Abstract
The complexity of a radionuclear event would be immense due to varying levels of radiation exposures and injuries caused by blast-associated trauma. With this scenario in mind, we developed a mouse model to mimic as closely as possible the potential consequences of radiation injury and radiation combined injury (RCI) on survival, immune system phenotype, and immune function. Using a mouse burn injury model and a 137CsCl source irradiator to induce injuries, we report that the immunological response to radiation combined injury differs significantly from radiation or burn injury alone. Mice that underwent radiation combined injury showed lower injury survival and cecal ligation and puncture (CLP) induced polymicrobial sepsis survival rates than mice with single injuries. As anticipated, radiation exposure caused dose-dependent losses of immune cell subsets. We found B and T cells to be more radiation sensitive, while macrophages, dendritic cells and NK cells were relatively more resistant. However, radiation and radiation combined injury did induce significant increases in the percentages of CD4+ regulatory T cells (Tregs) and a subset of macrophages that express cell-surface GR-1 (GR-1+ macrophages). Immune system phenotyping analysis indicated that spleen cells from radiation combined injury mice produced higher levels of proinflammatory cytokines than cells from mice with radiation or burn injury alone, especially at lower dose radiation exposure levels. Interestingly, this enhanced proinflammatory phenotype induced by radiation combined injury persisted for at least 28 days after injury. In total, our data provide baseline information on differences in immune phenotype and function between radiation injury and radiation combined injury in mice. The establishment of this animal model will aid in future testing for therapeutic strategies to mitigate the immune and pathophysiological consequences of radionuclear events.
INTRODUCTION
Following nuclear accidents or explosions, a high percentage of people near the event will be exposed to radiation and trauma caused by blast and burn injuries (1). This combination of radiation injury and additional trauma is commonly referred to as radiation combined injury (RCI). Radiation combined injury could occur from the forces of a blast, lacerations from flying debris, burns from fire and radiation, and other types of multiple traumatic injuries caused by collapsing structures or accidents from the chaotic nature of a disaster. History indicates that radiation combined injury will occur in upward of 70% of individuals affected by a radionuclear event (2, 3). Individual outcomes of such a disaster will be further complicated by variable radiation exposures and differing severity of injuries. The levels of susceptibility to opportunistic infection will depend on the level of radiation exposure and combined injury (4, 5). Currently, no effective treatments to achieve protection from secondary infections after RCI exist, partially due to a lack of understanding of the immunological sequelae encountered after RCI. Thus, it is imperative to gain a clearer understanding of the impact of radiation and radiation combined injury on immune system function and phenotype to aid in the development of medical countermeasures.
The primary goal of this research effort was to establish a working model for radiation and RCI to mimic as closely as possible the human response to these injuries. We propose that using outbred mice rather than inbred mice would provide a more realistic view of how human populations might respond to radiation or RCI. Radiation injury was induced by whole-body radiation of mice with 137Cs γ rays. A well-established mouse burn injury model was used to induce trauma in combination with radiation exposures. This mouse injury model has been shown by our group and others to closely mimic the immune and physiological response to severe injuries in humans (6, 7). To induce RCI, mice were exposed to varying levels of radiation and near-simultaneous 25% total body surface area (TBSA) burn injury. We measured survival responses and phenotypic changes in the immune system at time intervals ranging from 1 day to 28 days after injuries. In addition, we tested groups of single-injured or RCI mice for changes in their survival response to polymicrobial sepsis induced by the cecal ligation and puncture (CLP) approach. We show that RCI caused measureable differences in survival, sepsis survival, immune cell populations and immune cell phenotype responses to innate and adaptive immune stimuli. In total, these data provide comparative information about the immunological impacts of radiation, trauma, and RCI in an animal model to provide the groundwork needed for future therapeutic testing strategies. The objectives of future preclinical testing studies will be to ameliorate the life-threatening consequences of exposure to radiation and RCI in humans that may be exposed to these combined injury scenarios.
MATERIALS AND METHODS
Mice
Six-week-old male CD-1 mice purchased from Charles River Laboratories International, Inc. (Wilmington, MA) are a specialized outbred mouse line that is purposely bred to maintain outbred genotypes. Mice were maintained in our full-barrier animal facility under controlled temperature and humidity, and under a 12 h light/dark regimen. All mice were acclimatized for at least 5 days with a diet of standard chow and water ad libitum prior to use. Experiments were performed on mice aged 7–8 weeks. All animal protocols were approved by the Harvard Medical School Institutional Animal Care and Use Committee (Boston, MA) and were found to be in accordance with the guidelines of the National Institutes of Health (NIH, Bethesda, MD). Mice were anesthetized by i.p. injection with ketamine (125 mg/kg) and xylazine (10 mg/kg) purchased from Webster Veterinary (Devens, MA).
Reagents
Cells were prepared and cultured in RPMI-1640 that was supplemented with 5% heat-inactivated fetal calf serum, 1 mM glutamine, 10 mM Hepes, 100 μM nonessential amino acids, penicillin/streptomycin/fungiozone, and 2.5 × 10−5 M 2-Mercaptoethanol [referred to as complete-5 (C5)], all purchased from Life Technologies (Grand Island, NY). Dulbecco’s phosphate-buffered saline (PBS) was also purchased as a 1× solution from Life Technologies. Flow cytometry stains were performed in PBS containing 1% bovine serum albumin, Fraction V and 0.1% sodium azide, all purchased from Sigma Chemical Company (St. Louis, MO). Cells were fixed in PBS containing 0.3% paraformaldehyde. Cells were permeablized for intracellular staining using the Fix/Perm buffer set purchased from BioLegend (San Diego, CA).
Radiation Injury
Mice were exposed to whole-body γ irradiation at 0.5, 1, 2, 4, 6, 8, or 10 Gy with an average dose rate of 1.65 Gy/min using a 137Cs J.L. Shepherd & Associates Irradiator (San Fernando, CA). In brief, mice were anesthetized with ketamine (125 mg/kg)/xylazine (10 mg/kg), placed in a pie-plate holder on a turntable, and then exposed to 137Cs γ rays until the desired level of whole-body radiation exposure was achieved. Sham-irradiated mice were anesthetized with ketamine/xylazine, but were not irradiated (0 Gy group). For phenotyping and radiation dose-response survival studies, all mice received 5 ml Sulfatrim antibiotic (200 mg/ml solution) in their drinking water (250 ml).
Mouse Burn Injury Model
The mouse burn injury protocol was performed as described previously (8). Briefly, mice were anesthetized by i.p. injection with ketamine (125 mg/kg)/xylazine (10 mg/kg), their dorsal fur was shaved, and then placed in a plastic mold that exposed only 25% of their total body surface area (TBSA). Injury was induced by immersing the exposed part of the dorsum in 90°C water for 9 s. This approach causes a delineated full-thickness burn injury. Moreover, this injury is considered anesthetic due to nerve cell damage, therefore mice do not require post-injury analgesic treatment. Sham-injured mice underwent the same procedure, but were exposed to room temperature water for 9 s. All animals were resuscitated by i.p. injection with 1 ml of 0.9% pyrogen-free saline. The mortality from burn injury alone was <5%.
