Abstract
Background
After a prolonged period of increasing rates of lung cancer incidence and mortality for both men and women, incidence and mortality rates are decreasing in men and stabilizing in women. The goal of this study was to assess changes over 20 years in the prevalence of known risk factors for lung cancer and to elucidate possible predictors associated with lung cancer survival.
Methods
The study included a total of 908 patients with primary lung cancer referred to The University of Texas M. D. Anderson Cancer Center over three study periods 1985–1989 (N=392), 1993–1997 (N= 216), and 2000–2004 (N= 300). Detailed questionnaires were used to collect information from the patients. Hazard ratios were estimated by fitting a Cox proportional hazards model. Using the Kaplan Meier method, survival in months was calculated up to 2 years from the date of diagnosis to achieve comparability in the three groups.
Results
We observed a decrease in the proportion of patients who are current cigarette smokers and an increase in the proportion of patients who present with adenocarcinoma of the lung, are obese and patients who present with localized disease. We also found an increase in the number of patients who report a family history of lung cancer. The overall median survival duration has increased over the years from 12.0 months in 1985–1989 to 17.5 months in 2000–2004. Also, the probability of survival of patients who were alive at 2 years after diagnosis has also increased (26.5% in 1985–1989 to 40.8% in 2000–2004). Overall, women had a better median survival than men.
Conclusions
The results show that the demographic, histologic, clinical, and outcome variables of patients with lung cancer have changed over the past 20 years. Most important, the survival of patients with lung cancer has improved.
Author's Key words: Lung cancer survival, time-trends, predictors for lung cancer survival
Introduction
Lung cancer (LC) is the second most common incident cancer and the leading cause of cancer mortality in the United States [1]. In 2008, it is estimated that lung cancer will account for 90,810 deaths in men and 71,030 deaths in women [1]. The prevalence rates of lung cancer have increased over time for both men and women; whereas recent US trends show that lung cancer incidence and mortality rates are decreasing in men and in women, after long periods of increase, a stable trend (plateau phase) can be observed [1,2,3]. Also, a recent study has shown that, although the survival rates have increased for both men as well as women with survival rates for women being higher than men, the survival rates have shown greater improvements in men; thereby resulting in a narrowed gender gap [4]. The 2-year survival rate gap narrowed from 5.2% in 1975–1987 to 4.5% in 1988–1999 and 5-year survival rate gap decreased from 4.2% in 1975–1987 to 3.5% 1988–1999; with the results being more pronounced for local disease.
To date, few studies have evaluated changes over time in demographic, histologic, and clinical factors as related to outcome of patients with lung cancer. The goal of this study was to assess changes over 20 years in the prevalence of known risk factors for lung cancer and to elucidate possible predictors associated with lung cancer survival. We present data from 908 patients with primary lung cancer referred to The University of Texas M. D. Anderson Cancer Center over three study periods: 1985–1989, 1993–1997, and 2000–2004.
Materials and Methods
The criteria for including patient data in this retrospective study were (1) the patients should be US residents with histologically diagnosed primary lung cancer, (2) the patients should have received their primary treatment at M. D. Anderson Cancer Center, and (3) the patients should have completed detailed self-administered or interviewer-administered risk factor questionnaires. The Institutional Review Board of M. D. Anderson approved this study.
Selection of Time Periods
The three five-year time periods selected for analysis were 1985–1989, 1993–1997, and 2000–2004. Each study period included five years of data with a two- to three-year gap between two consecutive time periods, which resulted in a total assessment time of 20 years.
For the study period 1985–1989, data were collected from Patient Risk Evaluation Program (PREP) questionnaires completed by a cross-section of patients who were registered at M. D. Anderson from January 1985 through December 1993. All newly registering adult patients were asked to complete a self-administered demographic and epidemiologic questionnaire that included questions about sex, ethnicity, date of birth, education level, and smoking history.
For the period 1993–1997, data were collected as part of an ongoing molecular epidemiologic study of lung cancer. The participants were recruited from the Thoracic Center at M. D. Anderson since 1992 and included patients with newly diagnosed, histopathologically confirmed lung cancer who were enrolled in the study before the initiation of any treatment regimen. Participants were asked to fill out an informed consent form, and trained interviewers administered a detailed questionnaire to gather similar information on variables such as demographic characteristics, smoking history, occupational exposures, medical history, and family history of cancer.
