Nishida & Hosaka, 1996; deWaal, 1982) as well as increasing the likelihood of achieving paternity independently of social rank effects (Gilby et al., 2013). It is expected, therefore, to be a highly attractive commodity. Among chimpanzees, as with other species, agonistic support can be provided by more than one individual at the same time (rarely more than two supporters; S. S. K. Kaburu & N. E. Newton-Fisher, personal observations), and support can be mutual, whereby two individuals initiate joint aggression against a third party without any obvious solicitation. We considered all interactions on a dyadic basis. To determine whether agonistic support could be considered a rank-related commodity, we extracted all instances of agonistic support by adult males and determined (1) mean dominance rank of the supporter(s) and (2) the correlation between the frequency of support and dominance rank.
Social tolerance
Although tests of the grooming-trade model in cercopithecines often include tolerance in feeding contexts as a potential commodity (e.g. Carne et al., 2011; Ventura et al., 2006), we did not include this in our analysis because male chimpanzees rarely compete over access to food resources (except perhaps for meat): most chimpanzee food (leaves, fruits: Newton-Fisher, 1999b; Nishida & Uehara, 1983) is difficult to monopolize. Instead, we investigated general social tolerance, as indicated by the receipt of aggression from other adult males. We reasoned that individuals that are more tolerated would receive less aggression and that males may be able to trade grooming for greater levels of tolerance. We determined (1) mean dominance rank of an aggressor and (2) the correlation between the frequency of aggression performed and dominance rank.
Is grooming directed up the hierarchy?
To assess whether grooming is directed up the hierarchy, we extracted the amount of grooming that males directed to higher-ranking individuals and compared this to the amount we would expect under a null hypothesis of an even allocation of grooming, using a paired-samples t test. As the amount of grooming that a male can direct up the hierarchy depends on the number of individuals that outrank him, expected values were calculated by multiplying the total amount of grooming given by a male by the proportion of individuals ranking above him (after Newton-Fisher & Lee, 2011): an alpha male has no individuals ranked above him that he can groom; the lowest-ranked individual is constrained to groom only individuals that rank above him. Thus, we tested the hypothesis that male chimpanzees direct more of their grooming to those of higher rank than would be expected given their own rank within the hierarchy. Analysis of Sonso males’ grooming interactions was conducted using 539 bouts, totalling 44.75 h of grooming. Mean bout length was 299 ± 438 s. Analysis of M-group males’ grooming interactions used 659 bouts in the stable period (59.60 h of grooming) and 266 bouts in the unstable period (16.47 h of grooming). Mean bout lengths were 325 ± 449 s and 223 ± 279 s in the two periods, respectively.
Do dominants receive more grooming than subordinates?
To examine whether dominant males receive more grooming than they give, for each male, we divided the total amount of grooming they gave by the total amount of grooming they received (GG/GR) and tested the correlation between this ratio and the males’ rank by Spearman’s rank correlation.
Are closely ranked individuals more reciprocal in their grooming?
To test whether rank distance predicts the degree of reciprocity in grooming effort (dRI), we constructed an LMM with dRI as the dependent variable and rank distance as the fixed factor, with the identities of the dyad members and the within-dyad repeated measurements treated as the random factors. Rank distance varied over time for each dyad, so we determined the rank distance on the day of each of the respective grooming interactions. As a consequence, multiple rank distances existed for each male–male pair, and each dyadic rank distance value was therefore treated as the repeated measurement.
Is total grooming effort spread across all potential partners?
We assessed the relationship between grooming effort and rank distance by constructing an LMM in which the amount of grooming exchanged was treated as the dependent variable and the rank difference between groomers was entered as the fixed factor while the identity of the groomers was set as the random factor.
We assessed diversity of grooming partners using the standardized Shannon–Weaver index (Henzi, Lycett, & Weingrill, 1997; Krebs, 1999; Newton-Fisher & Lee, 2011; Silk et al., 1999):
in which n represents the number of potential grooming partners and pi the proportion of grooming directed to the ith individual. This index takes a value of 1 if an individual grooms all partners equally and tends towards 0 if grooming is directed to a subset of potential partners. The standardized Shannon–Weaver index is particularly useful when the number of available partners is constant across all individuals, and is identical to the equitability index (E) used by, for example, Dunbar (1984) and Watts (2000a). We calculated diversity for both grooming bouts (H′bout) and effort (H′duration) and compared these indices between communities using Mann–Whitney U tests.
What is the relationship between hierarchy steepness and grooming reciprocity?
To assess whether males of our two study communities differed in the amount of reciprocal grooming, we calculated RI values for each adult male dyad within each community using both duration (dRI) and the frequency of bouts (fRI). These values were compared between communities using independent-samples t tests. To further examine grooming trading patterns in these two communities, and to control for possible influence of recipient’s rank, dyadic association and meat transfers on grooming behaviour, further LMMs were created. In these models, duration of grooming given was entered as the dependent variable; duration of grooming received, support received, aggression received, and, for M-group males during the stable period, meat received (30 hunts, 31 meat-sharing sessions; meat sharing was too infrequent during the unstable period or among the Sonso males to be included) were entered as the fixed factors, while the identity of the males was set as a random factor.
RESULTS
Hierarchy Steepness
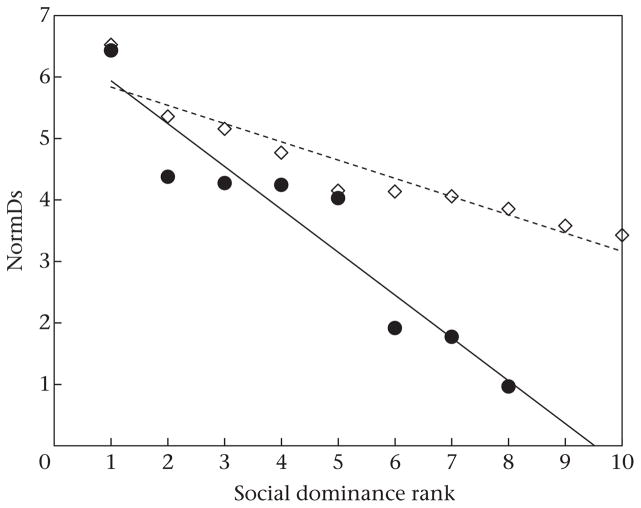
Our tests of the grooming-trade model were between two communities of chimpanzees with very different dominance hierarchies (Fig. 1). While the mean ± SD steepness value across communities was 0.40 ± 0.16, Sonso males in 2003/2004 formed a steep (despotic) hierarchy with a value of 0.70, somewhat steeper than they had shown in 1994/1995 (steepness = 0.40). By contrast, M-group males in 2011 formed a shallower (more egalitarian) hierarchy, with a dominance hierarchy steepness of 0.30 during the stable period. Males of this community also formed a shallow hierarchy (steepness = 0.22, P = 0.0013) in 1985 (Hayaki et al., 1989), but shallow hierarchies do not appear to be an inherent characteristic of M-group: data from 1992 revealed a more despotic hierarchy (steepness = 0.57). During the unstable period in 2011, our data on agonistic interactions produced a steepness value of 0.26, not significantly different from a random set of contest outcomes (P = 0.09). This suggests that the male dominance hierarchy essentially collapsed following the death of the alpha male. By way of comparison, chimpanzee males of North-group (Taï National Park) in 1993 and of Kanyawara (Kibale National Park) in 1998 both formed hierarchies with steepness values of 0.39. Except where indicated, for all steepness values P < 0.001. Multiple zeros in the 1985 aggression matrix probably explain why this period generated a steepness value that was lower, but still significant, than the nonsignificant value determined for the 2011 unstable period.
