Abstract
Background
Hepatoblastoma is a malignancy of young children. Low birth weight is associated with significantly increased risk of hepatoblastoma and neonatal medical exposures are hypothesized as contributors. This study represents the largest case–control study of hepatoblastoma to date and aimed to define the role of neonatal exposures in hepatoblastoma risk among low birth weight children.
Procedure
Incident hepatoblastoma cases who were born <2,500 g (N = 60), diagnosed between 2000 and 2008, were identified through the Children's Oncology Group. Controls were recruited through state birth registries (N = 51). Neonatal medical exposures were abstracted from medical records. Subjects from the Vermont Oxford Network were used for further comparisons, as were existing reports on neonatal medical exposures.
Results
Case–control comparisons were hindered by poor matching within birth weight strata. Cases were smaller and received more aggressive neonatal treatment compared to controls, and reflected high correlation levels between birth weight and treatments. Similar difficulty was encountered when comparing cases to Vermont Oxford Network subjects; cases were smaller and required more aggressive neonatal therapy. Furthermore, it appears hepatoblastoma cases were exposed to a greater number of diagnostic X-rays than in case series previously reported in the neonatal literature.
Conclusions
This study presents the largest case series of hepatoblastoma in <2,500 g birth weight infants with accompanying neonatal medical exposure data. Findings confirm that birth weight is highly correlated with exposure intensity, and neonatal exposures are themselves highly correlated, which hampers the identification of a causal exposure among hepatoblastoma cases. Experimental models or genetic susceptibility testing may be more revealing of etiology.
Keywords: case–control study, exposure, hepatoblastoma, low birth weight, NICU
INTRODUCTION
Hepatoblastoma is a rare embryonal tumor of early childhood, comprising approximately 1% of malignant neoplasms among children less than 20 years of age. The incidence rates of 8.7 and 5.1 cases per 1,000,000 children less than 1 year and 1–4 years of age, respectively, within the US population during 2005–2009 represents a twofold increased incidence since 1975 [1,2]. Inherited syndromes, such as Beckwith–Wiedemann, Familial Adenomatous Polyposis and Trisomy 18 (Edwards Syndrome) raise the risk of hepatoblastoma but account for only a small proportion of total cases [2,3]. Because hepatoblastoma is a malignancy of very young children and its moderately differentiated histology suggests developmental origins, special attention has been given to gestational and birth characteristics. The most striking and consistent association has been a strong inverse relationship between birth weight and hepatoblastoma risk [4–13]. In a large population-based US study, the relative risk of hepatoblastoma was increased in a dose-dependent fashion, based on birth weight classification: (1.56 [95% confidence interval (CI): 0.81–2.98], 3.37 [95% CI 1.44–7.88], and 17.18 [95% CI 7.46–39.54]) among birth weight categories <2,500 g, 1,500–2,500 g, and <1,500 g, respectively, compared to children >2,500 g at birth [11]. Similar findings have been reported in Japan, China, the United Kingdom, and the Nordic countries [5,9,12,13]. The association with low birth weight appears to be independent of gestational age, based on previous analysis [11]. Furthermore, the incidence of hepatoblastoma has increased in parallel with survival rates of children born <2,500 g, particularly those with birth weight <1,500 g [14,15].
We and others have hypothesized that medical exposures in the neonatal period may contribute to the association between hepatoblastoma and low birth weight [6–8,10,11,16–19]. The majority of infants <2,500 g birth weight, particularly those <1,500 g, will require high-level neonatal care, typically including admission to the neonatal intensive care unit (NICU) [20]. Infants are exposed to multiple potential environmental agents during NICU admission, including radiation, transfusions, erythropoietin, total parenteral nutrition (TPN) and intralipids, as well as multiple medications [21], many of which have potential mutagenic properties. Additionally, infants are exposed to high levels of the plasticizer di-(2-ethylhexyl)phthalate (DEHP), which is commonly used in medical devices and tubing and is a rodent hepatocarcinogen [19]. Previous case series and case–control studies of infants <1,500 g birth weight with hepatoblastoma, consisting of 5–12 cases, have investigated the role of NICU exposures in tumor development and have suggested associations with duration of oxygen exposure and furosemide therapy [17,18]. The goal of the present study was to better elucidate the role of neonatal treatment in the risk of hepatoblastoma among low birth weight children in the largest case–control study of hepatoblastoma to date.
METHODS
Cases were defined as incident hepatoblastomas, with pathologically confirmed disease, diagnosed between 0 and 6 years of age at United States Children's Oncology Group (COG) institutions between January 2000 and December 2008. Eligible cases were identified, both retrospectively and prospectively, through the COG central database beginning in 2005. Following local institutional review board approval, treating physicians were asked for permission to contact patients’ birth mothers. Once permission was received, patient families were traced and contacted, and consent was obtained for telephone interview and medical record release for children whose mothers reported birth weights <2,500 g at enrollment [22,23].