Cecal Ligation and Puncture Polymicrobial Sepsis Model
Cecal ligation and puncture was used to induce polymicrobial sepsis in mice (9, 10). At 7 days after irradiation or RCI, mice were anesthetized by i.p. injection with ketamine (125 mg/kg)/xylazine (10 mg/kg) and prepared for CLP surgery by shaving the abdomen with electric clippers, scrubbing the surgical site with Betadine and wiping with an alcohol swab. The skin was cut with surgical scissors, followed by the peritoneum. The cecum was gently withdrawn from the peritoneal cavity. The distal 1.5 cm of the cecum was ligated with 4-0 silk suture and a single, through and through puncture was made in the ligated portion of the cecum with a 21-gauge needle. The cecum was returned to the peritoneal cavity and the incision site was closed in layers with 5-0 LOOK suture purchased from Angiotech Pharmaceuticals, Inc. (Vancouver, BC, Canada). Mice were resuscitated with 1 ml of pyrogen-free saline immediately after surgery. All mice were treated by sc injection with Buprenorphine (0.05 mg/kg) twice daily for 2 days after surgery. Mice were observed daily to calculate survival rates for 14 days after CLP surgery.
Blood, Lymph Node and Spleen Cell Preparation
At time points after injuries, mice were sacrificed by CO2 asphyxiation. Blood was harvested by cardiac puncture into EDTA anti-coagulant buffer. The injury site draining lymph nodes (inguinal, axillary and brachial) and the spleens were harvested. The lymph nodes and spleens were immediately minced using a sterile wire mesh and cell suspensions were prepared in C5 medium. Red blood cells in blood and spleen cell preparations were lysed using our own formulated mouse red blood cell lysis buffer (designed for gentle lysis and preservation of fragile cells, e.g., neutrophils). Cell preparations were washed twice by centrifugation (200g, 10 min) in C5 medium and then strained in 70 μm cell strainers (BD Falcon, BD Biosciences, Bedford, MA) to remove debris. Cells were plated for flow cytometry stains in round-bottom 96-well plates. For ex vivo stimulation cultures, spleen cells were plated at 5 × 105 cells/well and were stimulated with 100 ng/ml E. coli lipopolysaccharide (LPS) O26:B6 (Difco, Detroit, MI), or 100 ng/ml of the synthetic bacterial lipopeptide (BLP) analog called Pam3CSK4 (Bachem Americas, Torrance, CA), or 1 μg/ml CpG oligodeoxynucleotide (ODN) sequence 2336 (Coley Pharmaceutical Group, Wellesley, MA), or soluble anti-CD3ε antibody at 0.5 μg/ml final (clone 145-2C11, BioLegend). Supernatants were harvested 48 h later and 20 μl samples were tested for cytokine levels by multiplex cytokine assays.
Multiplex Cytokine Assays
We developed a custom multiplex panel of Luminex bead assays to measure cytokine levels in 20 μl samples of culture supernatants. Our assays measured IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p40, IL-12p70, IL-13, IL-17, IL-18, IL-23, IL-33, TNFβ, MCP-1 and IFNγ. The multiplex assays were performed using a Luminex 200 instrument (Luminex Corporation, San Antonio, TX). We used the StarStation software program (Applied Cytometry Systems, Minneapolis, MN) to calculate supernatant cytokine levels by standard curve analysis using known concentrations of recombinant cytokines as standards.
Flow Cytometry (FACS)
Blood, lymph node, or spleen cells from individual mice were distributed into 96-well round-bottom plates. For each experiment, cells were resuspended at the same volume with C5 medium to aid in automated cell counting; 2 ml for blood cells added at 0.1 ml per well, 2 ml for lymph node cells added at 0.1 ml per well, 5 ml for spleen cells added at 0.05 ml per well of a round-bottom 96-well plate (Costar, Corning, NY). Cells were pelleted by centrifugation at 300g for 10 min. After agitation to suspend cells, 0.02 ml of FACS staining buffer (Dulbecco’s PBS containing 1% w/vol bovine serum albumin fraction V, and 0.1% w/vol sodium azide) with TruStain FC block reagent (BioLegend) was added for a 10 min incubation at 4°C in the dark. Mixtures of FACS staining antibodies were added (0.02 ml/well) for 20 min incubation at 4°C in the dark. For detection of FoxP3+ cells by intracellular stains, cells were surface stained for CD3 and CD4, then permeabilized using the fix/perm buffer set from BioLegend prior to staining with anti-FoxP3 specific antibody. We used a conventional multicolor FACS staining approach using fluorescently-tagged antibodies to identify immune cell subsets. The fluorescent-labeled antibodies used for FACS stains included FITC-labeled anti-CD3ε (145-2C11), -CD4 (GK1.5), -CD19 (6D5), and -NK1.1 (PK136) antibodies; PE-labeled anti-CD8 (53–6.7), -F4/80 (BM8), -B220 (RA3-6B2), and –CD5 (53–7.3) antibodies; APC-labeled anti-CD4 (GK1.5), -GR-1 (RB6-8C5), and CD11c (HL3) antibodies; and eFluor450 anti-FoxP3 (FJK-16s) antibody. All fluorescent-labeled antibodies were purchased from BioLegend. Cells were gated as follows to identify immune cell subsets: CD4 T cells (CD3+CD4+), CD8 T cells (CD3+CD8+), B cells (CD19+), monocytes/macrophages (F4/80+), dendritic cells (CD11c+), neutrophils (GR-1+), NK cells (NK1.1+), B1 B cells (CD19+CD5+), Gr-1+ macrophages/monocytes (F4/80+Gr-1+), plasmacytoid dendritic cells (CD11c+B220+), myeloid dendritic cells (CD11c+CD4+), lymphoid dendritic cells (CD11c+CD8+), and CD4+ regulatory T cells (CD4+FoxP3+). Cells were analyzed using a Miltenyi MacsQuant instrument (Cambridge, MA) and the FCS files were analyzed using FlowJo software version 7.6.5 (Ashland, OR). An example of the gating strategy used for immune cell subset analysis is illustrated in Supplementary Fig. 1 (http://dx.doi.org/10.1667/RR3120.1.S1).
Statistical Analysis
Data was analyzed for statistical significance using GraphPad Prism 5.0 Software (San Diego, CA). Survival data curves were analyzed by the log rank statistical test. Flow cytometry data and cytokine data were analyzed by one-way or two-way ANOVA and Tukey or Bonferroni multiple comparisons. P < 0.05 was considered significant.