Figure 1.
Dominance hierarchy steepness, indicated by the slope of the best-fit line of normalized David’s score (NormDs) against dominance ranks among adult male chimpanzees of the two study communities. Sonso chimpanzees (●, solid line, N = 8) show a steep (despotic) hierarchy, while for M-group chimpanzees (◇, dashed line, N = 10), the hierarchy is shallow (egalitarian).
We noted that in M-group in 2011, seven (70%) of the adult males were aged between 20 and 30, whereas only 3 (38%) of the Sonso 2003/2004 males were in this age range: across communities, we found that hierarchy steepness was negatively related to the number of adult males in this age bracket (Pearson correlation: r6 = −0.743, P = 0.03).
Tests of the Grooming-trade Model
Are there rank-related commodities?
Agonistic support
In the Sonso community, the provision and frequency of agonistic support was restricted largely to high-ranking individuals. We recorded 62 instances of agonistic support, with the alpha-, beta- and gamma-ranked males providing the majority of support (78%). The mean rank of a supporter was 2.63. Dominant male chimpanzees provided support significantly more frequently than did subordinates (Spearman rank correlation: rS = −0.833, N = 8, P = 0.015). In M-group during the period of social stability, we recorded 30 instances of agonistic support. The three top-ranking males provided only 44% of agonistic support, and the mean rank of a supporter was 4.77. During the period of social instability, we recorded 34 instances of agonistic support. The three top-ranking males provided only 32% of agonistic support, and the mean rank of a supporter was 4.88. There was no relationship among M-group males between support given and dominance rank (stable period: rS = 0.360, N = 8, P = 0.381; unstable period: rS = 0.071, N = 7, P = 0.906).
Social tolerance
We recorded 265 male–male aggressive interactions from Sonso community (mean winner’s rank = 2.71) and 76 male–male aggressive interactions from M-group chimpanzees in the stable period (mean winner’s rank = 3.61) and 72 from the unstable period (mean winner’s rank = 3.89). In neither community were rates of aggression given related to dominance rank (Sonso: rS = −0.71, N = 8, P = 0.058; M-group stable: rS = −0.64, N = 8, P = 0.096; M-group unstable: rS = −0.5, N = 7, P = 0.267). Note, however, that observations at Sonso occurred during a period when a mid-ranking adult male (NK) attempted to rise in social rank. Excluding this individual’s aggression produced a significant relation between aggression performed and rank (Sonso: rS = −0.93, N = 7, P = 0.007).
Is grooming directed up the hierarchy?
Sonso males directed a significant amount (60%) of their grooming effort up the hierarchy (paired-samples t test: t7 = 2.971, P = 0.021), while this was not the case among M-group males: in the stable period, 49% of grooming effort was directed towards dominants (t9 = 1.794, P = 0.106); in the unstable period, 52% of grooming effort was directed up the hierarchy (t8 = 0.980, P = 0.356). Thus, among Sonso males (despotic hierarchy), but not M-group males (egalitarian hierarchy), subordinates directed a greater proportion of their grooming effort towards dominants.
Do dominants receive more grooming than subordinates?
We found a significant relationship between the ratio of grooming given to grooming received (GG/GR) and social rank for Sonso males (Spearman rank correlation: rS = 0.905, N = 8, P = 0.002), but not for M-group males (stable period: rS = −0.030, N = 10, P = 0.934; unstable period: rS = 0.383, N = 9, P = 0.308). Thus, in Sonso (despotic) but not M-group (egalitarian), dominants received more grooming than they performed, which suggests the presence of outbidding competition among the Sonso males.
Are closely ranked individuals more reciprocal in their grooming?
The greater the rank distance, the less balanced was grooming effort within dyads for the Sonso (despotic hierarchy) males (LMM: dRI: β ± SE = −0.076 ± 0.026, t = −2.914, P = 0.005), while this was not the case for M-group (egalitarian hierarchy) males, for whom we found no evidence of any relationship between rank distance and reciprocity, either in the stable period (dRI: β ± SE = −0.026 ± 0.015, t = −1.700, P = 0.121) or the unstable period (dRI: β ± SE = 0.007 ± 0.019, t = 0.370, P = 0.672). Thus, under the despotic hierarchy, where agonistic support was available as a commodity, close-ranked individuals were more reciprocal in their grooming.
Is total grooming effort spread across all potential partners?
In both communities, adult males allocated some grooming to most available partners (mean ± SD: H′bout: Sonso: 0.64 ± 0.06; M-group: stable: 0.82 ± 0.12; unstable: 0.71 ± 0.14; H′duration: Sonso: 0.60 ± 0.18; M-group: stable: 0.78 ± 0.13; unstable: 0.65 ± 0.17). By way of comparison, mean H′bout for adult males of the Sonso community in 1994/1995 was 0.82 (Newton-Fisher & Lee, 2011). Sonso males directed grooming to fewer partners than did M-group males during the stable period (Mann–Whitney U test: H′bout: W = 69, N1 =8, N2 = 10, P = 0.008; H′duration: W = 64, N1 =8, N2 = 10, P = 0.034), but during the unstable period, males of the two communities did not differ in the number of grooming partners (H′bout: W = 50, N1 = 8, N2 = 9, P = 0.200; H′duration: W = 44, N1 =8, N2 = 9, P = 0.481).
We found an inverse relationship between grooming effort and rank distance in Sonso: the smaller the rank difference between groomers, the more grooming they exchanged (LMM: β ± SE = −0.435 ± 0.141, t = −3.092, P = 0.006), but adult males of this community did not direct most grooming towards partners of adjacent social rank (Fig. 2). There was no relationship between grooming effort and rank distance for the M-group males (stable: β ± SE = 0.017 ± 0.077, t = 0.219, P = 0.792; unstable: β ± SE = −0.052 ± 0.069, t = −0.749, P = 0.460).
Figure 2.
