Abstract
Using knockout and transgenic technology, genetically modified animal models allowed us to understand the role of glucagon signalling in metabolism. Mice with a global deletion of the glucagon receptor gene (Gcgr) were designed using gene targeting. The phenotype of Gcgr−/− mouse provided important clues about the role of Gcgr in foetal growth, pancreatic development and glucose and lipid homeostasis. The lack of Gcgr activation was associated with: (i) hypoglycaemic pregnancies, poor foetal growth and increased foetal–neonatal demise; (ii) altered cytoarchitecture of pancreatic islets; (iii) altered glucose, lipid and hormonal milieu; (iv) reduced gastric emptying; (v) altered body composition and protection from diet-induced obesity; (vi) altered energy state; (vii) impaired hepatocyte survival; (viii) altered metabolic response to prolonged fasting and exercise and (ix) prevented development of diabetes in insulin-deficient mice. In contrast, mice overexpressing the Gcgr on pancreatic β-cells displayed an increase insulin secretion, pancreatic insulin content and β-cell mass, and partially protected against hyperglycaemia and impaired glucose tolerance when fed a high-fat diet. These findings suggest that glucagon signalling plays a significant role in the regulation of glucose and lipid homeostasis. Treatment options designed to block Gcgr activation may have negative implications in the treatment of diabetes.
Keywords: diabetes, Gcgr knockout mouse model, glucagon, glucagon signalling, hypoglycaemia, islets, pancreas
Introduction
Glucagon is a 29-amino acid polypeptide secreted by the α-cell of the islet of Langerhans [1,2]. Initially synthesized as a larger precursor ‘proglucagon’, it is cleaved by a specific enzyme, proconvertase 2 to the active molecule ‘glucagon’ [3]. Glucagon is also produced by the central nervous system on which its actions may include the regulation of glucose production [1,2]. Glucagon secretion is stimulated by hypoglycaemia, arginine, gastric inhibitor polypeptide, gastrin and potassium chloride and inhibited by hyperglycaemia, insulin, zinc, GLP-1 (glucagon-like peptide 1) and somatostatin [4-12].
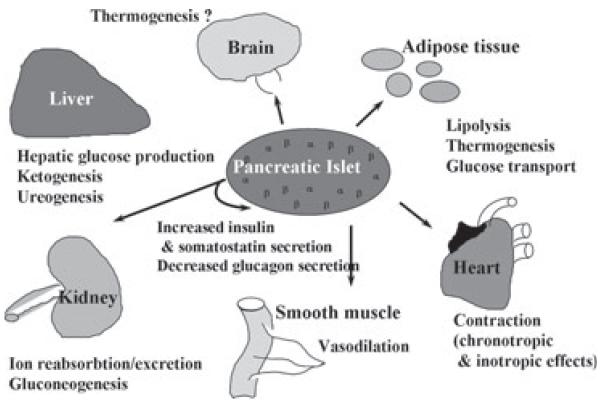
Glucagon action is transduced by a G-protein-coupled receptor (GCGR/Gcgr) that is a member of the class II GCGR superfamily of seven transmembrane spanning receptors that are coupled via GTP-binding proteins (G proteins) to adenyl cyclase [13]. Glucagon also mediates an increase in intracellular calcium in a phospholipase-C-dependent manner and it activates AMPK and JNK [14,15]. Binding sites for glucagon have been identified in liver, kidney, intestinal smooth muscle, brain, adipose tissue, heart and pancreatic islet β-cells (figure 1). Controversy exits about the presence of the Gcgr in pancreatic islet α-cells [16,17]. Gcgr expression is positively regulated by glucose and negatively regulated by glucagon and agents that increase intracellular cAMP [18].
Figure 1.
Glucagon action. Glucagon functions as a counterregulatory hormone, opposing the actions of insulin and maintaining blood glucose levels. Glucagon also elicits significant extrahepatic effects in tissues such as kidney, heart, adipose tissue, gastrointestinal tract (smooth muscle) and the central nervous system.
The mechanisms regulating the degradation and clearance of glucagon remain incompletely understood. Glucagon has been shown to be catabolized by the enzyme neutral endopeptidase 24.11 in pigs, as well as in vitro by dipeptidyl peptidase-4 at pharmacological levels [19,20]. Glucagon is cleared by the kidneys [21].
Glucagon generally functions as a counter-regulatory hormone, opposing the actions of insulin, and maintaining blood glucose levels by activating glycogenolysis and gluconeogenesis. Glucagon also elicits significant extrahepatic effects in tissues such as kidney, heart, adipose tissue, gastrointestinal tract, thyroid and central nervous system [1,2,16,22] (figure 1). Glucagon has potent hypolipaemic actions and reduces triglyceride(TG) and very low-density lipoproteinreleaseaswell asthe deposition of triacylglycerol in the liver, in addition to reducing cholesterol levels and stimulating fatty acid oxidation [23,24].
