Abstract
Objectives
Effective pharmacotherapy for Acanthamoeba keratitis has been hampered because of the marked resistance of various stains to a variety of antimicrobial agents. In view of the fact that topical Brolene (propamidine isethionate) and neosporin are currently considered to be the first-line medical treatment of choice in Europe, we sought to determine whether Alexidine is equally effective, because the latter drug is more readily available in the United States.
Methods
Trophozoites and cysts from 3 pathogenic corneal isolates (A. castellanii, A. polyphaga, and A. rhysodes) were incubated in peptone-yeast extract-glucose medium containing different concentrations of Alexidine for 24 hr. The number of trophozoites was counted by hemocytometer. The cysts were plated in to nonnutrient agar plates precoated with Escherichia coli and observed for viability or excystment over a period of 2 weeks. The capacity of different concentrations of Alexidine to induce cytolysis of corneal epithelial cells was tested in vitro. Chinese hamster corneas were treated with 5 μL of Alexidine topically, every hour; 6 times a day and the corneas were stained with fluorescein to asses the epithelial defects in vivo.
Results
Alexidine was effective in killing the trophozoites at a concentration of 10 μg/mL. However, a higher concentration of Alexidine (100 μg/mL) is required to kill Acanthamoeba cysts and the cytotoxic activities of Alexidine are comparable with chlorhexidine. We have also demonstrated that both Alexidine and chlorhexidine at 100 μg/mL induced significant cytopathic effect on the corneal epithelial cells in vitro. In vivo results indicate that Alexidine at a concentration of 100 μg/mL is less toxic than chlorhexidine when applied topically to the Chinese hamster cornea.
Conclusions
Our study has identified Alexidine as a novel anti-Acanthamoeba drug and suggests that Alexidine may be an effective therapeutic option because of its potency and low toxicity to the corneal tissues when applied topically in vivo.
Keywords: Acanthamoeba, Alexidine, Cytolysis, Cytotoxicity, Corneal epithelial cells
Acanthamoeba is a genus of ubiquitous, free-living protozoa. Acanthamoeba species have been isolated from soil and aquatic environments, including domestic water, swimming pools, eye wash stations, and from the air.1 Acanthamoeba exists in 2 distinct phases: motile metabolically active trophozoites capable of multiplication and are sensitive to antimicrobial agents, and a dormant cyst, resistant to desiccation, disinfection, and extremes of temperature.2,3 Acanthamoeba keratitis is a sight-threatening corneal infection caused by the pathogenic Acanthamoeba species.4 Despite the ubiquitous nature of Acanthamoebae, Acanthamoeba keratitis is most commonly seen in contact lens wearers who have experienced some sort of trauma to the corneal epithelium.4
Acanthamoeba keratitis is characterized by a ring-like corneal infiltrate, epithelial destruction, ocular pain, and resistance to antimicrobial agents.4 Treatment of the disease is very demanding, consisting of hourly applications of Brolene, polyhexamethyl biguanide, or chlorhexidine for several weeks.1 Despite such treatments, Acanthamoeba can cause severe damage to the corneal epithelium and the stroma and patients may eventually require a corneal transplant to restore vision.1 Acanthamoeba cysts are believed to be the source of recurrent infections in Acanthamoeba keratitis patients. Because the cysts can survive in the conjunctiva for several months and excyst under favorable conditions and reinfect the cornea or a corneal transplant, it is important to develop treatment options that are more effective in killing the Acanthamoeba cysts.
Bisbiguanides are a class of chemically related compounds known for their bactericidal properties.5 These compounds include the antiseptics Alexidine and chlorhexidine. Alexidine is the trade name for 1,1′-hexamethylene-bis[5-(2-ethylhexyl) biguanide] and differs chemically from chlorhexidine in possessing ethylhexyl end groups.5 Alexidine is more rapidly bactericidal and produces a significantly faster alteration in bactericidal permeability.6 Both Alexidine and chlorhexidine target the cytoplasmic membrane of the organism.6 It has been proposed that the nature of the ethylhexyl end group in Alexidine, as opposed to the chlorophenol in chlorhexidine, might influence the ability of a biguanide to produce lipid domains in the cytoplasmic membrane.6
Alexidine is a bisbiguinide, containing 2 active sites, and is used in a concentration of 0.0045% in the contact lens solutions.7 Similar to polyhexamide, these biguanide groups interact with and disrupt the acidic phospholipid groups in the amoebae’s cytoplasmic membrane, causing membrane disintegration and cell lysis.5 Although Alexidine has been effectively used as an antimicrobial agent,8 its effect on Acanthamoeba has not been investigated. In this study, we evaluate the effect of Alexidine on Acanthamoeba trophozoites and cysts and compare its effect with chlorhexidine.