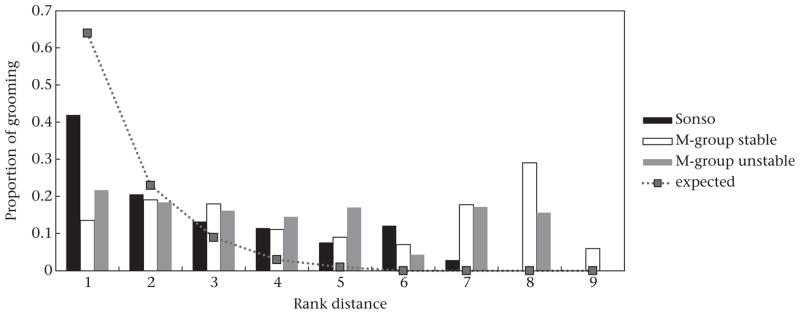
Mean allocation of grooming effort to individuals of increasing rank distance from the groomer, assessed across all adult male chimpanzees of both Sonso and M-group. Proportion of grooming allocation expected under Seyfarth’s (1977) model of social grooming was determined using the negative exponential function f(x) = 1.73e−x, a mathematical interpretation of the model’s prediction that grooming should be allocated mostly to individuals of adjacent dominance ranks. The constant (1.73) scales the function such that it allocates 100% of grooming across potential rank distances, while assigning the majority of this to adjacently ranked individuals (rank distance = 1). M-group chimpanzees did not allocate grooming according to this model (stable period: rS = 0.28, N = 9, P = 0.460; unstable period: rS = 0.55, N = 8, P = 0.160); for Sonso chimpanzees there was a significant correlation between observed and predicted values (rS = 0.89, N = 7, P = 0.007), but contrary to the model most grooming was not allocated to adjacently ranked individuals.
What is the relationship between hierarchy steepness and grooming reciprocity?
We found no statistically significant difference between communities in the degree to which male chimpanzees reciprocated grooming effort (independent-samples t test: Sonso versus M-group stable: t68 = 0.721, P = 0.475; Sonso versus M-group unstable: t51 = 0.417, P = 0.679), although M-group males were more reciprocal in the frequency of bouts exchanged during the stable period (mean fRI: Sonso = 0.45; M-group = 0.66; t68 = −2.458, P = 0.019).
For the Sonso males, distribution of grooming effort was predicted by both rates of support (LMM: β ± SE = 8.029 ± 1.888, t = 4.251, P = 0.001) and grooming effort received (β ± SE = 0.679 ± 0.088, t = 7.718, P < 0.001). Both variables exerted a significant influence: the reduced model that excluded either grooming received or support received showed a significantly inferior fit compared to the complete model (LRT, without grooming received: , P < 0.001; without support received: , P < 0.001). By contrast, rates of aggression received did not predict the distribution of grooming given (β ± SE = 0.187 ± 0.447, t = 0.418, P = 0.678). The model that included dyadic association and recipient’s dominance ranks showed that grooming received and recipient’s ranks were the only significant predictors of grooming given (grooming: β ± SE = 0.655 ± 0.079, t = 8.317, P < 0.001; recipient’s rank: β ± SE = −0.132 ± 0.034, t = −3.851, P < 0.001).
For the M-group males during the period of social stability, grooming given was significantly predicted by grooming received (β ± SE = 0.721 ± 0.083, t = 8.641, P < 0.001), whereas no relationship between grooming given and the receipt of other services was found: agonistic support (β ± SE = 2.487 ± 1.790, t = 1.389, P = 0.169), meat sharing (β ± SE = −0.435 ± 0.300, t = −1.450, P = 0.151) or aggression received (β ± SE = 0.396 ± 0.602, t = 0.657, P = 0.513). The reduced model that did not include grooming received had a significantly inferior fit compared to the complete model (LRT: , P < 0.001). When dyadic association and recipient’s dominance ranks were included in the model, grooming received still had a positive effect on grooming given (β ± SE = 0.576 ± 0.094, t = 6.152, P < 0.001). While grooming given was not significantly predicted by recipient’s dominance rank (β ± SE = −0.060 ± 0.043, t = −1.803, P = 0.075), it was positively influenced by dyadic association (β ± SE = 7.452 ± 2.708, t = 2.7511, P = 0.007).
Similar results were found during the period of social instability: grooming received was a significant predictor of grooming given (β ± SE = 0.515 ± 0.134, t = 3.841, P < 0.001), and removing grooming received from the model resulted in a significantly inferior fit (LRT: , P < 0.001); rerunning the analysis including zeros in the dependent variable did not change the results (with grooming received: β ± SE = 0.650 ± 0.093, t = 6.955, P < 0.001; LRT of the model without grooming received: , P < 0.001). In the model that included dyadic association and recipient’s dominance rank, grooming received continued to have a significant effect (β ± SE = 0.40 ± 0.15, t = 2.59, P = 0.01). Recipient’s dominance rank did not exert a significant effect (β ± SE = −0.35 ± 1.76, t = −0.20, P = 0.84), and in contrast to the results from the period of social stability, dyadic association was no longer a predictor of grooming given (β ± SE = 69.19 ± 54.90, t = 1.26, P = 0.22).
Thus, the analysis using LMM confirmed that for males in both communities the amount of grooming given was predicted by the amount of grooming received; that there was a strong reciprocity of grooming regardless of whether the dominance hierarchy could be characterized as despotic or egalitarian. It also showed that agonistic support was only a predictor of grooming given among males of the despotic Sonso community and not among males of the egalitarian M-group, for whom association was a significant predictor.
DISCUSSION
We found considerable variation in hierarchy steepness within and across communities. In accord with the grooming-trade model, our comparison between the two communities at the extremes of the hierarchy steepness distribution showed that adult male chimpanzees will trade grooming for agonistic support where this (or at least its likely effectiveness) is a rank-related commodity. Among Sonso males, which showed steep rank relationships: (1) dominants provided more agonistic support than subordinates; (2) males directed grooming up the hierarchy; (3) the ratio of grooming given to that received was lower for dominants than for subordinates. These results confirm previous findings that the tendency of Sonso male chimpanzees to groom up the hierarchy (Newton-Fisher,1997; Newton-Fisher & Lee, 2011) is best explained as a subordinate’s strategy to obtain rank-related benefits from dominants. Accordingly, our results suggest that Sonso males were more likely to provide support to partners that groomed them more and that this relationship between grooming given and support received was not a by-product of grooming reciprocity. By contrast, among M-group males with a shallow hierarchy during the study period, high-ranking individuals did not offer agonistic support more frequently than low-ranking males and dominance rank did not appear to affect grooming distribution. Furthermore, (4) in Sonso, but not M-group, males of similar dominance rank showed stronger reciprocity than those who held more distant ranks. In these respects male chimpanzees behaved much like female cercopithecoid monkeys, and their behaviour matched the predictions of the grooming-trade model. However, in contrast to the predictions of the model, males of the more egalitarian M-group did not exchange grooming more reciprocally than Sonso males: grooming reciprocity was not influenced by hierarchy steepness.