Studies Using the Gcgr Knockout Mouse Model (Gcgr−/−) have Revealed Important Insight into the Role of the Glucagon in Glucose and Lipid Homeostasis
In order to investigate the role of glucagon in glucose and lipid homeostasis, mice with a targeted deletion within the glucagon receptor gene Gcgr−/− were generated. The strategy used involved use of the murine Gcgr gene and positive and negative selectable markers. Specifically, exons 3–6 were replaced with a phosphoglycerate kinase promoted neomycin resistance cassette and the negative selectable thymidine kinase gene was engineered into the 3′ and 5′ ends. Appropriate targeting of the mutant Gcgr allele in embryonic stem cells and germ line transmission was confirmed by Southern blot and PCR analysis as previously shown [25]. Additional studies showed that glucagon stimulated increases in cAMP were lacking in hepatocytes of Gcgr−/− mice [25].
Lack of Glucagon Signal Alters Foetal Growth and Development
To determine whether glucagon signalling has an effect on foetal growth and development, Gcgr−/−, Gcgr+/− and WT pregnancies were sacrificed on embryonic day (e) e10.5, e15.5 and e18.5 of gestation and postnatal day 1. Embryos and pups were examined and weighed. Gcgr−/− pregnancies were characterized by poor foetal growth and increased foetal demised during late gestation [26]. Gcgr−/− females displayed significantly lower glucose levels throughout the pregnancy, leading to severe neonatal hypoglycaemia followed by an increase in neonatal death during the first 24 h of life [26]. Interestingly, Gcgr+/− pregnancies were characterized by normal maternal glucose levels, litter size, foetal growth and neonatal survival [26]. These results indicate that lack of glucagon signalling in the pregnant dam alters the proper intra-uterine milieu, such as lowering glucose levels, during critical periods of development leading to poor foetal growth and increased foetal demise.
Lack of Glucagon Signal Alters Pancreatic Islet Development
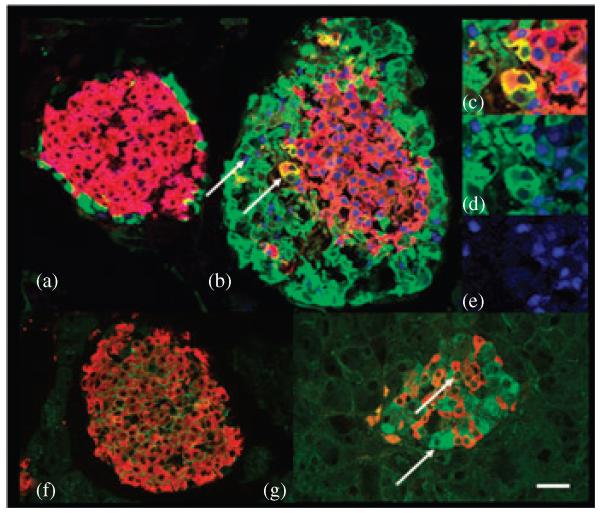
To determine whether glucagon signalling has an effect on pancreatic islet development, foetal and adult Gcgr−/− pancreata were studied as previously described by immunohistochemistry and gene expression analysis [26]. Deletion of the Gcgr delayed β-cell differentiation during foetal development [26]. Insulinexpressing cells were first observed in pancreata of Gcgr−/− mice at e13.5 in contrast to e11.5 in wild type (WT) littermates, and at e15.5, pancreata of Gcgr−/− embryos contained a similar number of insulin (β−) cells when compared to WT littermates. Insulin cell differentiation was not delayed because of alterations in the time line of expression of the peptide hormone processing enzymes which appeared at e10 in islet cell clusters [26]. Thus, ablation of Gcgr inhibited the differentiation of β-cells during the so-called protodifferentiated stage, which extends from early development until e day 14 in mice [27]. In addition, lack of glucagon signalling resulted in the presence of glucagon (α-cells) expressing embryonic markers [26] (figure 2). In WT islets, glucose transporter 2 (GLUT2) is usually found in the plasma membrane of β-cells and is absent from α-cells [28]; similarly, the transcription factor Pdx-1 is expressed by β-cells and by a subset of somatostatin (δ) cells throughout life and by α-cells of e10 but not of older embryos or adults [29-31]. Lack of glucagon signalling resulted in the expression of GLUT2 in the cytoplasm (figure 2g) and pancreatic duodenal homeobox-1 (PDX-1) in the nucleus and cytoplasm of α-cells (figure 2b-e) [26]. Similarly, cells co-expressing insulin and glucagon (GLU-IN cells), found only in the pancreas during the protodifferentiated stage of e10 to e15 WT mice, populated islets of adult Gcgr−/− mice (figure 2b-d) [32,33]. Double- and triple immunostaining studies of early pancreatic buds (data not shown), as well as adult islets, showed that the vast majority of GLU-IN cells are negative for Pdx-1 (figure 2b-d). It is generally believed that, during embryonic development, islet cells differentiate from precursors that first express Pdx-1, then initiate synthesis of GLUT2 and Neurogenin 3 (Ngn3) and is turned off by Ngn3 expression prior to the initiation of hormone expression [34]. Thus, the lack of PDX-1 expression in GLU-IN co-expressing cells suggests that those cells are generated by an independent set of precursor cells. In addition, β-cells of Gcgr−/− mice had low levels of Pdx-1, GLUT2 and mammalian homologues of avian A (MafA), which are molecules involved in the regulation of insulin expression [35-39]. These results indicated that the absence of glucagon signalling affects the initial stages of differentiation of islet precursor cells, may inhibit the progression of α-cells to maturity and alters the molecular profile of β-cells [26]. Thus, glucagon signalling during pancreatic development may have a local ‘paracrine’ role that either initiates or promotes the generation of other pancreatic endocrine progenitor cells.
