Abstract
Nestin, a marker of neural stem cells, is also expressed by cells located in the epithelium of the pancreatic primordium and by a subpopulation of exocrine cells but not by endocrine cells. These findings raised the possibility that the pancreatic epithelium is heterogeneous and comprised of subpopulations of exocrine/ nestin-positive and endocrine/nestin-negative precursor cells. We examined this issue in two mutant mouse models characterized by protracted expression of several embryonal properties in islet cells. One mutant line comprises mice lacking mature glucagon due to abrogation of proprotein convertase-2 (PC2−/−), responsible for the conversion of proglucagon into glucagon, while the second line consists of mice with a global deletion of the glucagon receptor (Gcgr −/−). We demonstrate that nestin is transiently expressed by acinar cells and by insulin and glucagon cells of islets of both lines of mice. In addition, the lack of glucagon signaling increased nestin mRNA levels in pancreas of mutant embryos and adult mice. We conclude that nestin+ cells located in the pancreatic primordium generate the cells of the endocrine and exocrine lineages. Furthermore, our results suggest that nestin expression is regulated by glucagon signaling.
Keywords: glucagon signaling, proprotein convertases, glucagon receptor, nestin, pancreatic islet cells, mouse development
INTRODUCTION
The pancreatic β cell has a pivotal role in the regulation of glucose homeostasis and its death leads to Type I diabetes. The search for sources of new β cells is driven by the shortage of islets of Langerhans suitable for replacement therapy for Type I diabetes. Recent advances in stem cell research have led to promising sources of insulin-producing cells. However, a key step in the generation of islet stem cells is the identification of progenitor cell markers for proper lineage isolation of insulin secreting cells (Soria, 2001).
The selection of appropriate lineage markers is likely to be derived from analysis of pancreatic islet cell development. The first indication of morphogenesis of the pancreas occurs around day 9.5 of embryonic development (e-9.5) in mice (22–25 somites) when the dorsal endoderm evaginates forming the epithelium of the pancreatic primordium and becomes surrounded by condensed mesoderm (Pictet and Rutter, 1972). This process is concomitant with the initiation of endocrine cell differentiation. The different endocrine cell types, derived from the pancreatic epithelium, appear sequentially during development. Glucagon (GLU+) and insulin (IN+) cells are first seen at e-9.5 and 10, respectively, SOM at day 15, and PP at postnatal day one (Alpert et al., 1988; Gittes and Rutter, 1992; Guz et al., 1995; Herrera et al., 1991). The epithelium of the pancreatic primordium also generates the exocrine component of the pancreas, which begins to differentiate at e-15 (Alpert et al., 1988; Gittes and Rutter, 1992; Pictet and Rutter, 1972).
Recent studies highlighted the role of several families of transcription and growth factors in islet cell differentiation (Jensen, 2004), many of which also play key roles in the development of neural tissues. The fact that islet cells and neurons have similar molecular phenotypes (Ahlgren et al., 1997; Alpert et al., 1988; Gradwohl et al., 2000; Naya et al., 1997; Sander et al., 2000; Sosa-Pineda et al., 1997; Sussel et al., 1998; Turque et al., 1994) raised the possibility that precursor cells of islets, like those of brain, express the intermediate filament nestin (Cattaneo and McKay, 1990; Lendahl et al., 1990), a classical marker of neuronal stem cells. This possibility was supported by the discovery of nestin+ cells in epithelial cells of the pancreatic bud of mouse embryos from e-10 to e-15 (Esni et al., 2004), raising the prospect that these cells were endocrine precursor cells. This possibility is controversial since several reports indicated that nestin expression was restricted to cells derived from the mesenchyme (Humphrey et al., 2003; Klein et al., 2003; Lardon et al., 2002; Selander and Edlund, 2002; Treutelaar et al., 2003). The derivation of pancreatic endocrine and exocrine cells from nestin precursors is further complicated by conflicting results obtained from cell lineage analysis of pancreatic cells using molecular marking techniques. The initial studies performed using this approach suggested that nestin precursors generated exocrine cells but not cells expressing insulin or glucagon (Delacour et al., 2004; Esni et al., 2004; Selander and Edlund, 2002) while more recent in vitro studies indicated that endocrine and exocrine cells of the embryonic pancreas derive from progenitors expressing the intermediate filament (Bernardo et al., 2006).
There is additional evidence from in vitro studies supporting the possibility that endocrine cells are derived from nestin+ precursors. Nestin was used as a marker to isolate a subpopulation of embryonic stem cells with the ability to initiate insulin expression in vitro (Blyszczuk et al., 2003; Lumelsky et al., 2001). Moreover, islets of adult mice and rats were found to contain nestin+ cells (Hunziker and Stein, 2000; Zulewski et al., 2001) and these cells were reported to differentiate into endocrine and exocrine cells in culture (Abraham et al., 2004; Hunziker and Stein, 2000; Klein et al., 2003; Seaberg et al., 2004; Zulewski et al., 2001). These observations suggest that embryonic islet precursor cells express nestin but that its expression in vivo is down-regulated and becomes undetectable during the initiation of endocrine differentiation.
In the present study, we sought to determine whether nestin is expressed in vivo by islet cells of two mutant mouse models characterized by protracted expression of several embryonal properties (Vincent et al., 2003; Vuguin et al., 2006), speculating that they may also retain expression of nestin after the initiation of hormone expression. One mutant line comprised mice with a deletion in the gene encoding for prohormone convertase-2 (PC2−/−), responsible for the conversion of proglucagon into the glucagon (Furuta et al., 1997; Steiner, 1998) while a second line consisted of mice with a global deletion of the glucagon receptor (Gcgr−/−) (Gelling et al., 2003). We found that nestin is transiently expressed by endocrine cells of Gcgr+/+ and PC2+/+ embryos during early development and by a significant number of insulin and glucagon cells of Gcgr−/− and PC2−/− mice during early and midgestation. Moreover, the lack of glucagon signaling increased nestin mRNA levels in pancreas of mutant embryos and adult mice. These observations support the view that nestin+ cells of the epithelium of the pancreatic primordium are multipotent stem cells that generate not only exocrine but also endocrine cells of the pancreas.
