Abstract
For effective cancer immunotherapy by vaccination, co-delivery of tumour antigens and adjuvants to dendritic cells and subsequent activation of antigen-specific cytotoxic T cells (CTLs) is crucial. In this study, a synthetic long peptide (SLP) harbouring the model CTL epitope SIINFEKL was encapsulated with the TLR3 ligand poly(inosinic-polycytidylic acid) (poly(I:C)) in cationic liposomes consisting of DOTAP and DOPC. The obtained particles were down-sized to about 140 nm (measured by dynamic light scattering) and had a positive zeta-potential of about 26 mV (according to laser Doppler electrophoresis). SLP loading efficiency was about 40% as determined by HPLC. Poly(I:C) loading efficiency was about 50%, as assessed from the fluorescence intensity of fluorescently labelled poly(I:C). Immunogenicity of the liposomal SLP vaccine was evaluated in vitro by its capacity to activate dendritic cells (DCs) and present the processed SLP to SIINFEKL-specific T cells. The effectiveness of the vaccine to activate CD8+ T cells was analysed in vivo after intradermal and subcutaneous immunisation in mice, by measuring antigen-specific T cells in blood and spleens and assessing their functionality by cytokine production and in vivo cytotoxicity. The liposomal formulation efficiently delivered the SLP to DCs in vitro and induced a functional CD8+ T cell immune response in vivo to the CTL epitope present in the SLP. The SLP-specific CD8+ T cell frequency induced by the poly(I:C)-adjuvanted liposomal SLP formulation showed an at least 25 fold increase over the T cell frequency induced by the poly(I:C)-adjuvanted soluble SLP. In conclusion, cationic liposomes loaded with SLP and poly(I:C) have potential as a powerful therapeutic cancer vaccine formulation.
Electronic supplementary material
The online version of this article (doi:10.1208/s12248-014-9686-4) contains supplementary material, which is available to authorized users.
KEY WORDS: cancer immunotherapy, cationic liposomes, CTL epitope, peptide antigen, Poly(I:C)
INTRODUCTION
Therapeutic vaccination has recently gained interest as a potential strategy to treat cancer and chronic infectious diseases, but it is not yet established as a standard treatment modality (1). It is obvious that treatment of an ongoing disease by vaccination to (re)activate the immune system is more demanding as compared to classical prophylactic vaccines and requires optimal formulation of the specific vaccines. Therefore, in contrast to preventive vaccination, for which the induction of neutralising antibodies can be adequate in most cases, therapeutic vaccination relies significantly on the induction of cell-mediated immunity. Cell types essential for a cellular response include CD8+ cytotoxic T lymphocytes (CTLs) and CD4+ T-helper (Th) cells, and activation of both cell types requires antigen presentation by specialised antigen-presenting cells (APCs) (1,2). Of these, dendritic cells (DCs) located in the lymphoid tissues (such as CD8α+ DC subset) or in the mucosa (CD103+) are the most efficient APCs, highly specialised at taking up exogenous antigens and presenting them to CD8+ T cells, as processed peptides in the groove of MHC class I molecules (3,4). Optimal vaccine formulations should therefore be targeted to DCs to be processed and presented to antigen-specific T cells. As CD8+ CTLs possess a central role in the response to viral infections and cancer, research strategies for the development of therapeutic vaccines are focusing on ways to induce a strong CTL response (5–8).
Therapeutic vaccination with overlapping synthetic long peptides (SLPs), including all potential MHC class I and II restricted epitopes of a tumour-associated antigen (TAA), has been proven superior to vaccination with protein antigen (9) or minimal synthetic peptide epitopes (10) for the induction of a long-term effector CD8+ T cell immune response in mouse models. Impressive clinical responses have been observed also in women with HPV16+ grade 3 vulvar intraepithelial neoplasia (VIN3 pre-malignant lesions) after therapeutic vaccination with a SLP-based vaccine against E6 and E7 oncoproteins in Montanide, an incomplete Freund’s adjuvant (IFA)-like mineral oil emulsion (11). However, end-stage HPV16+ cervical cancer patients did not benefit from this type of vaccine, despite induction of significant levels of HPV-specific T cells (11). These studies showed that SLP vaccines have functional potency when applied to pre-malignant-stage patients, but need to be further improved for use as a therapeutic vaccine against cancer. So far, SLPs have been administered in Montanide as the most common vehicle used for peptide vaccinations in clinical trials; however, the use of such water-in-oil formulations have some important drawbacks, such as their non-biodegradibility, low long-term stability and induction of local side effects (12). Therefore, there is an urgent need for replacement of Montanide emulsion-based formulations by more potent ones that, in addition, do not provoke serious side effects.