MATERIALS AND METHODS
Animals
Chinese hamsters were purchased from Cytogen Research and Development (West Roxbury, MA). All animals used were from 4 to 6 weeks of age, and all corneas were examined before experimentation to exclude animals with preexisting corneal defects. All animals were housed and cared for in accordance with the guidelines of the University Committee for the Humane Care of Laboratory Animals, National Institutes of Health Guidelines on laboratory animal welfare and the Association for Research in Vision and Ophthalmology statement for the use of animals in ophthalmic and vision research.
Acanthamoeba Cultures and Cell Lines
All Acanthamoeba species were obtained from American type culture collection, Manassas, VA. A. castellanii (ATCC-30868), A. polyphaga (ATCC-30461), and A. rhysodes (ATCC-50368) were isolated from human corneas.9 Amoebae were grown as axenic cultures in peptone-yeast extract-glucose medium (PYG) at 35°C with constant agitation as described previously.10
Chinese hamster corneal epithelial cells (HCORN) were immortalized with human papilloma virus E6 and E7 genes and cultured in complete minimum essential medium containing 1% L-glutamine(Bio-Whittaker, Walkersville, MD), 1% penicillin, streptomycin, amphotericin-B (Fungizone; Bio-Whittaker), 1%sodium pyruvate (Bio-Whittaker), and 10% fetal calf serum (Hyclone laboratories, Logan, UT).11
Acanthamoeba Cysts
Growth phase trophozoites were harvested from the culture flasks and washed 2 times with phosphate buffered saline (PBS) and resuspended in 10 mL of encystment medium containing 50 mm of MgCl2 at a final concentration of 1 × 106 trophozoites/mL.12 The trophozoites were incubated at 4°C for 1 week for encystment. Cyst purity was verified by calcofluor white staining to ensure 100% cyst population before use.12
Drugs
Alexidine dihydrochloride was obtained from Toronto Research Chemicals, Inc. (Toronto, Ontario, Canada). The drug was diluted in 10% methanol. Chlorhexidine was purchased from Sigma (Sigma Chemical Co., St Louis, MO).
Effect of Alexidine and Chlorhexidine on Acanthamoeba Trophozoites and Cysts
Trophozoites (1 × 106/mL) in the growth phase from A. castellanii, A. polyphaga, and A. rhysodes were incubated in PYG medium containing 10 and 20 μg/mL of Alexidine for 24 hr. Trophozoites were then counted by hemocytometer. The control cultures were trophozoites incubated for 24 hr in PYG medium without Alexidine.
Cysts from A. castellanii, A. polyphage, and A. rhysodes were incubated with different concentrations of Alexidine as 50, 80, and 100 μg/mL for 24 hr and counted by hemocytometer. The cysts were plated on to nonnutrient agar (NNA) plates precoated with Escherichia coli and observed for viability or excystment over a period of 2 weeks. After the samples were directly inoculated on NNA, plates were sealed, incubated at 35°C for 14 days, and investigated daily by inverted microscopy for amoebal growth.
Trophozoites and cysts from the 3 pathogenic strains were tested in a similar manner with chlorhexidine at 10, 20, 50, 80, 100, and 200 μg/mL concentrations.