We found that total grooming effort was not restricted to individuals of adjacent rank (contra Seyfarth’s (1977) model of social grooming) and that the number of grooming partners was lower among the more despotic Sonso chimpanzees, where adult males also exchanged more grooming with individuals closer to them in social rank (contra BMT). As restriction on the diversity of grooming partners is an expected outcome of the competitive mechanism in Seyfarth’s model, these results suggest that where agonistic support is available as a commodity, competitive restrictions may impinge on the free market envisioned under BMT: although our results do not support the strict priority of access mechanism in Seyfarth’s model, they may indicate agonistic competition with lower-ranking individuals being excluded from grooming opportunities.
Vehrencamp (1983) introduced the terms ‘despotic’ and ‘egalitarian’ to the study of animal behaviour to describe end points on a continuum of the degree of bias in which benefits are distributed within a social group. Similarly, van Schaik (1989) used the terms to refer to the relative steepness of a dominance hierarchy, for which de Vries et al. (2006) provided a measure. This is the way in which the terms have been used in this study. There is, however, a different usage of the same terminology. de Waal (1989) and Flack and de Waal (2004), for instance, use despotic and egalitarian to refer to behavioural style: the manner in which dominants and subordinates behave towards one another. In their terminology, despotic species are those in which dominant animals exploit subordinates, whereas in egalitarian species, dominants are more tolerant, levels of aggression are lower and subordinates can retaliate against dominants. While the two sets of terminology might in some cases coalesce, this is not necessarily the case in either theory or practice: de Waal’s (1989) usage refers to characteristics of relationships (the abstraction of interactions between particular pairs of individuals: Hinde,1976), whereas Vehrencamp’s (1983) and van Schaik’s (1989) usage refers to social structure (the abstraction of relationships: Hinde, 1976). Male chimpanzees show substantial tolerance towards subordinates, provide agonistic support and maintain strong and reciprocal grooming relationships, so tend towards ‘egalitarian’ by de Waal’s (1989) definition, yet they are ‘despotic’ by Vehrencamp’s (1983) definition as benefits are biased towards high-ranking individuals: for example, paternity for males (Boesch, Kohou, Nène, & Vigilant, 2006; Inoue, Inoue-Murayama, Vigilant, Takenaka, & Nishida, 2008; Newton-Fisher, Emery Thompson, Reynolds, Boesch, & Vigilant, 2010; Wroblewski et al., 2009) and resource quality for females (Pusey, Williams, & Goodall, 1997). The use of identical terminology to refer both to aspects of relationships and structure is unfortunate, but probably too ingrained to change.
In this study, we found that the number of adult males aged 20–30 years predicted hierarchy steepness. This is typically the period in which males compete for, or hold, high rank, although some males may claim alpha rank earlier (e.g. Nishida, 2012) and some can hold it through their thirties (e.g. Newton-Fisher et al., 2010; Nishida, 2012). For M-group in 2011, 70% of adult males were in this age range, twice the average proportion (0.37 ± 0.08) across the other communities. Together with the variation in hierarchy steepness found across and within communities, this finding suggests that the degree of structural despotism/egalitarianism is not an inherent characteristic of species, at least for chimpanzees, but reflects a sociodemographic process: where there are more equally matched competitors, each striving for high rank, their very competition constrains the ability of any one individual to establish a despotic position over the others. This could explain why captive studies have produced contradictory descriptions of chimpanzee dominance style (despotic: Hare, Melis, Woods, Hastings, & Wrangham, 2007; egalitarian: Jaeggi et al., 2010) and we should be wary, therefore, of attempts to classify the social structure of a species as despotic or egalitarian without considerable empirical support from multiple social groups with varying demography.
Our finding that grooming reciprocity persists in the face of variation in hierarchy steepness is contrary to the grooming-trade model, which suggests that grooming reciprocity should be weaker when grooming can be traded for alternative services (Barrett et al., 2002, 1999). Our LMM analysis showed that the amount of grooming received was the primary predictor for the amount of grooming given for both M-group and Sonso, where support received was also significant. This suggests that grooming is sufficiently valuable to male chimpanzees that they will continue to trade for it when other services are available and for which they also trade. In the biological markets framework, services for which supply is high should have low value: our results suggest therefore that supply is restricted, or alternatively that demand is high. Grooming bestows hygienic (Akinyi et al., 2013; Tanaka & Takefushi, 1993; Zamma, 2002) and stress relief benefits (Boccia, Reite, & Laudenslager, 1989; Shutt, MacLarnon, Heistermann, & Semple, 2007). These may be important and sought after by male chimpanzees (Newton-Fisher, 2014), yet by grooming others, a male chimpanzee is providing these benefits to his main reproductive rivals: this represents a relative cost to the groomer, and males should, therefore, restrict the number of others with whom they exchange grooming. If the primary means of gaining access to the benefits of grooming is to provide grooming (as our results indicate), male chimpanzees should therefore engage in a limited number of reciprocal grooming arrangements (as shown by Mitani, 2009; Newton-Fisher & Lee, 2011). Supply is therefore restricted: if a male cannot offer effective agonistic support (we found no evidence for meat exchange or tolerance as services for which grooming is traded), he must rely on these reciprocal grooming partners unless he can coerce individuals into grooming him.
Within particular grooming dyads, the reciprocal exchange of grooming-derived benefits suggests chimpanzees are egalitarian, yet if these reciprocal grooming arrangements exist as a result of a need to minimize the provision of benefits to rivals, this egalitarianism seems forced. Where opportunities exist to exert dominance over others, such as when the number of competitive rivals is small, male chimpanzees strive to establish themselves at the top of a clear hierarchy, and so in M-group in 2011, the large number of more-or-less equally matched males may have created a situation in which efforts to impose dominance by one male created opportunities that could be exploited by equally well-placed rivals. Our results indicate that the social structure of chimpanzee males is best considered essentially despotic, in that they seek to dominate others and establish hierarchies with consequent rank-biased distribution of benefits, and that, despite showing somewhat egalitarian dominance styles (sensu deWaal, 1989), tendencies towards apparent structural egalitarianism are likely to be the result of competitive constraints on this despotism.
