Abstract
The behavioral effects of a selective A3 adenosine receptor agonist 3-IB-MECA (N6-(3-iodobenzyl)-5′-N-methylcarboxamidoadenosine) in mice and the localization of radioligand binding sites in mouse brain were examined. Low levels of A3 adenosine receptors were detected in various regions of the mouse brain (hippocampus, cortex, cerebellum, striatum), using a radioiodinated, high-affinity Aragonist radioligand [125I]AB-MECA (N6-(3-iodo-4-aminobenzyl)-5′-N-methylcarboxamidoadenosine). Scatchard analysis in the cerebellum showed that the Kd value for binding to A3 receptors was 1.39 ± 0.04 nM with a Bmax of 14.8 ± 2.1 fmol/mg protein. 3-IB-MECA at 0.1 mg/kg i.p. was a locomotor depressant with> 50% reduction in activity. Although selective A1 or A2a antagonists reversed locomotor depression elicited by selective A1 or A2a agonists, respectively, the behavioral depressant effects of 3-IB-MECA were unaffected. 3-IB-MECA also caused scratching in mice, which was prevented by coadministration of the histamine antagonist cyproheptadine. The demonstration of a marked behavioral effect of A3 receptor activation suggests that the A3 receptor represents a potential new therapeutic target.
Keywords: Adenosine receptor, Xanthine, Locomotor activity, Histamine, Radioligand binding
1. INTRODUCTION
The centrally-mediated locomotor depressant effects of selective A1 and A2a receptor agonists and their reversal by xanthine antagonists have been well documented [1–3,15]. Activation of both A1 and A2a receptors, by the A1-selective agonist CHA (N6-cyclohexyladenosine) and the A2a-selective agonist APEC (2-[(2-aminoethyl-amino)carbonylethylphenylethylamino]-5′-N -ethylcarboxamidoadenosine), results in synergistic locomotor depression [3]. Similarly, although non-selective xanthines, such as caffeine and theophylline, cause stimulation oflocomotor activity [1,5], A1- or A2a-selective xanthines, such as CPX (1,3-dipropyl-8-cyclopentylxanthine) and CSC (8-(3-chlorostyryl)caffeine), respectively, do not elevate locomotor activity except when coadministered [6]. Therefore, the functional roles of A1 and A2a receptors in the CNS appear to be interrelated. A1 receptors occur throughout the brain, and activation of presynaptic A1 receptors results in decreased release of many stimulatory neurotransmitters (see [7]). In contrast, A2a adenosine receptors occur mainly in the striatum and are closely associated with D2-dopamine receptors [7]. Activation of A2a receptors inhibits a dopaminergic pathway in the striatum.
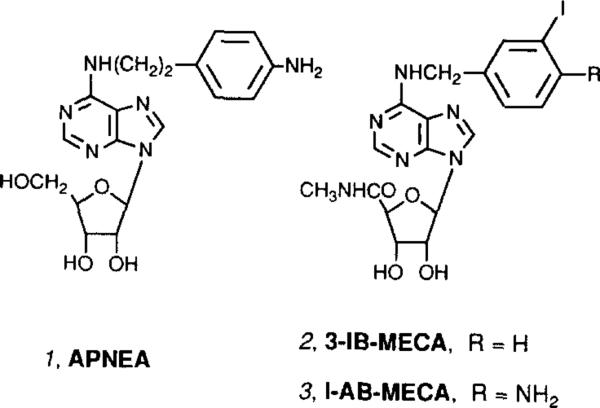
Recently, a third class of adenosine receptors (A3) has been cloned from rat brain [8]. A3 receptors appear responsible for well-documented stimulatory effects of adenosine agonists on release of inflammatory mediators from mast cells [12,20]. As with A1 receptors, cyclic AMP formation is inhibited by A3 receptor activation [8]. Phospholipid turnover is also stimulated by A3 receptor activation [9]. Unlike A1 and A2a receptors, most alkylxanthines are ineffective as competitors in binding experiments at rat A3 receptors [8,10] or as antagonists in functional assays [8,9]. The cardiovascular (hypotensive) effects of A3 receptor stimulation have been surmised indirectly [13], through coadministration of a non-selective agonist, APNEA (1, Fig. 1), and an antagonist that blocks action only at A1 and A2a receptors.
