Abstract
CD4+ T regulatory cells (Tregs) are activated during auto-immune, injury, and inflammatory responses, however, the molecular events that trigger Treg activation are poorly understood. The purpose of this study was to investigate whether Tregs (FoxP3+ CD4+ T cells) and non-Treg CD4+ T cells might display differences in T cell receptor (TCR) dependent signaling responses following in vitro or in vivo stimulation. This study used phospho-flow cytometry as a tool to profile the kinetics and extent of TCR signaling (ZAP-70 and PKC-θ phosphorylation and expression) in Tregs and non-Tregs. We found that in vitro stimulation with anti-CD3ε induces early and transient activation of ZAP-70 and PKC-θ in both Tregs and non-Tregs. However, the response in Tregs was more rapid and higher in magnitude than responses seen in non-Tregs. In contrast, bacterial superantigen or antigen-specific TCR stimulation did not significantly activate these signaling pathways in Tregs or non-Tregs. Additional experiments tested the kinetics of in vivo TCR signaling in Tregs and non-Tregs in mice challenged with bacterial superantigen. The results of these experiments showed that superantigen rapidly activated ZAP-70 and PKC-θ in lymph node Tregs, but not in non-Tregs. In summary, we demonstrate the versatility of using phospho-flow cytometry to measure cell signaling in CD4+ T cells. The results of these in vitro and in vivo studies demonstrate that Tregs and non-Treg CD4+ T cells show marked differences in their reactivity to TCR-dependent stimulation and contribute new insights into basic mechanisms that lead to Treg activation.
Keywords: T cell activation, CD4+ T regulatory cells, T cell receptor, intracellular signaling, differential cell activation
1. INTRODUCTION
The immune system functions to control the host response to infection, injury, and tissue damage to prevent the development of immune- and inflammatory-mediated diseases. In general, inflammatory reactions are driven by innate immune cell types, while adaptive immune cells regulate immune responses by amplifying or suppressing immune cell reactivity. A subset of CD4+ T cells called regulatory T cells (Tregs), which were first described by Sakaguchi and coworkers, are a CD4+ T cell subset that are known to play a central role in controlling the development of autoimmune reactivity and disease (Sakaguchi et al., 1995). Regulatory T cells express seemingly normal T cell receptor (TCR) diversity, but have been shown to develop as a distinct CD4+ T cell subset in the thymus by a self-antigen recognition mechanism (Josefowicz and Rudensky, 2009). Furthermore, their development depends on the expression and function of the Forkhead Protein 3 (FoxP3) transcription factor (Wan and Flavell, 2007). Thus, Tregs represent a distinct and unique CD4+ T cell subset, but express TCR characteristics similar to conventional CD4+ T cells.
It is presently unclear whether Tregs control auto-immune or inflammatory responses by TCR dependent or independent signaling mechanisms. Moreover, there have been limited studies addressing differences in TCR signaling mechanisms between Tregs and CD4+ T cells (Crellin et al., 2007a; Li et al., 2005; Tsang et al., 2006). This is likely due to the difficulty in measuring signaling responses in this relatively rare CD4+ T cells subset; Tregs constitute approximately 10% of the total CD4 T cell population in mice and humans. Therefore, we wished to investigate whether Tregs, as defined by FoxP3 expression, and ‘non-Treg’ CD4+ T cells (FoxP3−) might demonstrate differences in TCR-dependent signaling responses.
The activation of CD4+ T cells is initiated by cross-linking of the TCR to antigen-bound major histocompatibility complex (MHC) class II on the surface of antigen presenting cells (APCs). Costimulatory signals such as those provided by the CD28 family of costimulatory molecules contribute to TCR signaling by governing the level and type of signaling response (Delves and Roitt, 2000; Frauwirth and Thompson, 2002). Following TCR activation, the immunoreceptor tyrosine-based activation motifs (ITAMs) in the TCR subunits are phosphorylated and the ζ-associated protein of 70kDa (ZAP-70) is recruited to the TCR/CD3-complex (Mustelin and Tasken, 2003). ZAP-70 is then phosphorylated at Tyr-493 by lymphocyte cell-specific protein tyrosine kinase (Lck) (Mustelin and Tasken, 2003; Chan et al., 1995). The physiological substrate of phosphorylated ZAP-70 is linker for activation of T cells (LAT) (Mustelin and Tasken, 2003; Au-Yeung et al., 2009). Phosphorylated LAT binds to the supramolecular activation complex (SMAC) at the interface between APCs and T cells, which signals ERK and NF-κB pathway activation (Monks et al., 1998). The phosphorylation of protein kinase C-θ (PKC-θ) by phospholipase C-γ1 (PLCγ1) and diacylglycerol (DAG) and its recruitment to SMAC is also an integral part of the NF-κB-dependent TCR signaling response (Thebault and Ochoa-Garay, 2004; Isakov and Altman, 2002). Interestingly, it has been shown the activation and mobilization of PKC-θ to the SMAC is enhanced by CD28-B7 ligation and can occur independent of ZAP-70 activation (Hayashi and Altman, 2007; Bi et al., 2001). Thus PKC-θ activation can be triggered by both TCR-dependent and -independent mechanisms.
T cell receptor signaling pathways have most often been defined in mouse and human T cell lines or clones, which can be expanded in vitro to sufficient cell numbers for biochemical and protein-protein interaction assays. However, specific TCR signaling characteristics discovered in T cell lines may not be identical to signaling responses in freshly-prepared primary CD4+ T cells or in CD4+ T cell subsets, like Tregs. For instance, a prior report already has documented differences in TCR induced phosphatidyl inositide-3 kinase (PI3-K) and the Ras/MAPK signaling pathways between Tregs and conventional CD4+ T cells (Crellin et al., 2007a; Li et al., 2005; Tsang et al., 2006). Tregs also show a diminished capacity to phosphorylate AKT and PLCγ1 as compared to normal CD4+ T cells (Hickman et al., 2006; Crellin et al., 2007b). These described differences between Tregs and non-Treg CD4+ T cells justify the need to further characterize and compare TCR signaling responses in Tregs versus CD4+ T cells.
Traditional cell-signaling methods, like Western immunoblots, are not feasible for studying TCR signaling in relatively rare cell populations like Tregs because large numbers of cells are needed to perform these assays. Moreover, to accurately measure signaling responses in Tregs, the cells would need to be purified rapidly away from other cells following TCR stimulation. Due to these major limitations, we turned to using a flow cytometry based method called phospho-flow cytometry to compare TCR-dependent signaling responses in Tregs versus non-Treg CD4+ T cells. The advantages of flow cytometry to measure signal transduction as opposed to Western immunoblots are that it allows for accurate identification of cells, it is easy to quantify, and can be done in a relatively high-throughput fashion. The basic protocol involves fixation and permeablization of cells followed by staining with cell-surface or intracellular marker antibodies. Phosphorylated and un-phosphorylated epitopes of the signaling molecules can be stained using specific antibodies. Thus, in comparison to Western blot analysis, this approach allows for simultaneous and rapid analysis of multiple markers and signaling molecules in the same cell (Krutzik and Nolan, 2003; Chow et al., 2001).
The present report uses phospho-flow cytometry to directly measure the kinetics and nature of early ZAP-70 and PKC-θ activation in CD4+FoxP3+ Tregs versus non-Treg CD4+ T cells following TCR stimulation in vitro and in vivo. We used polyclonal TCR stimulation approaches – anti-CD3ε specific antibody (Ab) or bacterial superantigen – as well as antigen-specific TCR stimulation to compare and contrast TCR induced signaling profiles in Treg and non-Treg CD4+ T cells in vitro. Our results demonstrate novel differences in signaling responses between Tregs and non-Treg CD4+ T cells. First, we demonstrate clear differences in the reactivity of Tregs and non-Tregs to antigen-, CD3-, and superantigen-induced TCR stimulation. And second, we validate the feasibility and accuracy of using the phospho-flow cytometry technique for measuring TCR signaling in Tregs and CD4+ T cells by performing both in vitro and in vivo time-course studies using 3 different T cell stimulation conditions. The findings in this report provide a foundation for future studies to address Treg and CD4+ T cell signaling and activation in response to infections, injury, and other disease processes in animal models.