Overall, our findings support the assertion that grooming strategies of male chimpanzees have been shaped by natural selection as modelled by BMT. We show that under the conditions of a steep dominance hierarchy, agonistic support is a commodity largely restricted to high-ranking individuals, and that male chimpanzees will trade grooming in order to access this commodity; this pattern is not seen when hierarchy is flatter and provision of agonistic support is less related to male social rank. Reciprocal exchange of grooming (trading grooming to receive grooming in return) is important regardless of hierarchy steepness, so in contrast to the ‘grooming-trade’ model developed for cercopithecines, grooming among adult male chimpanzees cannot be regarded as a low-value commodity for which individuals will trade only when other commodities are not available. Our findings also hint at the possibility of agonistic competition, or at least exclusion, in relation to grooming opportunities compromising the free market envisioned by biological market theory. Our results build on previous findings across chimpanzee communities to emphasize the importance of reciprocal grooming exchanges among adult male chimpanzees, which can be understood in a biological markets framework if grooming, at least by or with particular individuals, is a valuable commodity.
Acknowledgments
This work was funded by the Wenner-Gren Foundation (grant number 8216), the Leverhulme Trust (grant number F/00236/Z) and the Harry Frank Guggenheim Foundation. We thank the Uganda National Council for Science and Technology, the President’s Office, the Forest Department and Vernon Reynolds for granting permission to work in the Budongo Forest and the Tanzania Commission for Science and Technology, the Tanzania Wildlife Research Institute and the Mahale Mountains Wildlife Research Centre for allowing research in the Mahale Mountains National Park. We are also very grateful to the local field assistants for their fundamental assistance during data collection both in Budongo and Mahale. Finally, we extend our gratitude to Ronald Noë, Jacinta Beehner and an anonymous referee for invaluable comments on a previous draft of the manuscript.
References
- Akinyi MY, Tung J, Jeneby M, Patel NB, Altmann J, Alberts SC. Role of grooming in reducing tick load in wild baboons (Papio cynocephalus) Animal Behaviour. 2013;85:559–568. doi: 10.1016/j.anbehav.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers PCH, de Vries H. Elo-rating as a tool in the sequential estimation of dominance strengths. Animal Behaviour. 2001;61:489–495. [Google Scholar]
- Altmann J. Observational study of behaviour: sampling methods. Behaviour. 1974;49:227–265. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Arnold K, Whiten A. Grooming interactions among the chimpanzees of the Budongo Forest, Uganda: tests of five explanatory models. Behaviour. 2003;140:519–552. [Google Scholar]
- Balasubramaniam KN, Berman CM, Ogawa H, Li J. Using biological markets principles to examine patterns of grooming exchange in Macaca thibetana. American Journal of Primatology. 2011;73:1269–1279. doi: 10.1002/ajp.20999. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam KN, Dittmar K, Berman CM, Butovskaya M, Cooper MA, Majolo B, et al. Hierarchical steepness, counter-aggression, and macaque social style scale. American Journal of Primatology. 2012;74:915–925. doi: 10.1002/ajp.22044. [DOI] [PubMed] [Google Scholar]
- Barrett L, Gaynor D, Henzi SP. A dynamic interaction between aggression and grooming reciprocity among female chacma baboons. Animal Behaviour. 2002;63:1047–1053. [Google Scholar]
- Barrett L, Henzi SP. The utility of grooming in baboon troops. In: Noë R, van Hooff JARAM, Hammerstein P, editors. Economics in nature: Social dilemmas, mate choice and biological markets. Cambridge, U.K: Cambridge University Press; 2001. pp. 119–145. [Google Scholar]
- Barrett L, Henzi SP. Monkeys, markets and minds: biological markets and primate sociality. In: Kappeler PM, van Schaik CP, editors. Cooperation in primates and humans: Mechanisms and evolution. New York, NY: Springer; 2006. pp. 209–232. [Google Scholar]
- Barrett L, Henzi SP, Weingrill T, Lycett JE, Hill RA. Market forces predict grooming reciprocity in female baboons. Proceedings of the Royal Society B: Biological Sciences. 1999;266:665–670. [Google Scholar]
- Boccia ML, Reite M, Laudenslager M. On the physiology of grooming in a pigtail macaque. Physiology & Behaviour. 1989;45:667–670. doi: 10.1016/0031-9384(89)90089-9. [DOI] [PubMed] [Google Scholar]
- Boesch C, Boesch-Achermann H. The chimpanzees of the Taï Forest. Oxford, U.K: Oxford University Press; 2000. [Google Scholar]
- Boesch C, Kohou G, Nène H, Vigilant L. Male competition and paternity in wild chimpanzees of the Taï. American Journal of Physical Anthropology. 2006;130:103–115. doi: 10.1002/ajpa.20341. [DOI] [PubMed] [Google Scholar]
- Brown GR, Almond REA, Bergen Y. Begging, stealing, and offering: food transfer in nonhuman primates. Advances in the Study of Behavior. 2004;34:265–295. [Google Scholar]
- Bshary R. The cleaner fish market. In: Noë R, van Hooff JARAM, Hammerstein P, editors. Economics in nature: Social dilemmas, mate choice and biological markets. Cambridge, U.K: Cambridge University Press; 2001. pp. 146–172. [Google Scholar]
- Bshary R, Grutter AS. Experimental evidence that partner choice is a driving force in the payoff distribution among cooperators or mutualists: the cleaner fish case. Ecology Letters. 2002;5:130–136. [Google Scholar]
- Bshary R, Noë R. The ubiquitous influence of partner choice on the dynamics of cleaner fish – client reef fish interactions. In: Hammerstein P, editor. Genetic and cultural evolution of cooperation. Cambridge, MA: MIT Press; 2003. pp. 167–184. [Google Scholar]
- Bshary R, Schäffer D. Choosy reef fish select cleaner fish that provide high-quality service. Animal Behaviour. 2002;63:557–564. [Google Scholar]
- Bygott JD. Agonistic behaviour, dominance, and social structure in wild chimpanzees of the Gombe National Park. In: Hamburg DA, McCown ER, editors. The great apes. Menlo Park, CA: Benjamin/Cummings; 1979. pp. 405–427. [Google Scholar]
- Carne C, Wiper S, Semple S. Reciprocation and interchange of grooming, agonistic support, feeding tolerance, and aggression in semi-free-ranging Barbary macaques. American Journal of Primatology. 2011;73:1127–1133. doi: 10.1002/ajp.20979. [DOI] [PubMed] [Google Scholar]