RESULTS
Mouse Survival Response to Radiation Combined Injury
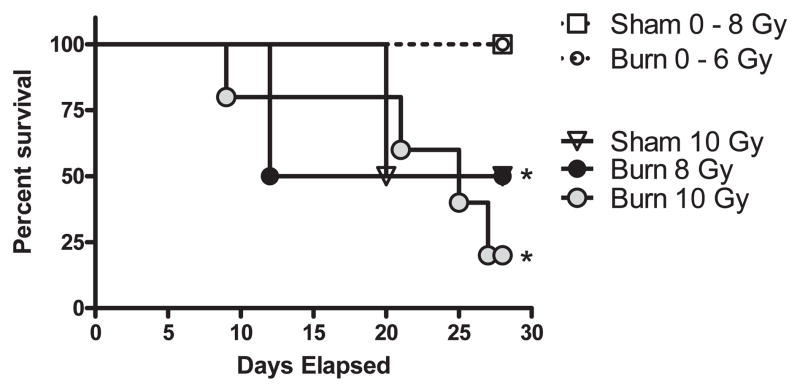
To establish a working model for radiation combined injury, we performed 28-day survival studies in mice that underwent whole-body radiation injury at graded doses between 0.5–10 Gy with or without combined 25% total body surface area (TBSA) burn injury. To best model a “near-simultaneous” combined injury, the burn injury procedure was performed immediately adjacent to the irradiator resulting in a time lag of approximately 3 min between injuries. As shown in Fig. 1, mice with combined injury succumbed to their injuries more readily than mice exposed to single injuries (P < 0.05 log-rank test). Significant mortality was not observed in radiation injury until 10 Gy exposure was reached, while RCI showed significant mortality at 6 Gy exposure. We intentionally used an outbred mouse strain called CD-1 mice for this model to better mimic the human response to radiation and combined injury. Additionally, mice were given oral antibiotics for the duration of these survival studies and for phenotyping experiments to more closely match the supportive care that would likely be given to injured patients. Though not unexpected, the results of these experiments indicate the more severe nature of combined injury compared to single injury at high whole-body radiation exposures.
FIG. 1.
Survival response curves for radiation and radiation combined injury. Groups of male CD-1 mice were exposed to 0, 0.5, 1, 2, 4, 6, 8 or 10 Gy radiation by timed exposure to 137CsCl without or with combined 25% TBSA burn injury. Mice were monitored daily to measure mortality over a 28 day time course. The (*) indicates significant mortality compared to sham mice by the log-rank statistical test for n = 16 mice per group.
The Impact of Radiation and Radiation Combined Injury on Immunity to Sepsis
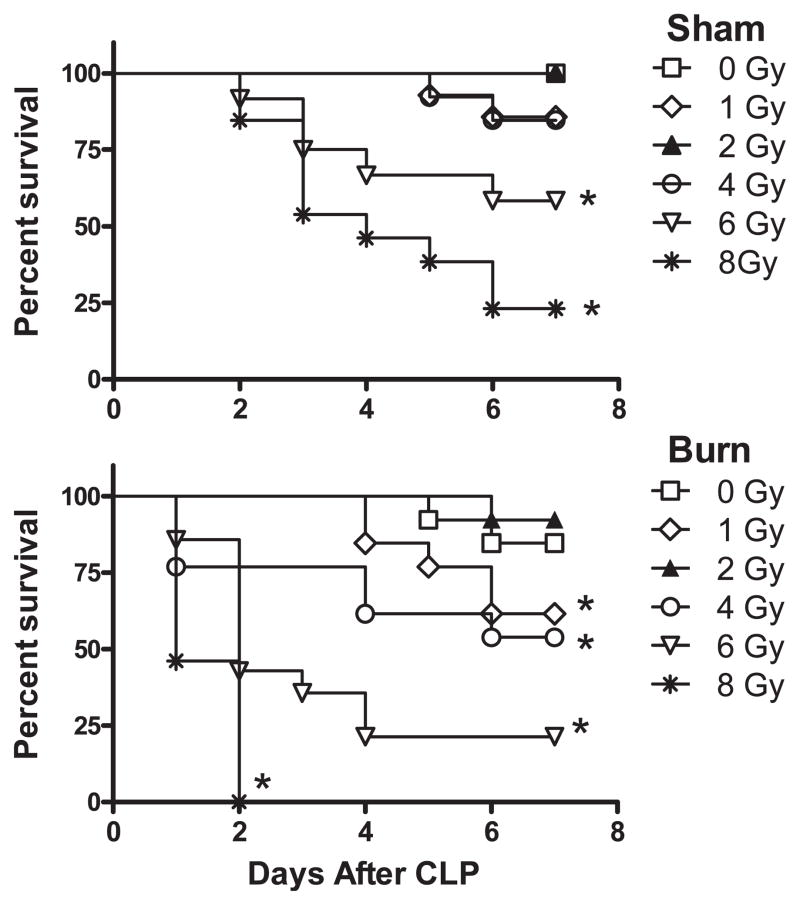
A significant complication of radiation combined injury will be the loss of effector immune function. In particular, the development of post-injury community- or hospital-acquired opportunistic infections would likely occur in patients exposed to radiation alone or radiation with combined trauma. To test the influence of radiation and radiation combined injury on antimicrobial immunity and sepsis survival, mice were exposed to a polymicrobial infection challenge by the cecal ligation and puncture technique. The CLP mouse model was used because it is considered a clinically relevant mouse model for acute and chronic microbial sepsis. Thus, survival rates in CLP mice will reflect a combination of antimicrobial immunity and the capacity of individual mice to survive sepsis and septic shock. Mice underwent irradiation and RCI at doses ranging from 0.5–8 Gy and oral antibiotics were withheld. After 7 days, mice underwent the CLP procedure to induce sepsis. Survival was monitored for 14 days after CLP challenge. The survival curves shown in Fig. 2 compare the differences in sepsis survival rates between mice exposed to radiation injury alone versus RCI. These data indicate that mice exposed to radiation doses lower than 4 Gy with combined burn injury showed a marked decrease in sepsis survival rates as directly compared to mice that underwent radiation injury alone. Mice exposed to 1 or 4 Gy irradiation with combined burn injury showed significantly lower sepsis survival rates than did sham-injured mice exposed to 1 or 4 Gy irradiation. Interestingly, exposure to 2 Gy irradiation caused an inversion in CLP survival response showing no significant difference between sham or burn injury alone. The severe nature of RCI on sepsis survival is demonstrated by the rapid death rate in RCI mice exposed to 6 or 8 Gy radiation levels. These findings demonstrate that combined injury results in a more severe loss of immune function resulting in a significant increase in sepsis-induced mortality.
FIG. 2.
Cecal ligation and puncture (CLP) sepsis survival response following radiation or radiation combined injury. Groups of male CD-1 mice were exposed to 0, 1, 2, 4, 6 or 8 Gy radiation by timed exposure to 137CsCl without or with combined 25% TBSA burn injury. One week later, mice underwent the CLP procedure to induce polymicrobial sepsis. Mice were monitored daily for 7 days to measure mortality. The (*) indicates significant mortality compared to sham or burn CLP mice by the log rank statistical test for n = 16 mice per group.
Phenotypic Changes in the Immune Cells Caused by Radiation Versus Radiation Combined Injury
We next wanted to determine the dose and time effects of radiation and RCI on immune cell changes in the blood, lymph nodes and spleen of mice. Groups of mice were exposed to varying levels of radiation with or without combined burn injury. At days 1, 7, 14 or 28, we prepared blood, spleen and lymph node cells for flow cytometry measurements of immune cell subsets. Flow cytometry was also used to measure cell numbers between injury groups. Specific cell-surface and intracellular markers were used to identify neutrophils, monocytes, macrophages, dendritic cells, NK cells, B cells, CD4 T cells and CD8 T cells in these immune cell compartments. We also measured additional subsets of these immune cell populations that are important in regulating inflammatory immune responses including CD4+ regulatory T cells (Tregs), GR-1+ macrophages, B1 B cells and dendritic cell subsets (myeloid, lymphoid and plasmacytoid subsets). The flow cytometry gating strategies used for immune cell subset analyses are shown in Supplementary Fig. 1 (http://dx.doi.org/10.1667/RR3120.1.S1).