Cytopathic Effect of Alexidine on HCORN Cells
Chinese hamster corneal epithelial (HCORN) cells were chosen as target cells for the cytopathic effect (CPE) assay based on known susceptibility of Chinese hamsters to Acanthamoeba corneal infection. The CPE assays were performed as described previously.13 Different concentrations of Alexidine (10, 20, 50, and 100 μg/mL) were added onto 96-well plates with a confluent monolayer of HCORN cells and incubated for 8 and 20 hr at 35°C. Control cells were not treated with the drug. After incubation, the plates were washed 3 times with the culture medium and stained with Giemsa stain. The remaining contents were solubilized in 200 μL of 5% sodium dodecyl sulfate (Sigma Chemical Co.) in PBS and transferred to a new 96-well plate, and the density of the residual epithelial cells was quantified by determining the optical density at 590 nm on a microplate reader (Molecular Devices, Menlo Park, CA). Results were expressed as mean optical density ± standard error for each group. All assays were performed in triplicate. The spectrophotometric results were confirmed by inspection of the wells with compound microscopy.
In Vivo Corneal Cytotoxicity
Chinese hamsters were anesthetized with ketamine and xylazine (Fort Dodge Laboratories, Fort Dodge, IA) injected peritoneally. Before manipulation, the corneas were anesthetized by topical application of Alcain (Alcon Laboratories, Fort Worth, TX).
Chinese hamster corneas were treated with 5 μL of Alexidine topically, every hour, 6 times a day, and the corneas were stained with fluorescein (FLU-GLO sterile strip, Akorn, Inc., IL) to assess the epithelial defect intensity. Control corneas were treated with the vehicle (1:4 dilution of 10% methanol). Positive controls consisted of corneas abraded with a cotton swab to produce an epithelial defect. Chinese hamster corneas were also treated with 5 μL of chlorhexidine and every hour, 6 times a day, and the epithelial defect was detected by the fluorescein strip.
Statistical Analysis
All statistical analyses were performed using the unpaired Student’s t test. A value of P<0.05 was considered statistically significant.
RESULTS
Effect of Alexidine and Chlorhexidine on Acanthamoeba Trophozoites
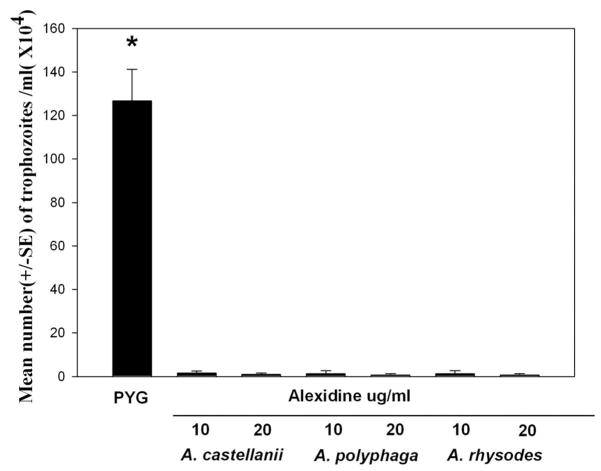
To test the effect of Alexidine on Acanthamoeba trophozoites, 1 × 106 trophozoites/mL from the 3 pathogenic strains (A. castellanii, A. polyphaga, and A. rhysodes) were incubated with 10 or 20 μg/mL of Alexidine dissolved in 1:4 dilution of 10% methanol. Trophozoites incubated with the vehicle alone served as control. After 24 hr of incubation, the numbers of trophozoites were counted with a hemocytometer using trypan blue to check for viability. Alexidine dihydrochloride was effective in killing the trophozoites at a concentration of 10 μg/mL. The vehicle alone did not have an effect on the viability of the trophozoites (Fig. 1).
FIG. 1.
Amoebicidal effect of alexidine against A. castellanii, A. polyphaga, and A. rhysodes trophozoites. Trophozoites (1 × 106/mL) in the growth phase were incubated in peptone-yeast extract-glucose (PYG) medium containing 10 μg/mL and 20 μg/mL of alexidine for 24 hr. Trophozoites were then counted by hemocytometer. The control cultures were trophozoites incubated for 24 hr in PYG medium without alexidine. After 24 hr of incubation, the numbers of trophozoites were counted with hemocytometer. *Significantly different from untreated controls (P<0.05).
Trophozoites from the 3 pathogenic strains were tested with same concentrations (10 or 20 μg/mL) of chlorhexidine. Chlorhexidine was effective in killing the trophozoites at a concentration of 10 μg/mL (data not shown).