- David HA. The method of paired comparisons. New York, NY: Hafner; 1988. [Google Scholar]
- Dunbar RIM. Reproductive decisions: An economic analysis of gelada baboon social strategies. Princeton, NJ: Princeton University Press; 1984. [Google Scholar]
- Eggeling WJ. Observations on the ecology of the Budongo rain forest, Uganda. Journal of Ecology. 1947;34:20–87. [Google Scholar]
- Fischer MK, Hoffmann KH, Völkl W. Competition for mutualists in an ant–homopteran interaction mediated by hierarchies of ant attendance. Oikos. 2001;92:531–541. [Google Scholar]
- Flack JC, de Waal FBM. Dominance style, social power, and conflict management: a conceptual framework. In: Thierry B, Singh M, Kaumanns W, editors. Macaque societies: A model for the study of social organization. Cambridge, U.K: Cambridge University Press; 2004. pp. 157–185. [Google Scholar]
- Frank RE, Silk JB. Impatient traders or contigent reciprocators? Evidence for the extended time course of grooming exchanges in baboons. Behaviour. 2009;146:1123–1135. [Google Scholar]
- Fruteau C, Voelkl B, van Damme E, Noë R. Supply and demand determine the market value of food providers in wild vervet monkeys. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:12007–12012. doi: 10.1073/pnas.0812280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilby IC. Meat sharing among the Gombe chimpanzees: harassment and reciprocal exchange. Animal Behaviour. 2006;71:953–963. [Google Scholar]
- Gilby IC, Brent LJ, Wroblewski EE, Rudicell RS, Hahn BH, Goodall J, et al. Fitness benefits of coalitionary aggression in male chimpanzees. Behavioral Ecology and Sociobiology. 2013;67:373–381. doi: 10.1007/s00265-012-1457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes CM, Boesch C. Reciprocity and trades in wild West African chimpanzees. Behavioral Ecology and Sociobiology. 2011;65:2183–2196. [Google Scholar]
- Gomes CM, Mundry R, Boesch C. Long-term reciprocation of grooming in wild West African chimpanzees. Proceedings of the Royal Society B: Biological Sciences. 2009;276:699–706. doi: 10.1098/rspb.2008.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall J. The chimpanzees of Gombe: Patterns of behaviour. Cambridge, MA: Belknap; 1986. [Google Scholar]
- Gumert MD. Payment for sex in a macaque mating market. Animal Behaviour. 2007;74:1655–1667. [Google Scholar]
- Gumert MD, Ho MH. The trade balance of grooming and its relationship to tolerance in Indonesian long-tailed macaques (Macaca fascicularis) Primates. 2008;49:176–185. doi: 10.1007/s10329-008-0089-y. [DOI] [PubMed] [Google Scholar]
- Harcourt AH, de Waal FBM. Coalitions and alliances in humans and other animals. Oxford, U.K: Oxford University Press; 1992. [Google Scholar]
- Hare B, Melis AP, Woods V, Hastings S, Wrangham R. Tolerance allows bonobos to outperform chimpanzees on a cooperative task. Current Biology. 2007;17:619–623. doi: 10.1016/j.cub.2007.02.040. [DOI] [PubMed] [Google Scholar]
- Hayaki H, Huffman MA, Nishida T. Dominance among male chimpanzees in the Mahale Mountains National Park, Tanzania: a preliminary study. Primates. 1989;30:187–197. [Google Scholar]
- Hemelrijk CK. Support for being groomed in long-tailed macaques, Macaca fascicularis. Animal Behaviour. 1994;48:479–481. [Google Scholar]
- Henzi SP, Barrett L. The value of grooming to female primates. Primates. 1999;40:47–59. doi: 10.1007/BF02557701. [DOI] [PubMed] [Google Scholar]
- Henzi SP, Barrett L, Gaynor D, Greeff J, Weingrill T, Hill RA. Effect of resource competition on the long-term allocation of grooming by female baboons: evaluating Seyfarth’s model. Animal Behaviour. 2003;66:931–938. [Google Scholar]
- Henzi SP, Lycett JE, Weingrill T. Cohort size and the allocation of social effort by female mountain baboons. Animal Behaviour. 1997;54:1235–1243. doi: 10.1006/anbe.1997.0520. [DOI] [PubMed] [Google Scholar]
- Hinde RA. Interactions, relationships and social structure. Man. 1976;11:1–17. [Google Scholar]
- Hoeksema JD, Schwartz MW. Modelling interspecific mutualisms as biological markets. In: Noë R, van Hooff JARAM, Hammerstein P, editors. Economics in nature: Social dilemmas, mate choice, and biological markets. Cambridge, U.K: Cambridge University Press; 2001. pp. 173–183. [Google Scholar]
- Inoue E, Inoue-Murayama M, Vigilant L, Takenaka O, Nishida T. Relatedness in wild chimpanzees: influence of paternity, male philopatry, and demographic factors. American Journal of Physical Anthropology. 2008;137:256–262. doi: 10.1002/ajpa.20865. [DOI] [PubMed] [Google Scholar]
- Jaeggi AV, Stevens JMG, van Schaik CP. Tolerant food sharing and reciprocity is precluded by despotism among bonobos but not chimpanzees. American Journal of Physical Anthropology. 2010;143:41–51. doi: 10.1002/ajpa.21288. [DOI] [PubMed] [Google Scholar]
- Kaburu SSK, Inoue S, Newton-Fisher NE. Death of the alpha: within-community lethal violence among chimpanzees of Mahale Mountains National Park. American Journal of Primatology. 2013;75:789–797. doi: 10.1002/ajp.22135. [DOI] [PubMed] [Google Scholar]
- Kaburu SSK, Newton-Fisher NE. Social instability raises the stakes during social grooming among wild male chimpanzees. Animal Behaviour. 2013;86:519–527. [Google Scholar]
- Kappeler PM, van Schaik CP. Cooperation in primates and humans: Mechanisms and evolution. Berlin, Germany: Springer; 2006. [Google Scholar]
- Koyama NF, Caws C, Aureli F. Supply and demand predict male grooming of swollen females in captive chimpanzees, Pan troglodytes. Animal Behaviour. 2012;84:1419–1425. [Google Scholar]
- Krebs CJ. Ecological methodology. 2. Menlo Park, CA: Benjamin/Cummings; 1999. [Google Scholar]
- Kutsukake N. Assessing relationship quality and social anxiety among wild chimpanzees using self-directed behaviour. Behaviour. 2003;140:1153–1171. [Google Scholar]
- Leimar O, Axén AH. Strategic behavior in an interspecific mutualism: interactions between lycaenid larvae and ants. Animal Behaviour. 1993;46:1177–1182. [Google Scholar]
- Leinfelder I, de Vries H, Deleu R, Nelissen M. Rank and grooming reciprocity among females in a mixed-sex group of captive hamadryas baboons. American Journal of Primatology. 2001;55:25–42. doi: 10.1002/ajp.1036. [DOI] [PubMed] [Google Scholar]
- Leiva D, de Vries H. Steepness: Testing steepness of dominance hierarchies. R package version 0.2. 2011 http://cran.r-project.org/web/packages/steepness/index.html.