Controls were randomly selected from rosters of state birth registries provided by 32 states. Eligibility criteria for controls included birth year between 1994 and 2008 and English or Spanish language fluency. States were grouped into six regions, with each region containing states that did and did not participate in control selection [24]. The participating states represent approximately 75% of the US population and non-participating states are not geographically clustered, so this group was felt to be representative of the overall US population. Controls were frequency matched to cases within two strata of birth weight (<1,500 g and 1,500–2,500 g), year of birth, sex, and birth region. Methods for contacting controls have been previously described [22,23].
There were 771 hepatoblastoma cases diagnosed between 0 and 6 years between 2000 and 2008 registered with COG. After receiving IRB approval and permission from treating physicians, letters were sent to 416 families without knowledge of birth weight. From this group, 408 consented for participation, and 87 of these families reported children as weighing <2,500 g at birth. Eighty hepatoblastoma case families completed the initial telephone interview and we received medical record release forms for 64 of these children.
Contact letters were mailed to mothers of 565 eligible controls identified from participating states. From this group, 155 consented to study participation, 147 mothers completed a telephone interview and 60 consented to release of medical records.
Medical records were requested for any case or control infant whose birth weight was reported by mothers at the time of enrollment as <2,500 g and for whom medical record release was obtained. Requested data included: all delivery records for the mother, all physician orders for medications, oxygen, transfusions, X-rays, phototherapy, TPN, and procedures while in the NICU or nursery, all flow sheets, including length, weight, and head circumference for each day in the NICU or nursery, and discharge summaries. Data were abstracted by a single neonatal nurse practitioner into a custom Filemaker database with blinding to case or control status. Abstracted data included birth date, time of birth, birth weight, birth length, gestational age, admission date and time, reasons for admission, discharge date and time, evidence of, and initiation and stop dates for: TPN, respiratory support modality, antibiotic(s), phototherapy, breast feeding, formula feeding, antenatal steroid exposure and postnatal steroid exposure, retinopathy of prematurity exam comments, radiographic data (imaging modality, body site imaged, dates), and transfusion data (product type, number of units, dates). Data for up to 180 days of NICU admission were abstracted.
Of the 64 cases for whom medical records were requested, 62 were obtained and 2 hospitals were not able to provide medical records. Sixty of these children had confirmed <2,500 g birth weight after review of their records; the two excluded cases had birth weights of 2,570 and 2,817 g. Of the 16 cases who were interviewed but did not consent to medical records, reported birth weights ranged from 652 to 2,495 g. Of the 60 potential <2,500 g birth weight controls, 57 records were received and for the 3 that were not, 1 infant died within an hour of birth, 1 mother did not provide the name of a treating institution and 1 hospital no longer had the records. Fifty-five of the control children had confirmed <2,500 g birth weight after medical record review and 51 survived to at least 180 days of age. Thus, our final analysis included 60 cases and 51 controls.
Descriptive statistics, including odds ratios and 95% CIs, were computed for cases and controls for all exposure data. Fisher's exact test was used to calculate P-values for categorical variables and two-sided t-tests were used for exposure variables. Additionally, in order to assess for differences in rates of intrauterine growth restriction between cases and controls, Z-scores of birth weight were calculated using the 2003 Fenton preterm infant growth chart; mean Z-scores for cases and controls were compared using a two-sided t-test [25].
We also compared our cases to national surveillance data, including birth weight statistics from the National Center for Health Statistics [26] and birth weight and exposure data from the Vermont Oxford Network [27]. The Vermont Oxford Network (VON) Expanded Database maintains surveillance data on infants weighing less than 2,500 g at birth, born between 2001 and 2008, from 279 participating U.S. NICUs [27]. VON data are primarily collected and utilized for quality improvement initiatives in NICU care. Exposure data from the VON data set that are directly comparable to data we collected included antenatal steroid exposure, exposure to any mechanical ventilation, and length of NICU stay. We compared cases to the overall VON cohort and also looked within narrow birth weight strata (<750, 751–1,000 g, 1,001–1,250 g, 1,251– 1,500 g, 1,501–2,000 g, 2,001–2,499 g). Descriptive comparisons and univariate analysis including chi-square tests of independence were performed to examine the relation between the VON Expanded Database subjects and our cases with specific exposures.
