Abstract
Cutaneous leishmaniases have persisted for centuries as chronically disfiguring parasitic infections affecting millions of people across the subtropics. Symptoms range from the more prevalent single, self-healing cutaneous lesion to a persistent, metastatic disease, where ulcerations and granulomatous nodules can affect multiple secondary sites of the skin and delicate facial mucosa, even sometimes diffusing throughout the cutaneous system as a papular rash. The basis for such diverse pathologies is multifactorial, ranging from parasite phylogeny to host immunocompetence and various environmental factors. Although complex, these pathologies often prey on weaknesses in the innate immune system and its pattern recognition receptors. This review explores the observed and potential associations among the multifactorial perpetrators of infectious metastasis and components of the innate immune system.
Keywords: cutaneous leishmaniasis, metastatic leishmaniasis, post-kala-azar dermal leishmaniasis, Leishmania RNA virus, pattern recognition receptor, Toll-like receptor
An ancient and emerging disease
Leishmaniases have persisted for centuries as life-threatening and disfiguring parasitic diseases affecting millions of people across the subtropics. Currently, 98 countries are listed as having endemic disease, amounting to an estimated 12 million cases with 2 million more each year [1]. Human disease is caused by sp. of Leishmania protozoan parasites and is cycled among hosts through the bite of a female sand fly vector. Symptoms range from single self-healing cutaneous lesions to fatal visceralization or chronic metastatic dissemination throughout the skin. However, despite its prevalence, persistence, and conspicuous symptoms, the disease remains largely uncontrolled, with few new treatment options and no comprehensively effective vaccine. Migration and densification of populations in subtropical regions are compounding with global warming and a growing HIV-positive (immunodeficient) demographic to class leishmaniasis as a serious emerging global threat [2]. Further, growing local and international instability has fuelled major outbreaks in new populations that spread quickly among the vulnerable of conflict zones, living in densely packed and poorly insulated shelters. These unsettled populations pose a risk of widening leishmanial geography during resettlement, as was the case after the Sudanese Civil War, the Gulf and Iraq wars, and currently among Syrian refugees [3,4].
The centuries of geographically isolated evolution have allowed each Leishmania spp. to develop intricate pathways of immune evasion, creating various symptomatic outcomes, and enabling parasites to persist under astounding immunological pressure, even existing as life-long infections after symptomatic resolution [5].
A common route of entry – widely different outcomes
Leishmania is generally transmitted through the bite of an infected sand fly. However, from this common origin, the same sp. can cause widely different outcomes. In most instances, disease is ‘asymptomatic’, without any obvious pathology, although still able to support life-long infection. The presence of persistent parasites in asymptomatic infections is a double-edged sword – on the one hand, potentially conferring immunity to superinfection, but on the other hand, creating the dangerous likelihood of reactivation, which is often associated with a more severe symptomatic outcome. In infections for which pathology is overt, outcomes can again vary widely. Localized cutaneous leishmaniasis (LCL) occurs in many cases, which can persist as chronic open lesions or resolve into hyperpigmented scars. For the more severe forms of leishmaniasis, pathology is not limited to the infection site but instead progresses in various ways that can be divided into metastatic leishmaniasis, diffuse CL (DCL) or a systemic visceralization (VL) that has an important cutaneous complication, post-kala-azar dermal leishmaniasis (PKDL). These forms can also appear following seemingly ‘asymptomatic’ infections without a prior cutaneous presentation.
Surprisingly, little is known about the basic mechanisms of symptomatic divergence. This review aims to assemble the current knowledge on the immunological, environmental, and phylogenetic perpetrators of persistent and metastatic outcomes, which significantly complicate the diagnosis, treatment, and control of leishmaniasis. We also use this opportunity to propose new potential risk factors that are supported by anecdotal evidence with the hope to stimulate much-needed further research.
Symptomatic outcomes of cutaneous leishmaniasis
Human infections are generally caused by species of two major Leishmania subgenera, namely Leishmania (Leishmania) and L. (Viannia). Although L. (Leishmania) is found worldwide, the majority of infections occur in the Paleotropics (Eurasia and Africa), where common infecting species are Leishmania major, Leishmania tropica, Leishmania aethiopica, and Leishmania donovani. Species of the Viannia subgenus, by contrast, are exclusively endemic in the Neotropics (the Americas), with common infections being caused by Leishmania braziliensis, Leishmania panamensis, and Leishmania guyanensis. Depending on the infecting species and the immune response in a susceptible host, Leishmania parasites can induce two major pathologies: VL or CL.
Although VL, or ‘kala-azar’ (see Glossary), is the most serious form of the disease, it is relatively rare, contributing to only 10% of all leishmaniasis worldwide. Leishmania parasites are mostly dermotropic, where even VL is often followed by the diffuse and difficult-to-treat cutaneous lesions of PKDL. This review focuses on the various forms of persistent CL.
Localized cutaneous leishmaniasis
CL is endemic in numerous regions of the subtropics. It is most frequently caused by L. major, L. tropica, and L. aethiopica in the Paleotropics, whereas in the Neotropics it is caused by Leishmania mexicana and Leishmania amazonensis, or L. (Viannia) braziliensis, L. (Viannia) panamensis, and L. (Viannia) guyanensis [6]. Although CL generally manifests as a lesion localized at the inoculation site (LCL), its various physical presentations and immunopathologies have complicated diagnosis and scuttled attempts of forming a universal therapeutic or vaccination strategy. Globally, lesions can vary from a small self-healing ulceration to granulomatous nodules and large, seeping, erythematous wounds. Chronic infection and inflammation can last for several months or years and often leads to significant tissue damage and permanent, hyperpigmented scarring. In certain cases, the infection metastasizes to sites beyond the inoculation and can be referred to as metastatic leishmaniasis, by analogy to tumor cell metastasis.
Metastatic leishmaniasis
Metastatic complications occur across all regions where leishmaniasis is endemic but are particularly prevalent and aggressive in L. (Viannia) infections of the Neotropics [7]. They may also present with various symptoms, seemingly dependent on differences in the immune response elicited by the various metastatic parasite species (Box 1). Particular symptomatic outcomes can be grouped geographically (Figure 1), where, for example, the Paleotropics hosts many forms of nonulcerative, papular, and herpetiform leishmaniasis spreading within a small radius of the primary lesion, whereas the Neotropics is better known for large ulcerative and granulomatous lesions, which often occur at sites distant from the primary lesion. Indeed, certain Neotropical parasites have a specific tropism for the delicate mucosal tissues of the nose and face, creating a particularly disfiguring disease known as mucosal leishmaniasis. The inflammation in the nasal mucosa is inordinately potent when contrasted with the sometimes undetectably low number of parasites. Lesion biopsies often reveal no infection. This phenomenon emphasizes the major role of the immune response in disease pathology and the potential of immunomodulatory agents in antileishmanial therapy. However, the immune involvement is diverse and opposing responses have been blamed for the various forms of infectious metastasis. For example, the mostly Paleotropical recidivans CL is described as a symptomatic reactivation of infection within the scars of a healed lesion; it is characterized by a potent cell mediated response in multiple nodules, which spread and coalesce to form significant tissue damage. Conversely, a total lack of a cell mediated response has been seen in DCL, where infection diffuses into hundreds of immunologically anergic nodules throughout the skin [8].
Box 1. Species-specific immunopathologies of metastatic leishmaniasis.
L. braziliensis
Mucosal leishmaniasis is most frequently observed with L. braziliensis, where metastasis occurs in 5–10% of CL patients. Although the manifestations of this disease can be complex, mucosal leishmaniasis generally metastasizes directly to the nasal mucosa with a limited number of secondary skin lesions. It most commonly affects the nasal septum, although infections have also been noted in the cartilaginous turbinates as well as the larynx. Mucosal lesional biopsies commonly reveal an unregulated hyperinflammatory response, where proinflammatory cytokines such as IFN-γ and TNF-α are produced without the dampening influence of IL-10 and transforming growth factor-β (TGF-β) [61,62]. Patients also produce significant quantities of inflammatory chemokines (CXCL10 and CCL4), resulting in the recruitment of intralesional CD8+ T cells [39]. Recently, the cytotoxic enzymes (granzyme B and perforin) produced by these intralesional CD8+ T cells were shown to mediate tissue damage in ulcerative CL [63,64]. However, it is also likely that tissue destructive enzymes such as matrix metalloproteases play important roles, although knowledge on their specific actions in metastatic leishmanial pathologies is sparse [65–67].
L. panamensis
More rarely, mucosal leishmaniasis can be caused by L. panamensis, a species that is endemic in western South America (Bolivia, Peru, and Colombia) and has also been found in Brazil. In Colombia, L. panamensis causes 55–80% of CL cases, 5% of which progress into mucosal leishmaniasis. Mucosal lesions due to L. panamensis have a tendency to be less destructive and less severe than those induced by L. braziliensis. Immunopathology in L. panamensis infection consists of a mixed Th1/Th2 immune response with high numbers of T regulatory cells [68]. Elements of this observation were recently replicated in a BALB/c murine model, which also revealed IL-13 and IL-4Ra as mediators of parasite persistence [69].