RESULTS
Transient Expression of Nestin by Endocrine Cells of Mutant Mice During Development
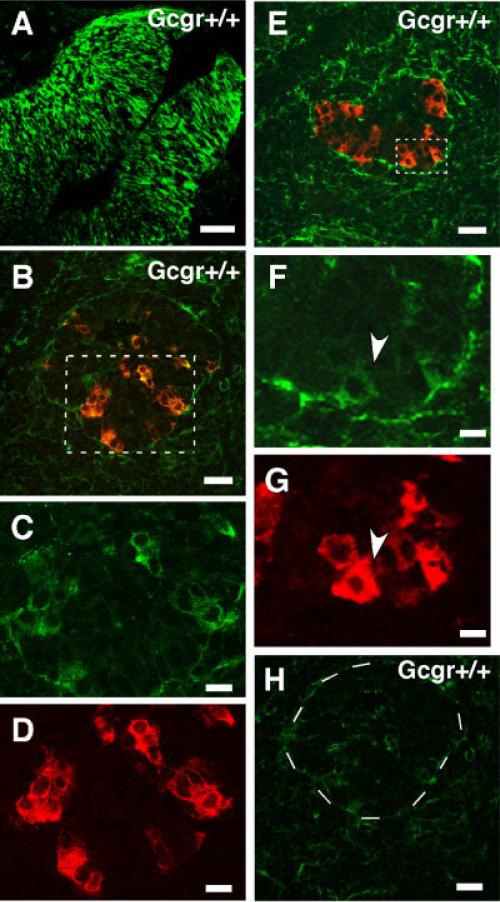
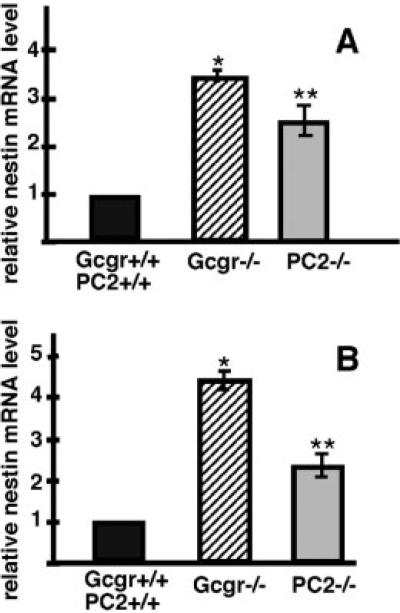
To determine whether nestin is expressed by pancreatic cells during development, pancreata of PC2−/−, PC+/+, Gcgr−/−, and Gcgr+/+ embryos were examined. At e-11, the earlier stage analyzed, the pancreatic primordium of Gcgr+/+ and PC2+/+ embryos contained clusters of glucagons+ (Glu+) cells and cells expressing low levels of nestin immunoreactivity. In these clusters, most cells coexpressed glucagon and nestin (Fig. 1B–D). At this stage, the pancreas contained few insulin+ (IN+) cells and an occasional IN+ cell expressed nestin (Fig. 1E–G). Nestin expression by endocrine cells of pancreas of Gcgr+/+ and PC2+/+ embryos was transient and was extinguished by e-15 of development (Fig. 2D). Similar to Gcgr+/+ and PC2+/+ embryos, the pancreatic primordia of e-11 Gcgr−/− and PC2−/− embryos contained nestin+ glucagon+ cells (results not shown). However, e-11 Gcgr−/− and PC2−/− embryos lacked IN+ cells (Vincent et al., 2003; Vuguin et al., 2006). Lack of glucagon signaling also affected the time-table of expression of nestin in islet cells. Thus, in contrast to e-15 Gcgr+/+ and PC2+/+ embryos, nestin+ cells do not disappear during midgestation but, rather, were abundant in pancreas of e-15 Gcgr−/− embryos and a significant number of these cells were IN+ or GLU+ (Fig. 2A and B). At this stage, 30% of IN+ cells expressed nestin (544 IN+ cells scored). Similar results were obtained with e15 PC2−/− embryos (not shown). The pattern of nestin staining observed in embryos and adults (see below) is cytoplasmic rather than that characteristic of cytoskeletal proteins. Our results and results by others (Koso et al., 2007; Yasuhara et al., 2006) suggests that the pattern of nestin staining is determined by the cellular environment. Cells in vitro that are attached to a surface have filamentous staining while those in vivo show uniformal cytoplasmic staining. The mutations also affected the level of expression of nestin mRNA, which were significantly higher in pancreas of e-15 Gcgr−/− and PC2−/− than in pancreas of Gcgr+/+ and PC2+/+ littermates (see Fig. 6A).
Fig. 1.