Fig. 1.
Structures of adenosine agonists that have been used to characterize A3 receptors.
We recently introduced a class of highly selective adenosine A3 agonists [11], including 3-IB-MECA (N6-(3-iodobenzyl)-5′ -N-methylcarboxamidoadenosine, 2, Fig. 1). A closely related iodinated radioligand, [125I]AB-MECA [9], 3, was found to bind to rat brain A3 receptors expressed in stably-transfected CHO cells with a Kd of 1 nM. However, this compound also binds to A1 receptors in mouse and bovine brain. The in vivo effects of selective A3 agonists have not been characterized. Since stimulation of A1 and A2a receptors produces demonstrable behavioral effects, the impact of acute administration of the A3 agonist 3-IB-MECA on locomotor activity in mice was investigated in this study. The presence of A3 receptors in regions of the mouse brain was also demonstrated using radioligand binding.
2. EXPERIMENTAL
2.1. Agents
3-IB-MECA and CSC were synthesized as described [6,11]. CPA and CPX were obtained from Research Biochemicals International (Natick, MA). The preparation and characterization of 1251-labeled AB-MECA (N6-(4-aminobenzyl)-5′-N-methylcarboxamidoadenosine) will be described elsewhere [9].
2.2. Locomotor activity
Adult male mice (NIH Swiss strain, 25–30 g) were housed in groups of 10 animals per cage with a light–dark cycle of 12:12 h. The animals were given free access to standard pellet food and water and were acclimatized to laboratory conditions for 24 h prior to testing. Each animal was used only once in the activity monitor.
Locomotor activity of individual animals was studied in an open field using a Digiscan activity monitor (Omnitech Electronics Inc., Columbus, OH) equipped with an IBM-compatible computer. The computer-tabulated measurements represent multivariate locomotor analysis with specific measures, such as simultaneous measurements of ambulatory, rearing, sterotypical, and rotational behaviors. Data was collected in the morning, for three consecutive intervals of 10 min each, and analyzed as a group. Statistical analysis was performed using Student's t-test. The results are reported as mean ± S.E.M. for each point. All drugs were dissolved in a vehicle consisting of a 20:80 v/v mixture of Alkamuls EL-620 (Rhone-Poulenc, Cranbury, NJ) and phosphate-buffered saline, except for CSC, which was dissolved initially in DMSO and diluted in at least 20 volumes of vehicle. Drugs were administered i.p. in a volume corresponding to 5 mllkg body weight. Where applicable, the antagonist was injected 10 min before the agonist. After injection of agonist, the mouse was placed in the activity monitor for 5 min before data collection was begun.
2.3. Membrane preparation
Twenty white male mice (NIH Swiss strain, 25–30 g) were killed by cervical fracture. Brains were rapidly removed, placed in ice-cold 50 mM Tris-HCl buffer (pH 7.4), and cortex, cerebellum, hippocampus, brain stem and striatum were dissected. Tissue was homogenized in 20 ml of ice-cold 50 mM Tris-HCl buffer (pH 7.4) using a Polytron (setting no. 6) for 10 s. The homogenates were centrifuged at 35,000 × g for 15 min at 4°c. The pellets then were resuspended in fresh volume of the same buffer, homogenized with the Polytron and re-centrifuged. The final pellet was stored at −70°C before use in the receptor binding assay. For the binding assay membranes were diluted at a protein concentration of 1–3 mg/ml. Protein concentrations were determined by the BCA protein assay reagents (Pierce Chemical Co., Rockford, IL) using bovine albumin as a standard.