Finally, since the VON database does not collect data on radiographic imaging or TPN, we examined published neonatal literature in order to make general comparisons with our case data. PubMed search terms for radiographic imaging included combinations of the following keywords: radiograph, exposure, low birth weight, and neonate. Search terms for TPN included: TPN, low birth weight, and neonate.
Institutional Review Board approval was obtained from the University of Minnesota, as well as participating COG institutions and health departments for states providing birth registry information. Informed consent was obtained from all participants prior to study participation.
RESULTS
Case and control characteristics are presented in Table I. Initial comparison of cases and controls revealed that cases were more likely to be born at a younger gestational age, to have lower birth weight and lower birth length, and to have longer NICU stays, as compared to controls. Notably, birth weight Z-scores did not differ significantly between cases (mean = −0.62, standard deviation (SD) = 1.14) and controls (mean = −0.56, SD = 0.95), t(109) = 0.31, P = 0.75. Examined exposures included respiratory support (including mechanical ventilator, continuous positive airway pressure and nasal cannula), antibiotics, TPN, and X-rays. Cases had significantly longer exposure to respiratory support, more exposure to antibiotics and more radiographic exams, as compared to controls. While a number of significant differences in exposures exist between cases and controls, this likely represents the lower birth weights among cases and the relative mismatch in size and gestational age between cases and controls. Despite achieving similar proportions of cases and controls within the previously defined birth weight strata at the time of subject recruitment, we found that because of the broad range of birth weights within each weight classification, cases and controls were poorly matched within strata, especially after including only those where medical record release was authorized by the mother. Given that the amount of neonatal treatment is highly correlated with birth weight [28], we noted strong associations of all neonatal treatments with case status.
TABLE I.
Characteristics and Exposure Data of <2,500 g Birth Weight Hepatoblastoma Cases (N = 60) and Controls (N = 51), Based on Medical Record Abstraction Data
| N (%) |
||||||
|---|---|---|---|---|---|---|
| Controls (All) | Cases (All) | Cases, <750g | Cases, 750–999 g | Cases, 1,000–1,499g | Cases, 1,500–2,500 g | |
| Count | 51 | 60 | 19 | 12 | 15 | 14 |
| Multiple gestation | ||||||
| No | 39 (76) | 46 (77) | 18 (95) | 6 (50) | 11 (73) | 11 (79) |
| Yes | 12 (24) | 14 (23) | 1 (5) | 6 (50) | 4 (27) | 3 (21) |
| Birth year | ||||||
| <2000 | 10 (20) | 10 (17) | 2 (10.5) | 2 (17) | 2 (13) | 4 (29) |
| 2000–2005 | 41 (80) | 29 (48) | 15 (79) | 7 (58) | 10 (67) | 7 (50) |
| >2005 | 0 | 21 (35) | 2 (10.5) | 3 (25) | 3 (20) | 3 (21) |
| Gestational age (weeks) | ||||||
| Mean (SE) | 32.5 (0.5) | 28.8 (0.5) | 26.3 (0.5) | 27.1 (0.7) | 28.9 (0.4) | 33.6 (1) |
| Median | 33 | 28 | 26 | 26.5 | 29 | 33.5 |
| Range | 24–40 | 23–40 | 23–30 | 24–31 | 26–32 | 29–40 |
| Birth weight (g) | ||||||
| Mean (SE) | 1,773 (74) | 1,147.9 (72.2) | 632.7 (20.2) | 886.3 (21) | 1,198.4 (34) | 2,017.4 (91.7) |
| Median | 1,873 | 992.5 | 645 | 867.5 | 1,163 | 1,953 |
| Range | 640–2,499 | 453–2,495 | 453–749 | 760–995 | 1,031–1,440 | 1,525–2,495 |