In this work, we proposed cationic liposomes, as a suitable alternative to Montanide, as adjuvant for SLP antigens. Liposomes have traditionally been used for the delivery of anti-cancer drugs like doxorubicin (13), but especially cationic liposomes can also efficiently target APCs, resulting in improvement of the induced humoral and cellular immune response, in particular when formulated with other adjuvants, such as Toll-like receptor (TLR) ligands (14–16). Advantages of liposomes as a vaccine delivery system include their excellent biocompatibility, their superior to Montanide biodegradability and their capacity for size and surface manipulations (17,18). The adjuvanticity of liposomes highly depends on their physicochemical properties such as lipid composition, size and surface charge (19). Size is a key factor for the adjuvanticity of liposomes. Particles with a size below 300 nm are efficiently taken up by DCs, whereas larger particles will most probably be taken up preferentially by macrophages, before they are scavenged by DCs (20–22).
In this study, we investigated the potential of a poly(inosinic-polycytidylic acid) (poly(I:C), a TLR3 ligand)-adjuvanted cationic liposomal formulation, as a delivery system for SLPs for cancer immunotherapy, upon vaccination. As a model peptide, a 24-amino acid-long SLP (referred to as OVA24) was used, covering the immunodominant SIINFEKL CTL epitope of ovalbumin (OVA). OVA24-loaded liposomes composed of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) and 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), with or without poly(I:C), were characterised for particle size, for zeta-potential and for peptide and TLR loading efficiencies. The obtained liposomal formulations were assessed in vitro and in vivo for their potency to induce a functional CD8+ T cell immune response. The observed T cell immune responses induced by our cationic adjuvanted SLP-loaded liposomal formulation were clearly superior to the one observed by the soluble SLP mixed with poly(I: C).
MATERIALS AND METHODS
Materials
The OVA-derived 24-mer SLP OVA24 [DEVSGLEQLESIINFEKLAAAAAK], including the immunodominant cytotoxic T lymphocyte (CTL) epitope [SIINFEKL] and the CTL OVA257-264 SIINFEKL (OVA8), was produced and purified at the GMP facility of the Clinical Pharmacy and Toxicology Department of Leiden University Medical Center (23). The lipids DOPC and DOTAP were purchased from Avanti Polar Lipids (Alabaster, AL, USA), and poly(I:C) and its rhodamine-labelled analogue were obtained from InvivoGen (Toulouse, France). Carboxyfluorescein succinimidyl ester (CFSE) was purchased from Invitrogen (Eugene, OR, USA). Acetonitrile (ACN), chloroform and methanol were obtained from Biosolve BV (Valkenswaard, the Netherlands), and Vivaspin 2 centrifuge membrane concentrators were purchased from Sartorius Stedim Biotech GmbH (Göttingen, Germany). Iscove’s modified Dulbecco’s medium (IMDM; Lonza Verniers, Belgium ) was supplemented with 8% (v/v) foetal calf serum (Greiner Bioscience, Alphen a/d Rijn, the Netherlands), 50 μM 2-mercaptoethanol (Sigma-Aldrich, Zwijndrecht, Netherlands) and 2 mM glutamine (Life Technologies, Bleiswijk, the Netherlands). Deionised water with a resistivity of 18 MΩ cm was produced by a Millipore water purification system (MQ water). Phosphate buffer was composed of 7.7 mM Na2HPO4·2H2O and 2.3 mM NaH2PO4·2H2O, pH 7.4 (10 mM phosphate buffer (PB), pH 7.4). MQ water and 10 mM PB, pH 7.4, were filtered through a 0.22-μm Millex-GP PES-filter (Millipore, Ireland) before use. Phosphate-buffered saline (PBS: 140 mM NaCl, 8.7 mM Na2HPO4·12H2O, 1.8 mM NaH2PO4·2H2O, pH 7.4), which was used for all the in vitro and in vivo assays, was purchased from B. Braun (Meslungen, Germany).
Mice
Female C57BL/6 (H-2b) mice were purchased from Charles River (L’Arbresle, France), and congenic CD45.1 (Ly5.1) mice were bred at the Leiden University Medical Center animal facility and used at 8–14 weeks of age according to the Dutch Experiments on Animal Act, which serves the implementation of “Guidelines on the protection of experimental animals” by the Council of Europe.