2.4. Radioligand binding
[125I]AB-MECA binding to A3 adenosine receptor in mouse brain membranes was performed in 50 mM Tris, 10 mM MgC12, 1 mM EDTA buffer (pH 7.4) containing 100 μl membrane suspension with adenosine deaminase (3 units/ml) added. Where applicable, the A1 component of binding was eliminated by the addition of 100 nM CPX to the medium. The final concentration of [125I]AB-MECA ranged from 0.1 to 4 nM, while the final volume of the preparation was 0.5 ml. Incubations were carried out in duplicate for 90 min at 25°C. Nonspecific binding was defined in the presence of 40 μM R-PIA (N6-R-phenylisopropyladenosine) and constituted approximately 30% of the total binding. Binding reactions were terminated by filtration through Whatman GFIB filters using a Brandel M24R cell harvester (Brandel Gaithersburg, MD). Filters were washed three times with 3 ml ice-cold buffer and placed in vials. Radioactivity was determined in a Beckman 5500B gamma-counter.
2.5. Analysis of data
Linear fitting of Scatchard plots and the saturation experiments, analyzed by nonlinear regression using computer program GraphPad lnPlot (Version 4.0, San Diego, CA), gave similar results for determination of Kd and Bmax values. Each experimental result is reported as mean ± S.E.M. from three or four experiments.
3. RESULTS
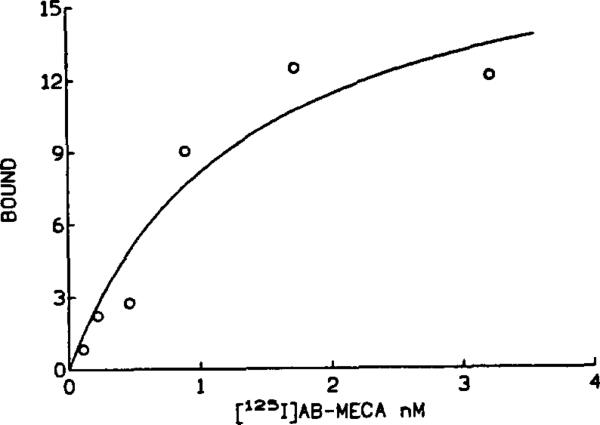
The high affinity radioligand, [125I]AB-MECA [12], bound specifically to both A1 and A3 adenosine receptors in membranes prepared from regions of NIH Swiss mouse brains. Specific binding represented ca. 70% of total binding, with Bmax values (expressed as fmol specifically bound/mg protein in parentheses): hippocampus (215 ± 17), cortex (159 ± 15), cerebellum (120 ± 5), and striatum (123 ± 23). Scatchard analysis indicated Kd values ranging from 1.9 to 2.8 nM. The A2a selective antagonist 8-(3-chlorostyryl)caffeine (CSC) failed to displace specific binding of [125I]AB-MECA. The specific binding consisted of two components, since the A1 selective antagonist 1 ,3-dipropyl-8-cyclopentylxanthine (CPX) completely displaced the most but not all of the binding with high affinity (sigmoidal competition curves had K1 values ranging from 2 to 8 nM). Thus, the majority of the specific binding of [125I]AB-MECA was to high-affinity A1 receptors. The residual binding, wich was not displaced by CPX at concentrations as high as 1 μM represented binding to A3 adenosine receptors [9]. Low levels of A3 adenosine receptors (18% of total specific binding using 0.4 nM (125I]AB-MECA in the presence of 100 nM CPX) were detected in the cerebellum and striatum, with even lower levels in the hippocampus and cortex (7–9% of total specific binding). A Scatchard analysis showed that in the presence of 100 nM CPX the Kd for binding of [125I]AB-MECA to cerebellar A3 receptors was 1.39 ± 0.04 nM with a Bmax of 14.8 ± 2.1 fmoVmg protein (n = 3) (Fig. 2).