| Birth length (cm) | ||||||
| Mean (SE) | 41.1 (0.9) | 36.3 (0.7) | 30.8 (0.5) | 33.6 (0.5) | 37.8 (0.7) | 43.8 (0.9) |
| Median | 42 | 35.5 | 30.5 | 34.5 | 38 | 44.8 |
| Range | 30.5–48 | 28–48 | 28–35.6 | 31–35.6 | 30.5–42.5 | 37–48 |
| Days in NICU | ||||||
| Mean (SE) | 33.1 (4.2) | 92.8 (9.6) | 131.8 (16.1) | 105.5 (13.1) | 79.4 (15.9) | 46.3 (22) |
| Median | 25 | 86 | 117 | 106 | 69 | 22.5 |
| Range | 1–135 | 2–352 | 44–352 | 51–186 | 16–217 | 2–325 |
| Total days of O2 | ||||||
| Mean (SE) | 16.4 (4) | 78.8 (8) | 111.6 (10.7) | 92.4 (16.3) | 51.7 (13.6) | 34.9 (19) |
| Median | 8 | 77 | 109 | 93 | 31.5 | 12 |
| Range | 0–127 | 0–180 | 40–180 | 4–180 | 0–180 | 2–180 |
| Days on TPN | ||||||
| Mean (SE) | 15.4 (3.8) | 27.4 (4.4) | 40.3 (10.6) | 33.9 (7.5) | 21.6 (6.9) | 8 (2.1) |
| Median | 8.5 | 21 | 32 | 27 | 16 | 6.5 |
| Range | 0–74 | 0–144 | 2–144 | 0–85 | 4–83 | 1–16 |
| Total days of antibiotics | ||||||
| Mean (SE) | 6.6 (1.4) | 29.1 (3.8) | 40.8 (5.9) | 27.2 (7.2) | 24.8 (9.4) | 17.1 (6.3) |
| Median | 3 | 19 | 44 | 18 | 9 | 5 |
| Range | 1–54 | 0–116 | 5–85 | 4–81 | 0–116 | 2–63 |
| Total X-rays (all sites) | ||||||
| Mean (SE) | 9.8 (1.6) | 43.8 (6.4) | 68.6 (13.8) | 48.1 (12.9) | 30.3 (8.2) | 14.6 (7.9) |
| Median | 7 | 23 | 56 | 33 | 15 | 5 |
| Range | 1–45 | 1–215 | 5–215 | 3–138 | 2–92 | 1–92 |
| Total chest X-rays | ||||||
| Mean (SE) | 7.3 (1.2) | 32.5 (4.9) | 52.2 (10.6) | 36.6 (10) | 20.2 (5.4) | 10.8 (6.8) |
| Median | 4 | 18 | 44 | 26 | 8 | 4 |
| Range | 1–41 | 0–192 | 4–192 | 2–94 | 1–53 | 0–78 |
| Total abdominal X-rays | ||||||
| Mean (SE) | 2.4 (0.6) | 11 (2) | 16.2 (4.7) | 11.4 (3.5) | 9.4 (3.2) | 3.6 (1.8) |
| Median | 1 | 6 | 11 | 8 | 5 | 1 |
| Range | 0–18 | 0–86 | 0–86 | 0–44 | 0–45 | 0–16 |
In an effort to examine better-matched subgroups, analyses comparing cases and controls weighing <1,500 g, as well as those >1,000 g, were performed. When only cases and controls with birth weights <1,500 g were considered (controls, n = 20, cases, n = 46), the mean birth weight among cases was significantly less than among controls, and thus, gestational age at birth was less, duration of NICU stay was longer, and cumulative exposure to oxygen, antibiotics and X-rays was greater; again, only TPN exposure was not different. When considering birth weights >1,000 g (controls, n = 49, cases, n = 29), the mean birth weights were not significantly different (control mean = 1,816 g, case mean = 1,594 g, P = 0.06); however, the cases were born at lower gestational age (P = 0.02), required longer NICU admissions (P = 0.03), and had greater total exposure to oxygen (P = 0.007), antibiotics (P = 0.01), and X-rays (P = 0.02). Cumulative TPN exposure was not different (P = 0.52).
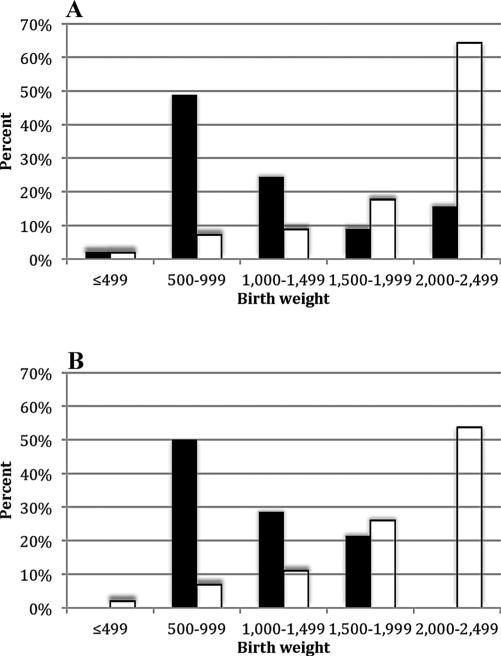
In order to better understand how the weight distribution of the cases in our study compared to the distribution of LBW infants in the general population, we accessed birth data from the National Center for Health Statistics [26] and graphed birth weights of cases and weighted averages of birth weights among low birth weight infants during the years 1996–2008, separated by plurality (Fig. 1). For both the single and multiple gestation infants, our cases were much smaller than the population infants, with the highest percent of single and multiple gestation cases falling within the 500–999 g category; whereas, for the population, the highest percent of infants were within the 2,000–2,499 g category.