Effect of Alexidine and Chlorhexidine on Acanthamoeba Cysts
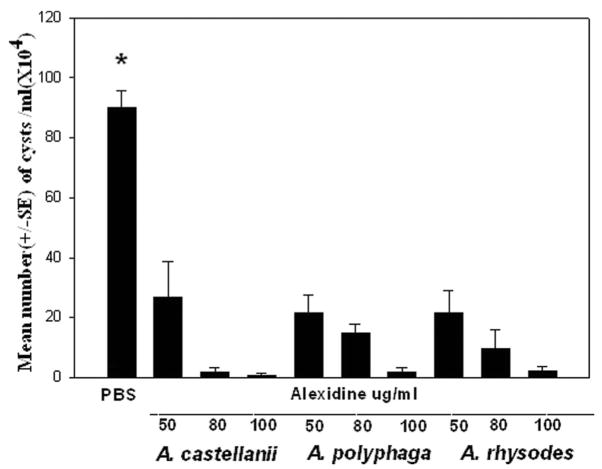
Trophozoites from the 3 pathogenic strains (A. castellanii, A. polyphaga, and A. rhysodes) were incubated in the encystment medium containing 50 mm MgCl2 for 1 week. Cysts (1 × 106) from each pathogenic strain were incubated with 50, 80, or 100 μg/mL of Alexidine or chlorhexidine for 24 hr and the numbers of cysts were counted with a hemocytometer. Cysts were plated onto NNA plates coated with E. coli and observed for excystment. Alexidine was effective in killing the cysts at a concentration of 100 μg/mL. No excystment was observed 2 weeks after incubation in NNA plates (Fig. 2). Cysts incubated with10, 20, 50, and 80 μg/mL of Alexdine excysted 14 days after incubation (Table 1).
FIG. 2.
Cysticidal activity of alexidine against A. castellanii, A. polyphaga, and A. rhysodes cysts. Cysts (1 × 106/mL) were incubated in phosphate buffered saline (PBS) containing different concentrations of alexidine for 24 hr and counted by hemocytometer. The control cultures were cysts incubated for 24 hr in PBS without alexidine. *Significantly different from untreated controls (P<0.05).
TABLE 1.
Cysticidal Effect of Alexidine on 3 Pathogenic Strains of Acanthamoeba Cysts
| Concentration of alexidine | 10 μg/mL | 20 μg/mL | 50 μg/mL | 80 μg/mL | 100 μg/mL |
|---|---|---|---|---|---|
| A. polyphaga | + | + | + | + | − |
| A. rhysodes | + | + | + | + | − |
| A. castellanii | + | + | + | + | − |
Cysts (1 × 106/mL) were incubated with the above concentrations of alexidine for 24 hr. The cysts were plated in nonnutrient agar (NNA) plates precoated with Escherichia coli and observed for viability or excystment over a period of 2 wk. Plates were sealed, incubated at 35°C for 14 days, and investigated daily by inverted microscopy for amoebal growth.
+, growth of trophozoites.
Cysts from the 3 pathogenic strains were incubated with 10, 20, 50, 80, 100, and 200 μg/mL of chlorhexidine for 24 hr. Chlorhexidine was effective in killing the cysts at concentrations of 100 and 200 μg/mL (Table 2).
TABLE 2.
Cysticidal Effect of Chlorhexidine on 3 Pathogenic Strains of Acanthamoeba Cysts
| Concentration of chlorhexidine | 10 μg/mL | 20 μg/mL | 50 μg/mL | 80 μg/mL | 100 μg/mL | 200 μg/mL |
|---|---|---|---|---|---|---|
| A. polyphaga | + | + | + | + | − | − |
| A. rhysodes | + | + | + | + | − | − |
| A. castellanii | + | + | + | + | − | − |
Cysts (1 × 106/mL) were incubated with the above concentrations of chlorhexidine for 24 hr. The cysts were plated in nonnutrient agar (NNA) plates precoated with E. coli and observed for viability or excystment over a period of 2 wk. Plates were sealed, incubated at 35°C for 14 days, and investigated daily by inverted microscopy for amoebal growth.
+, growth of trophozoites.
