Abstract
The hemodynamic effects of the novel, selective adenosine A3 receptor agonist 2-chloro-N6-(3-iodobenzyl)adenosine-5′-N-methyl-carboxamide (2-Cl-IB-MECA) were investigated in conscious rats. Intravenous administration of 200 μg/kg 2-Cl-IB-MECA resulted in a short-lasting hypotension, which was accompanied by a 50–100-fold increase in plasma histamine concentrations. Administration of a second dose of 2-Cl-IB-MECA did not elicit any hemodynamic effects. Also no histamine release was observed after the second dose. The observation of rapid tachyphylaxis in combination with histamine release suggests that mast cell mediator release plays a key role in the hemodynamic effects of adenosine A3 receptor agonists.
Keywords: Adenosine A3 receptor; Hemodynamics; Histamine release; Tachyphylaxis; 2-Chloro-N6-(3-iodobenzyl)adenosine-5-N-methylcarboxamide (2-Cl-IB-MECA); Rat, conscious
1. Introduction
Adenosine is an important regulatory metabolite that exerts pronounced biological effects in the cardiovascular and central nervous system. The actions of adenosine are mediated through cell-surface adenosine receptors. At present, adenosine A1, A2A, A2B and A3 receptors have been identified based on structure-activity relationships of agonist and antagonists and cloning of the four receptor subtypes from various species (for review, see Olah and Stiles, 1995).
The adenosine A3 receptor has only recently been discovered, with the cloning from rat, sheep and human sources (for review, see Linden, 1994). In vitro stimulation of this adenosine receptor subtype has been shown to inhibit adenylyl cyclase (Zhou et al., 1992) and to stimulate phospholipase C followed by formation of inositol-1,4,5-triphosphate (IP3) (Ramkumar et al., 1993; Abbracchio et al., 1995). In vivo activation of the A3 receptor has been shown to mediate heart rate-independent hypotension in rats (Fozard and Carruthers, 1993), vasoconstriction (Doyle et al., 1994) and depression of locomotor activity in mice (Jacobson et al., 1993).
Although selective agonists for the A1 and A2A receptors have been available for several years, highly selective A3 receptor agonists have been synthesized only recently. 2-Chloro-N6-(3-iodobenzyl) adenosine-5′-N-methyl-carboxamide (2-Cl-IB-MECA) is the first highly selective adenosine A3 receptor agonist (Kim et al., 1994). In vitro 2-Cl-IB-MECA has been shown to be a potent adenosine A3 receptor agonist with a 2500- and 1400-fold selectivity for A3 vs. A1 and A2A adenosine receptors, respectively.
In the present study the hemodynamic effects of 2-Cl-IB-MECA were investigated in conscious normotensive rats. The high selectivity of 2-Cl-IB-MECA enabled us to study A3 receptor-mediated effects in vivo, without the interference or blockade of the effects of other adenosine receptor subtypes. The role of mast cell mediator release in the cardiovascular response was investigated by measurement of free histamine concentrations in the blood and by assessment of the effectiveness of 2-Cl-IB-MECA upon repeated administration in the same rat.
2. Materials and methods
2.1. Animals and surgical procedures
Male Wistar rats (Crl:(WI)WU BR, Broekman Instituut, Someren, The Netherlands), weighing 300–350 g, were used. The animals were housed in plastic cages with a normal 12-h light-dark cycle and fed on laboratory chow (Standard Laboratory Chow, SRM-A, Hope Farms, Woerden, The Netherlands) and water ad libitum.
Four days before experimentation, indwelling cannulas were implanted into the right jugular vein (polythene, 13.5 cm, 0.6 mm i.d.) and the left and right femoral artery (Polythene, 18 cm, 0.58 mm i.d. +4.5 cm, 0.28 mm i.d.) under light ether anaesthesia. The cannula in the femoral artery was gently pushed through the artery until it reached the aorta abdominalis. The cannulas were used for drug administration, blood sample collection and recording of arterial blood pressure, respectively. The three cannulas were tunnelled subcutaneously to the back of the neck and exteriorized. After the operation the cannulas were filled with a 25% (g/v) solution of polyvinylpyrrolidone (PVP) (Brocacef, Maarssen, The Netherlands) in 0.9% (g/v) sodium chloride containing 50 IU heparin/ml (Hospital Pharmacy, Leiden, The Netherlands) to prevent clotting.