Figure 2.
α-Cells of Gcgr−/− mice express immature traits. Photomicrographs of islets of 3-month-old wild type mice (a and f) immunostained for visualization of (a) glucagon (green), PDX-1 (blue), insulin (red) and (f) GLUT2 (green) and insulin (red). Note that α-cells of WT mice lack GLUT2 and PDX-1 expression. Photomicrographs of islets of 3-month-old Gcgr−/− mice (b-e and g) immunostained for visualization of (b-e) glucagon (green), insulin (red), PDX-1 (blue) and (g) GLUT2 (green) and insulin (red). (b-e) In Gcgr−/− mice, a subset of α-cells coexpress Pdx-1 as indicated with arrowheads. (c-e) Similarly, coexpressing GLU-INS cells do not express PDX-1. (g) Most α-cells of Gcgr−/− mice express the GLUT2. Bars: 20 μm.
Lack of Glucagon Signal Increases the Number of Islets, Alters Islet Morphology and Increases Circulating GLP-1 Levels
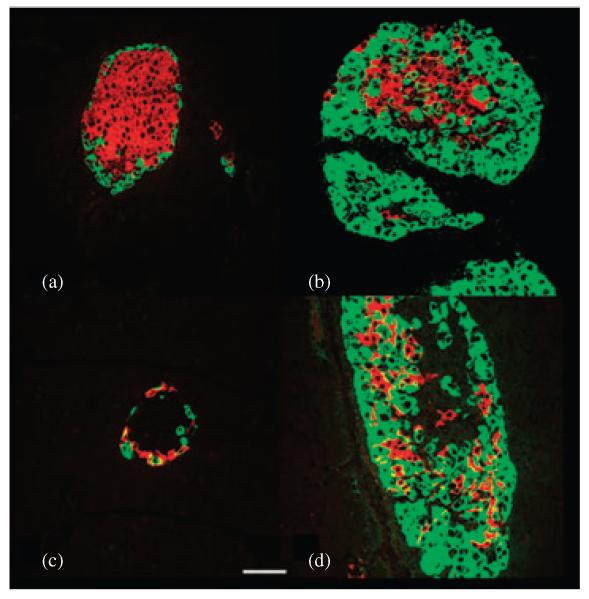
Loss of glucagon signalling resulted in an increase in the number of pancreatic islets [25,26]. Gcgr−/− islets were comprised of a thick mantle of α-cells and δ-cells surrounded by a core of β-cells (figure 3). In agreement with these findings, lack of glucagon signalling activated the expression of nestin-positive cells, which may represent endocrine precursor cells, in a subset of islets and exocrine cells of adult Gcgr−/− mice which suggested active islet neogenesis [40]. Morphometric analysis confirmed that the mean number of α-cells and somatostatin cells per section was more that twofold higher in Gcgr−/− than in WT littermates while the relative β-cell volume per section was similar in both lines. In addition to an increase in number, δ-cells in islets of Gcgr−/− mice exhibited an abnormal distribution as they were no longer localized to the mantle zone, but, rather, they were scattered in the core of the islet [26] (figure 3). The average polypeptide cell number per section was similar in islets of Gcgr−/− and WT mice [25,26]. In addition, pancreatic content of GLP-1 was significantly increased, and was accompanied by a 3- to 10-fold increase in circulating GLP-1 levels [25,41]. Interestingly, an animal model that lacks all the peptides derived from the glucagon gene (glucagon, GLP-1 and oxyntomodulin) displayed a similar phenotype of α-cell hyperplasia independent of improved glucose levels or absent GLP-1 levels [42].
Figure 3.
Islets of adult Gcgr−/− mice display non β-cell hyperplasia. Photomicrographs of islets of 3-month-old wild type mice immunostained for visualization of (a) insulin (red) and glucagon (green), (c) somatostatin (red) and glucagon (green). Photomicrographs of islets of 3-month-old Gcgr−/− mice immunostained for visualization of (b) insulin (red) and glucagon (green), (d) somatostatin (red) and glucagon (green). Note that the number of α-cells and δ-cells in islets of Gcgr−/− mice is higher than in WT littermates. Bar: 20 μm.
These data suggest that lack of either glucagon signalling or glucagon and GLP-1 gene expression increased the number of islets and lead to non-insulin cell hyperplasia, suggesting that an ‘autocrine/paracrine’ stimulus independently of glucose or GLP-1 levels is present in the Gcgr−/− mice. The precise mechanism underlying the regulation of the number of the different islet cells per islet and of the number of islets per pancreas by glucagon remains to be elucidated.
Lack of Glucagon Signal Reduces Gastric Emptying
The effect of the lack of glucagon signalling on the oral (gastrointestinal) absorption of d-xylose was investigated using Gcgr−/− and WT littermates [41]. The time curve responses suggested that the lack of glucagon signalling reduced xylose absorption, indicating that Gcgr−/− mice may have a slower rate of gastric emptying. This observation could be the result of the increased GLP-1 levels [25,41,43], which has been shown to reduce gastric emptying [44].