Liposome Preparation
Cationic liposomes loaded with OVA24 were prepared by using the thin film dehydration-rehydration method (24), followed by extrusion. In detail, DOTAP and DOPC in chloroform, in a 1:1 molar ratio, were mixed in a round-bottomed flask to reach a concentration of 10 mg lipid per mL of final liposome dispersion. A lipid film was formed by chloroform evaporation in a rotary evaporator for 1 h at 37°C. The film was then rehydrated either with filtered MQ water (2 mL, pH 5.5–5.8) for non-loaded (empty) liposomes or with 2 mL of a solution of 1 mg/mL OVA24 in ACN/H2O 1:1 (v/v) (OVA24-loaded liposomes); the dispersion was mixed briefly in the presence of glass beads and equilibrated for 1 h at room temperature. For poly(I:C)-loaded liposomes, the ligand including 0.5% fluorescently labelled equivalent was added dropwisely to the rehydrated lipid film until a concentration of 200 μg poly(I:C) per mL of liposome dispersion was reached. Next, the liposome dispersion was snap-frozen in liquid nitrogen, followed by freeze-drying in a Christ alpha 1–2 freeze-dryer (Osterode, Germany) overnight. Dehydrated-rehydrated liposomes were generated by gradually hydrating the freeze-dried lipid cake in 10 mM PB, pH 7.4. To prepare 2 mL of liposome dispersion, two volumes of 400 μL and one volume of 1,200 μL PB were successively added, with intervals of 20 min between each addition. The mixture was vortexed well during the rehydration steps, and the resulting dispersion was kept at room temperature for at least 1 h.
Sizing of the obtained multilamellar vesicles was performed by high-pressure extrusion at room temperature, by using LIPEX Extruder (Northern Lipids Inc., Canada). To obtain monodispersed liposomes, the liposome mixture was passed four times through a 400-nm-pore size membrane and four times through a 200-nm-pore size membrane (polycarbonate track-etched membranes, Nuclepore, Millipore, Kent, UK).
Purification (removal of free OVA24 peptide) and concentration of OVA24-loaded liposomes was performed by using a Vivaspin 2 centrifugation concentrator (PES membrane, molecular weight cut-off (MWCO) 300 kDa): 1,300 μL of the liposome dispersion was loaded in the concentrator, which was then centrifuged at 2,000 rpm and 20°C for 6–7 h, till the suspension was concentrated fivefold. The filtrate, containing the free OVA24, was collected, and the concentrated liposome dispersion was diluted by adding 1,000 μL PB, followed again by a fivefold concentration wash to remove most of the free soluble peptide. Samples of the liposome fraction and the free fractions were taken after each step for OVA24 analysis (see below). The concentrated-purified liposomes were physicochemically stable for at least 6 weeks in the refrigerator, as assessed by dynamic light scattering (DLS) and zeta-potential measurements.
Liposome Characterisation
Dynamic Light Scattering and Zeta-Potential
Average diameter (Zave) and polydispersity index (PDI) of the liposomes were determined by dynamic DLS using a Zetasizer (Nano ZS, Malvern Ltd., UK). The zeta-potential was determined by laser Doppler electrophoresis using the same device. For these measurements, liposome samples were diluted 100-fold in PB.
Peptide and Poly(I:C) Loading Efficiency
Peptide loading efficiency was determined by extracting OVA24 from the loaded liposomes and measuring the extracted fraction by reversed-phase HPLC. For that purpose, a modified Bligh-Dyer method was used: 250 μL of methanol (including 62.5 μg/mL of insulin as internal standard) and 125 μL of chloroform were added to 100 μL of aqueous liposomal dispersion. The mixture was vortexed well, followed by addition of 250 μL of 0.1 M HCl and another 125 μL of chloroform. After vortexing and centrifuging for 5 min at 1,000×g, the upper water-methanol phase containing the peptide OVA24 (as well as insulin) was carefully collected and analysed by HPLC (see below). In addition to the extracted samples from liposomes, the PB filtrates from the purification/concentration of the liposomes (before wash and after wash) containing the free OVA24 were also analysed by HPLC. The loading efficiency was expressed as a percentage (%LE) of the initial peptide amount that was loaded into the Vivaspin 2 centrifugation concentrator.