L. guyanensis
The clinical picture is slightly different for L. guyanensis, which is possibly the most prevalent parasite species in the Amazon basin. Although mucosal leishmaniasis has been described for L. guyanensis, the more common presentation is a large number of secondary lesions, which are nonulcerated, granulomatous, and display a weak response to treatment [70]. In general, L. guyanensis are less sensitive to antimonials than L. braziliensis [17] and parasites isolated from secondary lesions are more resistant to oxidative stress [15]. The intralesional immune response involves a strong role for T regulatory cells, which have been shown to underlie the chronicity of infection [71].
L. aethiopica
In the highlands of Ethiopia, there is a high incidence of CL caused by L. aethiopica. Generally, the cutaneous lesions are able to self-heal but persistent infections commonly metastasize to other parts of the body. Two major clinical presentations have been described: mucocutaneous leishmaniasis and DCL. DCL patients are poor responders to antimony treatment and are anergic to parasite antigens, whereas mucocutaneous patients develop chronic inflammation and are sensitively reactive to parasite antigens. These clinical presentations are independent of parasite genotype [72] but may still be dependent on extranuclear parasite factors such as LRV, which has recently been discovered in the region [26].
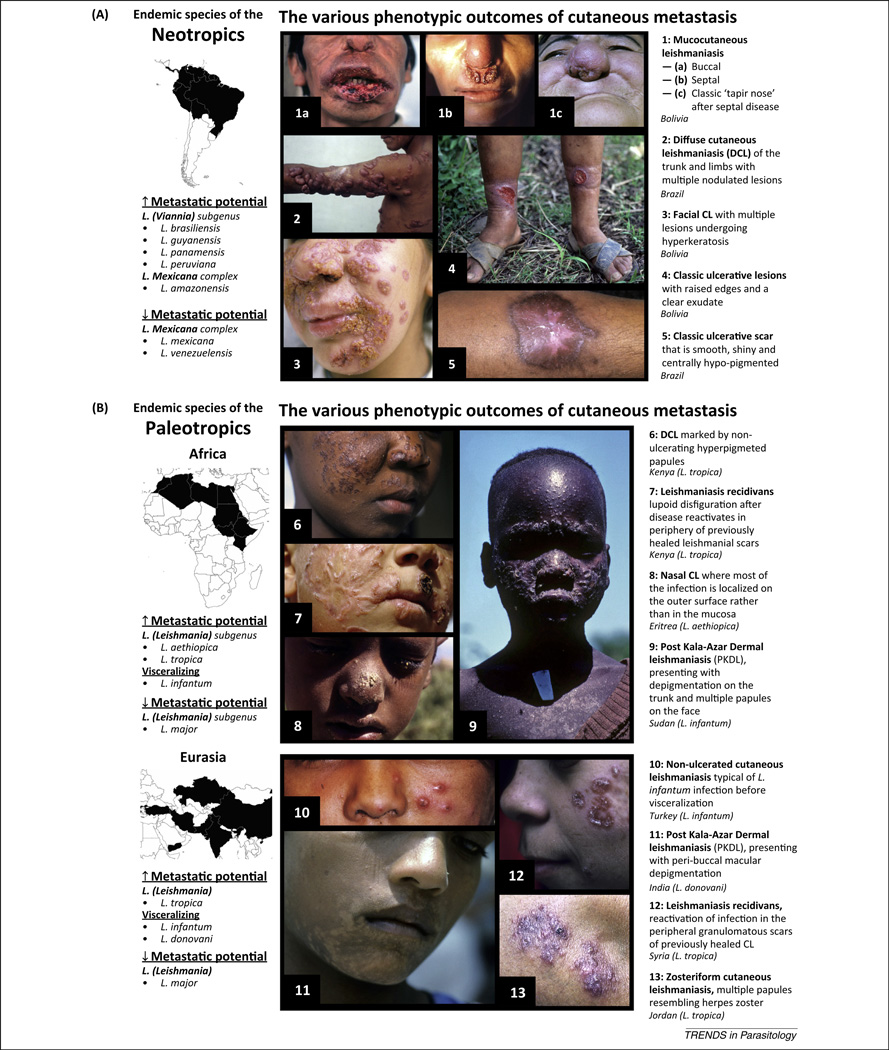
Figure 1.
Geographical context of the various outcomes of metastatic leishmaniasis. Metastatic cutaneous leishmaniasis (MCL) occurs across all Leishmania endemic regions but is much more prevalent in the Neotropics (A). Neotropical infections are mostly caused by the variously ‘metastatic’ species of the Leishmania (Viannia) subgenus. Species with high metastatic potential cause some form of MCL in approximately 20% of their infections. Neotropical MCL has a particular predilection for facial mucosa (1a–c) and are known for forming large ulcerative lesions (4–5) or, less frequently, diffuse granulomatous disease (2–3). The Paleotropics (B) have a much lower incidence of classic MCL. Post-kala-azar dermal leishmaniasis (PKDL), however, can occur in as many as 60% of all infections. MCL presentations vary from diffuse papular infections (6) to the rare leishmaniasis recidivans (12). PKDL presentations differ slightly between Eurasia and Africa, where India sees more progressive hypopigmented and macular rashes (11), whereas African PKDL has higher incidences of self-healing papular and ulcerative forms (9). All figures were kindly provided by Dr Philippe Desjeux. Geographically representative images were chosen from a global photographic catalog depicting the various symptomatic outcomes of cutaneous leishmaniasis.
Post-kala-azar dermal leishmaniasis
PKDL is an important dermal complication of Paleotropical VL, occurring in 10–20% of VL patients in India and up to 60% in Eastern African states such as Sudan and Ethiopia [9]. Although treatment is essential in Indian PKDL, African disease is generally self-healing. In most cases, patients feel otherwise well and present with a range of slowly evolving painless macular or papular rashes over large body surfaces, generally radiating from facial mucosa to large surfaces of the trunk and limbs. Nevertheless, the persistence of this seemingly harmless rash may play a more insidious role in the life cycle of its causative agent, L. donovani: functioning as a reservoir phase to shelter the parasite during the interseasonal periods of the sand fly. Indeed, the disease is mostly anthroponotic in these regions and only a few isolated studies have identified a potential animal reservoir. Despite the fact that the disease is caused by the same parasite, slight differences are seen between presentations in Africa and India. African PKDL patients have a higher tendency for ulcerative nodular forms, whereas Indian patients more commonly experience hypopigmented and maculopapular rashes with large plaque formations (Figure 1B). Interestingly, PKDL occurs almost exclusively in patients that were previously cured of VL and may appear up to 20 years after the initial infection; the average inter VL–PKDL period is 6 years on the Indian subcontinent and less than 1 year in Africa [9]. Although PKDL in Sudan can appear concurrently with, or sometimes in the absence of, VL, in general the disease is highly uncommon in patients who have not yet presented with VL and received treatment. Thus, certain therapies are actually considered a significant risk factor for developing the disease [10]. Because the therapies often restore or boost the patient’s inflammatory T helper 1 (Th1) immune response, PKDL is widely accepted as being an immunologically mediated disease (Box 2).
Box 2. Species-specific immunopathologies of PKDL.
Interestingly, the onset of PKDL is strongly associated with the successful treatment of VL in which the restoration of the patient’s antileishmanial immune response reduces the visceral parasite load and results in symptomatic resolution [30] (Figure I). Antimonial therapy carries a specific risk of developing PKDL. However, cases have also been reported after treatment with amphotericin B and miltefosine [73]. Although the sudden shift from a Th2 response during VL to Th1 after treatment has been implicated in numerous studies, specifically being seen through the restoration of leishmanin skin test (LST) positivity [29], the mechanism seems to rely on a more complex underlying immune response. Indeed, high serum IL-10 and upregulated intralesional IL-6 and TNF-α are predictive of, and essential to, the development of PKDL [9]. These high levels of local inflammatory markers could be compensatory for the malfunctioning IFN-γ signaling pathway, because although intralesional IFN-γ levels are high, its signaling seems to be corrupted by the low expression of the IFN-γ receptor [74]. The first line antileishmanials used for VL are known to induce or rather restore such cytokine environments and may thus explain the association of antileishmanial therapy to the onset of PKDL. Indeed, PKDL has recently been recognized as a form of paradoxical IRIS that emerges as a new disease entity following successful VL treatment and immune recovery [29].
Importantly, the appearance of any one of these forms of leishmanial metastasis can occur several months or years of the initial infection and often appear after the resolution of the initial infection with seemingly no predictive factors. The next part of this review describes a few circumstantial risk factors that may identify patients at higher risk of developing metastatic disease.