2. MATERIALS AND METHODS
2.1. Animals
Experiments were performed using cells prepared from 6–8 week old male, C57BL/6J mice or OT-II TCR transgenic mice. C57BL/6J mice were obtained from the Jackson Laboratories (Bar Harbor, ME). OT-II mice (transgenic mice with TCR specific for OVA323–339 peptide) were bred and maintained on C57BL/6J background. These mice were originally provided to us by Drs. Francis Carbone and William Heath (Department of Microbiology and Immunology, The University of Melbourne, Australia, and the Division of Immunology, The Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia) (Barnden et al., 1998). Mice were maintained in our VAF full-barrier animal facility with strictly controlled temperature, relative humidity and a 12-hour light/dark regimen. Purchased mice were acclimatized for at least 1 week prior to use. All mice were fed with standard lab mouse chow and provided water ad libitum. The maintenance and all procedures performed in this report have been reviewed and are in accordance with the guidelines of the National Institutes of Health (NIH; Bethesda, MD) and the Harvard Medical School Standing Committee on Animal Research.
2.2. Reagents
Culture medium, complete-5 (C5), used for cell preparation and for incubation of cells with stimuli was prepared by supplementing RPMI 1640 with 5% heat-inactivated FCS, 1 mM glutamine, 10 mM HEPES, 100 µM nonessential amino acids, penicillin/streptomycin/fungiozone, and 2.5 × 10−5 M 2-Mercaptoethanol, all purchased from Gibco-Invitrogen (Grand Island, NY, USA). Highly purified Staphylococcal Enterotoxin B (SEB) was obtained from Toxin Technology (Saratosa, FL, USA). Direct stimulation of the TCR/CD3 complex was achieved with anti-CD3ε mAb (clone 145-2C11), purchased from R&D Systems (Minneapolis, MN, USA). Ovalbumin peptide of amino acids 323–339 (OVA323–339) was obtained from New England Peptide LLC (Gardner, MA, USA). The fluorescent labeled antibodies for flow cytometry anti-CD4 [GK1.5] and -FoxP3 [FJK-16s] as well as the Fc block reagent (CD16/32 mAb) were purchased from BioLegend and eBioscience (San Diego, CA, USA). Primary antibodies, source rabbit, to stain intracellular signaling molecules included anti-ZAP-70 [99F2] and anti-phospho-ZAP-70 [Tyr493] purchased from Cell Signaling Technology (Danvers, MA, USA). Anti-PKC-θ [C-18] and anti-phospho-PKC-θ [Thr538] were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Flow cytometry detection for intracellular signaling molecules was performed using Alexa Fluor 555 conjugated F(ab’)2 fragment of goat anti-rabbit IgG, purchased from InVitrogen (Eugene, OR, USA). The CellTrace™ CFSE cell proliferation kit (Life Technologies; Grand Island, NY, USA) was used to assess T cell proliferation. For cytokine multiplex assay using Luminex technology, detection beads were purchased from Bio-Rad (Hercules, CA, USA) and customized for detection of IL-1α, IL-2, IL-6, IL-10, IFN-γ, and TNF-α (coating antibodies: BioLegend; San Diego, CA, USA / eBioscience; San Diego, CA, USA). Biotinylated antibodies and streptavidin-RPE for analyte detection were purchased from BioLegend (San Diego, CA, USA). The multiplex assay was performed using washing buffer (PBS supplemented with 0.05% Tween and 0.05% sodium azide) and incubation buffer (PBS supplemented with 0.05% Tween, 0.05% sodium azide, and 1% BSA).
2.3. In vitro T cell stimulation
C57BL/6J or OT-II transgenic mice were euthanized by CO2 asphyxiation and spleens were harvested. Using a sterile wire mesh, spleens were minced and whole spleen cell suspensions were prepared in C5. Following RBC lysis, cells were plated in sterile 96-well plates at 5 × 105 cells per well and allowed to rest for 1 hour prior to stimulation at 37° C, 5 % CO2. Cell stimulation was conducted by adding different concentrations (0 µg/ml, 0.01 µg/ml, 0.1 µg/ml, 1 µg/ml) of either CD3ε mAb, SEB, or OVA-peptide to the cell suspension for different incubation times (30 min, 1 hour, and 2 hours). In an additional set of experiments, very early signaling was investigated using incubation times earlier than 30 min (2 min, 5 min, 10 min, and 15 min). To assess intracellular signaling, cell stimulation was terminated by direct addition of PBS with 1.5% paraformaldehyde (PFA). Cells were cultured for 72 hours for CFSE proliferation assays and for 48 hours to generate supernatants for multiplex cytokine assays.
2.4. In vivo T cell stimulation
Mice were given SEB (25µg/mouse in 250 µl PBS) by intraperitoneal (i.p.) injection. Control mice received 250 µl PBS by i.p. injection. Following different stimulation times (30 min, 1, or 2 hours), mice were sacrificed by CO2 asphyxiation and lymph nodes and spleens were harvested. Cell suspensions were prepared as described above. After plating the cell suspension at 5 × 105 cells per well in round-bottom 96-well plates, cells were immediately fixed for 10 minutes in 1.5% PFA.
2.5. Flow cytometry
Intracellular T cell receptor signaling detection
Cells were fixed in 1.5% PFA, pelleted by centrifugation at 300 × g for 10 minutes and then permeabilized in 100 µL ice-cold methanol for 10 min. After fixing, cells were pelleted by centrifugation at 300 × g for 10 minutes and resuspended in 20 µL of flow staining buffer [phosphate buffered saline containing 1 % bovine serum albumin and 0.05% sodium azide (PBA)] and anti-CD16/32 mAb to block non-specific binding of Ab by Fc receptors. Cells were then stained using APC-labeled anti-CD4 mAb and FITC-labeled anti-FoxP3 mAb. Prior testing was performed to assure that these antibodies could still detect CD4 and FoxP3 levels in fixed and methanol-treated cells. After washing by centrifugation, cells were then stained for the intracellular signaling molecules, ZAP-70, p-ZAP-70, PKC-θ, or p-PKC-θ, in triplicate using rabbit anti-mouse primary Ab followed by Alexa555-labeled secondary goat anti-rabbit IgG (F(ab’)2 fragment). Negative controls were generated by performing the staining procedure without the primary Ab. Stained samples were fixed in 0.3% PFA buffer, washed and reconstituted in PBS for flow cytometry analysis on a FACSCalibur instrument (Becton-Dickinson, San Jose, CA, USA). Prior to running samples, the instrument was calibrated using calibration beads (Becton-Dickinson, San Jose, CA). Instrument settings and compensation were checked to minimize variability between experiments. Data analysis was achieved using the accompanying CELLQuest Pro software program. Mean fluorescence intensity (MFI) for each intracellular signaling molecule was detected and the relative fluorescence intensity (RFI) was determined as MFI minus MFI of negative control. Data analysis was based on an average of 1500–2000 Tregs per sample.
Proliferation assay
Splenocytes from C57BL/6J or OT-II transgenic mice were stained with the CellTrace™ CFSE cell proliferation kit following the manufacturer’s suggested protocol. Cells were plated in sterile 96-well plates at 5 × 105 cells per well in C5 medium and stimulated with anti-CD3ε mAb, SEB, or OVA-peptide. After 3 days incubation at 37° C, cells were pelleted by centrifugation, anti-CD16/32 was added to block non-specific Ab staining, and then stained using APC-labeled anti-CD4 mAb. Stained samples were fixed in 0.3% PFA buffer, washed and reconstituted in PBS for flow cytometry analysis on a MACSQuant® Analyzer (Miltenyi Biotec, Auburn, CA, USA). Data analysis was achieved using FlowJo software (Tree Star, Ashland, OR, USA).