Cytolysis of HCORN Cells by Alexidine
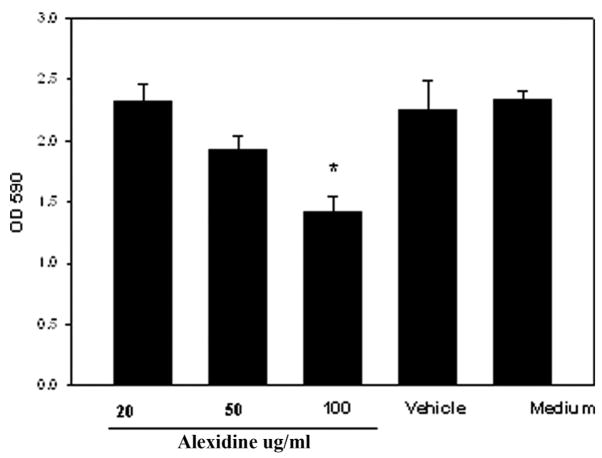
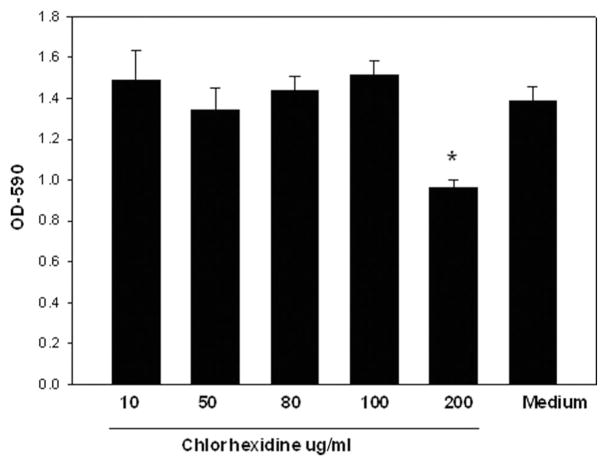
It is possible that Alexidine treatment induces cytolysis of the corneal epithelial cells. It was therefore important to investigate whether Alexidine could induce cytolysis of corneal epithelial cells in vitro. Chinese hamster corneal epithelial cells were incubated with medium with or without different concentrations of Alexidine (20, 50, and 100 μg/mL). The CPE assay was performed 8 hr later. Alexidine at concentrations of 100 μg/mL produced significant CPE on the HCORN cells when incubated for 8 hr (Fig. 3). However, at lower concentrations (20 and 50 μg/mL) Alexidine did not produce significant CPE on the HCORN cells. Similar results were obtained with chlorhexidine (Fig. 4).
FIG. 3.
Cytolytic activity of different concentrations of alexidine on Chinese hamster corneal epithelial cells (HCORN). HCORN cells were incubated with medium or vehicle (1:4 dilution of 10% methanol) with or without different concentrations of alexidine. The cytopathic effect assay was performed 8 hr later. After incubation, the plates were washed 3 times with the culture medium and stained with Giemsa stain. The remaining contents were solubilized in 200 μL of 5% sodium dodecyl sulfate in phosphate buffered saline and transferred to a new 96-well plate, and the density of the residual epithelial cells was quantified by determining the optical density at 590 nm on a microplate reader.*Significantly different from untreated controls (P<0.05).
FIG. 4.
Cytolytic activity of different concentrations of chlorhexidine on Chinese hamster corneal epithelial cells (HCORN). HCORN cells were incubated with medium, vehicle (1:4 dilution of 10% methanol) with or without different concentrations of chlorhexidine. The cytopathic effect assay was performed 8 hr later. After incubation, the plates were washed 3 times with the culture medium and stained with Giemsa stain. The remaining contents were solubilized in 200 μL of 5% sodium dodecyl sulfate in phosphate buffered saline and transferred to a new 96-well plate, and the density of the residual epithelial cells was quantified by determining the optical density at 590 nm on a microplate reader.*Significantly different from untreated controls (P<0.05).