Lack of Glucagon Signal Alters Glucose Homeostasis
To determine the effect of glucagon signalling on glucose homeostasis during the fed and fasting state, Gcgr−/− mice underwent glucose monitoring, intraperitoneal glucose tolerance test, glucagon challenge and insulin tolerance test [25]. One salient feature of the Gcgr−/− mice was the presence of lower blood glucose levels during the day and the development of hypoglycaemia during a prolonged fast [25]. Furthermore, despite similar insulin sensitivity during an insulin tolerance test, Gcgr−/− mice displayed a blunted blood glucose response to a glucose bolus when compared to WT littermates suggesting that glucose-stimulated insulin release was increased [25]. Ambient hepatic glycogen levels were significantly increased in Gcgr−/− compared to WT littermates but were not different during the fasting state [25]. Furthermore, lack of glucagon signalling led to a late-onset loss of retinal function, loss of visual acuity and eventual death of retinal cells [45]. Anatomical changes in the retina were seen at 10 months of age. Interestingly, the decreases in retinal function and visual acuity correlated directly with the degree of hypoglycaemia. When challenged with a high-fat diet (45% of energy from fat) Gcgr−/− did not develop hyperinsulinaemia, and displayed a blunted glucose excursion when challenged with a glucose bolus [41].
These data suggest that glucagon signalling is essential to maintain glucose homeostasis. Lack of glucagon signalling does not alter insulin action and may increase glucose-stimulated insulin secretion. Furthermore, lack of glucagon signalling may improve glycaemic control in models of high fat induced obesity.
Lack of Glucagon Signal Alters the Hormonal Milieu
To determine whether glucagon signal impact the hormonal milieu Gcgr−/− mice underwent a full metabolic profile in which several hormones and lipids were measured using standard methods [25]. Gcgr−/− mice display hyperglucagonaemia, similar insulin levels, fed and fasted serum free fatty acids (NEFA), lactate, cholesterol and high-density lipoprotein and increased plasma low-density lipoprotein and decreased leptin levels, when compared to a WT littermate [25]. In addition, female mice displayed decreased TG levels when compared to male Gcgr−/− littermates. Gcgr−/− mice have a small decrease in IGF-1 and a twofold increase in corticosterone during fasting, with low corticosterone levels in the afternoon, and an increased responsiveness to epinephrine [25]. In Gcgr−/− mice, high-fat diet does not alter plasma glucagon, GLP-1, TG, NEFA or corticosterone levels when compared to WT littermates [41]. Thus, lack of glucagon signalling does not alter insulin levels or corticosteroid release as well as circadian rhythm, increases counterregulatory hormone responsiveness and increases GLP-1 levels secondary to the overproduction of proglucagon. Furthermore, when challenged with a high-fat diet, Gcgr−/− mice displayed an improved hormonal milieu.
Lack of Glucagon Signal Alters Fat Deposition
To determine whether glucagon signal impacts growth or body composition, growth rates, food intake, indirect calorimetry and MRI analysis were performed using Gcgr−/− mice fed a control as well as a high-fat diet [25,41]. Gcgr−/− mice displayed similar growth rates, food intake and resting O2 consumption and energy expenditure when compared to WT littermates [25,41]. MRI results revealed a significant decrease in total adipose mass that was compensated for by an increase in lean body mass [25]. Interestingly, Gcgr−/− mice were resistant to a 12-week high fat feeding regime [41]. WT littermates consumed more fat and gained about 30% more weight compared to Gcgr−/−. In addition, Gcgr−/− mice were resistant to the high-fat diet-induced hepatic steatosis as determined by Oil Red O staining of liver sections and by measuring hepatic lipid content [41]. These data suggest that the lack of glucagon signalling leads to a lean phenotype, independent of the diet, by either a direct action of the Gcgr or by the increased GLP-1 levels in the central nervous system or the periphery.
Lack of Glucagon Signal Prevents the Development of Diabetes in Insulin-Deficient Mice
To determine the effect of glucagon signalling in murine models of insulin deficiency, Gcgr−/− mice have been treated with streptozotocin (STZ) [41,46]. Interestingly, a single dose of 125 mg/kg STZ (Sigma Chemical, St Louis, MO, USA) did not induce hyperglycaemia and pancreatic β-cell destruction when the mice were followed for 20 days after the treatment [41]. In contrast, it has been subsequently shown that two intravenous injections of STZ (100 mg/kg body weight followed 7 days later by 80 mg/kg), in Gcgr−/− mice results in a significant reduction in the islet β-cell mass (morphometric analysis and insulin levels followed a glucose challenge) with no clear metabolic or biochemical signs of diabetes in mice that were followed for 6 weeks [46]. These results suggest that blocking glucagon action in insulin-deficient diabetics will lead to an asymptomatic, benign, non-catabolic state.