- Millar RB, Anderson MJ. Remedies for pseudoreplication. Fisheries Research. 2004;70:397–407. [Google Scholar]
- Mitani JC. Reciprocal exchange in chimpanzees and other primates. In: Kappeler PM, van Schaik CP, editors. Cooperation in primates and humans: Mechanisms and evolution. New York, NY: Springer; 2006. pp. 107–119. [Google Scholar]
- Mitani JC. Male chimpanzees form enduring and equitable social bonds. Animal Behaviour. 2009;77:633–640. [Google Scholar]
- Mitani JC, Merriwether DA, Zhang C. Male affiliation, cooperation and kinship in wild chimpanzees. Animal Behaviour. 2000;59:885–893. doi: 10.1006/anbe.1999.1389. [DOI] [PubMed] [Google Scholar]
- Mitani JC, Watts DP. Why do chimpanzees hunt and share meat? Animal Behaviour. 2001;61:915–924. [Google Scholar]
- Muller MN, Wrangham RW. Dominance, cortisol and stress in wild chimpanzees (Pan troglodytes schweinfurthii) Behavioral Ecology and Sociobiology. 2004;55:332–340. doi: 10.1007/s00265-020-02872-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Nishida T. Long-term field studies of chimpanzees at Mahale Mountains National Park, Tanzania. In: Kappeler PM, Watts DP, editors. Long-term field studies of primates. Berlin, Germany: Springer; 2012. pp. 339–356. [Google Scholar]
- Neumann C, Duboscq J, Dubuc C, Ginting A, Irwan AM, Agil M, et al. Assessing dominance hierarchies: validation and advantages of progressive evaluation with Elo-rating. Animal Behaviour. 2011;82:911–921. [Google Scholar]
- Newton-Fisher NE. Social interactions of the Sonso (Budongo) chimpanzees 1994–1995. Unpublished raw data n.d [Google Scholar]
- Newton-Fisher NE. Doctoral dissertation. Cambridge, U.K: University of Cambridge; 1997. Tactical behaviour and decision making in wild chimpanzees. [Google Scholar]
- Newton-Fisher NE. Association by male chimpanzees: a social tactic? Behaviour. 1999a;136:705–730. [Google Scholar]
- Newton-Fisher NE. The diet of chimpanzees in the Budongo Forest Reserve, Uganda. African Journal of Ecology. 1999b;37:344–354. [Google Scholar]
- Newton-Fisher NE. Hierarchy and social status in Budongo chimpanzees. Primates. 2004;45:81–87. doi: 10.1007/s10329-003-0064-6. [DOI] [PubMed] [Google Scholar]
- Newton-Fisher NE. Roving females and patient males: a new perspective on the mating strategies of chimpanzees. Biological Reviews. 2014;89:356–374. doi: 10.1111/brv.12058. [DOI] [PubMed] [Google Scholar]
- Newton-Fisher NE, Emery Thompson M, Reynolds V, Boesch C, Vigilant L. Paternity and social rank in Budongo Forest chimpanzees. American Journal of Physical Anthropology. 2010;142:417–428. doi: 10.1002/ajpa.21241. [DOI] [PubMed] [Google Scholar]
- Newton-Fisher NE, Lee PC. Grooming reciprocity in wild male chimpanzees. Animal Behaviour. 2011;81:439–446. [Google Scholar]
- Nishida T. Alpha status and agonistic alliance in wild chimpanzees (Pan troglodytes schweinfurthii) Primates. 1983;24:318–336. [Google Scholar]
- Nishida T. Development of social grooming between mother and offspring in wild chimpanzee. Folia Primatologica. 1988;50:109–123. doi: 10.1159/000156335. [DOI] [PubMed] [Google Scholar]
- Nishida T. The chimpanzees of the Mahale Mountains: Sexual and life history strategies. Tokyo, Japan: Tokyo University Press; 1990. [Google Scholar]
- Nishida T. Chimpanzees of the Lakeshore. Cambridge, U.K: Cambridge University Press; 2012. [Google Scholar]
- Nishida T, Hosaka K. Coalition strategies among adult male chimpanzees of the Mahale Mountains, Tanzania. In: McGrew WC, Marchant LF, Nishida T, editors. Great ape societies. Cambridge, U.K: Cambridge University Press; 1996. pp. 114–134. [Google Scholar]
- Nishida T, Kano T, Goodall J, McGrew WC, Nakamura M. Ethogram and ethnography of Mahale chimpanzees. Anthropological Science. 1999;107:141–188. [Google Scholar]
- Nishida T, Uehara S. Natural diet of chimpanzees (Pan troglodytes schweinfurthii): long-term record from the Mahale Mountains, Tanzania. African Study Monographs. 1983;3:109–130. [Google Scholar]
- Noë R. A Veto game played by baboons: a challenge to the use of the Prisoner’s Dilemma as a paradigm for reciprocity and cooperation. Animal Behaviour. 1990;39:78–90. [Google Scholar]
- Noë R. Biological markets: partner choice as the driving force behind the evolution of mutualisms. In: Noë R, van Hooff JARAM, Hammerstein P, editors. Economics in nature: Social dilemmas, mate choice, and biological markets. Cambridge, U.K: Cambridge University Press; 2001. pp. 93–118. [Google Scholar]
- Noë R. Digging for the roots of trading. In: Kappeler PM, van Schaik CP, editors. Cooperation in primates and humans: Mechanisms and evolution. New York, NY: Springer; 2006. pp. 233–261. [Google Scholar]
- Noë R, Hammerstein P. Biological markets: supply and demand determine the effect of partner choice in cooperation, mutualism and mating. Behavioral Ecology and Sociobiology. 1994;35:1–12. [Google Scholar]
- Noë R, Hammerstein P. Biological markets. Trends in Ecology & Evolution. 1995;10:336–340. doi: 10.1016/s0169-5347(00)89123-5. [DOI] [PubMed] [Google Scholar]
- Norscia I, Antonacci D, Palagi E. Mating first, mating more: biological market fluctuation in a wild prosimian. PLoS One. 2009;4:e4679. doi: 10.1371/journal.pone.0004679. http://dx.doi.org/10.1371/journal.pone.0004679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM. Mixed effects models in S and S-PLUS. New York, NY: Springer-Verlag; 2000. [Google Scholar]
- Plumptre AJ. Changes following 60 years of selective timber harvesting in the Budongo Forest Reserve, Uganda. Forest Ecology and Management. 1996;89:101–113. [Google Scholar]
- Pusey A, Williams J, Goodall J. The influence of dominance rank on the reproductive success of female chimpanzees. Science. 1997;277:828–831. doi: 10.1126/science.277.5327.828. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. www.R-project.org. [Google Scholar]
- Reynolds V. The chimpanzees of the Budongo Forest: Ecology, behaviour, and conservation. Oxford, U.K: Oxford University Press; 2005. [Google Scholar]