For the period 2000–2004, the self-administered questionnaire, the Patient History Database Questionnaire (PHDB), was distributed to all new patients at the time of initial presentation as part of the clinical intake assessment process. The PHDB form provides a template for clinicians to complete the primary medical evaluation of patients. Data are abstracted and entered into a web-based Oracle database (an institution-wide core epidemiologic database) by certified clinical coding specialists. The database is maintained by the Department of Epidemiology at M. D. Anderson Cancer Center. The information that is gathered routinely from every new patient registering at M. D. Anderson includes standardized and uniform epidemiological data such as exposures to potential carcinogens, family cancer history, history of previous cancers, and quality of life indices. This data collection process was launched in December 1999. Quality control checks are performed regularly to maintain the integrity of the data. PHDB data from all patients with histopathologically confirmed lung cancer were included in the current risk analysis.
The questionnaires that were used to collect data in all three study periods were developed under the direction of Dr. Margaret Spitz. Therefore, there is strong continuity among the three questionnaires with respect to questionnaire structure, data query structure and format. Furthermore, all patients are routinely followed-up by scheduled appointments and by letters and/or phone calls. The Bureau of Vital Statistics from Texas, New Mexico, and Oklahoma is also checked every four months to verify patient vital status.
Clinical data, including cancer site, histologic type, date of diagnosis, treatment, and survival, were obtained from the Tumor Registry at M. D. Anderson.
We only included data for patients who had undergone treatment at M. D. Anderson Cancer Center, as we believe that patients who undergo some type of treatment provide more meaningful estimates for survival and are truly “analyzable” patients. Also, excluded were patients who received no treatment due to advanced disease (and were probably referred for hospice care) and patients who had come to M. D. Anderson Cancer Center for a “second opinion only” and may have received their treatment at other institutions closer to their place of residence. Including these patients could have resulted in a selection bias. For time period 2000–2004, data were available for 2336 patients; however to make groups comparable, we randomly selected 300 patients from this group for analyses.
Study Variables and Data Collection
The M. D. Anderson Tumor Registry uses the staging system of the Surveillance, Epidemiology and End Results (SEER) [5] program cancer registries that are required data elements for registries participating in the National Program of Cancer Registries of the Centers for Disease Control and Prevention (CDC). Stage is classified as localized, extensive (including direct extensive, lymph node involvement, and combined), and distant disease. Patients were further classified by histological lung cancer type (squamous, adenocarcinoma, other non-small cell lung carcinoma (NSCLC other) and small cell carcinoma) and treatment (surgery-based therapy, chemotherapy with no radiation, radiation with no chemotherapy, and chemotherapy and radiation combined).
Current smokers (CS) were defined as active smokers and included those who had stopped smoking less than 1 year before lung cancer diagnosis, former smokers (FS) were defined as those who had not smoked for one or more years before diagnosis, and never smokers (NS) were defined as those who had smoked fewer than 100 cigarettes in their lifetime.
Self-reported weight and height were available to compute body mass index (BMI) that was divided into three categories based on CDC criteria: underweight to normal (BMI < 25), overweight (BMI, 25.0–29.9), and obese (BMI ≥ 30). For analysis purposes, the overweight and obese BMI groups were combined, as very few patients were in the obese group. The presence of a family history of lung cancer was defined as reporting at least one first-degree relative (parent, sibling or offspring) with lung cancer. The overall pack-years variable for the three time periods was combined and dichotomized by median pack-years (46 pack-years) and smoking was characterized as light smokers (≤46 pack-years) and heavy smokers (>46 pack-years) versus never smokers.
Statistical Analysis
We used the Pearson chi-square test to determine if statistically significant differences existed between the three time groups in the distribution of all categorical variables and one-way analysis of variance (ANOVA) to test for differences in all continuous variables. Survival in months was calculated up to 2 years from the date of lung cancer diagnosis. This time period was selected to make the three groups comparable, since data for the last group (2000–2004) were available for only 2 years after entry into the study. Those who were alive or lost to follow-up after 2 years were censored. For calculating the survival rate, patients who were censored within the 1-year or 2-year periods were counted as alive. The Kaplan Meier method was used to calculate and compare survival between the groups, and the log-rank statistic was used to evaluate significant (two-sided alpha=0.05) differences in survival time. Hazard ratios (HRs) (1985–1989 group used as the reference) were estimated by fitting a Cox proportional hazards model adjusted for sex, ethnicity, smoking status, histology, stage, treatment, family history, and BMI. We used the Schoenfeld residuals plot (and added time-varying covariates to the model) to test the hypothesis of a proportional hazard. Those variables that did not meet the proportional hazard assumption were treated as time-dependent variables. For the survival analyses, age at diagnosis was categorized as ≤ 45 years, 46–60 years, and > 60 years. We used the SAS for Windows software package (version 9.1) to complete all statistical analyses. Patients with missing values for a variable were excluded from analysis for that variable.