Expression of nestin by pancreatic endocrine cells of Gcgr+/+ embryos at e-11.5. A: Immunolocalization of nestin in radial glial cells of brain of e-11 mouse embryos. This photomicrograph documents the specificity of the nestin antibody from Chemicon. Similar results were obtained with the nestin antisera from Developmental Studies Hybrid-oma Bank (not shown). B: Pancreas of e-11 Gcgr+/+ embryo immuno-stained for glucagon (red) and nestin (green). Note the presence of glucagons+nestin+ cells (yellow). C,D: Area indicated with dotted lines is shown in higher magnification, which illustrates single label staining for nestin and glucagon respectively. Scale bars for B–D = 35 and 20 μm. E: Pancreas of e-11 Gcgr+/+ embryo immunostained for insulin (red) and nestin (green). F,G: Area indicated with dotted lines is magnified, which illustrates single label immunostaining for nestin and insulin, respectively. Cell indicated with arrowhead is positive for nestin and insulin. Scale bars for E–G = 35 and 10 μm. H: Photomicrograph of a section of pancreas of an e-11 Gcgr+/+ embryo incubated with a green fluorescent goat antimouse IgG (incubation with antibodies to nestin was omitted). Dotted circle indicates area containing an islet. This picture documents the absence of immunopositive nestin cells. Scale bar = 40 μm.
Fig. 2.
Expression of nestin by pancreatic endocrine cells of Gcgr−/− embryos at e-15. A: Photomicrograph of a section of pancreas of an e-15 Gcgr−/− embryo immunostained for nestin (green) and insulin (red) documents the presence of a large number of nestin+ insulin+ cells (yellow). Also note that many exocrine cells are nestin positive. Scale bar = 40 μm. B: Photomicrograph of a section of pancreas of an e-15 Gcgr−/− embryo immunostained for nestin (green) and glucagon (red) demonstrates the presence of nestin+glucagon+ cells (yellow). Scale bar = 50 μm. C: Pancreas of e-15 Gcgr−/− embryos immunostained for insulin (red) using a guinea pig anti-insulin sera and a secondary anti-guinea pig IgG to visualize the pancreas and a green fluorescent goat anti-mouse IgG (incubation with antibodies to nestin was omitted). This picture documents the absence of immunopositive nestin cells. Scale bar = 40 μm. D: Photomicrograph of a section of pancreas of an e-15 Gcgr+/+ embryo immunostained for insulin (red) and nestin (green) demonstrates the absence of immunopositive double-labeled cells in control mice. Similar results were obtained with e-15 PC2+/+. Scale bar= 40 μm.
Fig. 6.
Pancreas of embryonic and adult mutant mice have high levels of nestin mRNA. Real time RT-PCR analysis of nestin expression in pancreas of (A) e-15 and (B) adult Gcgr−/−, PC2−/−, Gcgr+/+, and PC2+/+ mice, respectively. Note that that at both stages the levels of nestin mRNA are higher in pancreas of Gcgr−/− and PC2−/− mice than in islets of littermate controls. A: *WT vs. Gcgr−/− P < 0.0001; **WT vs. PC2−/− P < 0.0008. B: *WT vs. Gcgr−/− <0.0002; **WT vs. PC2−/− <0.0001.
The number of cells co-expressing nestin and either insulin or glucagon decreased abruptly during the perinatal period and were not observed in islets of postnatal (P) day 1(P-1) PC2−/− pups (Fig. 3A,B) or Gcgr−/− pups (not shown). Few nestin cells were observed in the exocrine compartment of postnatal PC2+/+ or Gcgr+/+ (not shown). In contrast, a large number of cells expressing nestin were observed in the exocrine pancreas of P1 PC2−/− mice (Fig. 3D) and of Gcgr−/− mice (not shown) and these cells co-expressed amylase (Fig. 3C– E). These observations indicate that nestin was transiently expressed by endocrine cells during prenatal development and that the duration and level of nestin expression increased in pancreas of mice lacking glucagon signaling.
Fig. 3.
Decreased number of nestin+ cells in pancreas of perinatal PC2−/− mice. Photomicrographs illustrate islets of P1 PC2−/− immuno-stained for nestin and either insulin (A) or glucagon (B). Note that GLU+ or IN+ cells (red) do not coexpress nestin (green). C–E: Photomicro-graphs illustrate pancreas of P1 PC2−/− mice immunostained for visualization of amylase (C, red) and nestin (D, green). E = C+D. Note expression of nestin (green) by amylase+ cells (red) located in a pancreatic lobe; coexpressing cells are yellow. Scale bar: 40 μm. F: Photomicrograph of pancreas of P1 PC2−/− incubated with green fluorescent goat antimouse IgG (incubation with antibodies to nestin was omitted). This picture documents the absence of immunopositive nestin cells. Comparable results were obtained with Gcgr−/− mice.
Reappearance of Nestin Expression in Pancreatic Cells of Adult Mutant Mice
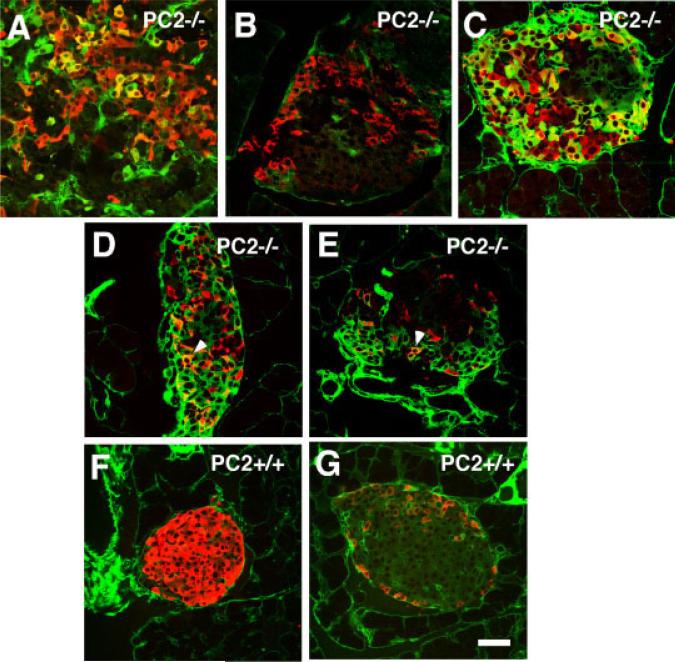
Since our previous results indicated that pancreas of adult PC2−/− and Gcgr−/− contain subsets of islet cells expressing traits characteristic of endocrine cells of embryos (Vincent et al., 2003; Vuguin et al., 2006), we sought to determine whether these cells also expressed nestin. Large numbers of nestin cells were found in approximately 10% of the islets in both strains of mutant mice and some of these cells co-expressed insulin (Fig. 4A), glucagon (Fig. 4C), somatostatin (Fig. 4D), or PP (Fig. 4E). Islets containing cells coexpressing nestin and a pancreatic hormone were not distributed throughout the pancreas but, rather, were localized to a subset of pancreatic lobules (Fig. 5A). In addition to these subsets of nestin+ endocrine and exocrine cells, all islets of Gcgr+/+ mice (Fig. 4F,G) and of PC2−/−, PC2+/+, and Gcgr−/− mice (not shown) contained nestin+ cells that did not express hormones. These cells are likely to be the mesenchymal nestin+ cells described by others (Delacour et al., 2004; Esni et al., 2004; Humphrey et al., 2003; Klein et al., 2003; Lardon et al., 2002; Treutelaar et al., 2003).