Lack of Glucagon Signal Alters Hepatic Energy State
Based on the findings that Gcgr−/− mice have an attenuated energy response to fasting and lacked an exercise-induced energy change (hepatic AMP/ATP ratios) when compared to littermate controls, it has been hypothesized that glucagon signalling plays a major role in controlling hepatic energy state [47]. To test this hypothesis experiments were designed using conscious mouse models that display increased glucagon levels under stressful conditions (prolonged fasting, hypoglycaemia, diabetes, exercise, high-fat diet) [47]. In these models, PEPCK expression was found to be essential to reduce the hepatic energy changes during fasting. Similarly, increased glucagon levels decreased the hepatic energy state under euglycaemic conditions and resulted in increased AMPK activation [47]. These results suggest that Gcgr activation stimulates gluconeogenic fluxes through PEPCK which lead to a low hepatic energy state that is sufficient to activate AMPK.
Lack of Glucagon Signal Alters Hepatocyte Survival
The role of the Gcgr in hepatocyte and cell survival was studied in Gcgr−/− mice in vivo (susceptibility to hepatocyte apoptosis by Jo2 injection or diets deficient in methionine and choline) and in vitro (murine hepatocyte cell culture) [48]. Lack of glucagon signalling increased the susceptibility to experimental hepatocellular injury. Injured hepatocytes displayed signs of apoptosis, such as blebbing, chromatin condensation and cell lysis. These morphological alterations were accompanied by an increase of serum levels of transaminases. When the Gcgr−/− mice were challenged with a methionine and choline-deficient diet the liver developed morphological changes characteristic of non-alcoholic steatohaepatitis. The cytoprotective effects of glucagon were independent of the activation of protein kinase A (PKA), phosphatidylinositol-3 kinase (PI3K) and MAPK, but appeared dependent upon cAMP-mediated activation of adenylate cyclase [48]. These data suggest that glucagon signalling may have a critical role in the regulation of hepatocyte cell survival and challenges the idea of modulating glucagon action in patients with type 2 diabetes mellitus as a treatment option.
Lack of Glucagon Signal Alters the Metabolic Response to Fasting and Exercise
To determine the role Gcgr signalling in lipid metabolism (synthesis, secretion and oxidation) during short and long-term (5 or 16 h) fasting, Gcgr−/− mice were studied in vivo (hepatic TG secretion) and in vitro (lipid synthesis, secretion and fatty acid oxidation in liver homogenates and in primary murine hepatocyte culture) [24]. After a prolonged fast, the lack of Gcgr signalling is associated with an increase in free fatty acid (FFA) and TG secretion independent of insulin and glucose levels and a decrease in FFA oxidation. Glucagon was shown to have no effect in the synthesis of newly synthesized FFA, but it significantly reduced TG synthesis by increasing FFA oxidation, and secretionas a result of a decreased availability of FFA and TG. Glucagon stimulates FFA oxidation by activating PPARα in an AMPK- and p38 MAPK-dependent pathway [24]. To further understand the role of Gcgr signalling in the regulation of PPARα and its subsequent effects on fibroblast growth factor 21 (FGF21), the hypothesis that the glucagon effects on AMPK and PPARα signalling are amplified by an increased availability of lipids was tested [15]. Gcgr−/− mice that underwent an 18-h fast, exhaustive treadmill exercise, conditions that have previously shown to increase glucagon action [49,50]; and a hyperglucagonaemic–euglycemic clamp with or without a simultaneous lipid infusion [15] were evaluated. Gcgr activation stimulated the expression of PPARα and FGF21, which was further amplified by an elevation in FFAs. These data suggest that glucagon, in addition to playing a major role in the regulation of glucose metabolism in the fasted state (glycogenolysis and gluconeogenesis), plays a central role in fatty acid oxidization during prolonged fasting and in response to exercise.
Studies Using the RiP-Gcgr Transgenic Mice have Revealed Important Insights into the Role of the Gcgr in β-Cell Function
To study the functional role of Gcgr in β-cell function transgenic mice were engineered that over-express the Gcgr in insulin cells using rat insulin II promoter (RiP-Gcgr) [51]. To examine whether the RiP-Gcgr transgene increased glucagon-stimulated insulin release, islets were isolated from RiP-Gcgr mice and incubated in glucose in the presence or absence of glucagon concentrations that were subthreshold for insulin secretion in control islets. RiP-Gcgr islets displayed a significant increase in insulin secretion in response to glucagon compared to that of controls suggesting that the regulatory role of glucagon on insulin release may be associated with the number of glucagon binding sites on insulin cells. Furthermore, RiP-Gcgr mice displayed enhanced glucose tolerance compared to controls. Morphological studies of pancreata did not indicate any gross differences in the islet morphology of RiP-Gcgr mice when compared to controls. However, quantification of the islet cell population by stereology indicated a small, but significant increase (approximately 20%) in insulin cell volume compared to littermate controls. Given the enhanced glucose homeostasis displayed by RiP-Gcgr mice, it was hypothesized that insulin cell overexpression of the Gcgr may protect against the induction of diabetes when the mice were fed a highfat diet. When challenged with insulin, both RiP-Gcgr and littermate controls fed the high-fat diet displayed impaired glucose tolerance compared to low-fat-fed control and RiP-Gcgr mice. However, pancreatic islet overexpression of Gcgr resulted in a significant decrease in the glucose excursion in RiP-Gcgr mice fed the high-fat diet compared to controls fed the same diet [51]. These data suggest that increased glucagon action at the level of the insulin cell, similar to GLP-1 and GIP both in regulating insulin cell mass and in potentiating glucose-stimulated insulin secretion, by increasing insulin cell competency. These data were strengthened by the findings of low levels of Pdx-1, GLUT2 and MafA, which are molecules involved in the regulation of insulin expression, in insulin cells of Gcgr−/− mice [26,35-39].