Results
A total of 908 patients were included: 1985–1989 (N=392), 1993–1997 (N= 216), and 2000–2004 (N= 300). This represents 28.3% of all eligible lung cancer patients at M. D. Anderson for the period 1985–1989, 15.1% of eligible lung cancer patients for the period 1993–1997, and 10.8% of eligible lung cancer patients for the period 2000–2004. The data that were used in 1985–1989 (PREP) and 2000–2004 (PHDB) were from self-administered questionnaires, whereas the data collected in 1993–1997 were part of an ongoing lung cancer study for which research interviewers collected the data.
The observed differences in prevalence of key variables between the three time periods are summarized in Table 1. Over the three time periods, the percentage of women (36.2% to 40.6%) and nonwhites (10.5% to 20.8%) presenting with lung cancer increased (P=0.08 and P=0.0009, respectively). The percentage of current smokers decreased (56.9% to 22.2%), while that of former smokers (35.7% to 68.9%) and never smokers (7.4 % to 8.9%) increased (P<0.0001). A decrease in the percentage of current smokers and an increase in the percentage of former smokers were also observed when the analysis was stratified by gender (P<0.0001, supplementary data Table A). Among ever smokers (current and former smokers) there was a decreasing, yet non-significant, trend for mean pack-years smoked among the three groups (P=0.09). We also observed an increase in age at diagnosis across the three time periods (P=0.0004). This trend was evident in both sexes (P<0.0001), current smokers (P=0.0072), whites (P<0.0001), and all histologic groups (P<0.001) (supplementary data Table B). Age at smoking initiation also increased from 16.9 years in 1985–1989 to 17.5 years in 1993–1997 and to 18.2 years in the most recent time period (P=0.006).
Table 1.
Changes in prevalence of lung cancer variables over three time periods spanning 20 years
| Time Period | |||||
|---|---|---|---|---|---|
| Variable | N | 1985–1989 Total N=392 N (%) | 1993–1997 Total N=216 N (%) | 2000–2004 Total N=300 N (%) | P-value (2 sided) |
| Gender: | |||||
| Female | 359 | 142 (36.2) | 98(45.4) | 119 (40.6) | |
| Male | 542 | 250 (63.8) | 118(54.6) | 174(59.4) | 0.08 |
| Age at Diagnosis: | |||||
| Mean±SD | 901 | 60.25± 9.6 | 62.3±9.97 | 63.18±10.4 | 0.0004 |
| Ethnicity: | |||||
| White | 765 | 351 (89.5) | 182 (84.3) | 232 (79.2) | |
| Nonwhite | 136 | 41 (10.5) | 34 (15.7) | 61 (20.8) | 0.0009 |
| Smoking Status: | |||||
| Never | 66 | 29 (7.4) | 11 (5.1) | 26 (8.9) | |
| Former | 448 | 140 (35.7) | 106 (49.1) | 202 (68.9) | |
| Current | 387 | 223 (56.9) | 99 (45.8) | 65 (22.2) | <0.0001 |
| Age at Smoking Initiation: | |||||
| Mean±SD | 821 | 16.88±4.72 | 17.45±5.1 | 18.2±5.46 | 0.006 |
| Pack-years: | |||||
| Mean±SD | 864 | 52.17±33.5 | 51.44±35.1 | 46.37±37.3 | 0.09 |
| Histology: | |||||
| Squamous | 209 | 91 (23.2) | 45 (20.8) | 73 (24.9) | |
| Adenocarcinoma | 406 | 174 (44.4) | 106 (49.1) | 126 (43.0) | |
| NSCLC, other | 120 | 63 (16.1) | 21 (9.7) | 36 (12.3) | |
| Small Cell | 166 | 64 (16.3) | 44 (20.4) | 58 (19.8) | 0.22 |
| Stage: | |||||
| Localized | 145 | 53 (14.0) | 48 (23.7) | 44 (15.4) | |
| Extensive | 273 | 135 (35.5) | 62 (30.5) | 76 (26.7) | |
| Distant | 450 | 192 (50.5) | 93 (45.8) | 165 (57.9) | 0.004 |
| Treatment: | |||||
| Surgery | 238 | 142(36.2) | 52 (24.1) | 44 (15.0) | |
| Chemotherapy | 257 | 89(22.7) | 62 (28.7) | 106 (36.2) | |
| Radiation | 231 | 103 (26.3) | 54 (25.0) | 74 (25.3) | |
| Chemotherapy & Radiation | 175 | 58(14.8) | 48(22.2) | 69 (23.5) | <0.0001 |
| Family History of Lung Cancer | |||||
| None | 633 | 335 (90.6) | 158 (73.1) | 120 (67.8) | |
| 1+ | 152 | 37 (9.4) | 58 (26.9) | 57 (32.2) | <0.0001 |
| BMI: | |||||
| ≤24.9 | 397 | 171 (51.0) | 101 (47.0) | 125 (44.5) | |
| 25–29.9 | 306 | 127 (38.0) | 82 (38.1) | 97 (34.5) | |
| ≥30 | 128 | 37 (11.0) | 32 (14.9) | 59 (21.0) | 0.02 |
The percentage of patients who presented with small cell carcinoma has increased (16.3% to 19.8 %), while the percentages of patients with adenocarcinoma (about 44%) and squamous cell carcinoma (about 23%) remained fairly constant over the three time points (P=0.22). When the data were stratified by sex, we observed that the proportion of adenocarcinoma in women remained consistent across the time points and in men decreased over time (P=0.11, supplementary data Table A). The percentage of patients presenting with localized cancer increased in the second time period and decreased thereafter (14.0%, 23.7%, and 15.4% respectively), and the percentage of extensive-stage cancer decreased (35.5% to 26.7%; P=0.004). Such a trend in presentation was observed in men (P=0.01, supplementary data Table A). In addition, there was a decrease in the percentage of patients undergoing surgery and radiation use has remained stable, while the use of chemotherapy, and a combination of chemotherapy and radiation increased (P<0.0001).