Fig. 1.
Birth weight distribution for <2,500 g birth weight hepatoblastoma cases with medical record release versus US births <2,500 g [26]. A: Single gestation births. B: Multiple gestation births. Legend: ■, case; □, population.
We next compared our hepatoblastoma cases to the VON Expanded Database; abstracted variables that matched the Database included “any ventilation” and “antenatal steroids.” Any ventilation included exposure to either conventional ventilation or high frequency ventilation following initial resuscitation, and antenatal steroid exposure included corticosteroids administered to the mother at any time prior to delivery. The relation between antenatal steroid exposure and case status, as well as the relation between any ventilation and case status, were both significant when combining all weight strata (χ2AS (1, N = 208,971) = 8.54, P <2vent (1, N = 206,130) = 43.82, P < 0.0005). Comparisons within weight strata were also attempted, but results are limited based on very small numbers of cases in each category and a very large number of VON subjects. Overall, cases were more likely to have received antenatal steroids and were more likely to have been exposed to some mechanical ventilation as compared to the VON population. The weight distribution of the VON Expanded Database subjects shows that within both the single and multiple gestation categories, >60% of infants had birth weight >1,500 g (mean birth weight ± standard error (SE) = 1,679.5 ± 1.2 g); whereas, <25% of cases within either single or multiple gestation categories fell into those birth weight strata (1,148 ± 72 g). Additionally, the mean total length of stay for VON Expanded Database infants was 33.8 ± 0.08 days, as compared to average length of stay of 92.8 ± 9.6 days for our cases. Similar to comparison with the initial control group, these findings are likely attributable to the fact that the hepatoblastoma cases are overall smaller and sicker compared to the VON Expanded Database cohort.
Lastly, we searched the available neonatal literature to find exposure data for both diagnostic radiographs and TPN. There is a small body of literature on diagnostic X-ray exposure in neonates [29–32]; relevant data from available studies are presented in Table II. Birth weights of our cases were comparable with those of previous case series; however, the mean number of diagnostic images was greater among our cases compared to previous reports (despite only having X-ray data recorded through the first 180 days of NICU admission for our cases, and even when compared to a study with similar mean length of stay [29]). These studies were conducted in a variety of sites (US, UK, France, and Germany) over a broad period of time, which may reflect regional differences in standard practice or changing trends in utilization. Thus we are unable to make direct comparisons of our data with that previously reported. Relevant data regarding TPN exposure in neonates was also identified [33–36] and is presented in Table III. Of available studies reporting duration of parenteral nutrition exposure, they typically involve interventions to supplement TPN formulations with additional nutrients or to standardize the approach to nutrition in low birth weight infants; for the purpose of comparison to our cases, we utilized data from control or standard care infants within these studies. Infants from these studies had similar birth weights to our cases and TPN exposure was variable across studies, with some showing very similar exposure to our cases [33,34] and some with shorter duration of exposure [35,36]. Similar to the diagnostic imaging data, these similarities and differences may reflect differences in practice across sites or may be reflective of the influence of the interventional trials they were a part of; whatever the case, our low birth weight hepatoblastoma cases appear to fall within the typical range for TPN exposure in the low birth weight neonatal population.
TABLE II.
Diagnostic X-Ray Exposure in Neonates; <2,500 g Birth Weight Hepatoblastoma Cases and Published Literature
| Author | Year | N | Mean birth weight, g (range) | Median birth weight, g | Mean length of stay, day (range) | Median length of stay, day | Mean number of images (range) | Median number of images |
|---|---|---|---|---|---|---|---|---|
| This study | 2013 | 60 | 1,148 (453–2,495) | 992.5 | 92.8 (2–352) | 86 | 43.8 (1–215) | 23 |
| Wilson-Costello et al. [29] | 1996 | 25 | 671 (490–745) | NA | 115 (77–175) | NA | 30.8 (12–59) | NA |
| Sutton et al. [30] | 1998 | 55 | 1,110 (540–1,480) | NA | 60 (4–239) | NA | 9.1 (0–54) | 5 |
| Donadieu et al. [31] | 2006 | 450 | NA (520–2,760) | 1,250 | NA (1–246) | 16 | NA (0–95) | 10.6 |
| Puch-Kapst et al. [32] | 2009 | 212 | NA (445–1,500) | 1,100 | NA (2–291) | 56 | NA (0–62) | 4 |
TABLE III.