Metastatic risk factors
The mechanisms behind metastatic potential are currently unknown. Although immunological and environmental vulnerabilities in the host and various parasite phylogenies have been linked to the onset of infectious metastasis (Figure 2), there is no consensus on which of these factors is essential. Conflicting evidence is probably an indication that the process is multifactorial and dependent on complex interactions among parasite, host, and environmental factors, including genetic and nongenetic factors.
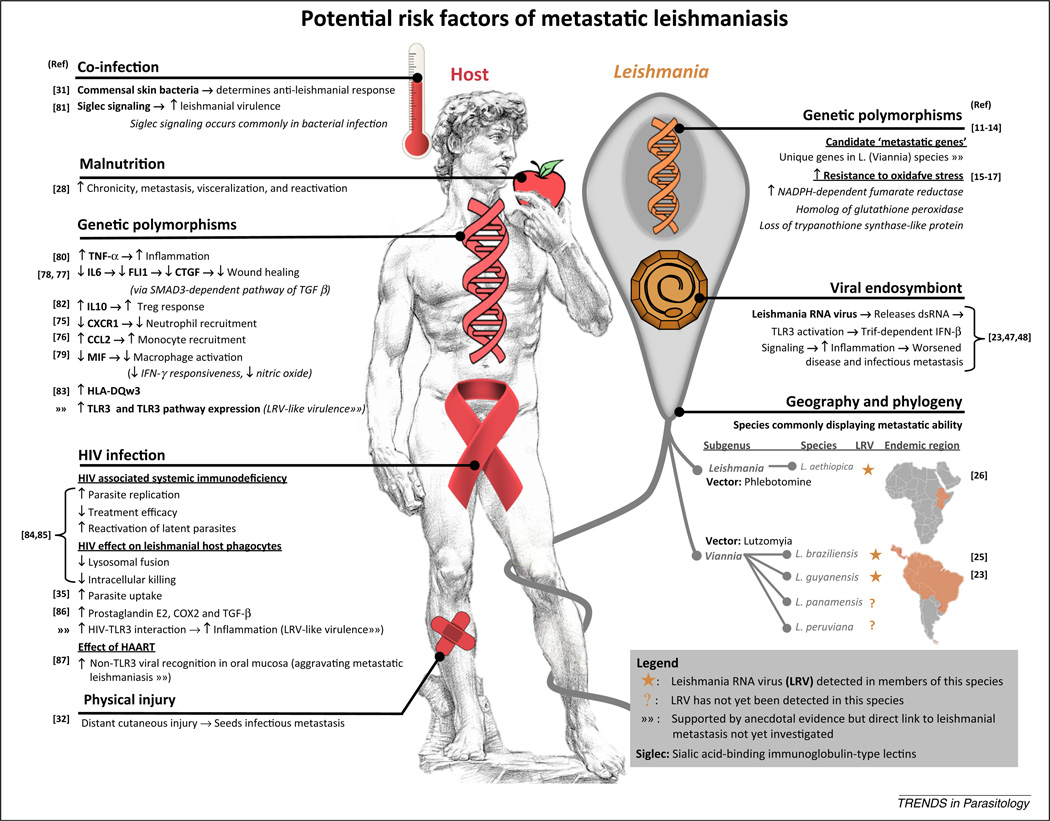
Figure 2.
The potential risk factors of metastatic leishmaniasis. A summary of the anecdotal or direct evidence for risk factors that may predispose metastatic complications in cutaneous leishmaniasis [11–17,23,25,26,28,31,35,47,48,75–87].
Metastatic risk factors in the parasite
Phylogeny and polymorphisms
Geographical boundaries remain the clearest delineation among symptomatic outcomes, and because they parallel parasite-specific endemic regions, it is often assumed that disease outcomes branch with parasite phylogeny. So far, comparative analysis across the genomes of LCL, metastatic CL, and VL Leishmania spp. has not revealed a universal ‘metastatic gene’ [11–13]. Nevertheless, these studies cannot yet include the effect of differential protein expression achieved through changes in gene regulation, copy number, single nucleotide polymorphisms, or the presence of pseudogenes [14]. Although many of the differentially expressed genes are ‘hypotheticals’ of unknown function, some may have putative virulence activity. For instance, metastatic L. braziliensis is known to carry supplementary copies of NADPH-dependent fumarate reductase and a homolog of glutathione peroxidase (as well as having lost a trypanothione synthase-like protein): all are important enzymes in the detoxification of oxidative stress. Whether these have any physiological importance is unknown. Hopefully, the rapidly growing database of Leishmania genomes (www.tritrypdb.org) will soon provide more insight into the role of parasite phylogeny in symptomatic outcome.
Although differences at the genetic level are poorly identified, various physiological differences have already been described, where metastatic parasites seem to have improved survival capabilities attributable to improved resistance to oxidative stress [15] and antileishmanial drugs such as antimony [16,17]. Interestingly, some studies have found heterogeneity among parasites isolated from the metastatic and primary lesions within the same patient. The most striking example was found in PKDL, where visceralizing parasites had significant genetic differences to those found later in the skin during PKDL [18,19]. Further, the cutaneous parasites showed an upregulation of certain surface proteins associated with virulence [20], implying that metastasis is a result of divergent parasite evolution or that, rather than metastasis, patients are actually reinfected with a variant, for which their existing immunity to previous infection acts as a susceptibility factor for the onset of PKDL.
Overall, findings support the hypothesis that infectious metastasis arises from low levels of persistent infection, where slowly dividing or ‘dormant’ parasites are possibly reactivated by antimony treatment or some similar type of immunological stress [16,17]. Indeed, parasites can often be detected in histologically ‘normal’ mucosal tissues of LCL patients [21], thereby indicating that this state of dormancy is probably achieved through a tightly controlled immunological tolerance rather than by transforming into a specialized ‘dormant’ morphology, for which there is no substantial evidence. Convincing support of immune tolerance is seen repeatedly where disruptions to immune functioning initiate symptomatic reactivations, such as after organ transplants or herpes zoster infection [22]. So far, some links to reactivation have been found in variations of parasite immunogenicity, where the concentration or combination of certain pattern recognition receptor (PRR) ligands is able to determine the course of infection. A striking example of this arises from a surprising nongenetic source, discussed below.
Nongenetic factors
We recently provided evidence that cytoplasmic pathogens of the Leishmania parasite can influence the course of leishmaniasis. Here, strains of L. guyanensis were found to be infected by a cytoplasmic virus, Leishmania RNA virus (LRV). These LRV-bearing parasites repeatedly metastasized in a hamster model of infection, in contrast to their LRV-negative counterparts [23,24]. The process was shown to be immunologically mediated, where the viral double-stranded RNA (dsRNA) provoked a potent inflammatory response after engaging endosomal Toll-like receptor 3 (TLR3), resulting in the production of interferon (IFN)-β, which inflamed and worsened leishmanial lesions in mice as well as prolonging parasite survival. This situation of improved parasite survival in spite of a potent inflammatory response is reminiscent of what is observed in human mucosal leishmaniasis patients. This variant of LRV (LRV1) has since been found in various isolates of Neotropical metastatic cutaneous leishmaniasis (MCL) from species of L. guyanensis and L. braziliensis [25]. Further, a depletion of LRV1 in genetically identical L. guyanensis clones confirmed the role of LRV1 in disease severity [23]. Similar to other Totiviruses, which are generally neither shed nor infectious and thus inherited only vertically or during genetic exchange, the relationships between the LRVs closely parallel the relationships between the parasite species within which they reside. Recently, confirmation of the presence in L. aethiopica of a new LRV variant of the single (and exceptional) LRV2 isolate of L. major [26], indicates that LRV infection may have a much wider global reach. As described in Box 1, L. aethiopica is one of the few Paleotropical species causing metastatic complications such as mucosal leishmaniasis and DCL. So far, however, LRV has only been proven to act as a virulence factor in murine models of L. guyanensis infections. Its clinical role in L. braziliensis and L. aethiopica infections is yet to be defined. Unfortunately, no reliable clinical or epidemiological data exist to assess the correlation between LRV presence and metastatic leishmaniasis. Current reports show that infectious metastasis for L. braziliensis is not exclusively associated with LRV presence [27]. Additionally, LRV has not yet been detected in L. panamensis, which can also cause mucosal leishmaniasis. These facts suggest that infectious metastasis is a complex multifactorial process, in which the host and its environment also play a major role.
Metastatic risk factors in the host
Although fair assumptions can be made on host risk factors from approximations in animal models, the lack of large-scale or systematic human studies leaves many questions regarding the effect of human-specific or ‘real-life’ factors, such as risks stratified with socioeconomic status, ethnicity, genetics, or occupation. The lack of these studies are probably a major contributing factor to the conflicting data found in studies on leishmanial metastasis.