The percentage of patients self-reporting a family history of cancer was 9.4% in 1985–1989, 26.9% in 1993–1997, and 32.2% in 2000–2004 (P<0.0001). Over time the prevalence of obesity has increased (11.0% in 1985–1989 to 21.0% in 2000–2004; P=0.02).
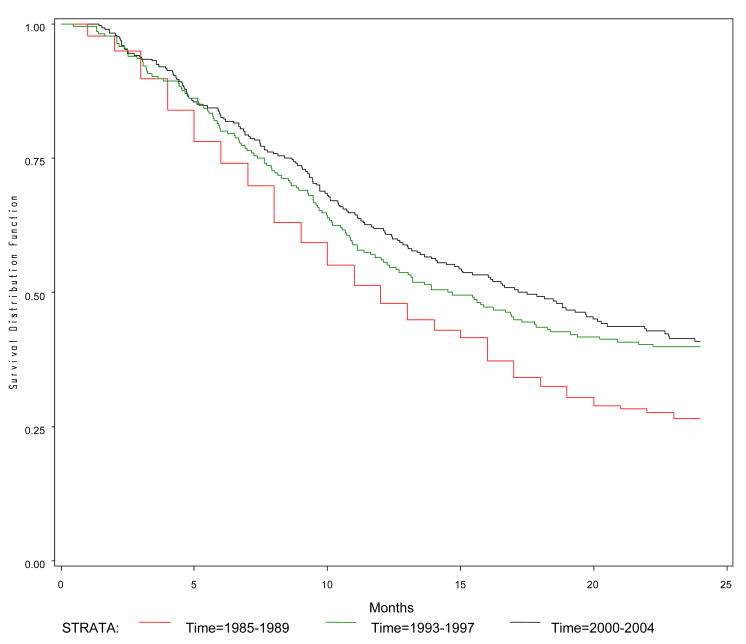
The survival interval (in months) and probability of survival at 1- and 2-year for the three study periods are summarized in Table 2. The overall median survival was 12.0 months (95% CI, 11.0–13.0) for 1985–1989, 14.6 months (95% CI, 11.8–18.3) for 1993–1997, and 17.5 months (95% CI, 14.1–20.5) for 2000–2004 (P=0.0001). The probabilities of survival at 2-year for the three groups were 26.5%, 39.8%, and 40.8% (P<0.0001). The Kaplan-Meier curve for 2-year survival confirms that the 2000–2004 group had the best 2-year survival rate compared to the other two groups (Figure 1).
Table 2.
Median survival time (in months) and the probability of survival at 1- and 2-years
| Year | 1985–1989 | 1993–1997 | 2000–2004 | P value |
|---|---|---|---|---|
| Overall Median Survival Months (95% CI) | 12.0 (11.0–13.0) | 14.6 (11.8–18.3) | 17.5 (14.1–20.5) | 0.0001 |
| Probability of survival at1-year (95% CI) | 47.9 (42.9–52.8) | 56.0 (49.6–62.8) | 61.5 (55.5–66.9) | <0.0001 |
| Probability of survival at2-year (95% CI) | 26.5 (22.3–31.0) | 39.8 (33.2–46.2) | 40.8 (34.8–46.8) | <0.0001 |
Figure 1.