In Vivo Cytotoxicity of Alexidine on Chinese Hamster Cornea
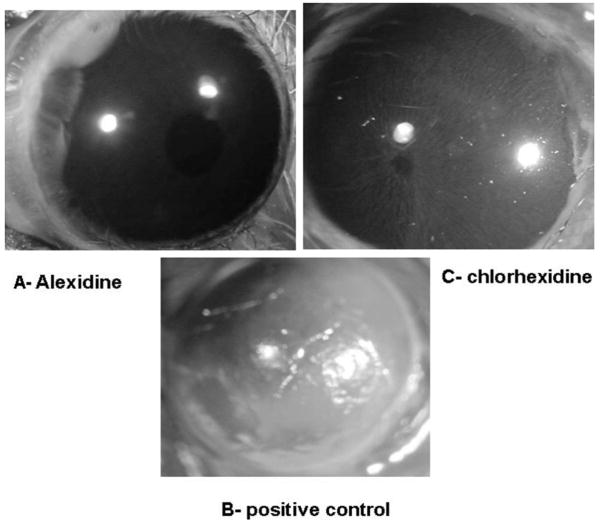
In vitro results indicate that Alexidine at a concentration of 100 μg/mL had a profound effect on cytolysis of corneal epithelial cells. Therefore, it was important to determine the cytotoxicity of Alexidine and chlorhexidine on corneal epithelia cells in vivo. Chinese hamster eyes were treated with 100μg/mL of Alexidine or chlorhexidine topically every hour, 6 times a day. As a control Chinese hamster eyes were treated topically with the vehicle (1:4 dilution of 10% methanol). At the end of 6 hr, corneas were tested for epithelial defect by staining with a fluorescein strip. The positive control consisted of Chinese hamster corneas abraded with a cotton swab to produce an epithelial defect. Corneas treated with Alexidine or vehicle in vivo did not show any visible epithelial defects (Fig. 5A). By contrast, Chinese hamster corneas treated with the same concentration of chlorhexidine (100μg) for 6 times showed mild epithelial defects when stained with fluorescein (Fig. 5C). Corneas abraded with a cotton swab (positive control) showed profound epithelial defects when stained with fluorescein (Fig. 5B).
FIG. 5.
In vivo cytotoxicity of alexidine and chlorhexidine on Chinese hamster cornea. Chinese hamster corneas were treated with 5 μL of alexidine topically, every hour, 6 times a day, and the corneas were stained with fluorescein to assess the epithelial defect (A). Control corneas were treated with the vehicle (1:4 dilution of 10% methanol). Positive control consisted of corneas abraded with a cotton swab to produce an epithelial defect (B). Chinese hamster corneas were also treated with 5 μL of chlorhexidine and every hour, 6 times a day, and the epithelial defect was detected by a fluorescein strip (C).
DISCUSSION
The cytotoxic activities of Alexidine against 3 pathogenic strains of Acanthamoeba were studied. The results showed that Alexidine dihydrochloride was effective in killing the trophozoites at a concentration of 10 μg/mL. However, a higher concentration of Alexidine (100 μg/mL) is required to kill Acanthamoeba cysts, and the cysticidal activity of Alexidine is comparable with chlorhexidine. We also demonstrated that both Alexidine and chlorhexidine at 100 μg/mL induced significant CPE on the corneal epithelial cells in vitro, however, in vivo results indicate that Alexidine at a concentration of 100 μg/mL is less toxic than chlorhexidine when applied topically to the Chinese hamster cornea. Alexidine at a concentration of 0.0045% is currently used as a disinfectant in a commercial contact lens care solution.7 More recently, it has been shown that ~30% to 60% of the polyhexamethylene biguanide and Alexidine were depleted when soft, hydrophilic contact lenses (group IV) were soaked in contact lens solutions for 6 hr.14 The lens uptake of these preservatives may result in a decreased anti-Acanthamoeba activity in such contact lens solutions and concomitantly increase the toxicity of the contact lens solutions on the corneal surface. These results indicate that the safe use of Alexidine as an antimicrobial agent in contact lens solutions seems to be precluded in type IV soft contact lenses.