On the day of the experiment the PVP solution was removed and the canulas were flushed with saline (0.9% (g/v) sodium chloride solution) containing 20 IU/ml heparin. All experiments were performed in conscious and freely moving rats.
2.2. Study design
Rats were randomly assigned to two treatment groups of 6 animals, which received either two subsequent intravenous (i.v.) infusions of 200 μg/kg 2-chloro-N6-(3-iodobenzyl)adenosine-5′-N-methylcarboxamide (2-Cl-IB-MECA) during 15 min or two subsequent infusions of the vehicle during 15 min. 2-Cl-IB-MECA was dissolved in dimethylsulfoxide (DMSO) and diluted with a sodium chloride solution to a final 30% (v/v) DMSO solution (a total volume of 765 μl). Fresh solutions were prepared prior to each experiment.
The effect measurements were started at least 45 min prior to the first drug infusion and lasted until 4.5 h after this infusion. Heart rate and blood pressure were recorded continuously and serial arterial blood samples were drawn for determination of plasma histamine concentrations. 2 h after the first administration the second dose of 2-Cl-IB-MECA (or vehicle) was administered.
2.3. Cardiovascular measurements
To minimize the contribution of diurnal rhythm in the hemodynamic parameters all experiments were started between 09:00 and 10:00 h in the morning. Arterial blood pressure was measured from the cannula in the left femoral artery using a miniature strain gauge P10EZ transducer (Viggo-Spectramed, Bilthoven, The Netherlands), equipped with a plastic diaphragm dome (TA1017, Disposable Critiflo Dome, Viggo-Spectramed). The pressure transducer was connected to a polygraph amplifier console (RMP6018, Nihon Kohden Corporation, Tokyo, Japan). Heart rate was captured from the blood pressure signal. The signals were passed through a CED 1401 interface (Cambridge Electronic Design, Cambridge, UK) into a 80486 computer using the data acquisition program Spike 2 (Spike 2 Software, Version 3.1, Cambridge, UK) and stored on diskette.
During the experiments the catheter connected to the pressure transducer was continuously flushed with saline containing 20 IU/ml heparin at a flow rate of 500 μl/h (Syringe infusion pump 22, Harvard apparatus; Plato, Diemen, The Netherlands) to ensure a continuous recording of the pharmacodynamic effect.
2.4. Determination of histamine levels
At predefined time points 20 or 50 μl arterial blood samples were drawn, added to 50 μl of a chilled EDTA/saline solution (5% (g/v) EDTA in 0.9% NaCl) and kept on ice. After centrifugation at 5000 rpm for 10 min, 50 μl plasma was carefully pipetted and transferred to a clean eppendorf tube. Depending on the expected histamine concentrations some samples were diluted with millepore water. The samples were stored at −20°C until analysis. Histamine concentrations in plasma were determined by a radioimmunoassay developed by Immunotech (Marseille, France) (Morel and Delaage, 1988).
2.5. Statistical analysis
Student’s t-tests were used to compare the different treatments and the two infusions. The corresponding non-parametric tests were used in case of non-homogeneity of the data. A significance level of 5% was selected. All data are reported as mean ± S.E., unless indicated otherwise.