Two-year survival curve by time period for patients with lung cancer
Table 3 presents the hazard ratios (HRs) from the Cox proportional hazard model. Treatment did not meet the proportional hazard assumption and was included as a time-dependent variable. Overall, men exhibited poorer survival outcomes than women (HR 1.25, 95% CI 1.01–1.54). There was no significant difference in survival among never smokers (HR 0.74, 95% CI 0.49–1.12) and light smokers (HR 1.06, 95% CI 0.86–1.31) compared to heavy smokers. Predictably, patients who presented with extensive- or distant-stage disease had 4.41 to 9.69 times poorer survival compared to patients presenting with local-stage disease. Compared to those patients who were treated with surgery alone, patients who received chemotherapy, radiation, or a combination of both had approximately 4-fold rates of poorer survival. Patients who presented with adenocarcinoma (HR 1.15, 95% CI 0.88–1.51), squamous cell carcinoma (HR 1.09, 95% CI 0.80–1.49) or NSCLC Other (HR 1.21, 95% CI 0.87–1.68) had poorer survival compared to patients who presented with small cell carcinoma, although none of the HR’s were statistically significant. Taking into account the covariates above, survival significantly improved across the three study periods, as observed in decreasing HRs for 1993–1997 (HR 0.51, 95% CI 0.38–0.67) and 2000–2004 (HR 0.74, 95% CI 0.58–0.93), compared to the earliest study period.
Table 3.
Multivariate Cox Model of Lung Cancer Prognostic Factors
| Variable | Reference Group | Hazard Ratio | 95% CI |
|---|---|---|---|
| Time Period | |||
| 1993–1997 | 0.51 | 0.38–0.67 | |
| 2000–2004 | 1985–1989 | 0.74 | 0.58–0.93 |
| Sex | Female | 1.25 | 1.01–1.54 |
| Ethnicity | NonWhite | 0.96 | 0.71–1.29 |
| Histology | |||
| Adenocarcinoma | 1.15 | 0.88–1.51 | |
| Squamous | 1.09 | 0.80–1.49 | |
| NSCLC, Other | Small Cell Carcinoma | 1.21 | 0.87–1.68 |
| Stage | |||
| Extensive | 4.41 | 2.50–7.75 | |
| Distant | Local | 9.69 | 5.53–16.97 |
| Treatment* | |||
| Chemotherapy | 3.89 | 0.99–15.29 | |
| Radiation | 3.87 | 1.12–13.33 | |
| Chemo & Radiation | Surgery | 4.09 | 1.03–16.17 |
| Family History of Lung Cancer | None | 0.74 | 0.57–0.96 |
| BMI < 25 | BMI ≥ 25 | 0.97 | 0.80–1.19 |
| Smoking | |||
| Light Smoker | 1.06 | 0.86–1.31 | |
| Never Smoker | Heavy Smokers | 0.74 | 0.49–1.12 |
For time-dependant variables, the HRs and 95% CIs are calculated for 24 months of follow-up. Given different follow-up time, HRs will change accordingly.
Discussion
This comparison from three time-periods of patient registrations at a single cancer center shows that the demographic, histologic, clinical, and outcome variables of patients with lung cancer have changed over the past 20 years. Most importantly, two-year survival of patients with lung cancer treated at this institution has improved.
Nationally, the population prevalences of never and former smokers have increased, and the percentage of current smokers has decreased over time (P<0.0001). Similar trends were observed for both men and women in our data (P=0.08). Women were more likely to be never smokers and to start smoking at a later age compared to men; however, they also tended to be diagnosed at earlier ages (supplementary data Tables A and B). Previous reports have also reported gender differences in age at diagnosis, cigarette smoking history, histologic type, and survival [6–9]. We noted that a higher percentage of men compared with women were current and former smokers for all three time-periods. In a study of 20,561 lung cancer patients (2875 women and 17 686 men), women presented at younger ages, smoked less intensively, consumed fewer cigarettes per day, and were more likely to be lifetime nonsmokers [10]. Visbal et al. also reported that men smoked at a higher intensity compared to women [11].
Higher percentages of both men and women were diagnosed with adenocarcinoma compared with other histologies, although over the years, the percentage of men with adenocarcinoma has gradually decreased, whereas in women, it has remained fairly constant (supplementary data Table A). Travis et al. reported that the incidence rates for adenocarcinoma rates plateaued in white men between 1989–1991 and continued to increase among women [12]. In contrast, some studies reported a higher proportion of men diagnosed with squamous cell carcinoma [11,13,14]. The percentage of patients who presented with small cell carcinoma at our institution has increased; however national reports show a decline in the incidence rate [15]. This could represent referral bias.
The percentage of patients with localized cancer (14.0% to 15.4%) and distant-stage (50.5% to 57.9%) also increased over the time period of our study, and concomitantly the percentage of extensive-stage disease has decreased (P=0.004). The increase in localized cancer for the second time group compared to other two groups for that period could be explained by our focus on targeting surgical patients and thus this steep increase could be due to over-sampling and not a true trend. Furthermore, M. D. Anderson is a comprehensive cancer hospital and many patients are referred here for tertiary care.