Fig. 4.
Reappearance of nestin+ cells in islets of adult PC2−/− mice. Photomicrographs of islets from 3-month-old PC2−/− mice immunostained for visualization of nestin (green) and insulin (red) (A,F), glucagon (red) (C,G), somatostatin (red) (D), or PP (red) (E). A–E: PC2−/− mice; F–G: PC2+/+ mice. Coexpressing cells are labeled yellow. Note that nestin is coexpressed by all four endocrine cell types of the islets. B: Pancreas of PC2−/− mice immunostained for glucagons (red) using a rabbit anti-glucagon sera and a secondary anti-rabbit IgG to visualize islets and a green fluorescent goat anti-mouse IgG (incubation with antibodies to nestin was omitted). Note that nestin cells are not stained. Similar results were obtained with 3-month-old Gcgr−/− mice. Scale bar = 40 μm.
Fig. 5.
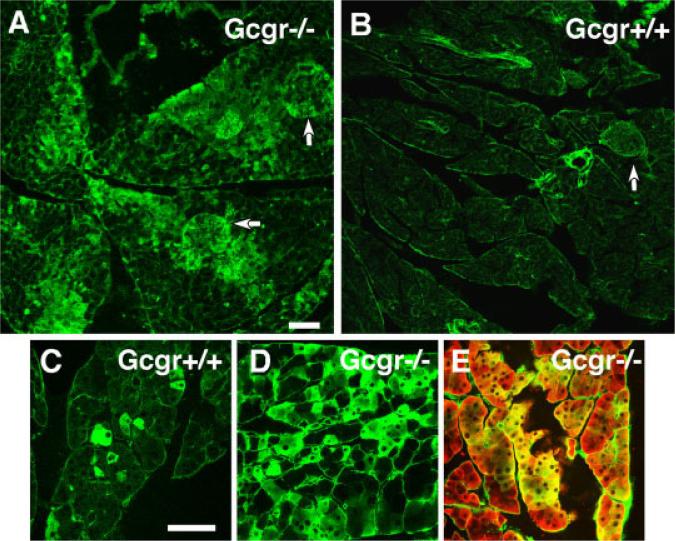
Increased number of nestin+ amylase+ cells in pancreas of adult Gcgr−/− mice. A: Low-power photomicrograph of pancreas of 3-month-old Gcgr−/− mice immunostained for visualization of nestin. Note that nestin is expressed by many exocrine cells and islets (indicated by arrows). B: Pancreas of Gcgr+/+ mice immunostained for nestin. Note the near absence of nestin-positive cells in exocrine and endocrine tissue. Arrow indicates an unlabeled islet. Scale bar for A and B = 100 μm. C: Photomicrogaph illustrate the presence of scattered nestin+ cells in pancreas of Gcgr+/+. D: In contrast, pancreas of Gcgr−/− mice contain a large number of nestin+ cells. E: Many nestin-positive cells coexpress amylase. Scale bar = 40 μm. Comparable results were obtained with PC2−/− and PC2+/+ mice.
The exocrine compartment of pancreas of 3-month-old Gcgr−/− mice (Fig. 5A) and of PC2−/− mice (not shown) contained a large number of nestin+amylase+ cells. Some of these cells were scattered within the exocrine tissue, resembling the pattern found in the exocrine tissue of pancreas of Gcgr+/+ (Fig. 5C) and PC2+/+ mice (not shown). However, abundant expression of nestin was found clustered in some lobules of the pancreas (Fig. 5A,D) where most of the nestin+ cells coexpressed amylase (Fig. 5E). The regions of the pancreas with abundant nestin expression in the exocrine tissue also contained islets harboring nestin+ endocrine cells (Fig. 5A). In agreement with the immunocytochemical results, the levels of nestin mRNA were also upregulated in pancreas of Gcgr−/− and PC2−/− adults as compared to that of Gcgr+/+ and PC2+/+ littermates (Fig. 6B).