TPN Exposure in Neonates; <2,500 g Birth Weight Hepatoblastoma Cases and Published Literature
| Author | Year | N | Included birth weights (g) | Mean birth weight (SE) (g) | Mean days on TPN (SE) |
|---|---|---|---|---|---|
| Current study | 2013 | 60 | <2,500 | 1,148 (72) | 27.4 (4.4) |
| Poindexter et al. [33] | 2004 | 712 | 401–1,000 | 768 (5.2) | 29.8 (0.8) |
| Trintis et al. [34] | 2010 | 88 | < 1,500 | 1,013 (33) | 26.8 (2.5) |
| Wang et al. [35] | 2010 | 15 | < 1,500 | 1,260 (40) | 17.1 (4.4) |
| Butler et al. [36] | 2013 | 69 | < 1,500 | NA | 13 (NA) |
DISCUSSION
This study was initiated to determine whether exposures in the neonatal period influenced risk of developing hepatoblastoma in early childhood. Hepatoblastoma is a very rare disease, and while the rate among low birth weight infants is disproportionately high the absolute number of cases among infants with birth weight <2,500 g is small. Based on previous national estimates, there are approximately 100 new hepatoblastoma cases diagnosed per year in the United States [37], and, of those, we would anticipate approximately 7% to be birth weight 1,500–2,500 g and 15% <1,500 g [11]; this implies there were approximately 800 eligible cases during 2000–2008 and approximately 56 of those were 1,500– 2,500 g and 120 were <1,500 g. Thus, this report includes about one third of <2,500 g birth weight hepatoblastoma cases diagnosed in 2000–2008. In addition to small sample size, our control series was imperfectly matched by birth weight. We note that even had we recruited all <2,500 g birth weight hepatoblastoma cases during the study period and perfectly matched controls on birth weight, the high correlation of NICU treatment and small absolute sample size would be a challenge to the identification a causative exposure in multivariate analysis.
In an effort to better match cases and controls and to fully appreciate possible causative exposures, subgroup analyses were performed on two separate weight groups (<1,500 and >1,000 g). In the <1,500 g group, cases remained smaller than controls, and exposure differences were possibly attributable to the high levels of correlation between birth weight and NICU treatment intensity. Interestingly, when birth weight >1,000 g was considered and the mean birth weights of cases and controls were similar, exposure to oxygen, antibiotics and X-rays remained higher among cases. While this does not point to a single causative exposure, it raises concern that either something is intrinsically different about these children, causing them to be sicker at birth, or that the cumulative impact of the multiple aggressive therapies is increasing their risk for subsequent hepatoblastoma; however, given that this observation was limited to a single subgroup, this is purely speculative. Although these children do appear to be sicker than their control group counterparts, it is notable that their mean age at diagnosis of hepatoblastoma was 1.8 years (median 1.3 years), and is not significantly different from children with >2,500 g birth weight, suggesting that although neonatal exposures or underlying biology may be different in these children, it does not appear to change the timing of tumor development.
As with other malignancies diagnosed in early childhood, the precise timing of tumor development is not clear, and in many cases tumorigenesis may occur in utero. Unfortunately, it is not possible to determine which cases within our series may have been truly congenital in nature. The ages at diagnosis for this case series ranged from 3 weeks to 5.1 years. We acknowledge, as an additional limitation to our case series, that congenital cases would not be impacted by neonatal exposures and their inclusion within this case series may dilute potential effects of neonatal exposure on non-congenital cases; however, based on the available data, it is not possible to separate out congenital from non-congenital cases.
Consequently, we have presented these data primarily as the largest series of <2,500 g birth weight hepatoblastoma cases with detailed accompanying data on neonatal exposures for future reference. Based on the best comparable exposures and length of stay, our data suggest that these children are overall sicker during the neonatal period, as compared to the general low birth weight neonatal population. A single causative exposure is possible, as are multiple neonatal exposures working in combination. Given the difficulty of isolating one or more medical exposures, it would be worthwhile to investigate whether low birth weight hepatoblastoma cases have an underlying genetic susceptibility, and whether use of an animal model might be possible to better understand this relationship.
This study presents the largest case series of hepatoblastoma in children with birth weight <2,500 g, with data on several important NICU exposures. Low birth weight hepatoblastoma cases in this study were smaller, requiring longer NICU admission and more intensive medical interventions, compared both to controls, as well as to the comparable variables in the VON Expanded Database. While it is possible that NICU environmental exposures in the neonatal period may be associated with increased risk of hepatic tumorigenesis, the high level of correlation, both between the exposures and birth weight, as well as with each other, make it difficult to draw conclusions. Future investigation on underlying genetic susceptibility and development of subsequent malignancy may contribute more to our understanding of hepatoblastoma in <2,500 g birth weight infants.