Over the years of our study, the percentages of patients treated with chemotherapy and a combination of chemotherapy and radiation have increased, and the percentage of patients undergoing surgery alone has decreased (P<0.0001). This may reflect changes in the therapeutic standards, but could also indicate an increased practice of using neo-adjuvant therapy. Two meta-analyses, one by Pignon et al [16] and the other by Warde and Payne [17], have reported that combination of chemotherapy and thoracic radiation results in a significant, albeit small, improvement in survival and thus an increased referral to radiotherapy centers as compared to patients undergoing chemotherapy alone in the community centers.
A family history of lung cancer is a known risk factor for lung cancer [18]. We observed that the percentage of patients reporting at least one first-degree family member with lung cancer has increased three-fold over the three study periods (9.4% to 32.2%, P<0.0001). This increase may suggest growing awareness of the importance of knowing one’s family history.
Reflecting national trends, the percentage of obese patients increased approximately twofold (11.0% to 21.0%), whereas the percentage of underweight to normal patients significantly decreased over time (51.0% to 44.5%) (P=0.02). There was however no association demonstrable between BMI and lung cancer survival.
The survival of patients with lung cancer has gradually improved over the years. However, the hazard ratios we report represent survival up to two years only. The overall median survival duration increased over the years from 12.0 months in 1985–1989 to 17.5 months in 2000–2004. The probability of survival at 2 years after diagnosis likewise increased (26.5% in 1985–1989 to 40.8% in 2000–2004). Few studies have analyzed the survival of patients with lung cancer [19,20] or other types of cancer [21–23] by similar trends. Kitajima et al compared the survival of patients with lung cancer between the study periods 1976–1984 (early period) and 1989–1997 (late period) and reported an increase in both median survival time (late period: 49.8 months vs. early period: 27.8 months) and 5-year survival (late period: 47.8% vs. early period: 34.8%) (P=0.0054) [19]. Ungat et al divided their data into five groups to analyze trends in cancer survival from 1969 to 1991 and observed no change in lung cancer survival [20]. However, they analyzed survival trends up to 1991 only, and their study groups were consecutive (for example, 1973–1976, 1977–1980, etc.). In our analyses, the three groups were spaced in such a way (approximately five years of data in each group) that they covered a span of approximately 20 years. Also, we only analyzed 2-year survival for the three groups. Data after two years were censored to increase the comparability of the three groups.
For periods- 1985–1989 and 2000–2004, women had better median survival than men. The median survival duration for women was 13 months (95% CI: 11–16) for 1985–1989, 12.45 months (95% CI: 10.18 – 18.27) for 1993–1997, and 22.83 months (95% CI: 18.13 - NA) for 2000–2004, whereas for men the median survival duration was 11 months (95% CI: 9–13), 16.05 months (95% CI: 12.09 – 20.21), and 12.84 months (95% CI: 1056 – 17.51) respectively (supplementary data Table C). In multivariate Cox analysis, we observed that being male was a poor prognostic factor for lung cancer (HR 1.25, 95% CI 1.01–1.54). A cohort study of 4618 patients with non-small cell lung cancer also reported better survival rates in women (19%) as compared to men (15%) [11].
In the multivariate Cox model, never smokers had a better, albeit non-significant, survival advantage compared to heavy smokers (HR 0.74, 95% CI 0.49–1.12), but there was no significant difference in survival between light and heavy smokers.
Predictable, extensive-stage disease (HR 4.41, 95% CI 2.50–7.75) and distant-stage disease (HR 9.69, 95% CI 5.53–16.97) were significant prognostic factors for poor survival compared to localized carcinoma; with the risk being highest in the distant-stage category. Tumor stage is a well-documented prognostic and predictive factor [24–26]. We also observed that patients whose treatment only involved surgery had better survival compared to other treatment groups. This is not unexpected since surgery is the treatment of choice for patients with early stage non-small cell lung cancer [27].
We observed that patients with a family history of lung cancer had better survival compared to patients with no family history (HR 0.74, 95% CI 0.57–0.96). One possible reason for this observation could be due to lead-time bias. Patients with a family history of lung cancer had a mean age of diagnosis of 60.95 years (SD ± 9.1) compared to 61.75 years (SD ± 10.2) for patients with no family history (P=0.27, supplementary data Table B). This may indicate that patients with a family history of lung cancer are well aware of increased risks and may be more likely to undergo screening.