2.6. Cytokine multiplex assay
The concentration of IL-1α, IL-2, IL-6, IL-10, IFN-γ, and TNF-α in supernatants following cell stimulation was assessed using the cytokine multiplex assay technology by Luminex®. The assay was conducted using 20 µL of sample and cytokine levels were determined by standard curve analysis. The plate was read on a Luminex® 200™ instrument (Luminex, Austin, TX, USA). Data aquisition and analysis was conducted using StarStation software v2.3 (Applied Cytometry Systems, Dinnington, UK).
2.7. Statistical analysis
Statistical analysis was conducted using the software package SigmaPlot 11.0 (Systat Software, San Jose, CA, USA). The Kruskal-Wallis ANOVA on ranks test was followed by the nonparametric Student-Newman-Keuls post test for multiple comparisons. The data were plotted as mean values ± SEM. P<0.05 was considered significant.
3. RESULTS
Measuring T cell receptor signaling in FoxP3+ and FoxP3− CD4+ T cells by phospho-flow cytometry
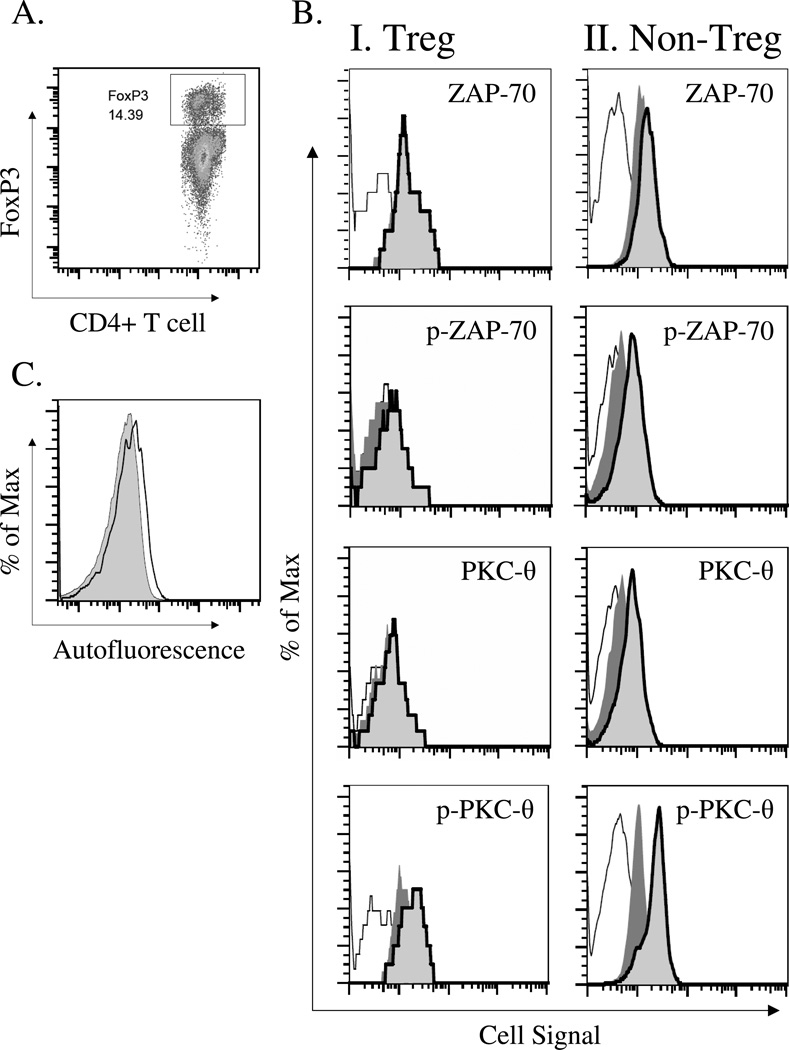
While much of what is known about TCR signaling has been gained from studies using T cell lines and tumor cells, we and others have become increasingly interested in studying signaling responses in primary CD4+ T cell subsets stimulated in vitro or in vivo by TCR dependent stimuli. Due to the difficulty in performing similar type studies in primary CD4+ T cells, we wished to optimize a high-throughput flow cytometry approach, commonly referred to as phospho-flow cytometry, to measure TCR-induced signaling in two developmentally distinct CD4+ T cell subsets, FoxP3+ CD4+ T cells (Tregs) and FoxP3− CD4+ T cells (non-Tregs). To accomplish this, we first tested whether we could detect changes in the level and phosphorylation of two well-described TCR signaling molecules in FoxP3+ and FoxP3− CD4 T cells. We chose ZAP-70 and PKC-θ as molecular targets for this study because they have been shown to be activated by phosphorylation at early time points following T cell activation (Williams et al., 1999; Monks et al., 1997). ZAP-70 is known to be activated at the TCR complex and PKC-θ activation is distal to the TCR complex and is also modulated by the CD28 pathway (Coudronniere et al., 2000). Spleen cells were prepared from C57BL/6J mice and stimulated with anti-CD3ε antibody. At specific time points after stimulation, cells were fixed, permeabilized with methanol, and then stained with anti-CD4 and anti-FoxP3 antibodies. Cells were counterstained with antibodies specific for the un-phosphorylated and phosphorylated forms of ZAP-70 or PKC-θ. Stained cells were analyzed by flow cytometry on gated FoxP3+ or FoxP3− CD4+ T cells. The histogram FACS plots shown in Figure 1 demonstrate the level of activation in anti-CD3 antibody stimulated FoxP3+ and FoxP3− CD4+ T cells. By gating CD4+ T cells as FoxP3+ or FoxP3− (Figure 1A), we were able to detect relative changes in un-phosphorylated and phosphorylated ZAP-70 and PKC-θ expression in Treg and non-Treg CD4+ T cells (Figure 1B). This was judged by changes in mean fluorescence intensity (MFI) of signaling molecule stains. These data indicate that anti-CD3 antibody stimulation caused significant increases in both un-phosphorylated and phosphorylated levels of ZAP-70 and PKC-θ in Tregs and non-Treg CD4+ T cells. They also provide a positive control for subsequent studies using different TCR dependent or independent stimulation conditions. While performing these studies, we also found that un-phosphorylated and phosphorylated signaling molecules showed higher staining in Tregs as compared to non-Tregs, as judged by mean fluorescence intensity (MFI) measurements (Figure 1C). Therefore, to directly compare Treg signaling with non-Treg signaling we took this differential autofluorescence of these cell types into account by using the relative fluorescence intensity (RFI) for quantitative analysis of phospho-flow data.
Figure 1. Analysis of intracellular signaling by phospho-flow cytometry in anti-CD3ε antibody stimulated FoxP3+CD4+ T cells (Tregs) and FoxP3−CD4+ T cells (non-Tregs).
The expression and phosphorylation of the intracellular signaling molecules, ZAP-70 and PKC-θ was assessed in splenic CD4+FoxP3+ T cells (Tregs) and CD4+FoxP3− T cells (non-Tregs) following stimulation with anti-CD3ε-specific antibody. Splenocytes were harvested from C57BL/6J mice. After 1 hour stimulation without or with 1 µg/ml anti-CD3ε mAb, cells were fixed with paraformaldehyde buffer and permeablized with ice-cold methanol. Cells were then stained with anti-CD4 and anti-FoxP3 antibody as well as antibodies specific for the un-phosphorylated or phosphorylated forms of ZAP-70 and PKC-θ. Stained cells were analyzed by flow cytometry. A) Gating strategy to identity CD4+FoxP3+ T cells (Tregs) in gated CD4+ T cells. B) Representative histograms displaying the expression/phosphorylation levels of ZAP-70/p-ZAP-70 and PKC-θ/p-PKC-θ in gated FoxP3+ CD4+ T cells (Tregs) and FoxP3− CD4+ T cells (non-Tregs) (negative control, solid line [=background]; stimulation with 0 µg/ml anti-CD3ε mAb, dark gray filling [constitutive expression / phosphorylation]; stimulation with 1 µg/ml anti-CD3ε mAb, solid bold line [induced expression / phosphorylation]. C) Differential autofluorescence between CD4+FoxP3+ T cells (Tregs) (solid line) and CD4+FoxP3− T cells (non-Tregs) (gray filling). For quantitative analysis of cell signaling in Tregs vs. non-Tregs, the RFI (relative fluorescence intensity) was therefore calculated (described in Material & Methods). This enabled us to directly compare signaling in Tregs vs. non-Tregs.