Conclusion
It has been postulated that in patients with diabetes, excess glucagon secretion plays a primary role in the metabolic perturbations associated with diabetes, such as hyperglycaemia and ketonuria [52]. Here we present evidence that glucagon signalling plays important roles in pancreatic development, insulin cell function and metabolic response to prolonged fasting, exercise, lipid metabolism, hepatic energy state and hepatocyte survival. Thus, the present findings further suggest that antagonizing glucagon action as a possible therapy for diabetes may have several unintended effects that may further compromise the regulatory response to an altered glucose metabolic state such as that seen in patients with diabetes.
Acknowledgements
We thank Drs R. Gelling, R. Burcelin, S. Ouhilal, all our collaborators and members of Charron and Vuguin laboratories who have contributed to this project over the years. This work was supported by the National Institutes of Health (Grants DK47425 and HL58119 to M. J. C. and KO8 HD-042172 to P. V.), the American Diabetes Association, Albert Einstein College of Medicine (AECOM) Comprehensive Cancer Center, the AECOM Diabetes Center and Danish Medical Research Council.
Footnotes
Conflict of Interests
The authors declare no conflicts of interests.
References
- 1.Burcelin R, Katz EB, Charron MJ. Molecular and cellular aspects of the glucagon receptor: role in diabetes and metabolism. Diabetes Metab. 1996;22:373–396. [PubMed] [Google Scholar]
- 2.Lefebvre PJ. Glucagon and its family revisited. Diabetes Care. 1995;18:715–730. doi: 10.2337/diacare.18.5.715. [DOI] [PubMed] [Google Scholar]
- 3.Rouille Y, Westermark G, Martin SK, Steiner DF. Proglucagon is processed to glucagon by prohormone convertase PC2 in alpha TC1-6 cells. Proc Natl Acad Sci USA. 1994;8:3242–3246. doi: 10.1073/pnas.91.8.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawamori D, Kurpad AJ, Hu J, et al. [Google Scholar]
- 5.Ravier MA, Rutter GA. Glucose or insulin, but not zinc ions, inhibit glucagon secretion from mouse pancreatic alpha-cells. Diabetes. 2005;6:1789–1797. doi: 10.2337/diabetes.54.6.1789. [DOI] [PubMed] [Google Scholar]
- 6.Zhou H, Zhang T, Harmon JS, Bryan J, Robertson RP. Zinc, not insulin, regulates the rat alpha-cell response to hypoglycemia in vivo. Diabetes. 2007;4:1107–1112. doi: 10.2337/db06-1454. [DOI] [PubMed] [Google Scholar]
- 7.Hauge-Evans AC, King AJ, Carmignac D, et al. Somatostatin secreted by islet delta-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes. 2009;2:403–411. doi: 10.2337/db08-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boushey RP, Abadir A, Flamez D, et al. Hypoglycemia, defective islet glucagon secretion, but normal islet mass in mice with a disruption of the gastrin gene. Gastroenterology. 2003;4:1164–1174. doi: 10.1016/s0016-5085(03)01195-8. [DOI] [PubMed] [Google Scholar]
- 9.Salehi A, Vieira E, Gylfe E. Paradoxical stimulation of glucagon secretion by high glucose concentrations. Diabetes. 2006;8:2318–2323. doi: 10.2337/db06-0080. [DOI] [PubMed] [Google Scholar]
- 10.Yanaihara C, Matsumoto T, Kadowaki M, Iguchi K, Yanaihara N. Rat pancreas contains the proglucagon(64-69) fragment and arginine stimulates its release. FEBS Lett. 1985;2:307–310. doi: 10.1016/0014-5793(85)81265-5. [DOI] [PubMed] [Google Scholar]
- 11.de Heer J, Rasmussen C, Coy DH, Holst JJ. Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, inhibits glucagon secretion via somatostatin (receptor subtype 2) in the perfused rat pancreas. Diabetologia. 2008;12:2263–2270. doi: 10.1007/s00125-008-1149-y. [DOI] [PubMed] [Google Scholar]
- 12.Hoy M, Olsen HL, Bokvist K, et al. Tolbutamide stimulates exocytosis of glucagon by inhibition of a mitochondrial-like ATP-sensitive K+ (KATP) conductance in rat pancreatic A-cells. J Physiol. 2000;527:109–120. doi: 10.1111/j.1469-7793.2000.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burcelin R, Li J, Charron MJ. Cloning and sequence analysis of the murine glucagon receptor-encoding gene. Gene. 1995;164:305–310. doi: 10.1016/0378-1119(95)00472-i. [DOI] [PubMed] [Google Scholar]
- 14.Jiang Y, Cypess AM, Muse ED, et al. Glucagon receptor activates extracellular signal-regulated protein kinase 1/2 via cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 2001;98:10102–10107. doi: 10.1073/pnas.131200398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berglund ED, Kang L, Lee-Young RS, et al. Glucagon and lipid interactions in the regulation of hepatic AMPK signaling and expression of PPARalpha and FGF21 transcripts in vivo. Am J Physiol Endocrinol Metab. 2010;4:E607–E614. doi: 10.1152/ajpendo.00263.2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev. 1999;6:876–913. doi: 10.1210/edrv.20.6.0385. [DOI] [PubMed] [Google Scholar]