3. Results
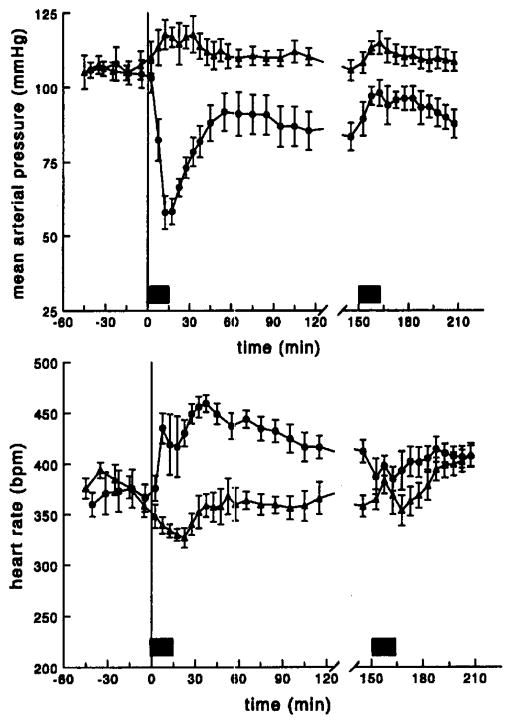
The time profiles of mean arterial pressure and heart rate after administration of 200 μg/kg 2-Cl-IB-MECA to conscious rats are depicted in Fig. 1. The first infusion of 2-Cl-IB-MECA resulted in a rapid fall of the blood pressure to a value which was about 50% of the pre-administration level. The hypotension was short-lasting. After approximately 15 min the blood pressure started to recover and rapidly returned to stable values, which were significantly lower than the blood pressure values in the control group. Administration of the second dose of 2-Cl-IB-MECA, at 150 min after the first infusion, did not produce any hypotension at all. The slight increase in the mean arterial pressure during the second infusion was not specific for 2-Cl-IB-MECA and was observed after administration of the vehicle as well.
Fig. 1.
Averaged time profiles of mean arterial pressure (upper panel) and heart rate (lower panel); conscious rats received two subsequent i.v. infusions of 200 μg/kg 2-Cl-IB-MECA in 15 min (●) or vehicle in 15 min (▲); the filled bars represent the two infusion periods; data are presented as mean ± S.E. (n = 6).
The effect of 2-Cl-IB-MECA on heart rate was more complex. Infusion of 200 μg/kg 2-Cl-IB-MECA resulted in an increase in heart rate, which was independent of the blood pressure. Near the end of the infusion a short-lasting decrease in heart rate was observed in some of the animals. However, as soon as the blood pressure started to normalize heart rate markedly increased again. The tachycardia was maintained over a long period of time and returned to baseline values slowly. Again, no increase in heart rate was observed upon the second infusion of the A3 agonist.
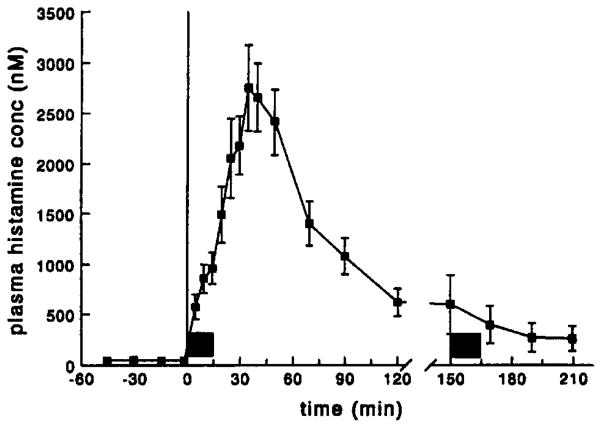
The time course of the plasma histamine concentrations is shown in Fig. 2. Following administration of 2-CI-IB-MECA a massive release of histamine was observed. This release resulted in a 50–100-fold increase in plasma histamine concentrations, which reached a maximum at approximately 35 min and slowly decreased to normal plasma levels. In line with the cardiovascular effects, the second infusion of 2-Cl-IB-MECA did not result in release of histamine. Administration of the vehicle did not affect plasma histamine concentrations. During the entire control experiment histamine levels remained stable at a concentration of 32 ± 3.2 nM (mean ± S.E., n = 6).
Fig. 2.
Plasma histamine concentration vs. time profile for rats which received two subsequent i.v. infusions of 200 μg/kg 2-Cl-IB-MECA in 15 min; the filled bars represent the two infusion periods. In the vehicle-treated group plasma histamine concentrations remained stable (32 ± 3.2 nM); data are presented as mean ± S.E. (n = 6).