ZAP-70 activation in Tregs and non-Treg CD4+ T cells following in vitro TCR stimulation by anti-CD3ε mAb, bacterial superantigen, or specific peptide antigen
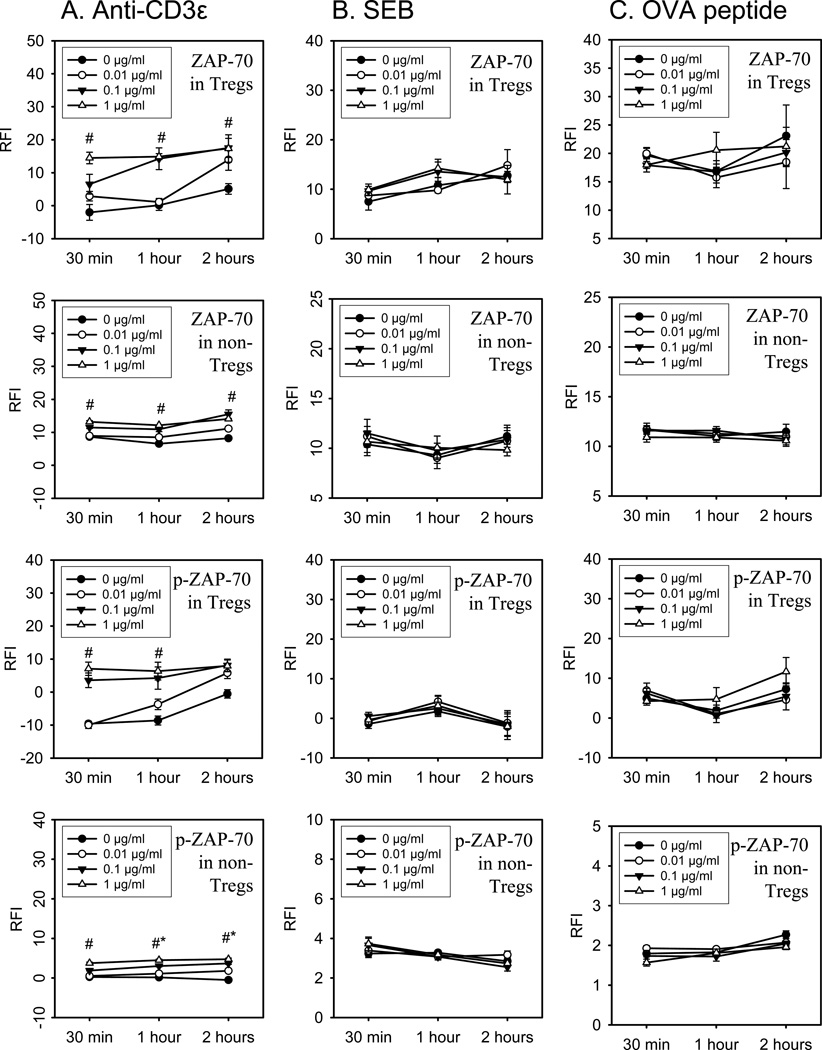
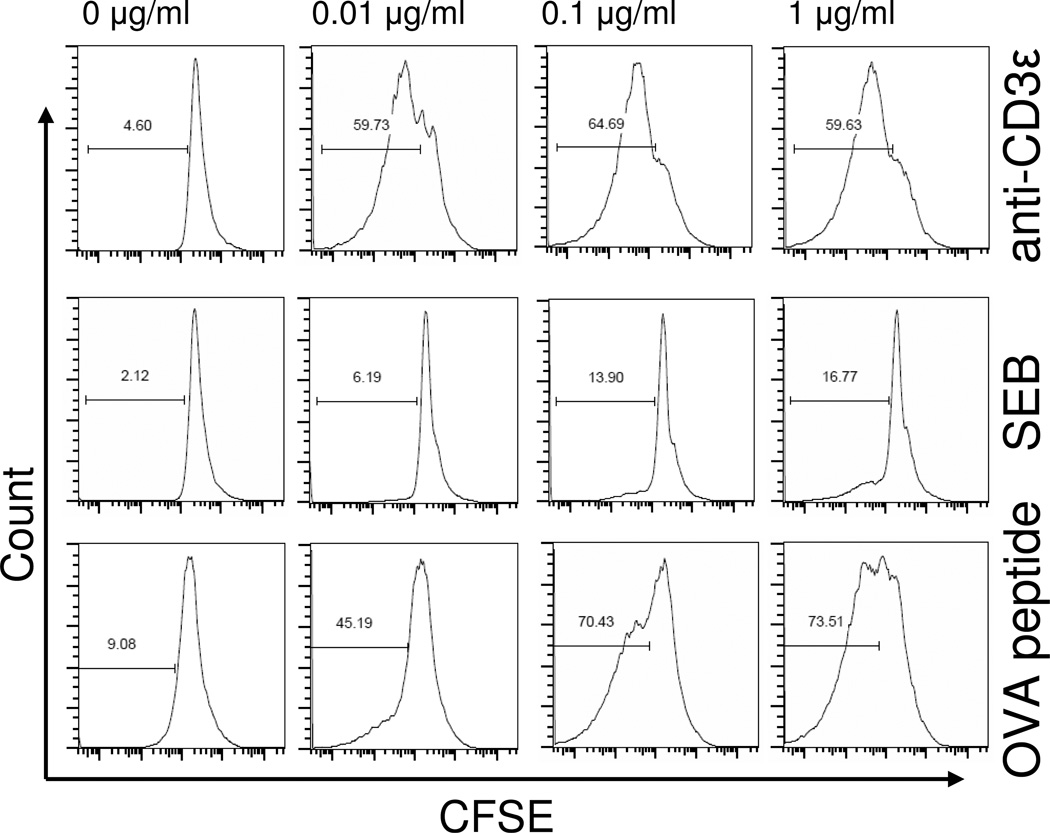
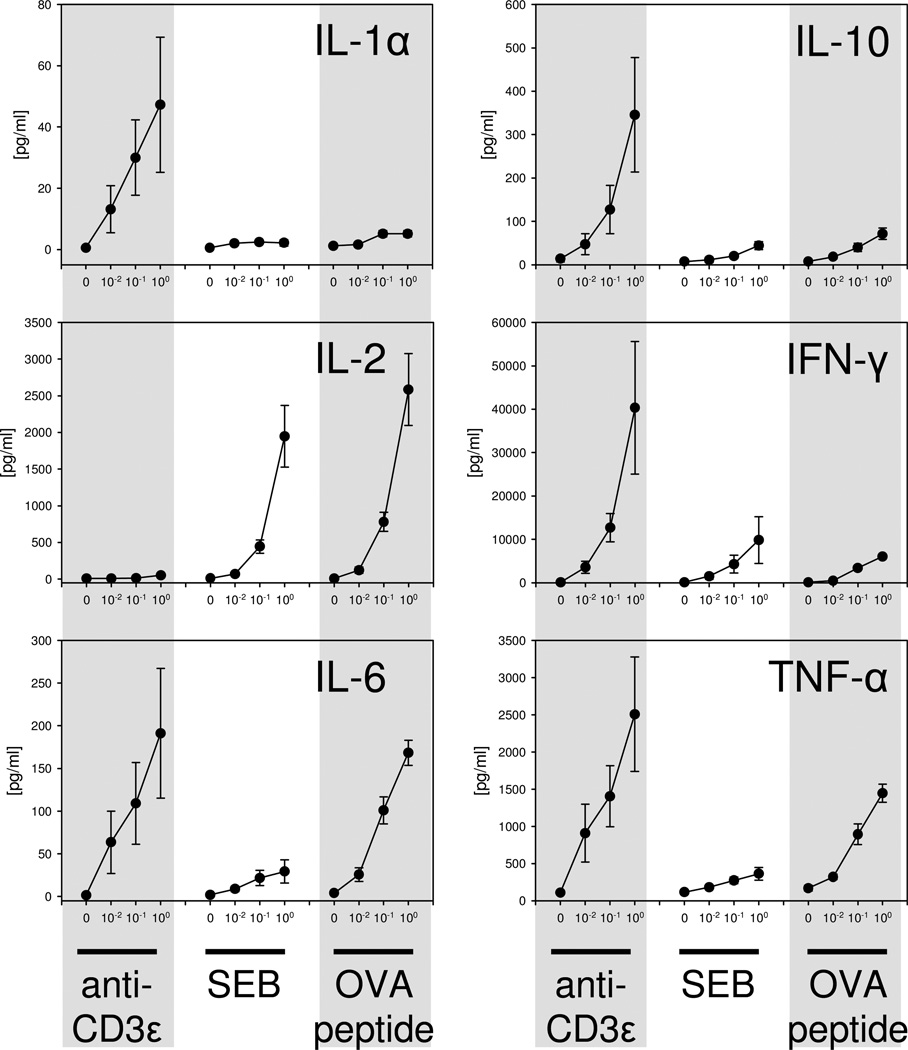
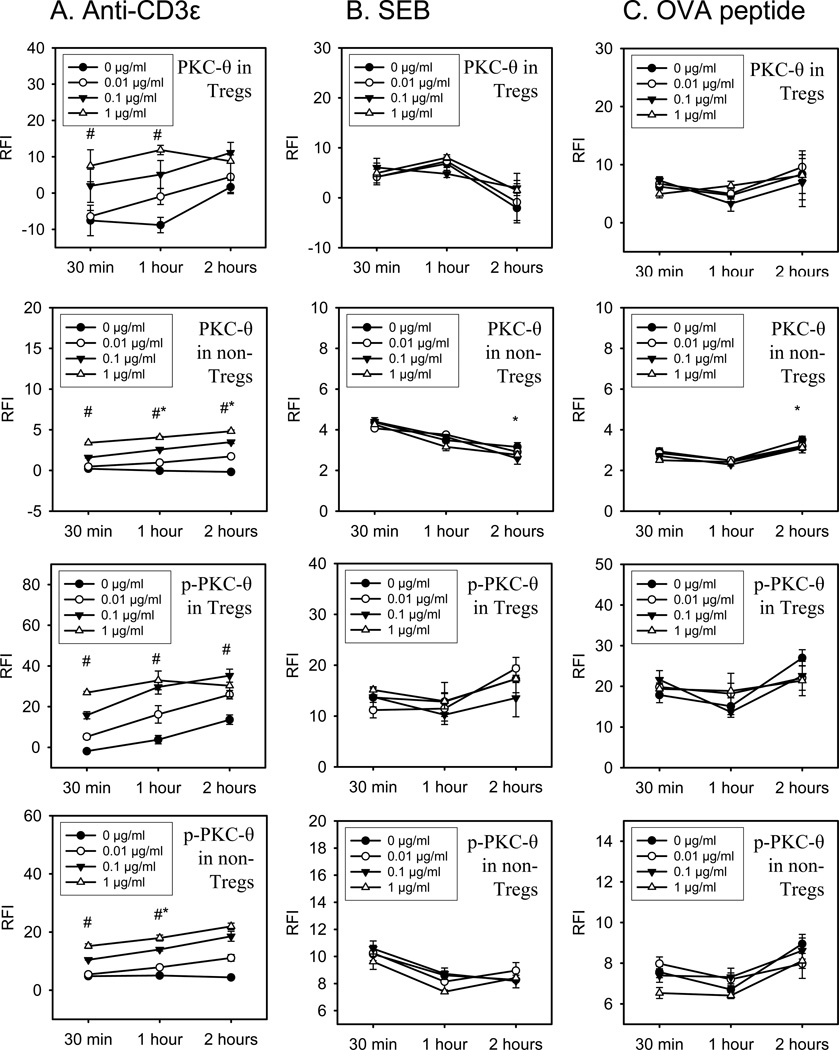
Potential differences in ZAP-70 activation in response to different types of TCR stimuli was assessed in vitro in FoxP3+ and FoxP3− CD4+ T cells by phospho-flow cytometry. As shown in Figure 2, anti-CD3ε mAb stimulation induced a significant dose and time dependent increase in both the un-phosphorylated and phosphorylated forms of ZAP-70 in Tregs and non-Treg CD4+ T cells. Of note, anti-CD3ε mAb induced ZAP-70 phosphorylation in Tregs more rapidly and to a higher magnitude than non-Tregs as evidenced by wide differences in p-ZAP-70 staining in Tregs versus non-Tregs at 30 minutes and 1 hour after stimulation (Figure 2). Stimulating cells with the bacterial superantigen, staphylococcal enterotoxin B (SEB), failed to show increased ZAP-70 expression or phosphorylation in Tregs or non-Treg CD4+ T cells as shown in Figure 2. We next tested if stimulating FoxP3+ or FoxP3− CD4+ T cells from OT-II TCR transgenic mice with antigen [ovalbumin peptide, AA sequence 323–339 (OVA323–339)] could induce significant ZAP-70 activation. Surprisingly, we found that OVA323–339 peptide stimulation also failed to induce significant ZAP-70 activation in Tregs and non-Tregs (Figure 2). To confirm that signaling changes were not occurring earlier than 30 minutes, we measured signaling in Tregs and non-Tregs at 2, 5, 10, and 15 minutes after anti-CD3ε, SEB or OVA-peptide stimulation. We did not detect ZAP-70 upregulation or phosphorylation at these earlier time points (Supplemental Data Figure 1). To confirm that the SEB and OVA323–339 peptide used in these signaling studies could activate CD4 T cells, we decided to measure SEB or OVA323–339 peptide induced CD4 T cell proliferation by spleen cells prepared from C57BL/6J or OT-II mice, respectively. As shown