ACKNOWLEDGMENTS
This research was supported by NIH R01 CA111355, T32 CA099936, K05 CA157439, the Macy Easom Fund for Childhood Cancer Research, and the Children's Cancer Research Fund, Minneapolis, MN. The authors wish to thank the Vermont Oxford Network and its members for providing summary tables for the purposes of this publication.
Footnotes
Conflict of interest: Nothing to declare.
REFERENCES
- 1.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) National Cancer Institute; Bethesda, MD: [July 10, 2012]. http://seer.cancer.gov/csr/1975_2009_pops09/, based on November 2011 SEER data submission, posted to the SEER web site, April 2012. [Google Scholar]
- 2.Darbari A, Sabin KM, Shapiro CN, et al. Epidemiology of primary hepatic malignancies in U.S. children. Hepatology. 2003;38:560–566. doi: 10.1053/jhep.2003.50375. [DOI] [PubMed] [Google Scholar]
- 3.Spector LG, Birch J. The epidemiology of hepatoblastoma. Pediatr Blood Cancer. 2012;59:776–779. doi: 10.1002/pbc.24215. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin CC, Baptiste MS, Schymura MJ, et al. Maternal and infant birth characteristics and hepatoblastoma. Am J Epidemiol. 2006;163:818–828. doi: 10.1093/aje/kwj104. [DOI] [PubMed] [Google Scholar]
- 5.Ikeda H, Matsuyama S, Tanimura M. Association between hepatoblastoma and very low birth weight: A trend or a chance? J Pediatr. 1997;130:557–560. doi: 10.1016/s0022-3476(97)70239-7. [DOI] [PubMed] [Google Scholar]
- 6.Tanimura M, Matsui I, Abe J, et al. Increased risk of hepatoblastoma among immature children with a lower birth weight. Cancer Res. 1998;58:3032–3035. [PubMed] [Google Scholar]
- 7.Spector LG, Feusner JH, Ross JA. Hepatoblastoma and low birth weight. Pediatr Blood Cancer. 2004;43:706. doi: 10.1002/pbc.20122. [DOI] [PubMed] [Google Scholar]
- 8.Reynolds P, Urayama KY, Von Behren J, et al. Birth characteristics and hepatoblastoma risk in young children. Cancer. 2004;100:1070–1076. doi: 10.1002/cncr.20061. [DOI] [PubMed] [Google Scholar]
- 9.Ansell P, Mitchell CD, Roman E, et al. Relationships between perinatal and maternal characteristics and hepatoblastoma: A report from the UKCCS. Eur J Cancer. 2005;41:741–748. doi: 10.1016/j.ejca.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 10.Spector LG, Johnson KJ, Soler JT, et al. Perinatal risk factors for hepatoblastoma. Br J Cancer. 2008;98:1570–1573. doi: 10.1038/sj.bjc.6604335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spector LG, Puumala SE, Carozza SE, et al. Cancer risk among children with very low birth weights. Pediatrics. 2009;124:96–104. doi: 10.1542/peds.2008-3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pu CL, Guo CB, Jin XQ, et al. Retrospective analysis of maternal and infant birth features of hepatoblastoma patients. Zhonghua Gan Zang Bing Za Zhi. 2009;17:459–461. [PubMed] [Google Scholar]
- 13.de Fine Licht S, Schmidt LS, Rod NH, et al. Hepatoblastoma in the Nordic countries. Int J Cancer. 2012;131:E555–E561. doi: 10.1002/ijc.27351. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention Infant mortality and low birth weight among black and white infants: United States, 1980–2000. MMWR Morb Mortal Wkly Rep. 2002;51:589–592. [PubMed] [Google Scholar]
- 15.Wilson-Costello D, Friedman H, Minich N, et al. Improved survival rates with increased neuro-developmental disability for extremely low birth weight infants in the 1990s. Pediatrics. 2005;115:997–1003. doi: 10.1542/peds.2004-0221. [DOI] [PubMed] [Google Scholar]
- 16.Maruyama K, Ikeda H, Koizumi T, et al. Prenatal and postnatal histories of very low birthweight infants who developed hepatoblastoma. Pediatr Int. 1999;41:82–89. doi: 10.1046/j.1442-200x.1999.01022.x. [DOI] [PubMed] [Google Scholar]
- 17.Maruyama K, Ikeda H, Koizumi T, et al. Case-control study of perinatal factors and hepatoblastoma in children with an extremely low birthweight. Pediatr Int. 2000;42:492–498. doi: 10.1046/j.1442-200x.2000.01287.x. [DOI] [PubMed] [Google Scholar]
- 18.Oue T, Kubota A, Okuyama H, et al. Hepatoblastoma in children of extremely low birth weight: A report from a single perinatal center. J Pediatr Surg. 2003;38:134–137. doi: 10.1053/jpsu.2003.50027. [DOI] [PubMed] [Google Scholar]