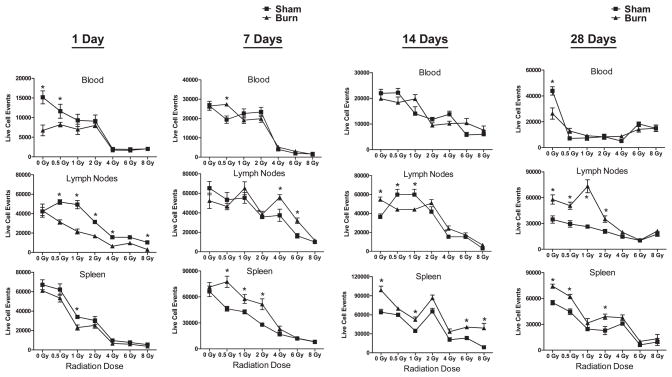
The dose and time effects of radiation and RCI on immune cell losses in the blood, lymph nodes and spleen of mice are shown in Fig. 3. The radiation-induced immune cell loss is evident from these data in all immune compartments. The combined burn injury caused higher cell losses at 1 day after injury at radiation doses lower than 4 Gy. However, at later time points, RCI mice showed significantly higher immune cell numbers in the spleen at 1 week and in the lymph nodes at 1 month after injury. These results suggest that combined injury may amplify early hematopoietic system injury, but may then promote later hematopoietic cell recovery.
FIG. 3.
Radiation and radiation combined injury effects on immune cell numbers in blood, lymph nodes and spleen of mice. Groups of male CD-1 mice were exposed to 0, 0.5, 1, 2, 4, 6 or 8 Gy radiation by timed exposure to 137CsCl without or with combined 25% TBSA burn injury. At 1, 7, 14 or 28 days after injuries, cells were prepared from blood, lymph nodes, or spleen of mice to be analyzed by flow cytometry. Cell counts were determined by flow cytometry and plotted as mean ± SEM for 5–6 mice per group. The (*) indicates significant differences at P < 0.05 between sham and burn injury or sham/burn injury with radiation exposure by one-way ANOVA and Tukey multiple comparisons for n = 5–6 individual mice per group.
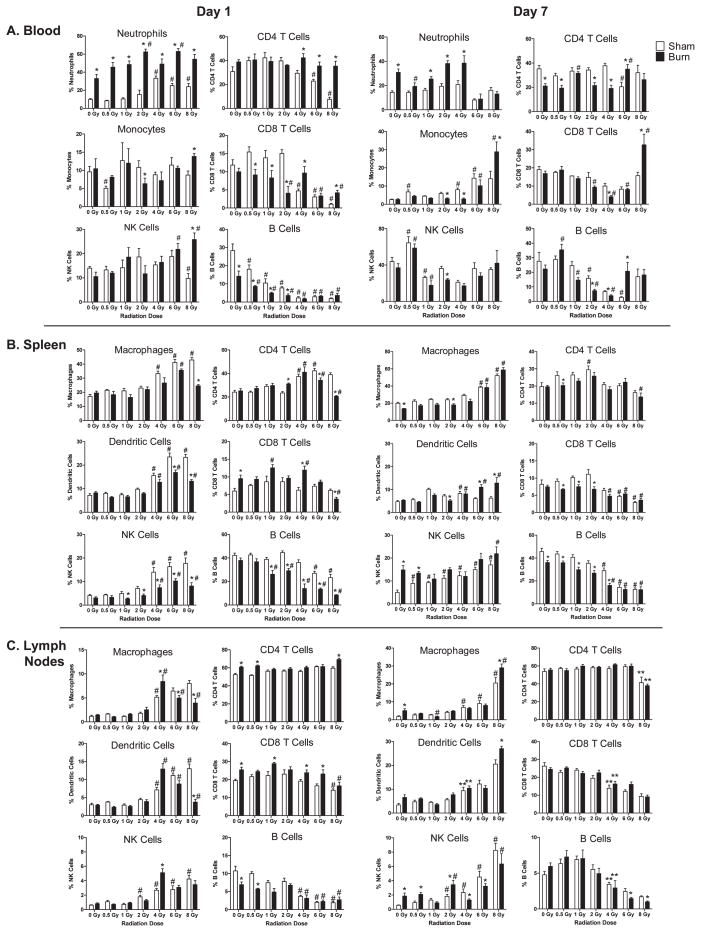
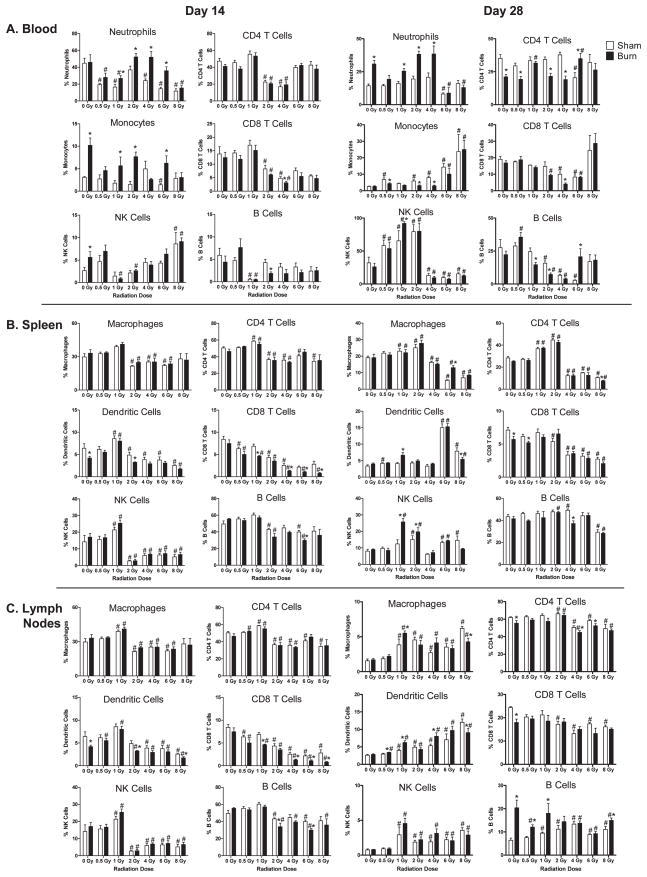
The effects of radiation and RCI on specific immune cell subsets were documented by multicolor flow cytometry studies using specific antibody reagents. Immune cell subsets were measured in cell suspensions by flow gating approaches. To standardize the flow cytometry detection, we suspended cells in equal volumes and equivalent amounts of subsamples were plated for flow cytometry analysis. This allowed us to compare specific immune cell subset losses and changes from different time points and conditions. The data plotted in Figs. 4 and 5 illustrates the effects of radiation and RCI on major immune cell subset changes at days 1, 7, 14 and 28 after injuries. It is apparent that radiation and radiation combined burn injury significantly affects immune subset percentages in the blood, lymph nodes and spleen of mice at these time points post-injuries. In general, burn injury promoted higher circulating levels of cells at all time points examined. This is especially evident in mice exposed to radiation doses lower than 4 Gy. Burn injury also promoted higher levels of circulating monocytes and CD8 T cells at 7 days after 8 Gy radiation exposure. In the spleens and lymph nodes, we found radiation-induced increases in NK cells at radiation doses lower than 2 Gy at all time points examined, while high doses significantly reduced their percentages. The percentages of CD4 T cells remained relatively constant at all time points, while CD8 T cells and B cells showed reductions at radiation doses greater than 4 Gy up to 14 days after radiation injury and RCI. Dendritic cells showed a variable time dependent response. There was an early increase in percentages in the spleen and lymph nodes, but a decrease at 14 days and an increase at 28 days. Overall, radiation injury alone had a dominant effect on these major immune cell subset percentages. The exception was the obvious increase in neutrophil percentages caused by RCI.