DISCUSSION
The large overlap between neurons and pancreatic endocrine cells in the molecular program of differentiation (Ahlgren et al., 1997; Alpert et al., 1988; Gradwohl et al., 2000; Naya et al., 1997; Sosa-Pineda et al., 1997; Sussel et al., 1998; Turque et al., 1994) and the knowledge that nestin is a marker of neuronal stem cells (Cattaneo and McKay, 1990; Johansson et al., 1999; Lendahl et al., 1990) drove the search in the pancreas for a nestin-positive population that would generate the cells of the endocrine and exocrine lineages. Previous reports indicated that nestin was expressed by mesenchymal cells of pancreas during development (Selander and Edlund, 2002), by the epithelium of the pancreatic primordium, and by acinar but not endocrine cells produced by this embryonic layer (Delacour et al., 2004; Esni et al., 2004). In contrast to those reports, the observations reported in this study demonstrate that nestin is expressed during early development by IN+ and GLU+ cells of Gcgr+/+ and PC2+/+ and that its expression increased significantly in mice lacking glucagon signaling.
The difference between our results and those reported by others cannot be due to the source of the nestin antibody, since the antisera we used were from the same source as that utilized by other laboratories (Delacour et al., 2004). We believe that the discrepancy is related to the technique used to process the tissues. The tissues used in this study were embedded in sucrose, frozen and sectioned in a cryostat microtome while other groups examined tissues that were embedded in paraffin, which generally decreases the sensitivity of the immunostaining procedure (Guz and Teitelman, unpublished data). This is particularly important when the antigen level is very low, as is the case for nestin in e-11 embryos. In addition, while sections of older embryos and adults were incubated overnight with antisera to nestin, sections of e-11 embryos were incubated for 3 days, a length of time that improved the visualization of the fluorophore linked to the secondary antibody.
The persistence of nestin expression in differentiated endocrine cells of PC2−/− and Gcgr−/− embryos follows the pattern of other embryonal traits that have protracted expression in islet cells of these two mutant lines (Vincent et al., 2003; Vuguin et al., 2006). Thus, while the pancreatic specific transcription factor Pdx-1 is expressed by pancreatic cells of PC2+/+ and Gcgr+/+ embryos but only by beta cells of adults, non-beta cells of embryos and adult PC2−/− and Gcgr−/− mice expressed Pdx-1 (Vincent et al., 2003; Vuguin et al., 2006). Similarly, the glucose transporter 2 (GLUT2), which is transiently expressed by glucagon cells in control mice during development, is expressed by alpha cells of adult Gcgr−/− mice (Vuguin et al., 2006). Finally, cells co-expressing insulin and glucagon, found only in pancreas of e10 to e-15 control mice, populate islets of adult Gcgr−/− mice (Vuguin et al., 2006). These observations suggested that the normal pattern of activation and inactivation of genes that occurs during normal development (Jensen, 2004; Wilson et al., 2003) is perturbed in mice lacking glucagon signaling. Nestin expression was down-regulated in islet cells of Gcgr+/+ and PC2+/+ mice soon after the initiation of endocrine cell differentiation. The lack of glucagon signaling in PC2−/− and Gcgr−/− mice resulted in the presence of higher levels of nestin in islet and exocrine tissue and in its protracted expression following the appearance of the hormones at e-15.
Nestin expression in pancreas was downregulated in PC2−/− and Gcgr−/− mice during late prenatal and early postnatal life, a period presumed to be characterized by a nadir in the rate of islet growth (Bonner-Weir, 2000) but it reappeared in pancreas of adults. In both lines, nearly 10% of the islets in adults contain abundant nestin expression and these islets were located in areas of the pancreas characterized by a large number of nestin+ exocrine cells. The pattern in the exocrine tissue differs from that reported in transgenic mice (nestin-Cre/R26R) using a Cre-Lox system that resulted in the expression of a transgene comprised of the nestin promoter and the coding region for β galactosidase. In nestin-Cre/R26R mice, the transgene succeeded in permanently labeling cells that transcribe the nestin gene and their descendants (Delacour et al., 2004; Treutelaar et al., 2003). Exocrine cells expressing β galactosidase were observed scattered throughout the pancreas (Delacour et al., 2004), presumably due to subtle differences in levels of expression of the transgene in acinar cells. In contrast, in PC2−/− and Gcgr−/− mice, nestin+ cells were found clustered in a subset of pancreatic lobes.
The reappearance of nestin+ cells in pancreas of mature PC2−/− and Gcgr−/− mice is intriguing. If nestin expression in adults was activated by the absence of glucagon signaling, it would be expected that all acinar cells of PC2−/− and of Gcgr−/− mice would express nestin and all islets of these two strains would contain nestin+ endocrine cells. However, nestin-positive cells were found in only a subset of islets and of exocrine cells of adult PC2−/− and of Gcgr−/− mice. These observations do not support the view that the lack of glucagon signaling led to the reappearance of nestin in all pancreatic cells. Rather, they suggest the possibility that the areas of the pancreas containing nestin+ cells were newly formed lobes that recapitulate the stages of pancreatic cells differentiation of embryos. The possible presence of islet neogenesis in PC2−/− and Gcgr−/− mice would account for the increased number of islets found in pancreas of both mutant lines (Vincent et al., 2003; Vuguin et al., 2006).
In conclusion, our results indicate that cells of the exocrine and endocrine lineages express nestin, and support the hypothesis that these cells are generated by nestin-positive precursors located in the epithelium of the pancreatic primordia. Moreover, it suggests that transient inhibition of glucagon signaling in pancreas of embryos ex vivo will increase nestin expression and will facilitate the isolation of pancreatic islet progenitors useful for transplantation therapy in Type I Diabetes.