- 19.Latini G, Gallo F, De Felice C. Birth characteristics and hepatoblastoma risk in young children. Cancer. 2004;101:210. doi: 10.1002/cncr.20357. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention Neonatal intensive-care unit admission of infants with very low birth weigh—19 states, 2006. MMWR Morb Mortal Wkly Rep. 2010;59:1444–1447. [PubMed] [Google Scholar]
- 21.Lai TT, Bearer CF. Iatrogenic environmental hazards in the neonatal intensive care unit. Clin Perinatol. 2008;35:163–181. doi: 10.1016/j.clp.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puumala SE, Ross JA, Feusner JH, et al. Parental infertility, infertility treatment and hepatoblastoma: A report from the Children's Oncology Group. Hum Reprod. 2012;27:1649–1656. doi: 10.1093/humrep/des109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Musselman JR, Georgieff MK, Ross JA, et al. Maternal pregnancy events and exposures and risk of hepatoblastoma: A Children's Oncology Group (COG) study. Cancer Epidemiol. 2013;37:318–320. doi: 10.1016/j.canep.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spector LG, Ross JA, Puumala SE, et al. Feasibility of nationwide birth registry control selection in the United States. Am J Epidemiol. 2007;166:852–856. doi: 10.1093/aje/kwm143. [DOI] [PubMed] [Google Scholar]
- 25.Fenton TR. A new growth chart for preterm babies: Babson and Benda's chart updated with recent data and a new format. BMC Pediatr. 2003;3:13. doi: 10.1186/1471-2431-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention National Center for Health Statistics. [June 24, 2012];VitalStats. http://www.cdc.gov/nchs/vitalstats.htm.
- 27.Horbar JD, Soll RF, Edwards WH. The Vermont Oxford Network: A community of practice. Clin Perinatol. 2010;37:29–47. doi: 10.1016/j.clp.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Gutbrod T, Wolke D, Soehne B, et al. Effects of gestation and birth weight on the growth and development of very low birthweight small for gestational age infants: A matched group comparison. Ach Dis Child Fetal Neonatal Ed. 2000;82:F208–F214. doi: 10.1136/fn.82.3.F208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson-Costello D, Rao PS, Morrison S, et al. Radiation exposure from diagnostic radiographs in extremely low birth weight infants. Pediatrics. 1996;97:369–374. [PubMed] [Google Scholar]
- 30.Sutton PM, Arthur RJ, Taylor C, et al. Ionising radiation from diagnostic x rays in very low birthweight babies. Arch Dis Child Fetal Neonatal Ed. 1998;78:F227–F229. doi: 10.1136/fn.78.3.f227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donadieu J, Zeghnoun A, Roudier C, et al. Cumulative effective doses delivered by radiographs to preterm infants in a neonatal intensive care unit. Pediatrics. 2006;117:882–888. doi: 10.1542/peds.2005-0817. [DOI] [PubMed] [Google Scholar]
- 32.Puch-Kapst K, Juran R, Phys D, et al. Radiation exposure in 212 very low and extremely low birth weight infants. Pediatrics. 2009;124:1556–1564. doi: 10.1542/peds.2008-1028. [DOI] [PubMed] [Google Scholar]
- 33.Poindexter BB, Ehrenkranz RA, Stoll BJ, et al. Parenteral glutamine supplementation does not reduce the risk of mortality or late-onset sepsis in extremely low birth weight infants. Pediatrics. 2004;113:1209–1215. doi: 10.1542/peds.113.5.1209. [DOI] [PubMed] [Google Scholar]
- 34.Trintis J, Donohue P, Aucott S. Outcomes of early parenteral nutrition for premature infants. J Perinatol. 2010;30:403–407. doi: 10.1038/jp.2009.173. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Tao YX, Cai W, et al. Protective effect of parenteral glutamine supplementation on hepatic function in very low birth weight infants. Clin Nutr. 2010;29:307–311. doi: 10.1016/j.clnu.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Butler TJ, Szekely LJ, Grow JL. A standardized nutrition approach for very low birth weight neonates improves outcomes, reduces cost and is not associated with increased rates of necrotizing enterocolitis, sepsis or mortality. J Perinatol. 2013;33:851–857. doi: 10.1038/jp.2013.66. [DOI] [PubMed] [Google Scholar]
- 37.Bulterys M, Goodman MT, Smith MA, et al. Hepatic tumors. In: Ries LA, Smith MA, Gurney JG, et al., editors. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995. National Cancer Institute, SEER Program; Bethesda, MD: 1999. NIH Pub. No. 99-4649. [Google Scholar]