FIG. 4.
Radiation and radiation combined injury influences on specific immune cell subsets in blood, lymph nodes, and spleen of mice at days 1 and 7 after injuries. Groups of male CD-1 mice were exposed to 0, 0.5, 1, 2, 4, 6 or 8 Gy radiation by timed exposure to 137CsCl without or with combined 25% TBSA burn injury. At days 1 or 7 after injuries, cells were prepared from blood, lymph nodes, or spleen from individual mice. Cell suspensions were stained with fluorescently-labeled antibodies specific for the indicated immune cell subsets as described in Materials and Methods. Flow cytometry was performed, and flow data was analyzed by gating to identify relative changes in cell percentages induced by radiation and radiation combined injury. The (*) indicates significant differences between sham and burn injury and (#) indicates significant differences between 0 Gy radiation and radiation exposures at the indicated doses. P < 0.05 by two-way ANOVA and Bonferroni multiple comparisons for n = 5–6 individual mice per group.
FIG. 5.
Radiation and radiation combined injury influences on specific immune cell subsets in blood, lymph nodes, and spleen of mice at days 14 and 28 after injuries. Groups of male CD-1 mice were exposed to 0, 0.5, 1, 2, 4, 6 or 8 Gy radiation by timed exposure to 137CsCl without or with combined 25% TBSA burn injury. At days 1 or 7 after injuries, cells were prepared from blood, lymph nodes, or spleen from individual mice. Cell suspensions were stained with fluorescently-labeled antibodies specific for the indicated immune cell subsets, as described in Materials and Methods. Flow cytometry was performed and flow data was analyzed by gating to identify relative changes in cell percentages induced by radiation and radiation combined injury. The (*) indicates significant differences between sham and burn injury and (#) indicates significant differences between 0 Gy radiation and radiation exposures at the indicated doses. P < 0.05 by two-way ANOVA and Bonferroni multiple comparisons for n = 5–6 individual mice per group.
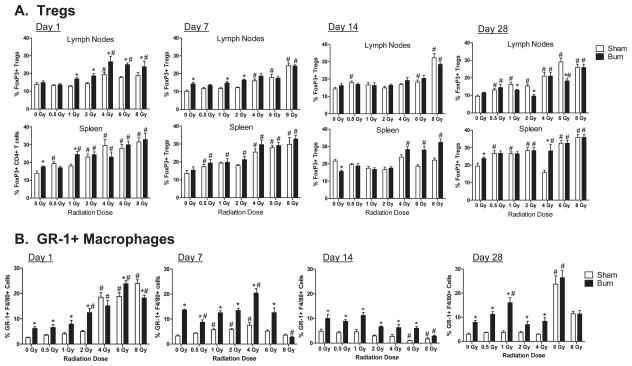
We assessed radiation and RCI effects on CD4+ regulatory T cells (Tregs), GR-1+ macrophages, B1 B cells and dendritic cell subsets (myeloid, lymphoid and plasmacytoid subsets). As shown in Fig. 6A, radiation, burn and RCI injuries increased the relative percentages of CD4+ Tregs, as measured by intracellular FoxP3 expression in CD4+ T cells. Radiation injury alone did not induce significant increases in Tregs until radiation exposure reached at least 2 Gy. Burn injury alone induced higher percentages of Tregs in the lymph nodes, but not the spleen at 7 days after injury. The effect of RCI on Tregs was most evident at 1 day after injuries in the lymph nodes and at 14 days after injuries in the spleen in burn-injured mice exposed to 4, 6 or 8 Gy radiation doses. Radiation, burn and RCI significantly increased the relative percentages of GR-1+ F4/80+ macrophages in the spleens of mice at days 1, 7, 14 and 28 after injuries (Fig. 6B). Burn injury alone significantly increased GR-1+ macrophage percentages in the spleen at all time points. In contrast, radiation injury alone did not increase GR-1+ macrophages until high-radiation exposure doses of 6 or 8 Gy. The combination of lower dose radiation injury and burn injury caused the greatest increases in GR-1+ macrophages. Thus, burn injury had a dominant effect on GR-1+ macrophage expansion in RCI. There were no significant changes in the percentages of the B1 B cell subset or dendritic cell subsets following radiation, burn or RCI (data not shown).
FIG. 6.
Radiation and radiation combined injury effects on CD4+ regulatory T cells and GR-1+ macrophages at days 1, 7, 14 and 28 after injuries. Groups of male CD-1 mice were exposed to 0, 0.5, 1, 2, 4, 6 or 8 Gy radiation by timed exposure to 137CsCl without or with combined 25% TBSA burn injury. At days 1, 7, 14 or 28 after injuries, cells were prepared from lymph nodes and spleens from individual mice. Cell suspensions were stained with anti-CD4 and anti-FoxP3 antibodies to identify CD4 regulatory T cells (Tregs) and anti-F4/80 and anti-GR-1 to identify the GR-1+ macrophage subset in spleens. The (*) indicates significant differences between sham and burn injury and (#) indicates significant differences between 0 Gy radiation and radiation exposures at the indicated doses. P < 0.05 by two-way ANOVA and Bonferroni multiple comparisons for n = 5–6 individual mice per group.