in Figure 4, we observed significant, dose-dependent SEB and OVA323–339 induced proliferation by CD4 T cells as judged using a FACS-based CFSE proliferation assay. We also measured cytokine production by SEB and OVA323–339 peptide stimulated spleen cell cultures and observed dose-dependent IL-2, IL-6, IFN-γ, and TNFα, production (Figure 5). Taken collectively, these findings indicate that the SEB and OVA323–339 peptide used to activate Tregs and non-Treg CD4 T cells for our TCR signaling studies were fully capable to stimulate CD4 T cells. We conclude that anti-CD3ε mAb induces significant ZAP-70 activation in both Tregs and non-Tregs. However, TCR stimulation by superantigen or cognate TCR-specific antigen does not deliver the needed signal for detectable ZAP-70 activation in FoxP3+ or in FoxP3− CD4 T cells. In addition, we report here the novel finding that Tregs demonstrate a more rapid and higher ZAP-70 phosphorylation than non-Treg CD4+ T cells in response to CD3ε stimulation.
Figure 2. Expression and phosphorylation of ZAP-70 in Tregs and non-Tregs following in vitro stimulation with CD3ε mAb, SEB, and OVA.
Splenocytes from WT or OT-II transgenic mice were incubated with anti-CD3ε mAb (column A), SEB (column B), or OVA peptide (column C) at different doses (0, 0.01, 0.1, or 1 µg/ml). The differential expression of the intracellular signaling molecule ZAP-70 was assessed in Tregs and non-Tregs by flow cytometry and analyzed in a time dependent manner (30 min; 1 hour; and 2 hours). Intracellular phosphorylation of ZAP-70 was assessed using an antibody specific for the phosphorylated epitope, Tyr493, of ZAP-70 in Tregs and non-Tregs. The relative fluorescence intensity (RFI) for stained intracellular ZAP-70 and p-ZAP-70 in Tregs and non-Tregs is displayed. Data shown represents one of three independent experiments. N=3 individual mice per group. Mean ± SEM given, #p<0.05 1 µg/ml vs. 0 µg/ml stimulation, *p<0.05 vs. 30 min stimulation at 1 µg/ml.
Figure 4. CD4+ T cell proliferation following stimulation with anti-CD3ε mAb, SEB, or OVA-peptide.