Our results further suggest that higher concentrations of Alexidine are required to kill cysts (100 μg/mL) and can be used in contact lens solutions without in vivo corneal cell cytotoxicity. Prolong treatment of Acanthamoeba keratitis is very intense, demanding hourly applications of Brolene, polyhexamethylene biguanide, or chlorhexidine for several weeks. Even with such triple therapies, Acanthamoeba spp. can cause severe damage to the corneal epithelium and stroma, ending in the need for corneal transplantation.1 Moreover, drug resistance can occur, even if corneal infections are brought under control. In addition, Acanthamoeba trophozoites can encyst in the stroma and produce recrudescent infections years later. Thus, alternative therapeutic agents are desperately needed. Alexidine dihydrochloride has been previously used in mouthwash as antiseptic.8 This compound is positively charged, and such cations are attracted to the negatively charged bacterial cell walls.5 In fact, gram-positive bacteria are more sensitive to cations because they are more negatively charged.5 Alexidine dihydrochloride is chemically similar to chlorhexidine, another bisbiguanide compound but is a better bacteriocidal agent. Chlorhexidine possesses chlorophenol end groups, as opposed to the ethylhexyl end groups in Alexidine.5 Cellular leakage induced by this compound is not responsible for cellular inactivation, but is a consequence of cell death.5 The difference in end groups between these 2 compounds is thought to be responsible for the more rapid bactericidal action of Alexidine dihydrochloride, as well as the ability of this biguanide to influence lipid domains.5,6
Several studies suggest that chlorhexidine has a similar effect on the trophozoites of Acanthamoeba castellanii, with the cysts being less sensitive.15–17 Unlike chlorhexidine but similar to Alexidine, polyhexamethylene biguanide causes domain formation of the acidic phospholipids of the cytoplasmic membrane.18–21 Recent studies have compared the responses of cysts and trophozoites of Acanthamoeba castellanii to disinfectants used in contact lens solutions and monitored the development of resistance during encystation and the loss of resistance during excystation.15–17 The lethal effects of chlorhexidine and of a polymeric biguanide were time and concentration dependent and mature cysts were more resistant than pre-encystment trophozoites or pre-excystment cysts. The cyst “wall” seemed to act as a barrier to the uptake of these agents, thereby presenting a classic type of intrinsic resistance mechanism.15–17 Our study has identified Alexidine as a novel anti-Acanthamoebae drug and suggests that Alexidine may be an effective therapeutic alternative to chlorhexidine because of its higher potency and low toxicity to the corneal tissues when applied topically in vivo.
Acknowledgments
This work was supported by NIH grants EY09756, R24 EY016664, The Pearl Vision Foundation, and an unrestricted grant from Research to Prevent Blindness, Inc., New York, NY.
References
- 1.Alizadeh H, Niederkorn JY, McCulley JP. Acanthamoeba keratitis. In: Pepose JS, Holland GN, Wilhelmus KR, editors. Ocular Infection and Immunity. St. Louis, MO: Mosby; 1996. pp. 1062–1071. [Google Scholar]
- 2.Aksozek A, McClellan K, Howard K, et al. Resistance of Acanthamoeba castellanii cysts to physical, chemical, and radiological conditions. J Parasitol. 2002;88:621–623. doi: 10.1645/0022-3395(2002)088[0621:ROACCT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Awwad ST, Petroll WM, McCulley JP, et al. Updates in Acanthamoeba keratitis. Eye Contact Lens. 2007;33:1–8. doi: 10.1097/ICL.0b013e31802b64c1. [DOI] [PubMed] [Google Scholar]
- 4.McCulley JP, Alizadeh H, Niederkorn JY. The diagnosis and management of Acanthamoeba keratitis. CLAO J. 2000;26:47–51. [PubMed] [Google Scholar]