Influences of Radiation, Burn, and RCI on Immune Cell Responses to Innate and Adaptive Immune Stimulants
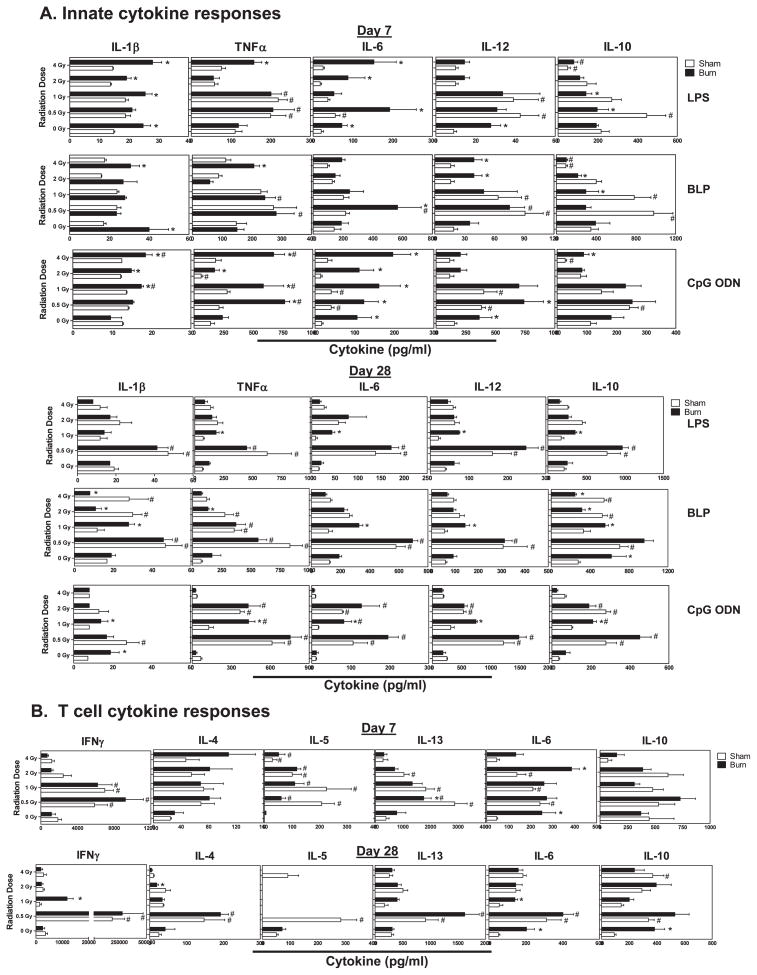
After characterizing immune cell losses and subset changes caused by radiation, burn and RCI, we wanted to compare how these injuries affect immune cell reactivity to innate and adaptive immune cell stimuli. To accomplish this, spleen cells were prepared from mice at 7 and 28 days after injuries and optimal stimulatory doses of E. coli lipopolysaccharide (LPS), bacterial lipopeptide (BLP), CpG oligodioxynucleotides (CpG ODN), or soluble anti-CD3ε antibody were added to stimulate cytokine production. We profiled cytokine production in these culture supernatants by multiplex bead-based assays. At 7 days after injuries, we observed clear and significant increases in LPS, BLP and CpG ODN-induced inflammatory cytokine production (Fig. 7A). Radiation injury at 0.5 and 1 Gy showed significant increases in LPS, BLP, and CpG ODN-induced IL-1β, TNFα, IL-6, IL-12 and IL-10. Burn injury alone caused significant increases in LPS-induced IL-1β, IL-6 and IL-12; BLP-induced IL-1β and IL-12; and CpG ODN-induced IL-6 and IL-10. Radiation exposure at 4 Gy with combined burn injury caused significant enhancement of LPS- and BLP-induced IL-1β, TNFα and IL-6. At 28 days post injuries, we observed significant increases in LPS, BLP and CpG ODN-induced cytokines in mice exposed to 0.5 Gy radiation or 0.5 Gy radiation with combined burn injury compared to unirradiated sham- or burn-injured mice (Fig. 7A). Cytokine production levels remained higher in mice exposed to 1 or 2 Gy radiation with or without burn injury, but the levels were lower than those observed in mice exposed to 0.5 Gy radiation.
FIG. 7.
Radiation and radiation combined injury effects on cytokine production by spleen cells stimulated with innate or T cell stimuli. Groups of male CD-1 mice were exposed to 0, 0.5, 1, 2 or 4 Gy radiation by timed exposure to 137CsCl without or with combined 25% TBSA burn injury. At days 7 or 28 after injuries, cells were prepared from the spleen of individual mice and cultured at 37°C in the presence of LPS, BLP, CpG ODN or anti-CD3ε antibody. After 48 h, supernatants were tested for cytokine levels using Luminex bead-based assays as described in Materials and Methods. Data is plotted as mean ± SEM. The (*) indicates significant differences between sham and burn injury and (#) indicates significant differences between 0 Gy radiation and radiation exposures at the indicated doses. P < 0.05 by two-way ANOVA and Bonferroni multiple comparisons for n = 5 individual mice per group.
Cytokine production by T cells was assessed by polyclonal T cell receptor activation with anti-CD3ε antibody. At day 7, radiation injury at 0.5, 1 and 2 Gy caused significant increases in IFNγ, IL-4, IL-5, IL-6 and IL-13, while combined burn injury did not appear to modulate T cell cytokine production; the exception was augmented IL-6 production by burn injury and RCI (Fig. 7B). Interestingly, IL-10 production was not significantly affected by radiation, burn or RCI. By 28 days after injuries, we found enhanced IFNγ, IL-4, IL-5, IL-6 and IL-13 production by spleen cells from mice exposed to 0.5 Gy radiation or RCI. This enhanced cytokine production seen at this late time point after radiation injury and RCI was not observed in mice exposed to higher than 0.5 Gy radiation doses. Therefore, radiation injury caused a generalized increase in T cell cytokine production without a clear bias in Th1- or Th2-type cytokine expression profiles.
DISCUSSION
The findings in this report provide basic observational data on immune system phenotypic changes caused by radiation injury, burn injury and radiation combined burn injury. Since the principal goal of this work was to establish and validate a working mouse model for radiation and RCI, we attempted to measure as many phenotypic changes in the immune system as was feasible. As such, this systems biology-like approach provided a broad and unbiased overview of changes in immune system phenotypes caused by these complex types of injuries. Since a radionuclear event would cause wide-ranging radiation dose exposures, this mouse model was designed using radiation dose as a primary variable. Combined injury induced by 25% total body surface area (TBSA) burn injury was the other variable. The burn injury model was used to induce combined injury for several reasons. First, it is an injury model with high survival rates in mice to allow for long-term studies. Second, due to the nature of this type of traumatic injury, full-thickness third-degree burn injury that causes nerve damage, burn-injured mice do not require post-injury analgesic at early time points after injury. This avoids having to use analgesics after radiation injury alone to conduct a fully controlled study. Third, burn injury shows many of the features of other severe traumatic injuries in humans including changes in innate and adaptive immune response phenotypes and higher susceptibility to sepsis and its complications (11).
Our first experiments evaluated direct survival rates for radiation and RCI. We report radiation and RCI survival responses that are in agreement with other reports, which also showed higher mortality in RCI than radiation injury alone (5, 12). However, we did find that outbred CD-1 mice showed better overall survival responses than those reported in inbred mice (5). The reason for this difference is likely due to outbred mice being larger and more robust than inbred mice. The size difference is approximately 5 g higher for CD-1 mice than for C57BL6/J mice of equivalent ages (unpublished observation). Also, housing conditions could account for differences in mouse survival curves. Nevertheless, we were not surprised to find that RCI mice showed higher mortality rates than mice with radiation injury alone. Thus, outbred mice provide several advantages for these severe injury models. The better survival rates of outbred mice will allow for more robust future testing of long-term effects of treatments on survival and improved immune functions. And of course, the outbred nature of these mice makes them a superior model for preclinical drug and immune response modifier (IRM) testing since humans are not genetically identical.