- Richter C, Mevis L, Malaivijitnond S, Schülke O, Ostner J. Social relationships in free-ranging male Macaca arctoides. International Journal of Primatology. 2009;30:625–642. doi: 10.1007/s10764-009-9364-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell YI, Phelps S. How do you measure pleasure? A discussion about intrinsic costs and benefits in primate allogrooming. Biology & Philosophy. 2013;28:1005–1020. [Google Scholar]
- Schino G. Grooming, competition and social rank among female primates: a meta-analysis. Animal Behaviour. 2001;62:265–271. [Google Scholar]
- Schino G, Aureli F. Grooming reciprocation among female primates: a meta-analysis. Biology Letters. 2008;4:9–11. doi: 10.1098/rsbl.2007.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schino G, Aureli F. Reciprocal altruism in primates: partner choice, cognition, and emotions. Advances in the Study of Behaviour. 2009;39:45–69. [Google Scholar]
- Schino G, di Giuseppe F, Visalberghi E. The time frame of partner choice in the grooming reciprocation of Cebus apella. Ethology. 2009;115:70–76. [Google Scholar]
- Schino G, di Sorrentino EP, Tiddi B. Grooming and coalitions in Japanese macaques (Macaca fuscata): partner choice and the time frame reciprocation. Journal of Comparative Psychology. 2007;121:181–188. doi: 10.1037/0735-7036.121.2.181. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Hoeksema JD. Specialization and resource trade: biological markets as a model of mutualism. Ecology. 1998;79:1029–1038. [Google Scholar]
- Seyfarth RM. Social relationships among adult female baboons. Animal Behaviour. 1976;24:917–938. doi: 10.1006/anbe.1999.1131. [DOI] [PubMed] [Google Scholar]
- Seyfarth RM. A model of social grooming among adult female monkeys. Journal of Theoretical Biology. 1977;65:671–698. doi: 10.1016/0022-5193(77)90015-7. [DOI] [PubMed] [Google Scholar]
- Seyfarth RM. The distribution of grooming and related behaviors among adult female vervet monkeys. Animal Behaviour. 1980;28:798–813. [Google Scholar]
- Seyfarth RM, Cheney DL. Grooming, alliances and reciprocal altruism in vervet monkeys. Nature. 1984;308:541–543. doi: 10.1038/308541a0. [DOI] [PubMed] [Google Scholar]
- Shutt K, MacLarnon A, Heistermann M, Semple S. Grooming in Barbary macaques: better to give than to receive? Biology Letters. 2007;3:231–233. doi: 10.1098/rsbl.2007.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JB. The patterning of intervention among male bonnet macaques: reciprocity, revenge and loyalty. Current Anthropology. 1992;33:318–325. [Google Scholar]
- Silk JB, Seyfarth RM, Cheney DL. The structure of social relationships among female savanna baboons in Moremi Reserve, Botswana. Behaviour. 1999;136:679–703. [Google Scholar]
- Stevens JMG, Vervaecke H, de Vries H, van Elsacker L. The influence of the steepness of dominance hierarchies on reciprocity and interchange in captive groups of bonobos (Pan paniscus) Behaviour. 2005;142:941–960. [Google Scholar]
- Stopka P, Macdonald DW. The market effect in the wood mouse, Apodemus sylvaticus: selling information on reproductive status. Ethology. 1999;105:969–982. [Google Scholar]
- Tanaka I, Takefushi H. Elimination of external parasites (lice) is the primary function of grooming in free-ranging Japanese macaques. Anthropological Science. 1993;101:187–193. [Google Scholar]
- Tiddi B, Aureli F, di Sorrentino EP, Janson CH, Schino G. Grooming for tolerance? Two mechanisms of exchange in wild tufted capuchin monkeys. Behavioral Ecology. 2011;22:663–669. [Google Scholar]
- van Schaik CP. The ecology of social relationships amongst female primates. In: Standen V, Foley R, editors. Comparative socioecology: The behavioural ecology of humans and other mammals. Oxford, U.K: Blackwell Scientific; 1989. pp. 195–218. [Google Scholar]
- Vehrencamp SL. A model for the evolution of despotic versus egalitarian societies. Animal Behaviour. 1983;31:667–682. [Google Scholar]
- Ventura R, Majolo B, Koyama NF, Hardie S, Schino G. Reciprocation and interchange in wild Japanese macaques: grooming, cofeeding, and agonistic support. American Journal of Primatology. 2006;68:1138–1149. doi: 10.1002/ajp.20314. [DOI] [PubMed] [Google Scholar]
- de Vries H, Stevens JMG, Vervaecke H. Measuring and testing the steepness of dominance hierarchies. Animal Behaviour. 2006;71:585–592. [Google Scholar]
- de Waal FBM. Chimpanzee politics. Baltimore, MD: Johns Hopkins University Press; 1982. [Google Scholar]
- de Waal FBM. Dominance “style” and primate social organization. In: Sanden VF, editor. Comparative socioecology. Oxford, U.K: Blackwell Scientific; 1989. pp. 243–264. [Google Scholar]
- Watts DP. Grooming between male chimpanzees at Ngogo, Kibale National Park. I. Partner number and diversity and grooming reciprocity. International Journal of Primatology. 2000a;21:189–210. [Google Scholar]
- Watts DP. Grooming between male chimpanzees at Ngongo, Kibale. II. Influence of male rank and possible competition for partners. International Journal of Primatology. 2000b;21:211–238. [Google Scholar]
- Watts DP. Reciprocity and interchange in the social relationships of wild male chimpanzees. Behaviour. 2002;139:343–370. [Google Scholar]
- Wroblewski EE, Murray CM, Keele BF, Schumacher-Stankey JC, Hahn BH, Pusey AE. Male dominance rank and reproductive success in chimpanzees, Pan troglodytes schweinfurthii. Animal Behaviour. 2009;77:873–885. doi: 10.1016/j.anbehav.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia DP, Li JH, Garber PA, Matheson MD, Sun BH, Zhu Y. Grooming reciprocity in male Tibetan macaques. American Journal of Primatology. 2013;75:1009–1020. doi: 10.1002/ajp.22165. [DOI] [PubMed] [Google Scholar]
- Xia DP, Li JH, Garber PA, Sun L, Zhu Y, Sun B. Grooming reciprocity in female tibetan macaques Macaca thibetana. American Journal of Primatology. 2012;74:569–579. doi: 10.1002/ajp.21985. [DOI] [PubMed] [Google Scholar]
- Yerkes RM. Genetic aspects of grooming, a socially important primate behavior pattern. Journal of Social Psychology. 1933;4:4–25. [Google Scholar]
- Zamma K. Grooming site preferences determined by lice infection among Japanese macaques in Arashiyama. Primates. 2002;43:41–49. doi: 10.1007/BF02629575. [DOI] [PubMed] [Google Scholar]
- Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM. Mixed effects models and extensions in ecology with R. New York, NY: Springer; 2009. [Google Scholar]