Fig. 2.
A representative saturation curve for binding at A3 receptors in mouse cerebellar membranes using [125l]AB-MECA in the presence of 100 nM CPX. Conditions are described in section 2 (specific binding shown). The Kd value for binding to A3 receptors was 1.39 ± 0.04 nM with a Bmax of 14.8 ± 2.1 fmol/mg protein.
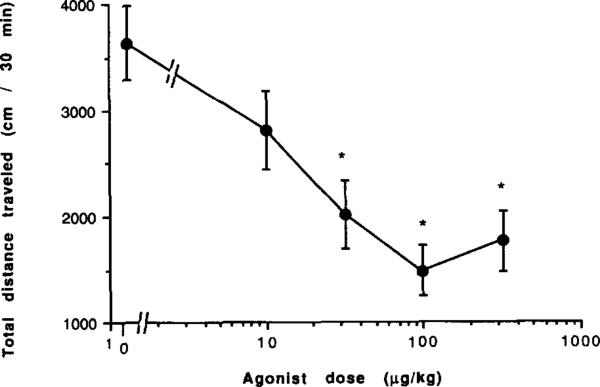
A potent (Ki 1.1 ± 0.3 nM at rat brain A3 receptors) and selective (50-fold less potent in binding to either A1 or A2a rat brain receptors) A3 agonist [11], 3-IB-MECA (2, Fig. 1), was selected for in vivo studies. The locomotor effects in mice of 3-IB-MECA alone or in combination with potent and selective A1 and A2a receptor antagonists were examined. 3-IB-MECA administered i.p. in a dose range of 0.01 to 0.3 mg/kg was found to be locomotor depressant (total distance travelled) with a threshold dose of 0.01 mg/kg and maximal depression reached at ~0.1 mg/kg (Fig. 3). Unlike depression elicited by selective A1 and A2a agonists, the depression did not exceed 90% at the higher doses, but reached a plateau at ca. 60% below control level (Fig. 3). Vertical activity, stereotypy counts, and rotational movement were depressed in a dose dependent manner following administration of 3-IB-MECA, however average distance per move and average speed were not changed significantly.
Fig. 3.
Locomotor activity in male NIH Swiss mice. Effect of the A3-selective adenosine agonist 3-IB-MECA. *P value is < 0.05 vs. vehicle control (n = 6–19).
Admmrstration of 3-IB-MECA also caused rapid scratching behavior, whose frequency appeared to increase with the dose of the A3 agonist. Since activation of A3 receptors causes release of histamine in cultured mast cells, it was proposed that the scratching could be related to histamine. Coadministration of a histamine H1-antagonist, cyproheptadine at 10 mg/kg i.p. eliminated this behavior, while at 1 mg/kg dose of cyproheptadme the effect was only partial.
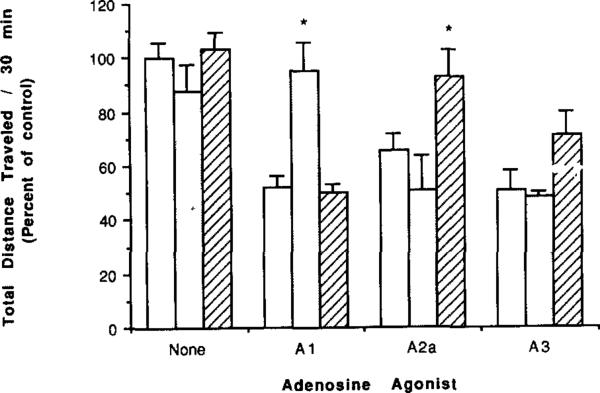
The highly A1-selective antagonist CPX (0.25 mg/kg) completely reversed locomotor depression elicited by the potent A1 agonist CPA at its determined ED50 value of 100 μg/kg i.p. (Fig. 4). CPX did not diminish the depressant effects of either the A2a-selective agonist APEC or 3-IB-MECA at doses chosen to cause comparable ca. 50% reduction in locomotor activity.