- 17.Kedees MH, Grigoryan M, Guz Y, Teitelman G. Differential expression of glucagon and glucagon-like peptide 1 receptors in mouse pancreatic alpha and beta cells in two models of alpha cell hyperplasia. Mol Cell Endocrinol. 2009;311:69–76. doi: 10.1016/j.mce.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abrahamsen N, Lundgren K, Nishimura E. Regulation of glucagon receptor mRNA in cultured primary rat hepatocytes by glucose and cAMP. J Biol Chem. 1995;26:15853–15857. doi: 10.1074/jbc.270.26.15853. [DOI] [PubMed] [Google Scholar]
- 19.Pospisilik JA, Hinke SA, Pederson RA, et al. Metabolism of glucagon by dipeptidyl peptidase IV (CD26) Regul Pept. 2001;3:133–141. doi: 10.1016/s0167-0115(00)00170-1. [DOI] [PubMed] [Google Scholar]
- 20.Hupe-Sodmann K, McGregor GP, Bridenbaugh R, et al. Characterisation of the processing by human neutral endopeptidase 24.11 of GLP-1(7-36) amide and comparison of the substrate specificity of the enzyme for other glucagon-like peptides. Regul Pept. 1995;3:149–156. doi: 10.1016/0167-0115(95)00063-h. [DOI] [PubMed] [Google Scholar]
- 21.Kahn CR. Elliot Proctor Joslin. In: Wilkins L-W, editor. Joslin’s Diabetes Mellitus. 14th edn. Williams & Wilkins; MA, USA: 2005. p. 183. [Google Scholar]
- 22.Porte D, Jr, Seeley RJ, Woods SC, Baskin DG, Figlewicz DP, Schwartz MW. Obesity, diabetes and the central nervous system. Diabetologia. 1998;41:863–881. doi: 10.1007/s001250051002. [DOI] [PubMed] [Google Scholar]
- 23.Eaton RP. Hypolipemic action of glucagon in experimental endogenous lipemia in the rat. J Lipid Res. 1973;3:312–318. [PubMed] [Google Scholar]
- 24.Longuet C, Sinclair EM, Maida A, et al. The glucagon receptor is required for the adaptive metabolic response to fasting. Cell Metab. 2008;5:359–571. doi: 10.1016/j.cmet.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gelling RW, Du XQ, Dichmann DS, et al. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. PNAS USA. 2003;3:1438–1443. doi: 10.1073/pnas.0237106100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vuguin PM, Kedees MH, Cui L, et al. Ablation of the glucagon receptor gene increases fetal lethality and produces alterations in islet development and maturation. Endocrinology. 2006;9:3995–4006. doi: 10.1210/en.2005-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pictet R, Rutter WJ. Development of the embryonic pancreas. In: Steiner DF, Frenkel N, editors. Handbook of Physiology. American Physiological Society; Washington, DC: 1972. pp. 25–66. [Google Scholar]
- 28.Ohtsubo K, Takamatsu S, Minowa MT, Yoshida A, Takeuchi M, Marth JD. Dietary and genetic control of glucose transporter 2 glycosylation promotes insulin secretion in suppressing diabetes. Cell. 2005;7:1307–1321. doi: 10.1016/j.cell.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Maechler P, Ritz-Laser B, et al. Pdx1 level defines pancreatic gene expression pattern and cell lineage differentiation. J Biol Chem. 2001;27:25279–25286. doi: 10.1074/jbc.M101233200. [DOI] [PubMed] [Google Scholar]
- 30.Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. Beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 1998;12:1763–1768. doi: 10.1101/gad.12.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Itkin-Ansari P, Demeterco C, Bossie S, et al. PDX-1 and cell-cell contact act in synergy to promote delta-cell development in a human pancreatic endocrine precursor cell line. Mol Endocrinol. 2000;6:814–822. doi: 10.1210/mend.14.6.0476. [DOI] [PubMed] [Google Scholar]
- 32.Pictet RL, Rall LB, Phelps P, Rutter WJ. The neural crest and the origin of the insulin-producing and other gastrointestinal hormone producing cells. Science. 1976;4223:191–192. doi: 10.1126/science.1108195. [DOI] [PubMed] [Google Scholar]
- 33.Pictet RL, Rutter W. Development of the embryonic pancreas. In: Steiner DF, editor. Handbook of Physiology, Section 7: Endocrinology. Vol. 1. American Physiological Society; Washington, DC: 1972. pp. 25–66. [Google Scholar]
- 34.Serafimidis I, Rakatzi I, Episkopou V, Gouti M, Gavalas A. Novel effectors of directed and Ngn3-mediated differentiation of mouse embryonic stem cells into endocrine pancreas progenitors. Stem Cells. 2008;1:3–16. doi: 10.1634/stemcells.2007-0194. [DOI] [PubMed] [Google Scholar]