To confirm the biological activity of stimuli used in this study, we tested the ability of anti-CD3ε mAb, SEB, or OVA323–339 peptide to induce CD4+ T cell proliferation. Proliferation was assessed by measuring the dilution of fluorescence intensity in CFSE-stained cells using flow cytometry. Representative histograms show the percentage of proliferated, FACS-gated CD4+ T cells following 72 hours of stimulation of splenocytes prepared from WT or OT-II transgenic mice. Stimulation with anti-CD3ε mAb or OVA-peptide caused a marked concentration-dependent proliferation of CD4+ T cells (rows 1 and 3), while stimulation with SEB induced only 17% (row 2) at the highest concentration tested (1 µg/ml). Plots are representative of spleen cells prepared from 3 individual mice per group.
Figure 5. Cytokine release following stimulation with anti-CD3ε mAb, SEB, or OVA-peptide.
Cytokine measurements in cell supernatants following stimulation were conducted to further validate the effectiveness of cell stimulation conditions used in our in vitro experiments. Splenocytes from WT or OT-II transgenic mice were incubated with anti-CD3ε mAb, SEB, or OVA-peptide at different doses (0, 0.01, 0.1, or 1 µg/ml) for 48 hours. Cytokines in the supernatant were measured using the Luminex cytokine multiplex assay. Stimulating cells with anti-CD3ε, SEB, or OVA-peptide caused a dose-dependent release of the cytokines IL-1α, IL-2, IL-6, IL-10, IFN-γ, and TNF-α. Data represent the mean ± SEM from 3 individual mice per group.
PKC-θ activation in Tregs and non-Treg CD4+ T cells following in vitro TCR stimulation by anti-CD3ε mAb, bacterial superantigen, or specific peptide antigen
In addition to examining the activation characteristics of ZAP-70 in Tregs and non-Treg CD4+ T cells, we wanted to profile another TCR signaling pathway, PKC-θ. Similar to the ZAP-70 studies, we examined dose- and time-dependent changes in anti-CD3ε mAb, SEB, or OVA323–339 peptide induced PKC-θ activation in FoxP3+ or FoxP3− CD4+ T cells by phospho-flow cytometry. Spleen cells from C57BL/6J mice were stimulated in vitro with anti-CD3ε mAb or SEB to test for changes in PKC-θ activation by these polyclonal TCR stimuli. Antigen-specific PKC-θ induction was measured in OVA323–339 peptide stimulated OT-II TCR transgenic FoxP3+ and FoxP3− CD4+ T cells. As shown in Figure 3, anti-CD3ε mAb stimulation induced a significant increase in PKC-θ expression and phosphorylation in FoxP3+ (Tregs) and FoxP3− (non-Treg) CD4+ T cells in a dose- and time-dependent manner. However, superantigen stimulation by SEB did not activate PKC-θ in Tregs or non-Treg CD4+ T cells. Similarly, we did not detect significant PKC-θ activation in ovalbumin peptide (OVA323–339) stimulated FoxP3+ or FoxP3− CD4+ T cells from OT-II TCR transgenic mice. Additional experiments investigating early signaling at 2, 5, 10, or 15 minutes after stimulation revealed, that anti-CD3ε, SEB, or OVA-peptide did not induce upregulation or phosphorylation of PKC-θ at these very early time points (Supplemental Figure 2).
Figure 3. Expression and phosphorylation of PKC-θ in Tregs and non-Tregs following in vitro stimulation with anti-CD3ε mAb, SEB, and OVA.
Using flow cytometry, the intracellular expression and phosphorylation of PKC-θ was assessed in Tregs vs. non-Tregs. The stimuli anti-CD3ε mAb (column A), SEB (column B), and OVA-peptide (column C) were added in different doses (0, 0.01, 0.1, or 1 µg/ml) to cultures of splenocytes harvested from WT or OT-II transgenic mice. Following incubation times from 30 min to 2 hours, the expression and phosphorylation of the intracellular signaling molecule PKC-θ was analyzed. The charts represent the quantitative data of the relative fluorescence intensity (RFI) for stained intracellular PKC-θ and p-PKC-θ in Tregs and non-Tregs. Data represent one of two independent experiments. N=3 individual mice per group. Mean ± SEM given, #p<0.05 1 µg/ml vs. 0 µg/ml stimulation at same time point, *p<0.05 vs. 30 min stimulation at 1µg/ml.
We made some other interesting observations regarding PKC-θ expression and activation in Tregs and non-Tregs made possible by the sensitivity and reproducibility of the phospho-flow cytometry technique. We consistently observed that Tregs underwent non-specific PKC-θ upregulation and phosphorylation while in culture. As shown in Figure 3, PKC-θ and p-PKC-θ expression levels in Tregs increased in a time-dependent manner without stimulation. Interestingly, this in vitro effect was not observed in non-Tregs or in Tregs from OT-II trangenic mice.
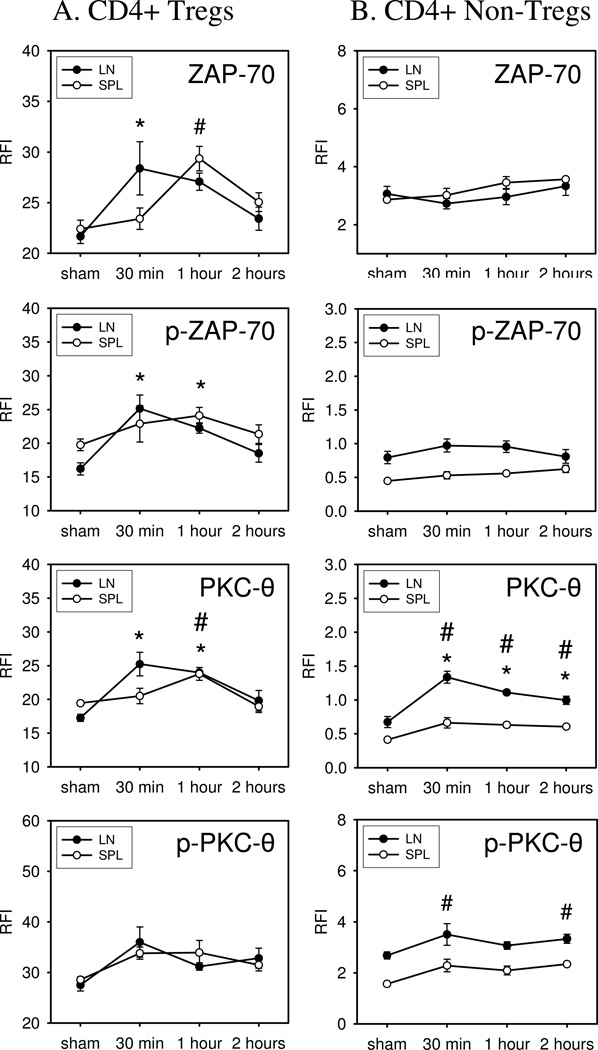
In vivo ZAP-70 and PKC-θ activation responses in Tregs and non-Treg CD4+ T cells in mice given SEB superantigen
We used phospho-flow cytometry to determine the kinetics and extent of ZAP-70 and PKC-θ signaling in lymph node and spleen FoxP3+ and FoxP3− CD4+ T cells in mice treated with SEB (1 mg/kg). At 30 minutes, 1 hour, or 2 hours after SEB challenge, lymph nodes (pooled inguinal, axillary, and brachial) and spleens were harvested to prepare cell suspensions for phospho-flow cytometry. The data shown in Figure 6 indicates that un-phosphorylated and phosphorylated ZAP-70 expression is significantly increased by 30 minutes after SEB injection in lymph node Tregs. However, SEB-induced ZAP-70 activation was delayed an additional 30 minutes in spleen Tregs showing significant increases by 1 hour after SEB challenge. We did not detect significant ZAP-70 activation in non-Treg CD4+ T cells prepared from SEB-treated mice. In contrast to ZAP-70 activation, we observed significant PKC-θ activation in both Tregs and non-Treg CD4+ T cells prepared from the lymph nodes of SEB-treated mice. PKC-θ activation showed similar kinetics to ZAP-70 activation in that PKC-θ activation was also delayed an additional 30 minutes in spleen Tregs. Non-Treg CD4+ T cells in the spleen did not show significant PKC-θ activation. Finally, we found that the un-phosphorylated and phosphorylated levels of ZAP-70 and PKC-θ returned to baseline by 2 hours after SEB treatment (Figure 6). The results of these in vivo studies demonstrate the feasibility of using phospho-flow cytometry to measure early time-dependent changes in TCR-induced signaling in FoxP3+ and FoxP3− CD4+ T cells. We document that SEB treatment caused rapid ZAP-70 and PKC-θ activation in lymph node Tregs. We also demonstrate that PKC-θ activation occurred in Treg and non-Treg CD4+ T cells following SEB challenge, but that ZAP-70 activation only occurred in Tregs. There was also a significant tissue specific difference in SEB-induced TCR signaling responses between lymph nodes and spleen.