Fig. 4.
Locomotor activity in mice following injection of an A1- (CPA, 100 μg/kg), A2a- (APEC, 16 μg/kg), or A3- (3-IB-MECA, 100 μg/kg) selective agonist and the effects of coadministration of selective antagonists (n = 6–19). Locomotor activity as a percent of control is shown for no antagonist (unshaded bars) or for coadministration of selective xanthine antagonists: CPX (shaded bars, AI, 0.25 mg/kg) or CSC (hatched bars, A2a, 1.0 mg/kg). * P value < 0.05 vs. adenosine agonist alone.
The xanthine antagonist CSC is highly A2a-selective in binding assays, functional adenylate cyclase assays, and in vivo with respect to locomotor activity [6]. A dose of CSC of 1 mg/kg caused a small, statistically insignificant reversal of the 3-IB-MECA-mediated locomotor depression (Fig. 4). Previously it was shown that the same dose of CSC caused a complete reversal of the behavioral depression elicited by APEC in the same experimental model [6].
4. DISCUSSION
A3 receptors were found to be present in the mouse brain, with highest density in the cerebellum and striatum. Thus, the striatum, which is critical to the locomotor depressant effects of A2a agonists [21], contains A3 receptors at a density at least 10-fold lower than A2a receptors. Since previously reported levels of mRNA coding for A3 receptors in rat brain indicated the greatest density in hippocampus and cerebellum [14], there is either a species difference between rat and mouse, or the level of message is not entirely predictive of the level of receptor density. A1 receptors are localized in high density in the hippocampus and appear to be involved in the cerebroprotective effects of adenosine [16]. A3 receptors were also detected in the hippocampus but at densities 1–2 orders of magnitude lower than A1 receptors.
The non-reversal oflocomotor depression by the A1-and A2a-adenosine antagonists is consistent with in vitro observations with rat A3-receptors, i.e. the inability of xanthmes to antagonize at this site [8, 10]. Both CPX and CSC are known to act centrally [6]. The lack of a selective A3 antagonist, to act peripherally or centrally, is a disadvantage in this study. Previous studies of locomotor depression by A1 and A2a-adenosine agonists [3] have utilized both centrally-active xanthines and other xanthines, such as 8-sulfophenyltheophylline, that do not penetrate the blood brain barrier, to demonstrate the central nature of the behavioral effects.
There is considerable interest in the therapeutic potential of selective A1-adenosine agonists and antagomsts to treat neurological and other CNS disorders. The cognition-enhancing [16, 17], cerebroprotective [18], and anti-convulsant [19] effects of selective A1-adenosine agents have frequently been demonstrated. A2a-antagomsts may be useful in treating Parkinson's disease [7]. The demonstration of a marked behavioral effect of A3 receptor activation suggests that the A3 receptor may also represent a unique therapeutic target.
Acknowledgements
The support for D.S. from the International Life Sciences Institute and for C. G.-R. from the Cystic Fibrosis Foundation is gratefully acknowledged.