4. Discussion
In the present study we have investigated the cardiovascular effects of the highly selective adenosine A3 agonist 2-chloro-N6-(3-iodobenzyl)adenosine-5′-N-methyl-carboxamide (2-Cl-IB-MECA). So far, in most in vivo studies non-selective ligands have been used, because selective adenosine A3 agonists have not been readily available. In several studies in rats, therefore, the A3 effect has been isolated by blockade of the A1 and A2A effects with antagonists (Fozard and Carruthers, 1993; Hannon et al., 1995). Due to its high selectivity, 2-Cl-IB-MECA is particularly useful in in vivo studies, for it allows to study adenosine A3-mediated responses without the need of co-administration of antagonists to block other adenosine receptors subtypes.
I.v. administration of 200 μg/kg 2-Cl-IB-MECA to conscious rats resulted in a decrease in blood pressure, which was – in contrast to A1 receptor-mediated hypotension – independent of heart rate (Mathôt et al., 1994) (Fig. 1). Interestingly, we observed a rather short-lasting hypotension, which was not consistent with the pharmacokinetic profile of 2-Cl-IB-MECA. Blood pressure values returned to baseline fast, while blood concentrations of 2-Cl-IB-MECA declined slowly with a half-life of approximately 30 min (unpublished observations). In addition to the hypotension, we observed an increase in heart rate. This was not a reflex tachycardia, because the heart rate effect developed prior to the decrease in blood pressure. Furthermore, heart rate remained significantly increased for a long time and returned to baseline values slowly. Upon repetition of the i.v. infusion, given 150 min after the first infusion, 2-Cl-IB-MECA was completely devoid of any cardiovascular actions. The small changes in blood pressure and heart rate after the second dose were presumably the result of irritation by the DMSO containing vehicle solution and were observed in the vehicle-treated animals as well.
The observations of the relatively short-lasting hypotension and the complete disappearance of the hemodynamic effects upon repeated administration suggested that the effects were not direct effects of the A3 agonist but indirect through an intermediate. In this respect mediator release from mast cells was likely to play a role (Linden, 1994). One of the mediators released upon activation of mast cells is histamine. After administration of the adenosine A3 agonist 2-Cl-IB-MECA plasma histamine concentrations increased substantially (Fig. 2). In the vehicle-treated animals histamine levels remained stable during the entire experiment (32 ± 3.2 nM), whereas after i.v. infusion of 2-Cl-IB-MECA histamine concentrations increased approximately 50–100-fold. Complete degranulation of mast cells occurred, since no release of histamine was observed during the second infusion of 2-Cl-IB-MECA.
These data are consistent with recent observations, in which administration of the non-selective adenosine agonist N6-2-(4-aminophenyl)ethyladenosine (APNEA) to anaesthetized rats resulted in an increase in histamine levels. Furthermore, APNEA was devoid of any cardiovascular effects after pretreatment of rats with the degranulating agent compound 48/80 (Hannon et al., 1995). Although adenosine A2B receptors may be involved in mast cell degranulation (Marquardt et al., 1994) as well, it is unlikely that these receptors contribute to the histamine release. At the cloned human A2B receptor 2-Cl-IB-MECA has been shown to be nearly inactive at 100 μM concentrations (A.P. IJzerman, unpublished observations).
After administration of 2-Cl-IB-MECA a long-lasting increase in histamine concentrations was observed, which declined slowly with a half-life of approximately 30 min. This kinetic profile suggests a gradual release of histamine from mast cells, since histamine itself has a half-life of seconds in the rat (for review, see Beaven, 1982). The hypotensive effect did not correspond to the time course of the histamine concentrations. At the time point of the maximal histamine concentrations blood pressure values were already more or less back to normal baseline levels. These observations may indicate that there is either rapid desensitisation of histamine receptors or that other mediators are involved in the response. Rat mast cells contain many other mediators, among which serotonin and leukotrienes. It may well be that the observed hypotension is elicited by other mediators and not by histamine alone.