A significant complication of radiation injuries and RCI will be the development of opportunistic infections post injuries (13). This is also a major complication of more conventional traumatic injuries from accidents, burns, falls, explosions and major surgery. We routinely use a mouse sepsis model called cecal ligation and puncture to measure the effects of interventional treatments on survival resistance to sepsis in injured mice (10). Thus, we adopted the CLP model to test sepsis survival responses in mice with radiation injury and RCI. We observed significant differences in sepsis survival rates between mice that underwent radiation injury alone versus RCI. Radiation injury caused a dose-dependent change in sepsis survival rates, but radiation combined injury caused higher mortality at lower radiation exposure doses than radiation injury alone. This finding supports that RCI causes a more severe reduction in antimicrobial immunity than radiation injury alone or increased susceptibility to septic shock and organ damage. Thus, we propose that differences in CLP sepsis survival will be useful as an outcome measure for testing therapies that might help restore immune system function in radiation and RCI.
Another intention of this study was to document changes in immune cell populations caused by radiation and RCI. Since we have a relatively high throughput approach to performing flow cytometry analysis of cell populations, we assessed many different immune cell subsets by multi-color FACS analysis. The immune cell subset data shows that radiation dose is a dominant factor contributing to cell loss after radiation and RCI. However, an unanticipated finding was that RCI did not cause higher cell number losses than radiation injury alone. Instead, we found slightly higher cell numbers in mice that underwent low-dose irradiation with combined burn injury. This suggests traumatic injuries might induce hematopoietic stem cell recovery or cell growth as suggested by other reports showing that injuries or infection can increase bone marrow or peripheral organ hematopoiesis (14–16). Nevertheless, this slight increase in immune cell numbers in burn mice did not protect them from the complications of RCI since RCI mice showed higher susceptibility to CLP sepsis. The key reason for performing extensive cell subset phenotyping in radiation and RCI was to identify which immune cell types persist after radiation and RCI and which cells show recovery. This information will be extremely useful for designing therapies to target immune cell populations. For example, treatments that target dendritic cells, macrophages, and NK cells might be more effective than treatments that target B cells or CD8 T cells, which are lost rapidly after radiation exposure and show slow recovery rates.
Another significant finding from these immune cell subset phenotyping studies was that radiation and RCI increased the relative percentages of several immune cell subsets: CD4+ FoxP3+ T cells (Tregs) and F4/80+ GR-1+ macrophages. Interestingly, these immune cell subsets have been shown to increase in animals and patients with cancer, trauma and severe infections (17–19). Recently, our group has described an expansion of GR-1+ macrophages after burn injury (20). Furthermore, Tregs have been described to play a decisive role in the immune response to injury (21). It appears that expansion of Tregs and the GR-1+ macrophage subset is a commonality shared by radiation injury, RCI, and conventional-type injuries. Burn injury alone caused direct increases in Tregs and GR-1+ macrophages, while radiation injury alone at doses higher than 2 Gy increased the relative percentages of these populations. This suggests that radiation-induced Treg increases could be due to Tregs being more resistant to radiation-induced cell death than conventional CD4+ T cells, or due to their activation and expansion from the cellular damage caused by radiation injury. The RCI induced increase in GR-1+macrophage percentages appeared to be much more dependent on burn injury compared to radiation injury. Interestingly, radiation injury did not suppress the burn-induced increase in GR-1+ macrophage expansion, which suggests that GR-1+ macrophages may be resistant to radiation. This observation is significant because GR-1+ macrophages are known to expand in cancer patients and are thought to suppress cancer immunity; GR-1+ macrophages are called myeloid suppressor cells by investigators studying cancer (17). Thus, these cells may persist or expand further after radiation treatments to suppress immune-mediated clearance of cancer cells.
The pro- and counter-inflammatory effects of traumatic injuries on the immune system have been well described, but less is known about how radiation and RCI influences innate and adaptive immune system phenotypes. In this report, we profiled changes in spleen cell reactivity to polyclonal innate and adaptive immune cell stimuli at 7 and 28 days after radiation or RCI. TLR2 (BLP), TLR4 (LPS), TLR9 (CpG ODN) and T cell (anti-CD3 antibodies) activating stimulants were used. We found evidence for sustained priming of innate immune system responses by radiation injury and RCI at 7 days. This demonstrates that radiation injury and RCI induce immune system priming responses similar to what has been reported in other more common traumatic injuries. For example, our group described that burn injury primes the innate immune system for enhanced TLR reactivity (22). Priming of the innate immune system for heightened reactivity to inflammatory stimuli is thought to be central to the development of systemic inflammatory response syndrome (SIRS) and the two-hit response immune phenotype (23). The two-hit response is associated with diminished resistance to secondary infections and organ damage after trauma and might serve as an explanation for the diminished survival responses to CLP sepsis after radiation and RCI.
Interestingly, we observed that radiation and RCI at 0.5 Gy exposures caused sustained innate immune system priming. This effect was also observed, albeit to a lesser extent, in mice that had been irradiated with 1 Gy and 2 Gy, but not in mice that were exposed to 4 Gy radiation. This finding suggests that cells that are damaged by low-to-moderate radiation doses might survive and cause continued proinflammatory immune system stimulation by producing alarmins, while higher radiation doses cause lethal cell damage and are cleared from the body at early time points after radiation injury (24–26). Furthermore, we found that radiation and RCI with 0.5–2 Gy caused a general increase in T cell responses without a clear Th1/Th2/Th17 bias. This observation suggests that radiation and RCI do not necessarily skew the adaptive immune system response, but that they do act as a general immune stimulant for T cells. This is in contrast to what has been reported in other types of traumatic injuries where there is induction of Th2-and T cell exhaustion-type responses (27–29). Taken together, our results show that radiation and RCI have profound and long-lasting phenotypic effects on the immune system that alters the host response to infections.
In summary, our study provides a broad description of immunological changes induced by radiation and RCI with a wide range of radiation doses. We describe radiation and RCI as distinct injury types that share commonalities with conventional-type injuries, but also lack some of the phenotypic features of traumatic injuries that occur without radiation exposure. The findings provide an unbiased view of how these complex types of injuries affect specific immune cell subsets and immunity. We intend to use these animal models as a way to test and develop medical countermeasures for alleviating the damaging effects of radiation and RCI on the immune system. Future work will focus on early interventions to restore immune system function and homeostasis to reduce the morbidity and mortality caused by radiation injury, trauma and RCI.
Acknowledgments
This research was supported by funding from NIH grant # R21/R33 AI080565 as part of Project Bioshield and the Medical Countermeasures for Radiological and Nuclear Threats Program.