EXPERIMENTAL PROCEDURES
Animals
The colony of PC2 mice used in this study and housed at Downstate was started with breeding pairs provided Dr. D.F. Steiner (Furuta et al., 1997). The generation and the procedure for determination of their genotype from tail biopsies by PCR of DNA is described elsewhere (Furuta et al., 1997; Vincent et al., 2003). Primers were obtained from Operon (Alameda, CA). The generation of Gcgr−/−mice has been previously reported (Gelling et al., 2003). Heterozygous (Gcgr+/−) and homozygous (Gcgr−/−) matings, conducted at AECOM, yielded null (Gcgr−/−) mice in a Mendelian ratio that were genotyped by PCR analysis as described (Vuguin et al., 2006). Heterozygous (Gcgr+/−) matings yielded null (Gcgr−/−), heterozygote (Gcgr+/−), and wild type (Gcgr+/+) mice in a Mendelian ratio. A similar mating protocol was used to generate PC2−/−, PC2+/− and PC2+/+ mice. At least 3 embryos/ stage/genotype, 3 neonates/genotype, and 6 adults/genotype were examined. For timed pregnancies, it is assumed that the morning of plug detection corresponds to day 0.5 of pregnancy and embryo ages are rounded to the nearest half-day.
Animals were fed ad libitum with free access to water and maintained in a murine hepatitis virus-free barrier facility on a 12-h light-12-h dark cycle. Pregnant mice were dissected and the embryos fixed overnight by immersion in a solution of 4% paraformaldehyde in 0.1M phosphate buffer (PF). Adult mice were perfused through the heart with a solution of 4% PF and postfixed for several hours in the same fixative. Fixed tissues were infiltrated in 30% sucrose and mounted in embedding matrix (Lipshaw Co., Pittsburgh, PA), and 10-μm cryostat sections were collected onto gelatin-coated slides. The animal protocols used in these studies were approved by the Downstate and AECOM Animal Care and Use Committees.
Source of Antibodies
Guinea pig antiserum to bovine insulin was purchased from Linco Research Inc (Eureka, MO). Rabbit antiserum to human glucagon (GLU) was purchased from Calbiochem (San Diego, CA). Rabbit antiserum to human pancreatic polypeptide (PP) and somatostatin (SOM) was supplied by Peninsula Labs (Belmont, CA). Monoclonal antibodies to nestin were purchased from Chemicon (Temecula, CA) and from the Developmental Studies Hybridoma Bank (U. of Iowa). Antibodies were used at the following dilutions: antiguinea pig insulin antibody at 1:400; rabbit antisera to human glucagon, somatostatin, and pancreatic polypeptide at 1:4,000, 1:8,000 and 1:10,000, respectively, and to nestin at 1:5,000. Secondary antibodies: Alexa fluor 488 anti-mouse and anti-rabbit IgG, Alexa fluor 594 anti-guinea pig, anti-rabbit, and anti-mouse IgG were purchased from Molecular Probes, Inc. (Eugene, OR). These antibodies were used at a 1:200 dilution.
Immunolabeling of Cryostat Sections
Sections were incubated overnight in an empirically derived optimal dilution of control serum or primary antibody raised in species “X” containing 1% goat serum in Tris-saline solution (TS; 0.9% NaCl in 0.1 M Tris, pH 7.4). Sections of e-11.5 pancreas were incubated for 72 hr with primary antibodies. Then, sections were rinsed in TS and incubated for 2 hr in a 1:200 solution of anti-(species x) fluorescent-IgG in 1% goat serum in TS. Sections labeled with fluorescent probes were washed in TS, covered with 2–3 drops of Prolong Antifade solution (Molecular Probes) after completion of the staining procedure, and dried at room temperature before examination. Immunostaining of embryos, neonates, and adult mutant and control litter-mates were processed in parallel. Number of slides examined: 12 for e-11 embryos/genotype, 24 for e-15/genotype, 20 for P-1 and over 30 for adults/genotype.
Confocal Microscopy
Confocal images were obtained using a Radiance 2000 confocal microscope (BioRad, Hercules, CA) attached to a Zeiss Axioskop microscope (Carl Zeiss Inc.). Images of 1,240 × 1,240 pixels were obtained and processed using Adobe Photoshop 6.0 (Adobe Systems, Mountain View, CA). All images of sections from embryonic, postnatal, and adult control and mutant mice were captured using similar settings of the confocal microscope.
Quantitative Real Time RTPCR
Total RNA was isolated from Pancreata of E15 embryos or adult control (Gcgr+/+ or PC2+/+), Gcgr−/−, and PC2−/−sing TRIzol reagent (Invitrogen). RNA was treated with DNase-1 (Ambion, Austin, TX), and 5 μg of total RNA was transcribed using Oligo (dT)20 and SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The reverse transcription was performed at 50°C for 45–50 min and stopped by incubating at 70°C for 15 min. PCR amplifications were performed using an ABI prism 7700 Sequence detection System (Applied Biosysytems, Foster City, CA). Reactions were performed in a 25-μl reaction using 2× solution of RT2 Real-Time™ SYBR green/ROX PCR Master Mix (SuperArray, Fredrick, MD). Primers for mouse nestin (Nes, Catalog Number: PPM04735A, RefSeq accession no. NM_016701.3) and 18S rRNA gene (18SrRNA, Catalog Number: PPM57735A, RefSeq accession no. K01364.1) were purchased from Super-Array (Fredrick, MD). Amplification was performed by initial polymerase activation for 10 min at 95°C and 40 cycles of 95°C for 15 s and 60°C for 60 s. Quantitative values were obtained as threshold PCR cycle number (Ct) when the increase in fluorescent signal of PCR product showed exponential amplification. Target gene mRNA level was normalized to that of 18sRNA in the same sample. In brief, the relative expression level of the target gene compared with that of 18SrRNA was calculated as 2–ΔCt, where ΔCt = Ct target gene – Ct (18sRNA). The ratio of relative expression of the target gene in Gcgr−/− and PC2−/− tissues to that in Gcgr+/+ and PC2+/+ tissues, respectively, was then calculated as 2–{Δ}{ Δ} Ct, where {Δ}{ Δ}Ct = {Δ}Ct Gcgr−/− islet – {Δ}Ct control Gcgr+/+ islet. Each sample was measured in triplicates.