Immunology and environment
Leishmaniasis has established itself as an emerging opportunistic infection in HIV-positive patients, where its occurrence is now used as a clinical indicator for performing an HIV test. Indeed, HIV predisposes the onset of less common leishmanial complications such as VL and diffuse metastatic infection by 1000-fold [2]. Similarly, the immunological dysfunction associated with severe malnutrition can also be exploited by leishmaniasis and stands as the most prevalent cause of immunodeficiency worldwide [28]. Equally, metastatic leishmanial complications have been associated with sudden immune-reconstitution such as after highly active antiretroviral therapy (HAART) [29] or Th1-boosting antimonial therapy [30], thus showing that diverse fluctuations in immunocompetence can influence CL. As immunocompetence also determines the composition of the host microbiome, a recent study has elegantly linked disruptions in skin commensals to the control of CL [31]. This study, however, did not investigate leishmanial metastasis, but we can postulate the potential importance of these potently immunomodulatory ‘co-infections’, especially considering the metastatic influence of the nested co-infection, LRV.
Local changes in skin immunity after physical injuries have already been shown to predispose metastatic reactivations. Here, secondary lesions were observed to develop in the scar tissue of unrelated wounds [32]: a phenomenon implying that, similar to cancer, metastatic sites are immunologically ‘seeded’ before the establishment of secondary lesions by local changes in the immune microenvironment. These local variations in immunological status remain as an interesting and underdeveloped avenue of study in leishmaniasis. The most common and potent facilitators for these local changes are the PRRs, which are at the frontline of innate pathogen recognition. Each PRR has an immunological arsenal specific to a range of signature pathogen ligands. Thus, concomitant infections stimulating a variety of PRRs induce a range of signaling cascades that not only blend together to create a unique immunological microenvironment but are also able to synergize or inhibit each other. Further, it has been shown that even a previous infection in the host can alter subsequent PRR activity, inducing homotolerance and heterotolerance or hyperactivity [33]. The potent effect of TLR3 signaling on disease severity, exemplified in the case of LRV nested co-infection, sheds light on the possible importance of the number, timing, and magnitude of innate immune responses in the evolution of leishmaniasis. Consequently, the mechanism whereby HIV infection acts as an aggravating factor in metastatic leishmaniasis may be far more complex than a simple collapse of the CD4 compartment. Indeed, Leishmania survive better in cells exposed to HIV and vice versa [34,35]. This creates a situation where PRR crosstalk as well as genetic polymorphisms in these PRRs (Box 3) should be considered as key parameters of leishmanial pathogenesis.
Box 3. Host PRR polymorphisms – a potential risk factor in metastatic leishmaniasis.
PRRs have been almost completely overlooked in studies relating genetic polymorphisms to symptomatic variants of leishmaniasis. For instance, there is no study yet correlating PRR polymorphisms and susceptibility or resistance to Leishmania infection, although it is likely to be a key parameter in the control of parasite burden. Various genetic polymorphisms, however, have revealed immune susceptibilities for cytokines and chemokines in the pathology of metastatic leishmaniasis [75–79,88]. A few potential candidates are suggested below.
TLR polymorphisms
Numerous TLR polymorphisms have already been identified in humans and associated with the development of various common inflammatory diseases [89]. In the context of LRV recognition, TLR3 polymorphisms could play a major role in leishmanial metastasis. Several polymorphisms of TLR3 have been associated with human disease [80,90,91]. For instance, an intronic polymorphism, elevating TLR3 expression, was correlated with improved viral clearance in hepatitis C [92]. TLR3 polymorphisms have been similarly linked to common coinfections of leishmaniasis. For example, natural resistance to HIV-1 was associated with a common polymorphism (Leu412Phe) of TLR3, where the presence of the 412Phe allele was further associated with increased production of inflammatory cytokines [93]. Interestingly, the distribution of this allele varies between Eurasia and Africa, being almost absent in the latter group [94].
NLR polymorphisms
NLRs have also been associated with several diseases. For example, NLRP3, was originally named Cryopyrin for its association with a class of inflammatory diseases induced by low temperatures. Since then, similar pathologies have been mapped to this gene and are now commonly known as the Cryopyrin-associated periodic syndromes. Symptoms are mediated by hyperactive NLRP3 and a resultantly high level of the proinflammatory cytokine IL-1β. Surprisingly, loss-of-function polymorphisms have also been associated with chronic inflammatory conditions. An example of which is genetically defective NLRC2 expression, now strongly associated with Crohn’s disease [95,96]. This, however, could be explained by the fact that NLRC2 is essential in controlling gut bacteria through its recognition of a component of bacterial cell wall, muramyl dipeptide [97]. This reduction in microbial control could subsequently drive the inflammation associated with the development of Crohn’s disease. Because NLRC2 is especially expressed in the skin, this polymorphism results in a similar environment of proinflammation through disruptions of the cutaneous microbiome, and thereby influences the local inflammatory microenvironment in leishmaniasis. Therefore, much potential lies in the study of PRR polymorphisms in leishmanial pathogenesis.
Pattern recognition receptors and Leishmania
The Leishmania parasite has several molecular patterns, which are sensitively detected by the innate PRRs of the host. The host cell of the obligate intracellular stage of Leishmania is the macrophage, a potent immune phagocyte notoriously coated and lined with various PRRs, and so it is clear that the parasite does not go by undetected. Indeed, inoculation results in a rapid initiation of inflammatory signaling cascades, where the collateral tissue damage of the resultant hyperinflammation is at the root cause of disease pathology. Three major families of signaling PRRs have been identified: the TLRs, the nucleotide-binding domain, leucine-rich repeat containing receptors (NLRs), and the retinoic acid-inducible gene 1 like receptors (RLRs). So far, the TLRs are the most extensively described.
Toll-like receptors
In Leishmania infection, the role of certain TLRs has been well documented and recently reviewed [36,37]. In most instances, studies were based on the common adaptor molecule MyD88, where deficient mice showed that TLRs play a generally protective role against Leishmania infection. This MyD88-dependent protection was mostly attributed to TLR2 and TLR4 signaling that have been repeatedly noted as beneficial across a broad range of Leishmania species [38–40]. Interestingly, however, the MyD88 pathway is not exclusive to TLRs, and thus these protective roles may also be explained by MyD88-dependent components of IL-1/IL-18 signaling, as will be discussed in the next section. Nevertheless, further protective roles were found for endosomal TLRs (sensing nucleic acids) through studies on the Unc93B1 chaperone protein: involved in the translocation of TLRs from the endoplasmic reticulum to endosomes [41]. Among the endosomal TLRs, TLR9 seems crucial to pathogenesis, because TLR9-deficient mice are rendered more susceptible to L. major [42,43], L. braziliensis [44], and L. guyanensis [45]. However, as mentioned previously, it is difficult to make generalizations about TLRs from these studies as Leishmania may engage multiple TLRs with intersecting pathways that are then able to either synergize with or reduce the efficacy of the co-stimulated pathway. For example, L. panamensis induces TLR1, TLR2, TLR3, TLR4, and TLR9 transcription in primary macrophages [46]. The TLR4 ligand in L. panamensis remains to be determined, however, as Viannia spp. lack the P-8 proteoglycolipid complex that is generally responsible for leishmanial TLR4 stimulation. Further, the classic TLR2 ligand, LPG, is also expressed in significantly lower quantities (10–20-fold less) in Viannia. It is possible that its activation and upregulation could be attributed to crosstalk, where nuclear factor-κB (NF-κB) activation, a common target of many PRRs, is known to upregulate TLR2.
Protection via TLRs is usually linked to the production of proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), C-X-C motif chemokine 10 (CXCL10), and chemokine ligand 5 (CCL5). Importantly, however, these same immunological mediators of protection have also been blamed for the extreme inflammation associated with mucosal and metastatic leishmaniasis. The model of LRV-dependent metastasis is one such example where TNF-α-mediated hyperinflammation not only induces tissue damage that worsens disease outcome but is also favorable to parasite survival [47], indicating that the magnitude as well as the combination of TLR signaling is important in determining disease outcome [48]. Immunological crosstalk has already been described among many TLRs [33]. TLR7 stimulation, for instance, can induce inhibitory heterotolerance on concomitantly stimulated TLR2, TLR4, and TLR9 pathways [49]. Further, TLR3 has shown inflammatory synergy with TLR9 and TLR2 [50,51], a phenomenon that may account for the hyperinflammatory response seen in TLR3 stimulatory Leishmania infections carrying LRV. These studies propose that TLR stimulation should be investigated in a multidimensional manner, taking into account not only the incidence of their stimulation but also the concomitantly stimulated receptors and the chronological order in which they are stimulated.