- 5.McDonnell G, Russell AD. Antiseptics and disinfectants: Activity, action, and resistance. Clin Microbiol Rev. 1999;12:147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chawner JA, Gilbert P. A comparative study of the bactericidal and growth inhibitory activities of the bisbiguanides alexidine and chlorhexidine. J Appl Bacteriol. 1989;66:243–252. doi: 10.1111/j.1365-2672.1989.tb02475.x. [DOI] [PubMed] [Google Scholar]
- 7.Atkins N. Developments in contact lens solutions. Optician. 2006;3:1–7. [Google Scholar]
- 8.Spolsky VW, Forsythe AB. Effects of alexidine. 2HCL mouthwash on plaque and gingivitis after six months. J Dent Res. 1977;56:1349–1358. doi: 10.1177/00220345770560111101. [DOI] [PubMed] [Google Scholar]
- 9.Hurt M, Neelam S, Niederkorn J, et al. Pathogenic Acanthamoeba spp secrete a mannose-induced cytolytic protein that correlates with the ability to cause disease. Infect Immun. 2003;71:6243–6255. doi: 10.1128/IAI.71.11.6243-6255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silvany RE, Dougherty JM, McCulley JP, et al. The effect of currently available contact lens disinfection systems on Acanthamoeba castellanii and Acanthamoeba polyphaga. Ophthalmology. 1990;97:286–290. doi: 10.1016/s0161-6420(90)32590-3. [DOI] [PubMed] [Google Scholar]
- 11.Leher H, Silvany R, Alizadeh H, et al. Mannose induces the release of cytopathic factors from Acanthamoeba castellanii. Infect Immun. 1998;66:5–10. doi: 10.1128/iai.66.1.5-10.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connell C, Rutter A, Hill B, et al. Encystation of Acanthamoeba castellanii: Dye uptake for assessment by flow cytometry and confocal laser scanning microscopy. J Appl Microbiol. 2001;90:706–712. doi: 10.1046/j.1365-2672.2001.01296.x. [DOI] [PubMed] [Google Scholar]
- 13.McClellan K, Howard K, Niederkorn JY, et al. Effect of steroids on Acanthamoeba cysts and trophozoites. Invest Ophthalmol Vis Sci. 2001;42:2885–2893. [PubMed] [Google Scholar]
- 14.Rosenthal RA, Dassanayake NL, Schlitzer RL, et al. Biocide uptake in contact lenses and loss of fungicidal activity during storage of contact lenses. Eye Contact Lens. 2006;32:262–266. doi: 10.1097/ICL.0b013e31802b413f. [DOI] [PubMed] [Google Scholar]
- 15.Khunkitti W, Lloyd D, Furr JR, et al. Aspects of the mechanisms of action of biguanides on trophozoites and cysts of Acanthamoeba castellanii. J Appl Microbiol. 1997;82:107–114. doi: 10.1111/j.1365-2672.1997.tb03304.x. [DOI] [PubMed] [Google Scholar]
- 16.Khunkitti W, Lloyd D, Furr JR, et al. The lethal effects of biguanides on cysts and trophozoites of Acanthamoeba castellanii. J Appl Bacteriol. 1996;81:73–77. doi: 10.1111/j.1365-2672.1996.tb03284.x. [DOI] [PubMed] [Google Scholar]
- 17.Lloyd D, Turner NA, Khunkitti W, et al. Encystation in Acanthamoeba castellanii: Development of biocide resistance. J Eukaryot Microbiol. 2001;48:11–16. doi: 10.1111/j.1550-7408.2001.tb00410.x. [DOI] [PubMed] [Google Scholar]
- 18.Broxton P, Woodcock PM, Gilbert P. A study of the antibacterial activity of some polyhexamethylene biguanides towards Escherichia coli ATCC 8739. J Appl Bacteriol. 1983;54:345–353. doi: 10.1111/j.1365-2672.1983.tb02627.x. [DOI] [PubMed] [Google Scholar]
- 19.Broxton P, Woodcock PM, Gilbert P. Binding of some polyhexamethylene biguanides to the cell envelope of Escherichia coli ATCC 8739. Microbios. 1984;41:15–22. [PubMed] [Google Scholar]
- 20.Broxton P, Woodcock PM, Heatley F, et al. Interaction of some polyhexa-methylene biguanides and membrane phospholipids in Escherichia coli. J Appl Bacteriol. 1984;57:115–124. doi: 10.1111/j.1365-2672.1984.tb02363.x. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda T, Ledwith A, Bamford CH, et al. Interaction of a polymeric biguanide biocide with phospholipid membranes. Biochim Biophys Acta. 1984;769:57–66. doi: 10.1016/0005-2736(84)90009-9. [DOI] [PubMed] [Google Scholar]