ACKNOWLEDGMENTS
This work was supported by the National Institute of Health (grants DK47425 and HL58119 to M.J.C., DK71949 to G.T., KO8 HD042172 to P.V., and DK 13914 and DK 020595 to D.F.S.), the Juvenile Diabetes Research Foundation (1-2005-181 to G.T.), Albert Einstein College of Medicine (AECOM) Comprehensive Cancer Center (to M.J.C.), the AECOM Diabetes Center (to M.J.C.), and the Howard Hughes Medical Institute (to D.F.S).
REFERENCES
- Abraham EJ, Kodama S, Lin JC, Ubeda M, Faustman DL, Habener JF. Human pancreatic islet-derived progenitor cell engraftment in immunocompetent mice. Am J Pathol. 2004;164:817–830. doi: 10.1016/S0002-9440(10)63170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlgren U, Pfaff S, Jessel TM, Edlund T, Edlund H. Independent requirement for ISL1 in the formation of the pancreatic mesenchyme and islet cells. Nature. 1997;385:257–260. doi: 10.1038/385257a0. [DOI] [PubMed] [Google Scholar]
- Alpert S, Hanahan D, Teitelman G. Hybrid insulin genes reveal a developmental lineage for pancreatic endocrine cells and imply a relationship with neurons. Cell. 1988;53:295–308. doi: 10.1016/0092-8674(88)90391-1. [DOI] [PubMed] [Google Scholar]
- Bernardo AS, Barrow J, Hay CW, McCreath K, Kind AJ, Schnieke AE, Colman A, Hart AW, Docherty K. Presence of endocrine and exocrine markers in EGFP-positive cells from the developing pancreas of a nestin/EGFP mouse. Mol Cell Endocrinol. 2006;253:14–21. doi: 10.1016/j.mce.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Blyszczuk P, Czyz J, Kania G, Wagner M, Roll U, St-Onge L, Wobus AM. Expression of Pax4 in embryonic stem cells promotes differentiation of nestin-positive progenitor and insulin-producing cells. Proc Natl Acad Sci USA. 2003;100:998–1003. doi: 10.1073/pnas.0237371100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner-Weir S. Perspective: postnatal pancreatic β cell growth. Endocrinology. 2000;141:1926–1929. doi: 10.1210/endo.141.6.7567. [DOI] [PubMed] [Google Scholar]
- Cattaneo E, McKay R. Proliferation and differentiation of neuronal stem cells regulated by nerve growth factor. Nature. 1990;347:762–765. doi: 10.1038/347762a0. [DOI] [PubMed] [Google Scholar]
- Delacour A, Nepote V, Trumpp A, Herrera PL. Nestin expression in pancreatic exocrine cell lineages. Mech Dev. 2004;121:3–14. doi: 10.1016/j.mod.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Esni F, Stoffers DA, Takeuchi T, Leach SD. Origin of exocrine pancreatic cells from nestin-positive precursors in developing mouse pancreas. Mech Dev. 2004;121:15–25. doi: 10.1016/j.mod.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Furuta M, Yano H, Zhou A, Rouille Y, Holst JJ, Carroll R, Ravazzola M, Orci L, Furuta H, Steiner DF. Defective prohormone processing and altered pancreatic islet morphology in mice lacking active SPC2. Proc.Natl Acad Sci USA. 1997;94:6646–6651. doi: 10.1073/pnas.94.13.6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelling RW, Du QX, Dichmann DS, Rømer J, Huang H, Cui L, Obici S, Tang B, Holst JJ, Fledelius C, Johansen PB, Rossetti L, Jelicks LA, Serup P, Nishimura E, Charron MJ. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc Natl Acad Sci USA. 2003;100:1438–1443. doi: 10.1073/pnas.0237106100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittes GK, Rutter WJ. Onset of cell-specific gene expression in the developing mouse pancreas. Proc Natl Acad Sci USA. 1992;89:1128–1132. doi: 10.1073/pnas.89.3.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradwohl G, Dierich A, Lemeur M, Guillemot F. Neurogenin 3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci USA. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guz Y, Montminy MR, Stein R, Leonard J, Gamer LW, Wright CVE, Teitelman G. Expression of murine STF-1, a putative insulin gene transcription factor, in beta cells of pancreas, duodenal epithelium and pancreatic exocrine and endocrine progenitors during ontogeny. Development. 1995;121:11–18. doi: 10.1242/dev.121.1.11. [DOI] [PubMed] [Google Scholar]
- Herrera PL, Huarte J, Sanvito F, Meda P, Orci L, Vassalli JD. Embryogenesis of the murine endocrine pancreas; early expression of the pancreatic polypeptidegene. Development. 1991;113:1257–1265. doi: 10.1242/dev.113.4.1257. [DOI] [PubMed] [Google Scholar]
- Humphrey RK, Bucay N, Beattie GM, Lopez A, Messam CA, Cirulli V, Hayek A. Characterization and isolation of promoter-defined nestin-positive cells from the human fetal pancreas. Diabetes. 2003;52:2519–2525. doi: 10.2337/diabetes.52.10.2519. [DOI] [PubMed] [Google Scholar]
- Hunziker E, Stein M. Nestin-expressing cells in the pancreatic islets of Langerhans. Biochem Biophys Res Commun. 2000;271:116–119. doi: 10.1006/bbrc.2000.2611. [DOI] [PubMed] [Google Scholar]
- Jensen J. Gene regulatory factors in pancreatic development. Dev Dyn. 2004;229:176–200. doi: 10.1002/dvdy.10460. [DOI] [PubMed] [Google Scholar]
- Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- Klein T, Ling Z, Heimberg H, Madsen OD, Heller RS, Serup P. Nestin is expressed in vascular endothelial cells in the adult human pancreas. J Histochem Cytochem. 2003;51:697–706. doi: 10.1177/002215540305100601. [DOI] [PubMed] [Google Scholar]
- Koso H, Satoh S, Watanabe S. c-kit marks late retinal progenitor cells and regulates their differentiation in developing mouse retina. Dev Biol. 2007;301:141–154. doi: 10.1016/j.ydbio.2006.09.027. [DOI] [PubMed] [Google Scholar]
- Lardon J, Rooman I, Bouwens L. Nestin expression in pancreatic stellate cells and angiogenic endothelial cells. Histochem Cell Biol. 2002;117:535–540. doi: 10.1007/s00418-002-0412-4. [DOI] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin secreting structures similar to pancreatic islets. Science. 2001;292:1389–1394. doi: 10.1126/science.1058866. [DOI] [PubMed] [Google Scholar]
- Naya FJ, Huang HP, Qui Y, Mutoh H, De-Mayo FJ, Leiter AB, Tsai MJ. Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2-NeuroD-deficient mice. Genes Dev. 1997;11:2323–2334. doi: 10.1101/gad.11.18.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pictet R, Rutter WJ. Development of the embryonic pancreas. In: Steiner DF, Frenkel M, editors. Handbook of physiology. American Physiological Society; Washington, DC: 1972. pp. 25–66. [Google Scholar]
- Sander M, Sussel L, Conners J, Scheel D, Kalamaras J, Dela Cruz F, Schwitzgebel V, Hayes-Jordan A, German M. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development. 2000;127:5533–5540. doi: 10.1242/dev.127.24.5533. [DOI] [PubMed] [Google Scholar]
- Seaberg RM, Smukler SR, Kieffer TJ, Enikolopov G, Asghar Z, Wheeler MB, Korbutt G, van der Kooy D. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nature Biotechnol. 2004;22:1115–1124. doi: 10.1038/nbt1004. [DOI] [PubMed] [Google Scholar]
- Selander L, Edlund H. Nestin is expressed in mesenchymal and not epithelial cells of the developing mouse pancreas. Mech Dev. 2002;113:189–192. doi: 10.1016/s0925-4773(02)00023-0. [DOI] [PubMed] [Google Scholar]
- Soria B. In-vitro differentiation of pancreatic beta-cells. Differentiation. 2001;68:205–219. doi: 10.1046/j.1432-0436.2001.680408.x. [DOI] [PubMed] [Google Scholar]
- Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. The Pax4 gene is essential for differentiation of insulin-producing β cells in the mammalian pancreas. Nature. 1997;386:399–402. doi: 10.1038/386399a0. [DOI] [PubMed] [Google Scholar]
- Steiner DF. The proprotein convertases. Curr Opin Chem Biol. 1998;2:31–39. doi: 10.1016/s1367-5931(98)80033-1. [DOI] [PubMed] [Google Scholar]
- Sussel L, Kalamaras J, Hartigan-O'Connor DJ, Meneses JJ, Pedersen RA, Ruben-stein JL, German MS. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development. 1998;125:2213–2221. doi: 10.1242/dev.125.12.2213. [DOI] [PubMed] [Google Scholar]
- Treutelaar MK, Skidmore JM, Dias-Leme CL, Hara M, Zhang L, Simeone D, Martin DM, Burant CF. Nestin-lineage cells contribute to the microvasculature but not endocrine cells of the islet. Diabetes. 2003;52:2503–2512. doi: 10.2337/diabetes.52.10.2503. [DOI] [PubMed] [Google Scholar]
- Turque N, Plaza S, Radvanyi F, Carriere C, Saule S. Pax-QNR/Pax-6, a paired box- and homeobox-containing gene expressed in neurons, is also expressed in pancreatic endocrine cells. Mol Endocrinol. 1994;8:929–938. doi: 10.1210/mend.8.7.7984154. [DOI] [PubMed] [Google Scholar]
- Vincent M, Guz Y, Rozenberg M, Webb G, Furuta M, Steiner D, Teitelman G. Abrogation of protein convertase 2 activity results in delayed islet cell differentiation and maturation, increased alpha-cell proliferation, and islet neogenesis. Endocrinology. 2003;144:4061–4069. doi: 10.1210/en.2003-0088. [DOI] [PubMed] [Google Scholar]
- Vuguin P, Kedees M, Gui L, Guz Y, Gelling R, Nejathaim M, Charron M, Teitelman G. Ablation of the glucagon receptor gene increases fetal lethality, and produces alterations in islet development and maturation. Endocrinology. 2006;147:3995–4006. doi: 10.1210/en.2005-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME, Scheel D, German MS. Gene expression cascades in pancreatic development. Mech Dev. 2003;120:65–80. doi: 10.1016/s0925-4773(02)00333-7. [DOI] [PubMed] [Google Scholar]
- Yasuhara T, Matsukawa N, Hara K, Yu G, Xu L, Maki M, Kim SU, Borlongan CV. Transplantation of human neural stem cells exerts neuroprotection in a rat model of Parkinson's disease. J Neurosci. 2006;26:12497–12511. doi: 10.1523/JNEUROSCI.3719-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulewski H, Abraham EJ, Gerlach MJ, Daniel PB, Moritz W, Muller B, Vallejo M, Thomas MK, Habener JF. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex-vivo into pancreatic endocrine, exocrine and hepatic phenotypes. Diabetes. 2001;50:521–533. doi: 10.2337/diabetes.50.3.521. [DOI] [PubMed] [Google Scholar]