NLRs and RLRs
Unlike the TLRs, NLRs are exclusively intracellular, enabling stressed, damaged, or infected cells to directly sense and respond to internal danger signals. Although the signals and activation complexes are very different from those of the TLR pathway, some family members (NOD1 and NOD2) can directly engage the common TLR nuclear factor, NF-κB, producing many of the same proinflammatory cytokines. Most NLR family members, however, signal through the formation of an inflammasome, a multiprotein complex that induces inflammation via caspase-1-mediated cleavage and activation of pro-IL-1β and pro-IL-18 [52]. The RLRs, by contrast, detect foreign cytoplasmic DNA and RNA to induce an antiviral immune response. The RLRs include RIG-I and MDA5, which work via interferon regulatory factor 3 (IRF3) and IRF7 by utilizing the mitochondrion-localized adaptor protein MAVS [53].
Because NLRs and RLRs are able to detect both pathogen-associated molecular patterns (PAMPs) as well as cellular danger signals (which are undoubtedly present in the destructive inflammatory environment of a typical leishmanial lesion), we expect to find a significant role for NLRs and RLRs in the evolution of disease. Despite this great potential, however, studies on their involvement in leishmaniasis are sparse. Thus far, there is no report on a possible role of RLRs in leishmaniasis and only a handful on NLR activation. The first study describing the role of NLRs in leishmaniasis correlated the clearance of various New World Leishmania species to an NLRP3-dependent production of nitric oxide (NO) and IFN-γ [54]. The authors showed that the resultant active IL-1β induced by NLRP3 signaling carried out this response in an autocrine manner, where the IL-1 receptor and its MyD88 adaptor protein were necessary and sufficient to trigger inducible nitric oxide synthase (iNOS). Interestingly, immunological cross-talk from TLR4 can increase the inactive IL-1β precursor, thus facilitating the NLRP3 response by ‘priming the system’ [55]. Indeed, TLR4 is an essential component of initiating parasitotoxic oxidative stress in L. major infection. Although these studies note NLRP3 inflammasome activation, they did not identify the stimulating ligand. In the ulcerative environment of a typical leishmanial lesion, we expect many cellular danger signals with the potential of NLRP3 stimulation. An example of such activation has been described, whereby nucleotides released by macrophages infected with L. amazonensis engaged the purinergic receptors (P2Y2 and P2Y4), often found upstream of the NLRP3 inflammasome. This drastically reduced parasite burden and even induced apoptosis of the host cell [56]. TLR crosstalk can also have a deleterious effect on this parasitotoxic inflammasome, for instance, IFN-β, such as is produced in response to TLR3 engagement, is known to disrupt oxidative parasite killing via the upregulation of superoxide dismutase (SOD1) [57]. This molecule decreases caspase-1 activation, which is an essential part of NLRP3 inflammasome function [58]. SOD1 upregulation is seen particularly in Neotropical Leishmania spp., where infectious metastasis (and IFN-β inducing LRV1) is endemic. Interestingly, IFN-β is described as having many contradictory roles in leishmaniasis (Box 4).
Box 4. The contradictory roles of IFN-β in leishmaniasis.
Type I interferons, such as IFN-β, were originally discovered and defined by their ability to ‘interfere’ with viral infections and, for a long time, it was the sole function to which they were attributed. In recent years, more diverse roles have been added, ranging from antineoplastic agents to regulators of the immune system. They are currently found to have various and sometimes contradictory roles in viral, bacterial, and protozoan infections [98,99]. For leishmaniasis, the roles of IFN-β seem to be particularly conflicting, where IFN-β treatment has been demonstrated to play both protective and detrimental roles during L. major infection by differentially modulating iNOS expression, depending on the dose and timing of its administration [100]. For example, low-dose IFN-β treatment in a murine model was noted as having a significantly protective effect against progressive CL, restoring natural killer cell cytotoxic activity, increasing lymphocyte proliferation, and upregulating parasitotoxic nitric oxide [101]. However, high concentrations of this proinflammatory cytokine increased L. braziliensis parasite load and worsened disease in a murine model of L. amazonensis infection: an observation analogous to the correlation of hyperinflammation with increased parasite infectivity in metastatic disease. Parasites evidently benefited from an IFN-β-mediated upregulation of superoxide dismutase in human macrophages, thereby detoxifying the oxidative radicals used to kill intracellular infections [57], whereas destructive inflammation was caused by an extreme recruitment of inflammatory monocytes [102].
The only other study on the inflammasome in leishmaniasis was a preliminary effort to profile the transcription of inflammasome components in a macrophage system of infection. Here, the authors quantified the mRNA of two inflammasomes (NLRP3 and NAIP5), as well as some common inflammasome adaptor/effector molecules (IPAF, ASC, caspase-1, IL-1β, and IL-18) in macrophages infected with L. major and found a significant upregulation for all the above-mentioned components except NAIP5 [59]. Again showing the potential for such studies in leishmaniasis. Anecdotal evidence widely supports a role for NLRs in leishmaniasis, for instance, a common NRLP3 stimulant, poly (lactic-co-glycolic) acid (PLGA), is an effective adjuvant in a KMP11-based antileishmaniasis vaccine [60].
The virus-recognizing RLRs would probably have the highest potential to play a role in Leishmania infected with the dsRNA virus, LRV. However, it is also likely that the length of the dsRNA genome of LRV may not fit the size restrictions for RLR activation.
Concluding remarks
Infectious metastasis in CL is a complex, multifactorial process involving various risk factors. Much research is still needed to definitively identify the exact causes of this disfiguring complication and, further, to gauge their relative contributions in the pathogenesis of disease. Outstanding questions for further research are proposed in Box 5. So far, anecdotal evidence has short-listed some candidate ‘metastatic risk factors’ in parasite phylogeny, host immunocompetence, and environmental influences, which were summarized here. The geographical isolation of metastatic outcomes insinuates that parasite-intrinsic factors are the overriding determinants of the complication. Indeed, metastatic parasites seem to have an increased resistance to oxidative stress and antileishmanials, prolonging their survival even in the more potently inflammatory environment that they tend to induce. Nevertheless, the small genetic differences between metastatic and nonmetastatic parasites make the presence of a ‘metastatic gene’ unlikely. This indicates that increased parasite survival is probably attributable to nongenetic factors or to a more complex situation of immune evasion in the host. A strong candidate for such a nongenetic, immunomodulatory metastatic factor is the presence of the highly immunogenic dsRNA virus within the cytoplasm of metastatic strains of L. braziliensis, L. guyanensis, and L. aethiopica parasites. This potently pathogenic role for an innate antiviral pathway strongly implicates PRRs and coinfecting pathogens in metastatic leishmaniasis. Already, encouraging studies have shown the substantial influence of the host skin microbiome and HIV viral particles in leishmanial lesion formation and parasite survival. Therefore, further studies on PRR polymorphisms and crosstalk in metastatic disease have great potential to reveal associations and ultimately stand as evidence for the use of innate immunomodulators in the treatment and prevention of metastatic disease.
Box 5. Outstanding questions.
Large-scale epidemiological studies are needed to understand the geographical extent and clinical relevance of the LRV nested coinfection in various Leishmania species.
A continued and more robust search for parasite-intrinsic metastatic factors could be achieved through broad-ranging comparative studies at the genetic and protein levels.
Genome-wide association studies on parasites and on leishmaniasis endemic human populations could reveal any immunological susceptibilities, which predispose leishmanial metastasis.
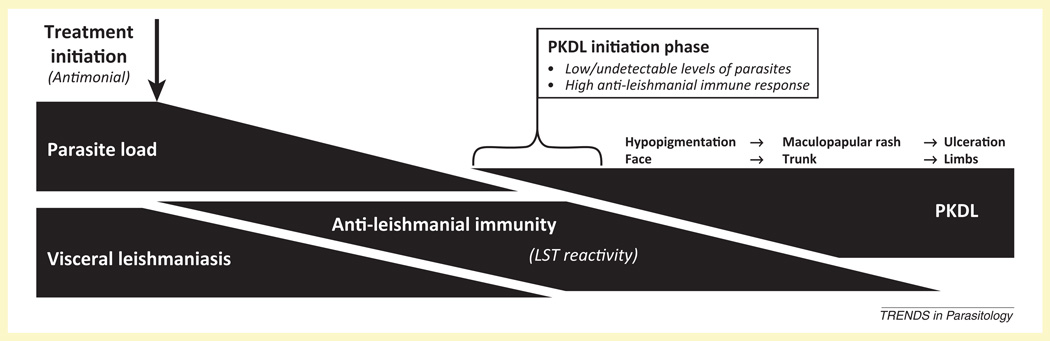
Figure I.
The clinical context of post-kala-azar dermal leishmaniasis (PKDL) development. Visceral leishmaniasis (VL) is marked by high visceral parasite loads and is often associated with a weak or negative result for the leishmanin skin test (LST). The absence of LST reactivity is indicative of low cellular immunity against leishmanial antigens. VL is often successfully treated with antimonials resulting in a reduction of parasite load and recuperation of antileishmanial response. This stage of disease resolution is often where PKDL is initiated. PKDL develops in three clinically relevant stages, spreading as a hypopigmented rash from the periorificial regions of the face to the periphery as a maculopapular or ulcerative rash.