References
- 1.DiCarlo AL, Hatchett RJ, Kaminski JM, Ledney GD, Pellmar TC, Okunieff P, et al. Medical countermeasures for radiation combined injury: radiation with burn, blast, trauma and/or sepsis. Report of an NIAID Workshop, March 26–27, 2007. Radiat Res. 2008;169(6):712–21. doi: 10.1667/RR1295.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kishi H. Effects of the “Special Bomb”: Recollections of a Neurosurgeon in Hiroshima, August 8–15, 1945. Neurosurgery. 2000;47(2):441–6. doi: 10.1097/00006123-200008000-00034. [DOI] [PubMed] [Google Scholar]
- 3.Ohkita T. Review of thirty years study of Hiroshima and Nagasaki atomic bomb survivors. II. Biological effects. A. Acute effects. J Radiat Res. 1975;(Suppl):49–66. doi: 10.1269/jrr.16.supplement_49. [DOI] [PubMed] [Google Scholar]
- 4.Brook I, Ledney GD, Madonna GS, DeBell RM, Walker RI. Therapies for radiation injuries: research perspectives. Military Med. 1992;157(3):130–6. [PubMed] [Google Scholar]
- 5.Palmer JL, Deburghgraeve CR, Bird MD, Hauer-Jensen M, Kovacs EJ. Development of a combined radiation and burn injury model. J Burn Care Res. 2011;32(2):317–23. doi: 10.1097/BCR.0b013e31820aafa9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holzheimer RG, Molloy RG, O’Riordain DS, Mendez MV, Curley P, Collins K, et al. Long-term immunotherapeutic intervention with pentoxifylline in a mouse model of thermal injury and infection. J Trauma. 1995;38(5):757–62. doi: 10.1097/00005373-199505000-00014. [DOI] [PubMed] [Google Scholar]
- 7.O’Sullivan ST, Lederer JA, Horgan AF, Chin DH, Mannick JA, Rodrick ML. Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann Surg. 1995;222(4):482–92. doi: 10.1097/00000658-199522240-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy TJ, Paterson HM, Kriynovich S, Zang Y, Kurt-Jones EA, Mannick JA, et al. Linking the “two-hit” response following injury to enhanced TLR4 reactivity. J Leuko Biol. 2005;77(1):16–23. doi: 10.1189/jlb.0704382. [DOI] [PubMed] [Google Scholar]
- 9.Goebel A, Kavanagh E, Lyons A, Saporoschetz IB, Soberg C, Lederer JA, et al. Injury induces deficient interleukin-12 production, but interleukin-12 therapy after injury restores resistance to infection. Ann Surg. 2000;231(2):253–61. doi: 10.1097/00000658-200002000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shelley O, Murphy T, Paterson H, Mannick JA, Lederer JA. Interaction between the innate and adaptive immune systems is required to survive sepsis and control inflammation after injury. Shock. 2003;20(2):123–9. doi: 10.1097/01.shk.0000079426.52617.00. [DOI] [PubMed] [Google Scholar]
- 11.Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, et al. A genomic storm in critically injured humans. J Experim Med. 2011;208(13):2581–90. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiang JG, Jiao W, Cary LH, Mog SR, Elliott TB, Pellmar TC, et al. Wound trauma increases radiation-induced mortality by activation of iNOS pathway and elevation of cytokine concentrations and bacterial infection. Radiat Res. 2009;173(3):319–32. doi: 10.1667/RR1892.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiCarlo AL, Maher C, Hick JL, Hanfling D, Dainiak N, Chao N, et al. Radiation injury after a nuclear detonation: medical consequences and the need for scarce resources allocation. Disast Med Public Health Prepar. 2011;5:S32–44. doi: 10.1001/dmp.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scumpia PO, Kelly-Scumpia KM, Delano MJ, Weinstein JS, Cuenca AG, Al-Quran S, et al. Cutting edge: bacterial infection induces hematopoietic stem and progenitor cell expansion in the absence of TLR signaling. J Immunol. 2010;184(5):2247–51. doi: 10.4049/jimmunol.0903652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacNamara KC, Jones M, Martin O, Winslow GM. Transient activation of hematopoietic stem and progenitor cells by IFNgamma during acute bacterial infection. PloS One. 2011;6(12):e28669. doi: 10.1371/journal.pone.0028669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosinski M, Yarmush ML, Berthiaume F. Quantitative dynamics of in vivo bone marrow neutrophil production and egress in response to injury and infection. Ann Biomed Engin. 2004;32(8):1108–19. doi: 10.1114/b:abme.0000036647.81372.ce. [DOI] [PubMed] [Google Scholar]
- 17.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182(8):4499–506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byrne WL, Mills KHG, Lederer JA, O’Sullivan GC. Targeting regulatory T cells in cancer. Cancer Res. 2011;71(22):6915–20. doi: 10.1158/0008-5472.CAN-11-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Souza-Fonseca-Guimaraes F, Parlato M, Fitting C, Cavaillon J-M, Adib-Conquy M. NK Cell tolerance to TLR agonists mediated by regulatory T cells after polymicrobial sepsis. J Immunol. 2012;188(12):5850–8. doi: 10.4049/jimmunol.1103616. [DOI] [PubMed] [Google Scholar]
- 20.O’Leary FM, Tajima G, Delisle AJ, Ikeda K, Dolan SM, Hanschen M, et al. Injury-induced GR-1+ macrophage expansion and activation occurs independently of CD4 T-cell influence. Shock. 2011;36(2):162–9. doi: 10.1097/SHK.0b013e31821af669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacConmara MP, Tajima G, O’Leary F, Delisle AJ, McKenna AM, Stallwood CG, et al. Regulatory T cells suppress antigen-driven CD4 T cell reactivity following injury. J Leuko Biol. 2011;89(1):137–47. doi: 10.1189/jlb.0210082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paterson HM, Murphy TJ, Purcell EJ, Shelley O, Kriynovich SJ, Lien E, et al. Injury primes the innate immune system for enhanced toll-like receptor reactivity. J Immunol. 2003;171(3):1473–83. doi: 10.4049/jimmunol.171.3.1473. [DOI] [PubMed] [Google Scholar]
- 23.Stoecklein VM, Osuka A, Lederer JA. Trauma equals danger—damage control by the immune system. J Leuko Biol. 2012 doi: 10.1189/jlb.0212072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425(6957):516–21. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 25.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81(1):1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 26.Oppenheim JJ, Tewary P, de la Rosa G, Yang D. Alarmins initiate host defense. Adv Experim Med Biol. 2007;601:185–94. doi: 10.1007/978-0-387-72005-0_19. [DOI] [PubMed] [Google Scholar]
- 27.Guo Z, Kavanagh E, Zang Y, Dolan SM, Kriynovich SJ, Mannick JA, et al. Burn injury promotes antigen-driven Th2-type responses in vivo. J Immunol. 2003;171(8):3983–90. doi: 10.4049/jimmunol.171.8.3983. [DOI] [PubMed] [Google Scholar]
- 28.Bandyopadhyay G, De A, Laudanski K, Li F, Lentz C, Bankey P, et al. Negative signaling contributes to T-cell anergy in trauma patients. Crit Care Med. 2007;35(3):794–801. doi: 10.1097/01.CCM.0000256847.61085.A5. [DOI] [PubMed] [Google Scholar]
- 29.Hsieh CH, Hsu JT, Hsieh YC, Frink M, Raju R, Hubbard WJ, et al. Suppression of activation and costimulatory signaling in splenic CD4+ T cells after trauma-hemorrhage reduces T-cell function: a mechanism of post-traumatic immune suppression. Am J Pathol. 2009;175(4):1504–14. doi: 10.2353/ajpath.2009.081174. [DOI] [PMC free article] [PubMed] [Google Scholar]