Our study has some limitations. First, the patient population was taken from a single tertiary care center. Hence, the results may not be generalizable to community practice. More generalizable results may be obtained from population based registries or surveys as compared to hospital-based registries. Also, the racial distribution varies from state-to-state and this may affect the generalizability of our findings. Second, the questionnaires that were administered in 1985–1989 and 2000–2004 were self-administered, whereas for the period 1993–1997, interviewers collected the data. Also, for the first study period, completion of the questionnaires was voluntary, in the second period patients were selected for enrollment in a case-control study and in the third study period the questionnaire was included in intake form and therefore the compliance rate was at least 96%. Response bias (especially among Black or Hispanic patients) for the first two time periods is a major concern. Third, because the time period for our third study group ended in 2004, we collected follow-up data through 2006 only, and hence, we had to censor the survival analysis of other groups to two years.
Nevertheless this analysis shows interesting secular changes in the demographic, histologic, clinical, and outcome variables of lung cancer patients over 20 years. The survival of patients with lung cancer has improved, but further studies will be required to observe the changes in survival over time in relation to variables such as ethnicity, stage and therapy.
Supplementary Material
Acknowledgments
Sources of Support: Our research was supported by a cancer prevention fellowship funded by National Cancer Institute grant K07CA093592, National Cancer Institute grants CA55769 and CA123208, and the Flight Attendant Medical Research Institute.
Footnotes
Conflict of Interest: none declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society. Cancer Facts and Figures 2008. Atlanta GA: American Cancer Society; 2008. cited 2008 February 21. Available from: http://www.cancer.org/downloads/STT/2008CAFFfinalsecured.pdf. [Google Scholar]
- 2.Zang EA, Wynder EL. Differences in lung cancer risk between men and women: examination of the evidence. J Natl Cancer Inst. 1996 Feb 21;88(3–4):183–92. doi: 10.1093/jnci/88.3-4.183. [DOI] [PubMed] [Google Scholar]
- 3.American cancer Society. Cancer Deaths Drop for Second Consecutive Year. Atlanta GA: American Cancer Society; 2007. [cited 2007 June 26]. Available from: http://www.cancer.org/docroot/MED/content/MED_2_1x_Cancer_Deaths_Drop_for_Second_Consecutive_Year.asp?sitearea=MED. [Google Scholar]
- 4.Fu JB, Kau TY, Severson RK, Kalemkerian GP. Lung cancer in women: analysis of the national Surveillance, Epidemiology, and End Results database. Chest. 2005 Mar;127(3):768–77. doi: 10.1378/chest.127.3.768. [DOI] [PubMed] [Google Scholar]
- 5.Surveillance, Epidemiology, and End Results (SEER) 1993–2003 Public-use Data. Bethesda MD: National Cancer Institute; 2007. [cited 2007 Sep 6]. Available from: http://seer.cancer.gov/ [Google Scholar]
- 6.Ferguson MK, Skosey C, Hoffman PC, Golomb HM. Sex-associated differences in presentation and survival in patients with lung cancer. J Clin Oncol. 1990;8:1402–7. doi: 10.1200/JCO.1990.8.8.1402. [DOI] [PubMed] [Google Scholar]
- 7.McDuffie HH, Klaassen DJ, Dosman JA. Female-male differences in patients with primary lung cancer. Cancer. 1987;59:1825–30. doi: 10.1002/1097-0142(19870515)59:10<1825::aid-cncr2820591024>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 8.Johnson BE, Steinberg SM, Phelps R, Edison M, Veach SR, Ihde DC. Female patients with small cell lung cancer live longer than male patients. Am J Med. 1988;85:194–6. doi: 10.1016/s0002-9343(88)80341-3. [DOI] [PubMed] [Google Scholar]
- 9.Wolf M, Holle R, Hans K, Drings P, Havemann K. Analysis of prognostic factors in 766 patients with small cell lung cancer (SCLC): the role of sex as a predictor for survival. Br J Cancer. 1991;63:986–92. doi: 10.1038/bjc.1991.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radzikowska E, Glaz P, Roszkowski K. Lung cancer in women: age, smoking, histology, performance status, stage, initial treatment and survival. Population-based study of 20 561 cases. Ann Oncol. 2002;13:1087–93. doi: 10.1093/annonc/mdf187. [DOI] [PubMed] [Google Scholar]