Acknowledgments
We are especially grateful to Dr Philippe Desjeux for kindly providing us with the photographic work presented in Figure 1 of this review. Further thanks are given to the artist, Abdón Romero (of Hildago-Romero Artists Studios, Miami, FL, USA) for his skilled sketch of ‘David’ that was used in Figure 2. We apologize to those not cited owing to space constraints. Our work is funded by grants FNRS No. 3100A0-116665/1, IZ70Z0-131421, FNRS No. 310030-153204/1, the association Institute for Arthritis Research (aIAR) (N.F.), National Institutes of Health (NIH) RO1 AI29646 and NIH R56 AI099364 (S.M.B.), and the Pierre Mercier Foundation (C.R.).
Glossary
- Damage- (or Danger-) associated molecular patterns (DAMPs)
molecules that are generally present due to a noninfectious threat to cellular integrity and are capable of initiating signaling cascades, through PRRs such as NLRs and TLRs. Some examples are proteins released during DNA damage such as cytosolic DNA and RNA fragments, or nucleotides such as ATP. Fragments of damaged tissue such as hyaluronan, uric acid, and heparin sulfate have also been described as potent DAMPs
- Highly active antiretroviral therapy (HAART)
a combination of at least three drugs proven to suppress HIV replication, thus prolonging and improving the quality of life for individuals with HIV. A combination is used to avoid the development of drug resistance
- Immune reconstitution inflammatory syndrome (IRIS)
a condition developing during immune recovery from a major immunosuppressive event (e.g., HIV infection, or VL) in which the immune system responds to previously acquired antigens with an overwhelming level of inflammation that paradoxically worsens the disease
- Kala-azar (KA)
a Hindi term for ‘Black Fever’, describing the unexplained cutaneous discoloration associated with end-stage VL, where parasites fatally (if left untreated) infest the liver, spleen, and bone marrow
- Leishmania RNA virus (LRV)
a cytoplasmic double-stranded RNA (dsRNA) virus residing within some strains of the Leishmania parasite, which may act as a virulence factor in metastatic leishmaniasis
- Nucleotide-binding oligomerization domain receptors (NLRs)
also called Nod-like receptors, are intracellular sensors of PAMPS and DAMPs able to cooperate with TLRs to regulate or potentiate the inflammatory and apoptotic response
- Pathogen-associated molecular patterns (PAMPs)
small molecular motifs common among certain pathogen groups that are recognized by PRRs in cells of the innate immune system and generally produce a cytokine signaling cascade. Some examples include bacterial lipopolysaccharide (LPS, specific to TLR4), flagellin (TLR5), dsRNA (TLR3), and unmethylated CpG DNA (TLR9)
- Retinoic acid-inducible gene 1 like receptors (RLRs)
also called RIG-1-like receptors, are cytoplasmic RNA helicase enzymes, which recognize viruses by binding their dsRNA. RIG-1, MDA5, and LGP2 are the currently described members of this PRR family
- T helper cell subsets (Th1/Th2/Th17/Treg)
subsets of a CD4 T cell lineage, which promote various types of immune response. Th1: cell mediated cytotoxic response (via IFN-γ). Th2: B cell mediated antibody response (via IL-4, IL-5, and IL-13). Th17: antifungal response (via IL-17A). Treg: anti-inflammatory response (via IL-10)
- Toll-like receptors (TLRs)
are PRRs able to detect a range of pathogenic patterns on the plasma membrane (TLR1, TLR2, TLR4, TLR5, and TLR6) or within the endosomal compartment (TLR3, TLR7/8, and TLR9)
References
- 1.World Health Organization. Control of the leishmaniases. WHO Tech. Rep. Ser. 2010;949:1–186. [PubMed] [Google Scholar]
- 2.van Griensven J, et al. Leishmaniasis in immunosuppressed individuals. Clin Microbiol. Infect. 2014;20:286–299. doi: 10.1111/1469-0691.12556. [DOI] [PubMed] [Google Scholar]
- 3.Hotez PJ, et al. Neglected tropical diseases of the Middle East and North Africa: review of their prevalence, distribution, and opportunities for control. PLoS Negl. Trop. Dis. 2012;6:e1475. doi: 10.1371/journal.pntd.0001475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobson RL. Leishmaniasis in an era of conflict in the Middle East. Vector Borne Zoonotic Dis. 2011;11:247–258. doi: 10.1089/vbz.2010.0068. [DOI] [PubMed] [Google Scholar]
- 5.Mendonca MG, et al. Persistence of Leishmania parasites in scars after clinical cure of American cutaneous leishmaniasis: is there a sterile cure? J. Infect. Dis. 2004;189:1018–1023. doi: 10.1086/382135. [DOI] [PubMed] [Google Scholar]
- 6.Alvar J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goto H, Lindoso JA. Current diagnosis and treatment of cutaneous and mucocutaneous leishmaniasis. Expert Rev. Anti Infect. Ther. 2010;8:419–433. doi: 10.1586/eri.10.19. [DOI] [PubMed] [Google Scholar]
- 8.Murray HW, et al. Advances in leishmaniasis. Lancet. 2005;366:1561–1577. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- 9.Zijlstra EE, et al. Post-kala-azar dermal leishmaniasis. Lancet Infect. Dis. 2003;3:87–98. doi: 10.1016/s1473-3099(03)00517-6. [DOI] [PubMed] [Google Scholar]
- 10.Mondal D, Khan MG. Recent advances in post-kala-azar dermal leishmaniasis. Curr. Opin. Infect. Dis. 2011;24:418–422. doi: 10.1097/QCO.0b013e32834a8ba1. [DOI] [PubMed] [Google Scholar]
- 11.Lynn MA, McMaster WR. Leishmania: conserved evolution – diverse diseases. Trends Parasitol. 2008;24:103–105. doi: 10.1016/j.pt.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Depledge DP, et al. Comparative expression profiling of Leishmania: modulation in gene expression between species and in different host genetic backgrounds. PLoS Negl. Trop. Dis. 2009;3:e476. doi: 10.1371/journal.pntd.0000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith DF, et al. Comparative genomics: from genotype to disease phenotype in the leishmaniases. Int. J. Parasitol. 2007;37:1173–1186. doi: 10.1016/j.ijpara.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogers MB, et al. Chromosome and gene copy number variation allow major structural change between species and strains of Leishmania. Genome Res. 2011;21:2129–2142. doi: 10.1101/gr.122945.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acestor N, et al. Resistance to oxidative stress is associated with metastasis in mucocutaneous leishmaniasis. J. Infect. Dis. 2006;194:1160–1167. doi: 10.1086/507646. [DOI] [PubMed] [Google Scholar]
- 16.Souza AS, et al. Resistance of Leishmania (Viannia) braziliensis to nitric oxide: correlation with antimony therapy and TNF-α production. BMC Infect. Dis. 2010;10:209. doi: 10.1186/1471-2334-10-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arevalo J, et al. Influence of Leishmania (Viannia) species on the response to antimonial treatment in patients with American tegumentary leishmaniasis. J. Infect. Dis. 2007;195:1846–1851. doi: 10.1086/518041. [DOI] [PubMed] [Google Scholar]
- 18.Dey A, Singh S. Genetic heterogeneity among visceral and post-Kala-Azar dermal leishmaniasis strains from eastern India. Infect. Genet. Evol. 2007;7:219–222. doi: 10.1016/j.meegid.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Subba Raju BV, et al. Genetic fingerprinting and identification of differentially expressed genes in isolates of Leishmania donovani from Indian patients of post-kala-azar dermal leishmaniasis. Parasitology. 2008;135:23–32. doi: 10.1017/S0031182007003484. [DOI] [PubMed] [Google Scholar]
- 20.Salotra P, et al. Upregulation of surface proteins in Leishmania donovani isolated from patients of post kala-azar dermal leishmaniasis. Microbes Infect. 2006;8:637–644. doi: 10.1016/j.micinf.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 21.Figueroa RA, et al. Detection of Leishmania in unaffected mucosal tissues of patients with cutaneous leishmaniasis caused by Leishmania (Viannia) species. J. Infect. Dis. 2009;200:638–646. doi: 10.1086/600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Postorino MC, et al. Visceral leishmaniasis reactivation in transplant patients: a minireview with report of a new case. J. Nephrol. 2011;24:530–534. doi: 10.5301/JN.2011.8343. [DOI] [PubMed] [Google Scholar]