Figure 6. Expression and phosphorylation of PKC-θ and ZAP-70 in Tregs vs. non-Tregs following in vivo challenge of mice with SEB.
WT mice were challenged by i.p injection with 25µg/mouse SEB, dissolved in 250µl of PBS, or sham-treated by injection of PBS alone. After 30 min, 1 hour, or 2 hours treated mice were sacrificed and lymph nodes (LN) and spleens (SPL) were harvested to generate cell suspensions. The expression and phosphorylation of PKC-θ and ZAP-70 was assessed in Tregs and non-Tregs by flow cytometry. Data shown represent one of two independent experiments. N=3 mice. Mean ± SEM given, #p<0.05 vs. sham (SPL), *p<0.05 vs. sham (LN).
4. DISCUSSION
Antigen-specific CD4+ T cell activation is initiated by cross-linking of the TCR α and β chains to antigen-bound MHC class II molecule. The strength and type of signaling response is modulated by costimulatory molecules at the interface between antigen presenting cells and T cells referred to as the immunological synapse (Delves and Roitt, 2000; Frauwirth and Thompson, 2002; Grakoui et al., 1999). We initiated this particular study as a first-step to develop an efficient and feasible approach to investigate early TCR signaling differences between FoxP3+ regulatory CD4+ T cells and conventional non-regulatory (FoxP3−) CD4+ T cells in vivo. We decided upon working with phospho-flow cytometry to achieve this objective due to its high throughput nature and its capacity to quantitatively measure signaling responses in cells. In addition, we knew that we would have to use a cell-based approach because it would not be possible to purify Tregs from non-Treg CD4+ T cells rapidly and effectively enough to accurately detect early signaling responses (Gavin et al., 2006; Wang et al., 2007). Unlike Western immunoblot analysis, flow cytometry detects signaling responses by changes in the mean fluorescence intensity (MFI) of stained signaling molecules. The MFI provides a measurable estimate of changes in relative expression levels of molecules, which are detected by specific antibody staining reagents.
These experiments focused on ZAP-70 and PKC-θ activation because these signaling molecules represent central TCR signaling molecules. ZAP-70 was chosen because it is membrane proximal and required for most signaling downstream of the TCR (Au-Yeung et al., 2009). PKC-θ was chosen for this study because it is required for TCR-induced activation of NF-κB and AP-1 transcription factors (Lucas et al., 2004; Monks et al., 1998) Furthermore, the expression profile or ZAP-70 and PKC-θ is more restricted to T cells than other TCR signaling molecules (Baier et al., 1993; Meller et al., 1999).
We first performed a series of optimization studies to refine the phospho-flow cytometry method to detect changes in ZAP-70 and PKC-θ levels and phosphorylation in FoxP3+ Tregs and non-Treg CD4+ T cells. Our optimized approach is outlined in the Materials and Methods section of this manuscript. We were initially pleased to find that we were able to detect nuclear FoxP3 expression in methanol-fixed T cells. This feature made the approach both feasible and efficient. This finding also indicated that methanol fixation was compatible for nuclear antigen detection. We then used our optimized approach to measure signaling kinetics in Tregs and non-Treg CD4+ T cells that were activated in vitro or in vivo by different types of TCR agonists. The results of in vitro and in vivo studies revealed striking differences in signaling responses by Tregs and non-Treg CD4+ T cells. The first major difference was that Tregs and non-Treg CD4+ T cells showed significant ZAP-70 and PKC-θ activation in response to CD3ε stimulation, while cells stimulated with SEB superantigen did not show any detectable ZAP-70 and PKC-θ activation. Of note, we detected up-regulation of ZAP-70 and PKC-θ, as early as 30 min following stimulation. This could be due to pre-formed mRNA, or in the case of ZAP-70 due to pre-existence of ZAP-70 in a complex that opens following TCR stimulation (Bacon et al., 1995). We also tested ZAP-70 and PKC-θ signaling responses by OT-II TCR transgenic CD4+ T cells stimulated with cognate OVA-peptide antigen. Surprisingly, we found that antigen-activated OT-II Tregs and non-Treg CD4+ T cells did not show detectable early ZAP-70 or PKC-θ phosphorylation, even at very early time points (Supplemental Figures 1 and 2). The finding that superantigen did not signal ZAP-70 activation is consistent with a single published report, which showed that superantigen stimulation did not induce significant ZAP-70 phosphorylation in memory T cells (Watson and Lee, 2006). The discovery that in vitro TCR stimulation by specific antigen does not induce significant early ZAP-70 and PKC-θ activation is novel and suggests that rapid ZAP-70 and PKC-θ activation may be more specific to direct CD3 complex stimulation. Of note, much of the published data describing ZAP-70 and PKC-θ was generated in T cells stimulated by anti-CD3ε and/or anti-CD28 antibody (Au-Yeung et al., 2009; Farber, 2009). Nevertheless, the data generated using anti-CD3ε antibody stimulation serves as a positive control for our ability to detect ZAP-70 and PKC-θ phosphorylation in Tregs and non-Treg CD4 T+ cells. Thus, we believe that the failure to detect ZAP-70 and PKC-θ activation in SEB- or antigen-stimulated CD4+ T cells is not due to a technical problem with our phospho-flow cytometry method. Additional control studies reported here also showed that the SEB and the OVA323–339 peptide used in our studies were capable of activating significant CD4 T cell proliferation and cytokine production (Figures 4 and 5).
The results of in vivo studies were markedly different than the results of in vitro experiments. We found that SEB treatment caused detectable and significant changes in ZAP-70 and PKC-θ levels and phosphorylation. The most interesting aspect of these in vivo experiments was that SEB could induce rapid TCR signaling response in Tregs when given to mice, while in vitro stimulation failed to activate detectable ZAP-70 or PKC-θ phosphorylation in Tregs or non-Treg CD4+ T cells. This selective in vivo response by Tregs may be due to the reported higher metabolic rate, a higher sensitivity to environmental stimuli, and higher rapidity of response by Tregs as compared to non-Treg CD4 T cells (Matarese et al., 2008). Thus, we conclude from these findings that a critical signal for SEB-induced Treg activation was not present when cells were stimulated ex vivo. Furthermore, these results highlight the importance of developing phospho-flow cytometry for investigating cell-mediated immune responses in vivo - the phospho-flow method allowed us to measure signaling in in vivo activated T cells.
In summary, this study used phospho-flow cytometry to investigate potential differences in TCR signaling responses between Tregs and non-Treg CD4+ T cells. We found that in vitro cell stimulation with anti-CD3ε antibody induces early and transient activation of ZAP-70 and PKC-θ in both Tregs and non-Tregs. However, we discovered that bacterial superantigen or stimulation of TCR transgenic CD4+ T cells with antigen did not significantly activate these signaling pathways in Tregs or non-Tregs. On the other hand, mice treated with superantigen showed ZAP-70 and PKC-θ activation in lymph node Tregs, but not in non-Tregs. The results of these in vivo studies provide new data to suggest that lymph node Tregs respond rapidly to strong TCR stimulation. We also demonstrate the versatility of using phospho-flow cytometry to measure in vivo T cell signaling responses. Taken collectively, the results of these experiments demonstrate that Tregs and non-Treg CD4+ T cells show marked differences in their reactivity to TCR-dependent stimulation and contribute new insights into basic mechanisms that lead to rapid TCR signaling in Tregs.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by NIH grant numbers 5R01GM057664 and 5R01GM035633.