In conclusion, activation of the adenosine A3 receptor in vivo with the selective adenosine A3 receptor agonist 2-Cl-IB-MECA resulted in a short-lasting hypotension, which was accompanied by histamine release from mast cells. The complete tachyphylaxis suggested that the A3 receptor-mediated cardiovascular responses were dependent on the release of mediators from mast cells. It remains, however, to be established which mediator is responsible for the hypotensive effect.
References
- Abbracchio MP, Brambilla R, Ceruti S, Kim HO, Von Lubitz DKJE, Jacobson KA, Cattabeni F. G-protein-dependent activation of phospholipase C by adenosine A3 receptors in rat brain. Mol Pharmacol. 1995;48:1038. [PubMed] [Google Scholar]
- Beaven MA. Factors regulating availability of histamine at tissue receptors. In: Gannelin CR, Parsons ME, editors. Pharmacology of Histamine Receptors. Wright; London: 1982. p. 103. [Google Scholar]
- Doyle MP, Linden J, Duling BR. Nucleoside induced arterial constriction: a mast cell-dependent response. Am J Physiol. 1994;266:H2042. doi: 10.1152/ajpheart.1994.266.5.H2042. [DOI] [PubMed] [Google Scholar]
- Fozard JR, Carruthers AM. Adenosine A3 receptors mediate hypotension in the angiotensin II-supported circulation of the pithed rat. Br J Pharmacol. 1993;109:3. doi: 10.1111/j.1476-5381.1993.tb13522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon JP, Pfannkuche HJ, Fozard JR. A role for mast cells in adenosine A3 receptor-mediated hypotension in the rat. Br J Pharmacol. 1995;115:945. doi: 10.1111/j.1476-5381.1995.tb15902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Nikodijević O, Shi D, Gallo-Rodriguez C, Olah ME, Stiles GL, Daly JW. A role for central A3-adenosine receptors; mediation of behavioral depressant effects. FEBS Lett. 1993;336:57. doi: 10.1016/0014-5793(93)81608-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HO, Ji XD, Siddiqi SM, Olah ME, Stiles GL, Jacobson KA. 2-Substitution of N6-benzyladenosine-5′-uronamides enhances selectivity for A3 adenosine receptors. J Med Chem. 1994;37:3614. doi: 10.1021/jm00047a018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden J. Cloned adenosine A3 receptors: pharmacological properties, species differences and receptor functions. TiPS. 1994;15:298. doi: 10.1016/0165-6147(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Marquardt DL, Walker LL, Heinemann S. Cloning of two adenosine receptor subtypes from mouse bone marrow-derived mast cells. J Immunol. 1994;152:4508. [PubMed] [Google Scholar]
- Mathôt RAA, Van Schaick EA, Langemeijer MWE, Soudijn W, Breimer DD, IJzerman AP, Danhof M. Pharmacokinetic-pharmacodynamic relationship of the cardiovascular effects of adenosine A1 receptor agonist N6-cyclopentyladenosine in the rat. J Pharmacol Exp Ther. 1994;268:616. [PubMed] [Google Scholar]
- Morel AM, Delaage MA. lmmunoanalysis of histamine trough a novel chemical derivatization. J Allerg Clin Immunol. 1988;82:646. doi: 10.1016/0091-6749(88)90978-5. [DOI] [PubMed] [Google Scholar]
- Olah ME, Stiles GL. Adenosine receptor subtypes: characterization and therapeutic regulation. Annu Rev Pharmacol Toxicol. 1995;35:581. doi: 10.1146/annurev.pa.35.040195.003053. [DOI] [PubMed] [Google Scholar]
- Ramkumar V, Stiles GL, Beaven MA, Ali H. The A3 adenosine receptor is the unique adenosine receptor which facilitates release of allergic mediators from mast cells. J Biol Chem. 1993;268:16887. [PubMed] [Google Scholar]
- Zhou QY, Olah ME, Johnson RA, Stiles GL, Civelli O. Molecular cloning and characterization of an adenosine receptor, the A3 adenosine receptor. Proc Natl Acad Sci USA. 1992;89:7432. doi: 10.1073/pnas.89.16.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]