- 23.Ives A, et al. Leishmania RNA virus controls the severity of mucocutaneous leishmaniasis. Science. 2011;331:775–778. doi: 10.1126/science.1199326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez JE, et al. Clonal diversity in the expression and stability of the metastatic capability of Leishmania guyanensis in the golden hamster. J. Parasitol. 2000;86:792–799. doi: 10.1645/0022-3395(2000)086[0792:CDITEA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 25.Zangger H, et al. Detection of Leishmania RNA virus in Leishmania parasites. PLoS Negl. Trop. Dis. 2013;7:e2006. doi: 10.1371/journal.pntd.0002006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zangger H, et al. Leishmania aethiopica field isolates bearing an endosymbiontic dsRNA virus induce pro-inflammatory cytokine response. PLoS Negl. Trop. Dis. 2014;8:e2836. doi: 10.1371/journal.pntd.0002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pereira Lde O, et al. Severity of tegumentary leishmaniasis is not exclusively associated with Leishmania RNA virus 1 infection in Brazil. Mem. Inst. Oswaldo Cruz. 2013;108:665–667. doi: 10.1590/0074-0276108052013021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oliveira AG, et al. Influence of the nutritional status in the clinical and therapeutical evolution in adults and elderly with American Tegumentary Leishmaniasis. Acta Trop. 2013;128:36–40. doi: 10.1016/j.actatropica.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Khalil EA, et al. Post-kala-azar dermal Leishmaniasis: a paradigm of paradoxical immune reconstitution syndrome in non-HIV/AIDS patients. J. Trop. Med. 2013;2013:275253. doi: 10.1155/2013/275253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mukhopadhyay D, et al. Post kala-azar dermal leishmaniasis: an unresolved mystery. Trends Parasitol. 2014;30:65–74. doi: 10.1016/j.pt.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naik S, et al. Compartmentalized control of skin immunity by resident commensals. Science. 2012;337:1115–1119. doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wortmann GW, et al. Cutaneous leishmaniasis following local trauma: a clinical pearl. Clin. Infect. Dis. 2000;31:199–201. doi: 10.1086/313924. [DOI] [PubMed] [Google Scholar]
- 33.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Mock DJ, et al. Leishmania induces survival, proliferation and elevated cellular dNTP levels in human monocytes promoting acceleration of HIV co-infection. PLoS Pathog. 2012;8:e1002635. doi: 10.1371/journal.ppat.1002635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lodge R, et al. HIV-1 promotes intake of Leishmania parasites by enhancing phosphatidylserine-mediated, CD91/LRP-1-dependent phagocytosis in human macrophages. PLoS ONE. 2012;7:e32761. doi: 10.1371/journal.pone.0032761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faria MS, et al. Toll-like receptors in Leishmania infections: guardians or promoters? J. Parasitol. Res. 2012;2012:930257. doi: 10.1155/2012/930257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soong L. Subversion and utilization of host innate defense by Leishmania amazonensis. Front. Immunol. 2012;3:58. doi: 10.3389/fimmu.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muraille E, et al. Genetically resistant mice lacking MyD88-adapter protein display a high susceptibility to Leishmania major infection associated with a polarized Th2 response. J. Immunol. 2003;170:4237–4241. doi: 10.4049/jimmunol.170.8.4237. [DOI] [PubMed] [Google Scholar]
- 39.Vargas-Inchaustegui DA, et al. CXCL10 production by human monocytes in response to Leishmania braziliensis infection. Infect. Immun. 2010;78:301–308. doi: 10.1128/IAI.00959-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Revaz-Breton M, et al. The MyD88 protein 88 pathway is differently involved in immune responses induced by distinct substrains of Leishmania major. Eur. J. Immunol. 2010;40:1697–1707. doi: 10.1002/eji.200939821. [DOI] [PubMed] [Google Scholar]
- 41.Schamber-Reis BL, et al. UNC93B1 and nucleic acid-sensing Toll-like receptors mediate host resistance to infection with Leishmania major. J. Biol. Chem. 2013;288:7127–7136. doi: 10.1074/jbc.M112.407684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carvalho LP, et al. Lymph node hypertrophy following Leishmania major infection is dependent on TLR9. J. Immunol. 2012;188:1394–1401. doi: 10.4049/jimmunol.1101018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abou Fakher FH, et al. TLR9-dependent activation of dendritic cells by DNA from Leishmania major favors Th1 cell development and the resolution of lesions. J. Immunol. 2009;182:1386–1396. doi: 10.4049/jimmunol.182.3.1386. [DOI] [PubMed] [Google Scholar]
- 44.Weinkopff T, et al. Role of Toll-like receptor 9 signaling in experimental Leishmania braziliensis infection. Infect. Immun. 2013;81:1575–1584. doi: 10.1128/IAI.01401-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ives A, et al. MyD88 and TLR9 dependent immune responses mediate resistance to Leishmania guyanensis infections, irrespective of Leishmania RNA virus burden. PLoS ONE. 2014;9:e96766. doi: 10.1371/journal.pone.0096766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramirez C, et al. Human macrophage response to L (Viannia) panamensis: microarray evidence for an early inflammatory response. PLoS Negl. Trop. Dis. 2012;6:e1866. doi: 10.1371/journal.pntd.0001866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hartley MA, et al. Leishmania RNA virus: when the host pays the toll. Front. Cell. Infect. Microbiol. 2012;2:99. doi: 10.3389/fcimb.2012.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hartley MA, et al. The therapeutic potential of immune cross-talk in leishmaniasis. Clin. Microbiol. Infect. 2013;19:119–130. doi: 10.1111/1469-0691.12095. [DOI] [PubMed] [Google Scholar]
- 49.Hotz C, Bourquin C. Systemic cancer immunotherapy with Toll-like receptor 7 agonists: timing is everything. Oncoimmunology. 2012;1:227–228. doi: 10.4161/onci.1.2.18169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vanhoutte F, et al. Toll-like receptor (TLR)2 and TLR3 synergy and cross-inhibition in murine myeloid dendritic cells. Immunol. Lett. 2008;116:86–94. doi: 10.1016/j.imlet.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 51.Whitmore MM, et al. Negative regulation of TLR-signaling pathways by activating transcription factor-3. J. Immunol. 2007;179:3622–3630. doi: 10.4049/jimmunol.179.6.3622. [DOI] [PubMed] [Google Scholar]
- 52.Martinon F, et al. The inflammasomes: guardians of the body. Annu. Rev. Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 53.Meylan E, et al. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 54.Lima-Junior DS, et al. Inflammasome-derived IL-1β production induces nitric oxide-mediated resistance to Leishmania. Nat. Med. 2013;19:909–915. doi: 10.1038/nm.3221. [DOI] [PubMed] [Google Scholar]
- 55.Becker CE, O’Neill LA. Inflammasomes in inflammatory disorders: the role of TLRs and their interactions with NLRs. Semin. Immunopathol. 2007;29:239–248. doi: 10.1007/s00281-007-0081-4. [DOI] [PubMed] [Google Scholar]
- 56.Marques-da-Silva C, et al. Infection with Leishmania amazonensis upregulates purinergic receptor expression and induces host–cell susceptibility to UTP-mediated apoptosis. Cell. Microbiol. 2011;13:1410–1428. doi: 10.1111/j.1462-5822.2011.01630.x. [DOI] [PubMed] [Google Scholar]
- 57.Khouri R, et al. IFN-β impairs superoxide-dependent parasite killing in human macrophages: evidence for a deleterious role of SOD1 in cutaneous leishmaniasis. J. Immunol. 2009;182:2525–2531. doi: 10.4049/jimmunol.0802860. [DOI] [PubMed] [Google Scholar]
- 58.Meissner F, et al. Superoxide dismutase 1 regulates caspase-1 and endotoxic shock. Nat. Immunol. 2008;9:866–872. doi: 10.1038/ni.1633. [DOI] [PubMed] [Google Scholar]
- 59.Mahmoudian Sani M, et al. Evaluation of the expression of the inflammasome pathway related components in Leishmania major-infected murine macrophages. Eur. J. Exp. Biol. 2013;3:104–109. [Google Scholar]