ABBREVIATIONS
- DAG
diacylglycerol
- ERK
extracellular signal-regulated kinases
- ITAM
immunoreceptor tyrosine-based activation motif
- LAT
linker for activation of T cells
- Lck
leukocyte specific protein tyrosine kinase
- LN
lymph node
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PKC-θ
protein kinase C-theta
- PLCγ1
phospholipase C-γ1
- RFI
relative fluorescence intensity
- SPL
spleen
- TCR
T cell receptor
- ZAP-70
ζ-associated protein of 70kDa
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Au-Yeung BB, Deindl S, Hsu LY, Palacios EH, Levin SE, Kuriyan J, Weiss A. The structure, regulation, and function of ZAP-70. Immunol. Rev. 2009;228:41. doi: 10.1111/j.1600-065X.2008.00753.x. [DOI] [PubMed] [Google Scholar]
- 2.Bacon KB, Premack BA, Gardner P, Schall TJ. Activation of dual T cell signaling pathways by the chemokine RANTES. Science. 1995;269:1727. doi: 10.1126/science.7569902. [DOI] [PubMed] [Google Scholar]
- 3.Baier G, Telford D, Giampa L, Coggeshall KM, Baier-Bitterlich G, Isakov N, Altman A. Molecular cloning and characterization of PKC theta, a novel member of the protein kinase C (PKC) gene family expressed predominantly in hematopoietic cells. J. Biol. Chem. 1993;268:4997. [PubMed] [Google Scholar]
- 4.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 1998;76:34. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 5.Bi K, Tanaka Y, Coudronniere N, Sugie K, Hong S, van Stipdonk MJ, Altman A. Antigen-induced translocation of PKC-theta to membrane rafts is required for T cell activation. Nat. Immunol. 2001;2:556. doi: 10.1038/88765. [DOI] [PubMed] [Google Scholar]
- 6.Chan AC, Dalton M, Johnson R, Kong GH, Wang T, Thoma R, Kurosaki T. Activation of ZAP-70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO J. 1995;14:2499. doi: 10.1002/j.1460-2075.1995.tb07247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow S, Patel H, Hedley DW. Measurement of MAP kinase activation by flow cytometry using phospho-specific antibodies to MEK and ERK: potential for pharmacodynamic monitoring of signal transduction inhibitors. Cytometry. 2001;46:72. doi: 10.1002/cyto.1067. [DOI] [PubMed] [Google Scholar]
- 8.Coudronniere N, Villalba M, Englund N, Altman A. NF-kappa B activation induced by T cell receptor/CD28 costimulation is mediated by protein kinase C-theta. Proc. Natl. Acad. Sci. U. S. A. 2000;97:3394. doi: 10.1073/pnas.060028097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crellin NK, Garcia RV, Levings MK. Altered activation of AKT is required for the suppressive function of human CD4+CD25+ T regulatory cells. Blood. 2007a;109:2014. doi: 10.1182/blood-2006-07-035279. [DOI] [PubMed] [Google Scholar]
- 10.Crellin NK, Garcia RV, Levings MK. Flow cytometry-based methods for studying signaling in human CD4+CD25+FOXP3+ T regulatory cells. J. Immunol. Methods. 2007b;324:92. doi: 10.1016/j.jim.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Delves PJ, Roitt IM. The immune system. Second of two parts. N. Engl. J. Med. 2000;343:108. doi: 10.1056/NEJM200007133430207. [DOI] [PubMed] [Google Scholar]
- 12.Farber DL. Biochemical signaling pathways for memory T cell recall. Semin. Immunol. 2009;21:84. doi: 10.1016/j.smim.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frauwirth KA, Thompson CB. Activation and inhibition of lymphocytes by costimulation. J. Clin. Invest. 2002;109:295. doi: 10.1172/JCI14941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gavin MA, Torgerson TR, Houston E, DeRoos P, Ho WY, Stray-Pedersen A, Ocheltree EL, Greenberg PD, Ochs HD, Rudensky AY. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc. Natl. Acad. Sci. U. S. A. 2006;103:6659. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi K, Altman A. Protein kinase C theta (PKCtheta): a key player in T cell life and death. Pharmacol. Res. 2007;55:537. doi: 10.1016/j.phrs.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hickman SP, Yang J, Thomas RM, Wells AD, Turka LA. Defective activation of protein kinase C and Ras-ERK pathways limits IL-2 production and proliferation by CD4+CD25+ regulatory T cells. J. Immunol. 2006;177:2186. doi: 10.4049/jimmunol.177.4.2186. [DOI] [PubMed] [Google Scholar]
- 18.Isakov N, Altman A. Protein kinase C(theta) in T cell activation. Annu. Rev. Immunol. 2002;20:761–794. 761. doi: 10.1146/annurev.immunol.20.100301.064807. [DOI] [PubMed] [Google Scholar]
- 19.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krutzik PO, Nolan GP. Intracellular phospho-protein staining techniques for flow cytometry: monitoring single cell signaling events. Cytometry A. 2003;55:61. doi: 10.1002/cyto.a.10072. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Godfrey WR, Porter SB, Ge Y, June CH, Blazar BR, Boussiotis VA. CD4+CD25+ regulatory T-cell lines from human cord blood have functional and molecular properties of T-cell anergy. Blood. 2005;106:3068. doi: 10.1182/blood-2005-04-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lucas PC, lister-Lucas LM, Nunez G. NF-kappaB signaling in lymphocytes: a new cast of characters. J. Cell Sci. 2004;117:31. doi: 10.1242/jcs.00904. [DOI] [PubMed] [Google Scholar]
- 23.Matarese G, De RV, La CA. Regulatory CD4 T cells: sensing the environment. Trends Immunol. 2008;29:12. doi: 10.1016/j.it.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Meller N, Elitzur Y, Isakov N. Protein kinase C-theta (PKCtheta) distribution analysis in hematopoietic cells: proliferating T cells exhibit high proportions of PKCtheta in the particulate fraction. Cell Immunol. 1999;193:185. doi: 10.1006/cimm.1999.1478. [DOI] [PubMed] [Google Scholar]
- 25.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 26.Monks CR, Kupfer H, Tamir I, Barlow A, Kupfer A. Selective modulation of protein kinase C-theta during T-cell activation. Nature. 1997;385:83. doi: 10.1038/385083a0. [DOI] [PubMed] [Google Scholar]
- 27.Mustelin T, Tasken K. Positive and negative regulation of T-cell activation through kinases and phosphatases. Biochem. J. 2003;371:15. doi: 10.1042/BJ20021637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151. [PubMed] [Google Scholar]
- 29.Thebault S, Ochoa-Garay J. Characterization of TCR-induced phosphorylation of PKCtheta in primary murine lymphocytes. Mol. Immunol. 2004;40:931. doi: 10.1016/j.molimm.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 30.Tsang JY, Camara NO, Eren E, Schneider H, Rudd C, Lombardi G, Lechler R. Altered proximal T cell receptor (TCR) signaling in human CD4+CD25+ regulatory T cells. J. Leukoc. Biol. 2006;80:145. doi: 10.1189/jlb.0605344. [DOI] [PubMed] [Google Scholar]
- 31.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Ioan-Facsinay A, van dV, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur. J. Immunol. 2007;37:129. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 33.Watson AR, Lee WT. Defective T cell receptor-mediated signal transduction in memory CD4 T lymphocytes exposed to superantigen or anti-T cell receptor antibodies. Cell Immunol. 2006;242:80. doi: 10.1016/j.cellimm.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams BL, Irvin BJ, Sutor SL, Chini CC, Yacyshyn E, Bubeck WJ, Dalton M, Chan AC, Abraham RT. Phosphorylation of Tyr319 in ZAP-70 is required for T-cell antigen receptor-dependent phospholipase C-gamma1 and Ras activation. EMBO J. 1999;18:1832. doi: 10.1093/emboj/18.7.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.