- 35.Stein R. Insulin gene transcription: the factors involved in cell-type-specific and glucose-regulated expression in islet β cells are also essential during pancreatic development. In: Jefferson LS, editor. Handbook of Physiology, Section 7. The Endocrine System II. American Physiology Society; Washington, DC: 2001. pp. 25–78. [Google Scholar]
- 36.Matsuoka T, Artner I, Henderson E, Means A, Sander M, Stein R. The MafA transcription factor appears to be responsible for tissue-specific expression of insulin. Proc Natl Acad Sci USA. 2004;101:2930–2933. doi: 10.1073/pnas.0306233101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuoka T, Zhao L, Artner I, et al. Members of the large Maf transcription family regulate insulin gene transcription in islet β cells. Mol Cell Biol. 2003;23:6049–6062. doi: 10.1128/MCB.23.17.6049-6062.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kataoka K, Han SI, Shioda S, Hirai M, Nishizawa M, Handa H. MAfA is a glucose-regulated and pancreatic beta-cell-specific transcriptional activator for the insulin gene. J Biol Chem. 2002;51:49903–49910. doi: 10.1074/jbc.M206796200. [DOI] [PubMed] [Google Scholar]
- 39.Guillam MT, Hummler E, Schaerer E, et al. Early diabetes and abnormal postnatal pancreatic islet development in mice lacking Glut-2. Nat Genet. 1997;17:327–330. doi: 10.1038/ng1197-327. [DOI] [PubMed] [Google Scholar]
- 40.Kedees MH, Guz Y, Vuguin PM, et al. Nestin expression in pancreatic endocrine and exocrine cells of mice lacking glucagon signaling. Dev Dyn. 2007;4:1126–1133. doi: 10.1002/dvdy.21112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Conarello SL, Jiang G, Mu J, et al. Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia. Diabetologia. 2007;1:142–150. doi: 10.1007/s00125-006-0481-3. [DOI] [PubMed] [Google Scholar]
- 42.Hayashi Y, Yamamoto M, Mizoguchi H, et al. Mice deficient for glucagon gene-derived peptides display normoglycemia and hyperplasia of islet { alpha}-cells but not of intestinal L-cells. Mol Endocrinol. 2009;12:1990–1999. doi: 10.1210/me.2009-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naslund E, Bogefors J, Skogar S, et al. GLP-1 slows solid gastric emptying and inhibits insulin, glucagon, and PYY release in humans. Am J Physiol. 1999;3:R910–R916. doi: 10.1152/ajpregu.1999.277.3.R910. [DOI] [PubMed] [Google Scholar]
- 44.Nauck MA, Niedereichholz U, Ettler R, et al. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol. 1997;5:E981–E988. doi: 10.1152/ajpendo.1997.273.5.E981. [DOI] [PubMed] [Google Scholar]
- 45.Umino Y, Everhart D, Solessio E, et al. Hypoglycemia leads to age-related loss of vision. Proc Natl Acad Sci USA. 2006;51:19541–19545. doi: 10.1073/pnas.0604478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee Y, Wang MY, Du XQ, Charron MJ, Unger RH. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes. 2011;2:391–397. doi: 10.2337/db10-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berglund ED, Lee-Young RS, Lustig DG, et al. Hepatic energy state is regulated by glucagon receptor signaling in mice. J Clin Invest. 2009;8:2412–2422. doi: 10.1172/JCI38650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sinclair EM, Yusta B, Streutker C, et al. Glucagon receptor signaling is essential for control of murine hepatocyte survival. Gastroenterology. 2008;6:2096–2106. doi: 10.1053/j.gastro.2008.07.075. [DOI] [PubMed] [Google Scholar]
- 49.Marliss EB, Aoki TT, Unger RH, Soeldner JS, Cahill GF., Jr. Glucagon levels and metabolic effects in fasting man. J Clin Invest. 1970;12:2256–2270. doi: 10.1172/JCI106445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wasserman DH, Spalding JA, Lacy DB, Colburn CA, Goldstein RE, Cherrington AD. Glucagon is a primary controller of hepatic glycogenolysis and gluconeogenesis during muscular work. Am J Physiol. 1989;1:E108–E117. doi: 10.1152/ajpendo.1989.257.1.E108. [DOI] [PubMed] [Google Scholar]
- 51.Gelling RW, Vuguin PM, Du XQ, et al. Pancreatic beta-cell overexpression of the glucagon receptor gene results in enhanced beta-cell function and mass. Am J Physiol Endocrinol Metab. 2009;3:E695–E707. doi: 10.1152/ajpendo.00082.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fanelli CG, Porcellati F, Rossetti P, Bolli GB. Glucagon: the effects of its excess and deficiency on insulin action. Nutr Metab Cardiovasc Dis. 2006;16(Suppl. 1):S28–S34. doi: 10.1016/j.numecd.2005.10.018. [DOI] [PubMed] [Google Scholar]