- 11.Visbal AL, Williams BA, Nichols FC, 3rd, Marks RS, Jett JR, Aubry MC, et al. Gender differences in non-small-cell lung cancer survival: an analysis of 4,618 patients diagnosed between 1997 and 2002. Ann Thorac Surg. 2004 Jul;78(1):209–15. doi: 10.1016/j.athoracsur.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 12.Travis WD, Lubin J, Ries L, Devesa S. United States lung carcinoma incidence trends: declining for most histologic types among males, increasing among females. Cancer. 1996 Jun 15;77(12):2464–70. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2464::AID-CNCR8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 13.Zheng T, Holford TR, Boyle P, Chen Y, Ward BA, Flannery J, et al. Time trend and the age-period-cohort effect on the incidence of histologic types of lung cancer in Connecticut, 1960–1989. Cancer. 1994 Sep 1;74(5):1556–67. doi: 10.1002/1097-0142(19940901)74:5<1556::aid-cncr2820740511>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 14.Morgan LC, Grayson D, Peters HE, Clarke CW, Peters MJ. Lung cancer in New South Wales: current trends and the influence of age and sex. Med J Aust. 2000 Jun 19;172(12):578–82. doi: 10.5694/j.1326-5377.2000.tb124122.x. [DOI] [PubMed] [Google Scholar]
- 15.Yoshimi I, Ohshima A, Ajiki W, Tsukuma H, Sobue T. A comparison of trends in the incidence rate of lung cancer by histological type in the Osaka Cancer Registry, Japan and in the Surveillance, Epidemiology and End Results Program, USA. Jpn J Clin Oncol. 2003 Feb;33(2):98–104. doi: 10.1093/jjco/hyg019. [DOI] [PubMed] [Google Scholar]
- 16.Pignon JP, Arriagada R, Ihde DC, Johnson DH, Perry MC, Souhami RL, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med. 1992 Dec 3;327(23):1618–24. doi: 10.1056/NEJM199212033272302. [DOI] [PubMed] [Google Scholar]
- 17.Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol. 1992 Jun;10(6):890–5. doi: 10.1200/JCO.1992.10.6.890. [DOI] [PubMed] [Google Scholar]
- 18.Cote ML, Kardia SL, Wenzlaff AS, Ruckdeschel JC, Schwartz AG. Risk of lung cancer among white and black relatives of individuals with early-onset lung cancer. JAMA. 2005 Jun 22;293(24):3036–42. doi: 10.1001/jama.293.24.3036. [DOI] [PubMed] [Google Scholar]
- 19.Kitajima T, Nishii K, Ueoka H, Shibayama T, Gemba K, Kodani T, et al. Recent improvement in lung cancer screening: a comparison of the results carried out in two different time periods. Acta Med Okayama. 2006 Jun;60(3):173–9. doi: 10.18926/AMO/30751. [DOI] [PubMed] [Google Scholar]
- 20.Ugnat AM, Xie L, Semenciw R, Waters C, Mao Y. Survival patterns for the top four cancers in Canada: the effects of age, region and period. Eur J Cancer Prev. 2005 Apr;14(2):91–100. doi: 10.1097/00008469-200504000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Helgesen F, Holmberg L, Johansson JE, Bergstrom R, Adami HO. Trends in prostate cancer survival in Sweden, 1960 through 1988: evidence of increasing diagnosis of nonlethal tumors. J Natl Cancer Inst. 1996 Sep 4;88(17):1216–21. doi: 10.1093/jnci/88.17.1216. [DOI] [PubMed] [Google Scholar]
- 22.Post PN, Kil PJ, Coebergh JW. Trends in survival of prostate cancer in southeastern Netherlands, 1971–1989. Int J Cancer. 1999 May 17;81(4):551–4. doi: 10.1002/(sici)1097-0215(19990517)81:4<551::aid-ijc8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 23.Du WB, Chia KS, Sankaranarayanan R, Sankila R, Seow A, Lee HP. Population-based survival analysis of colorectal cancer patients in Singapore, 1968–1992. Int J Cancer. 2002 May 20;99(3):460–5. doi: 10.1002/ijc.10333. [DOI] [PubMed] [Google Scholar]
- 24.Marchevsky AM. Problems in pathologic staging of lung cancer. Arch Pathol Lab Med. 2006 Mar;130(3):292–302. doi: 10.5858/2006-130-292-PIPSOL. [DOI] [PubMed] [Google Scholar]
- 25.Pelletier MP, Edwardes MD, Michel RP, Halwani F, Morin JE. Prognostic markers in resectable non-small cell lung cancer: a multivariate analysis. Can J Surg. 2001 Jun;44(3):180–8. [PMC free article] [PubMed] [Google Scholar]
- 26.Birim O, Kappetein AP, van Klaveren RJ, Bogers AJ. Prognostic factors in non-small cell lung cancer surgery. Eur J Surg Oncol. 2006 Feb;32(1):12–23. doi: 10.1016/j.ejso.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Noel G, Medioni J, Valery CA, Boisserie G, Simon JM, Cornu P, et al. Three irradiation treatment options including radiosurgery for brain metastases from primary lung cancer. Lung Cancer. 2003;41:333–43. doi: 10.1016/s0169-5002(03)00236-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.