- 60.Santos DM, et al. PLGA nanoparticles loaded with KMP-11 stimulate innate immunity and induce the killing of Leishmania. Nanomedicine. 2013;9:985–995. doi: 10.1016/j.nano.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 61.Carvalho LP, et al. Differential immune regulation of activated T cells between cutaneous and mucosal leishmaniasis as a model for pathogenesis. Parasite Immunol. 2007;29:251–258. doi: 10.1111/j.1365-3024.2007.00940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Faria DR, et al. Decreased in situ expression of interleukin-10 receptor is correlated with the exacerbated inflammatory and cytotoxic responses observed in mucosal leishmaniasis. Infect. Immun. 2005;73:7853–7859. doi: 10.1128/IAI.73.12.7853-7859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Santos Cda S, et al. CD8+ granzyme B+-mediated tissue injury vs. CD4+IFNγ+-mediated parasite killing in human cutaneous leishmaniasis. J. Invest. Dermatol. 2013;133:1533–1540. doi: 10.1038/jid.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Novais FO, et al. Cytotoxic T cells mediate pathology and metastasis in cutaneous leishmaniasis. PLoS Pathog. 2013;9:e1003504. doi: 10.1371/journal.ppat.1003504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maretti-Mira AC, et al. Therapeutic failure in American cutaneous leishmaniasis is associated with gelatinase activity and cytokine expression. Clin. Exp. Immunol. 2011;163:207–214. doi: 10.1111/j.1365-2249.2010.04285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maretti-Mira AC, et al. MMP-9 activity is induced by Leishmania braziliensis infection and correlates with mucosal leishmaniasis. Acta Trop. 2011;119:160–164. doi: 10.1016/j.actatropica.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 67.Silva-Almeida M, et al. Extracellular matrix alterations in experimental Leishmania amazonensis infection in susceptible and resistant mice. Vet. Res. 2012;43:10. doi: 10.1186/1297-9716-43-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Diaz YR, et al. T-bet, GATA-3, and Foxp3 expression and Th1/Th2 cytokine production in the clinical outcome of human infection with Leishmania (Viannia) species. J. Infect. Dis. 2010;202:406–415. doi: 10.1086/653829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Castilho TM, et al. Murine model of chronic L (Viannia) panamensis infection: role of IL-13 in disease. Eur. J. Immunol. 2010;40:2816–2829. doi: 10.1002/eji.201040384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Couppie P, et al. Disseminated cutaneous leishmaniasis due to Leishmania guyanensis: case of a patient with 425 lesions. Am. J. Trop. Med. Hyg. 2004;71:558–560. [PubMed] [Google Scholar]
- 71.Bourreau E, et al. Intralesional regulatory T-cell suppressive function during human acute and chronic cutaneous leishmaniasis due to Leishmania guyanensis. Infect. Immun. 2009;77:1465–1474. doi: 10.1128/IAI.01398-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schonian G, et al. Genetic variability within the species Leishmania aethiopica does not correlate with clinical variations of cutaneous leishmaniasis. Mol. Biochem. Parasitol. 2000;106:239–248. doi: 10.1016/s0166-6851(99)00216-9. [DOI] [PubMed] [Google Scholar]
- 73.Kumar D, et al. Post-kala-azar dermal leishmaniasis (PKDL) developing after treatment of visceral leishmaniasis with amphotericin B and miltefosine. Ann. Trop. Med. Parasitol. 2009;103:727–730. doi: 10.1179/000349809X12554106963438. [DOI] [PubMed] [Google Scholar]
- 74.Ansari NA, et al. Interferon (IFN)-γ, tumor necrosis factor-α, interleukin-6, and IFN-γ receptor 1 are the major immunological determinants associated with post-kala azar dermal leishmaniasis. J. Infect. Dis. 2006;194:958–965. doi: 10.1086/506624. [DOI] [PubMed] [Google Scholar]
- 75.Castellucci L, et al. CXCR1 and SLC11A1 polymorphisms affect susceptibility to cutaneous leishmaniasis in Brazil: a case–control and family-based study. BMC Med. Genet. 2010;11:10. doi: 10.1186/1471-2350-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ramasawmy R, et al. The –2518 bp promoter polymorphism at CCL2/MCP1 influences susceptibility to mucosal but not localized cutaneous leishmaniasis in Brazil. Infect. Genet. Evol. 2010;10:607–613. doi: 10.1016/j.meegid.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Castellucci L, et al. IL6-174 G/C promoter polymorphism influences susceptibility to mucosal but not localized cutaneous leishmaniasis in Brazil. J. Infect. Dis. 2006;194:519–527. doi: 10.1086/505504. [DOI] [PubMed] [Google Scholar]
- 78.Castellucci L, et al. FLI1 polymorphism affects susceptibility to cutaneous leishmaniasis in Brazil. Genes Immun. 2011;12:589–594. doi: 10.1038/gene.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Jesus Fernandes Covas C, et al. Candidate gene case–control and functional study shows macrophage inhibitory factor (MIF) polymorphism is associated with cutaneous leishmaniasis. Cytokine. 2013;61:168–172. doi: 10.1016/j.cyto.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 80.Al-Qahtani A, et al. Toll-like receptor 3 polymorphism and its association with hepatitis B virus infection in Saudi Arabian patients. J. Med. Virol. 2012;84:1353–1359. doi: 10.1002/jmv.23271. [DOI] [PubMed] [Google Scholar]
- 81.Ghoshal A, et al. 9-O-acetylated sialic acids enhance entry of virulent Leishmania donovani promastigotes into macrophages. Parasitology. 2009;136:159–173. doi: 10.1017/S0031182008005180. [DOI] [PubMed] [Google Scholar]
- 82.Salhi A, et al. Immunological and genetic evidence for a crucial role of IL-10 in cutaneous lesions in humans infected with Leishmania braziliensis. J. Immunol. 2008;180:6139–6148. doi: 10.4049/jimmunol.180.9.6139. [DOI] [PubMed] [Google Scholar]
- 83.Petzl-Erler ML, et al. Association of mucosal leishmaniasis with HLA. Hum. Immunol. 1991;32:254–260. doi: 10.1016/0198-8859(91)90088-q. [DOI] [PubMed] [Google Scholar]
- 84.Okwor I, et al. The immunology of Leishmania/HIV co-infection. Immunol. Res. 2013;56:163–171. doi: 10.1007/s12026-013-8389-8. [DOI] [PubMed] [Google Scholar]
- 85.Andreani G, et al. Mechanisms of interaction between protozoan parasites and HIV. Curr. Opin. HIV AIDS. 2012;7:276–282. doi: 10.1097/COH.0b013e32835211e9. [DOI] [PubMed] [Google Scholar]
- 86.Barreto-de-Souza V, et al. Increased Leishmania replication in HIV-1-infected macrophages is mediated by tat protein through cyclooxygenase-2 expression and prostaglandin E2 synthesis. J. Infect. Dis. 2006;194:846–854. doi: 10.1086/506618. [DOI] [PubMed] [Google Scholar]
- 87.Danaher RJ, et al. HIV protease inhibitors alter innate immune response signaling to double-stranded RNA in oral epithelial cells: implications for immune reconstitution inflammatory syndrome? AIDS. 2010;24:2587–2590. doi: 10.1097/QAD.0b013e32833f4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cabrera M, et al. Polymorphism in tumor necrosis factor genes associated with mucocutaneous leishmaniasis. J. Exp. Med. 1995;182:1259–1264. doi: 10.1084/jem.182.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Drexler SK, Foxwell BM. The role of Toll-like receptors in chronic inflammation. Int. J. Biochem. Cell Biol. 2010;42:506–518. doi: 10.1016/j.biocel.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 90.Moumad K, et al. Genetic polymorphisms in host innate immune sensor genes and the risk of nasopharyngeal carcinoma in North Africa. G3 (Bethesda) 2013;3:971–977. doi: 10.1534/g3.112.005371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li G, Zheng Z. Toll-like receptor 3 genetic variants and susceptibility to hepatocellular carcinoma and HBV-related hepatocellular carcinoma. Tumour Biol. 2013;34:1589–1594. doi: 10.1007/s13277-013-0689-z. [DOI] [PubMed] [Google Scholar]
- 92.Qian F, et al. Impaired Toll-like receptor 3-mediated immune responses from macrophages of patients chronically infected with hepatitis C virus. Clin. Vaccine Immunol. 2013;20:146–155. doi: 10.1128/CVI.00530-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sironi M, et al. A common polymorphism in TLR3 confers natural resistance to HIV-1 infection. J. Immunol. 2012;188:818–823. doi: 10.4049/jimmunol.1102179. [DOI] [PubMed] [Google Scholar]
- 94.Barreiro LB, et al. Evolutionary dynamics of human Toll-like receptors and their different contributions to host defense. PLoS Genet. 2009;5:e1000562. doi: 10.1371/journal.pgen.1000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ogura Y, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 96.Hugot JP, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 97.Girardin SE, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 98.Wang BX, Fish EN. The yin and yang of viruses and interferons. Trends Immunol. 2012;33:190–197. doi: 10.1016/j.it.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wilson EB, Brooks DG. Decoding the complexity of type I interferon to treat persistent viral infections. Trends Microbiol. 2013;21:634–640. doi: 10.1016/j.tim.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bogdan C, et al. The role of type I interferons in non-viral infections. Immunol. Rev. 2004;202:33–48. doi: 10.1111/j.0105-2896.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- 101.Mattner J, et al. Protection against progressive leishmaniasis by IFN-β. J. Immunol. 2004;172:7574–7582. doi: 10.4049/jimmunol.172.12.7574. [DOI] [PubMed] [Google Scholar]
- 102.Xin L, et al. Type I IFN receptor regulates neutrophil functions and innate immunity to Leishmania parasites. J. Immunol. 2010;184:7047–7056. doi: 10.4049/jimmunol.0903273. [DOI] [PMC free article] [PubMed] [Google Scholar]