REFERENCES
- 1.Snyder SH, Katims JJ, Annau Z, Bruns RF, Daly JW. Proc. Natl. Acad. Sci. USA. 1981;78:3260–3264. doi: 10.1073/pnas.78.5.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barraco RA, Aggarwal AK, Phillis JW, Moron MA, Wu PH. Neurosci. Lett. 1984;48:139–144. doi: 10.1016/0304-3940(84)90009-0. [DOI] [PubMed] [Google Scholar]
- 3.Nikodijević O, Sarges R, Daly JW, Jacobson KA. J. Pharmacol. Exp. Ther. 1991;259:286–294. [PMC free article] [PubMed] [Google Scholar]
- 4.van Galen PJM, Stiles GL, Michaels G, Jacobson KA. Medicinal Res. Rev. 1992;12:423–471. doi: 10.1002/med.2610120502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi OH, Shamim MT, Padgett WL, Daly JW. Life Sci. 1988;43:387–98. doi: 10.1016/0024-3205(88)90517-6. [DOI] [PubMed] [Google Scholar]
- 6.Jacobson KA, Nikodijević O, Padgett W, Gallo-Rodriguez C, Maillard M, Daly JW. FEBS Lett. 1993;323:141–144. doi: 10.1016/0014-5793(93)81466-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferre S, Fuxe K, von Euler G, Johansson B, Fredholm BB. Neuroscience. 1992;51:501–512. doi: 10.1016/0306-4522(92)90291-9. [DOI] [PubMed] [Google Scholar]
- 8.Zhou QY, Li CY, Olah ME, Johnson RA, Stiles GL, Civelli O. Proc. Nat!. Acad. Sci. USA. 1992;89:7432–7436. doi: 10.1073/pnas.89.16.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olah ME, Galla-Rodriguez C, Jacobson KA, Stiles GL. 125I-4-Aminobenzyl-5′-N-methylcarboxamidoadenosine, a high affinity radioligand for the rat A3 adenosine receptor. Mol. Pharmacal. 1994;45:978–982. [PMC free article] [PubMed] [Google Scholar]
- 10.van Bergen A, van Galen PJM, Stiles GL, Jacobson KA. ACS 206th National Meeting; Chicago, IL. Aug., 1993; 1993. Abstract MEDI217. [Google Scholar]
- 11.Gallo-Rodriguez C, Ji X-D, Melman N, Siegman BD, Sanders LH, Orlina J, Fischer B, Pu Q-L, Olah ME, van Galen PJM, Stiles GL, Jacobson KA. Structure-activity relationships of N6-benzyladenosine-5′-uronamides as A3-selective adenosine agonists. J. Med. Chem. 1994;37:636–646. doi: 10.1021/jm00031a014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramkumar V, Stiles GL, Beaven MA, Ali H. J. Bioi. Chern. 1993;268:16887–16890. [PubMed] [Google Scholar]
- 13.Fozard JR, Carruthers AM. Br. J. Pharmacol. 1993;109:3–5. doi: 10.1111/j.1476-5381.1993.tb13522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De M, Austin KF, Dudley MW. Differential distribution of A3 receptor in rat brain. Soc. for Neuroscience; Washington, DC: 1993. Abstr. 42.11. [Google Scholar]
- 15.Daly JW. In: Caffeine, Coffee, and Health. Garattini S, editor. Raven Press; New York: 1993. pp. 97–150. [Google Scholar]
- 16.Schingnitz G, Küfner-Mühl U, Ensinger H, Lehr E, Kuhn FJ. Nucleosides Nucleotides. 1991;10:1067–1076. [Google Scholar]
- 17.von Lubitz DKJE, Paul IA, Bartus RT, Jacobson KA. Eur. J. Pharmacol. 1993 doi: 10.1016/0014-2999(93)90522-j. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Lubitz DKJE, Dambrosia JM, Kempski O, Redmond DJ. Stroke. 1988;19:1133–1139. doi: 10.1161/01.str.19.9.1133. [DOI] [PubMed] [Google Scholar]
- 19.von Lubitz DKJE, Paul IA, Carter M, Ji X.-d., Jacobson KA. Eur. J. Pharmacol. 1993 doi: 10.1016/0014-2999(94)90762-5. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali H, Cunha-Melo JR, Saul WF, Beaven M. J. Bioi. Chern. 1990;265:745–753. [PubMed] [Google Scholar]
- 21.Nikodijević O, Daly JW, Jacobson KA. FEBS Lett. 1990;261:67–70. doi: 10.1016/0014-5793(90)80638-y. [DOI] [PMC free article] [PubMed] [Google Scholar]