For the OVA24 peptide quantification, the HPLC method described before (25) was used. In detail, 50 μL of the water-methanol extract (see above) was injected onto a 3-μm C18-AQ reversed-phase HPLC column (150 × 4 mm) (Dr. Maisch HPLC GmbH, Ammerbuch-Entringen, Germany) by using an HPLC system from Waters (Saint-QuentinEn Yvelines Cedex, France). The mobile phases 5% ACN in MQ water with 0.1% TFA (solvent A) and 95% ACN in MQ water with 0.1% TFA (solvent B) were applied in a linear gradient from 0 to 78% solvent B over 20 min at a flow rate of 1 mL/min. Detection of the peptide was achieved by measuring the absorbance at 220 nm through an ultraviolet system detector (Waters 486), and the peptide concentration for each sample was determined based on a calibration curve with known OVA24 concentrations (0.5–500 μg/mL).
The amount of poly(I:C) in the liposomes was determined by measuring the fluorescence (ex. 546 nm and em. 576 nm) of its rhodamine-labelled analogue in collected non-solubilised (i.e., intact) liposomal samples and the PB filtrates from the purification/concentration process (before wash and after wash) containing the free poly(I:C) (assuming that the 0.5% of the used labelled compound behaved the same as the non-labelled equivalent). A calibration curve of the fluorescently labelled poly(I:C) was made, ranging from 7.81 to 1,000 ng/mL, and all samples were measured using a fluorescence micro plate reader (Tecan, Salzburg, Austria).
DC Maturation Evaluated by IL-12 ELISA and Flow Cytometric Analysis of CD80 DC Marker
Immature D1 cells (5 × 104 cells/well) (i.e., a long-term growth factor-dependent dendritic cell line derived from spleens of C57BL/6 mice that was cultured as described before (26)) were incubated in 96-well round-bottomed plates at 37°C in supplemented IMDM with OVA24-loaded liposomes or soluble OVA24 (sOVA24) at different concentrations. After overnight incubation at 37°C, the plates were centrifuged and the supernatant was collected for IL-12p40 measurement by ELISA (BD Biosciences) according to the manufacturer’s protocol. In addition, the cells were stained in staining buffer (PBS +0.1% (w/v) bovine serum albumin (BSA) +0.05% (w/v) sodium azide) for 30 min with fluorescently labelled FITC antibody specific for the mouse CD80 DC surface marker (eBiosciences). 7-Aminoactinomycin D (7AAD) (Life Technologies) was used for the exclusion of dead cells, and data acquisition of samples was done with a BD LSR II flow cytometer. The data were analysed by using the FlowJo software (Tree Star).
In Vitro MHC Class I Antigen Presentation
The immunogenicity of the OVA24 formulations was initially tested in an in vitro readout, with regard to their efficiency to activate immature DCs and present SIINFEKL to CD8+ antigen-specific T cells, leading to their activation. Immature D1 cells were incubated in 96-well flat-bottomed plates at 37°C in supplemented IMDM with OVA24-loaded liposome formulation or plain OVA24 in PBS solution (sOVA24) at different concentrations. The stability of the liposomes did not change when diluted in PBS, as assessed by DLS analysis (unpublished data). After 2.5 h, the plates were washed three times with supplemented IMDM culture medium, in order to remove excess antigen. Subsequently, T cell hybridoma B3Z cells (50 × 105/well) were added followed by overnight incubation at 37°C (27). Chlorophenol red-β-galactopyranoside (CPRG) was used as lacZ substrate in cell lysates, and the colour conversion was measured by detecting absorbance at 590 nm.
Immunisation of Mice
Mice were immunised with OVA24-loaded liposomes or sOVA24 (with or without poly(I:C)) by intradermal injection in the abdominal skin area (28). All formulations were prepared on the day of injection. Vaccination dose was 5 nmol (12.5 μg) of peptide in a total volume of 30 μL, and immunisations were performed on day 0 (prime immunisation) and repeated after 2 weeks on day 14 (boost injection). Vaccinations with adjuvanted liposomes included 4 μg of poly(I:C)/mouse. Besides intradermal injection, which was chosen based on preliminary in vivo experiments, subcutaneous immunisation (in the flank) of the formulations in a total volume of 200 μL PBS served as a control (data not shown in all experiments). During the in vivo studies, blood samples were obtained from the tail vein at different time points. After the mice were sacrificed on day 28/29, the spleens were removed and processed for splenocyte isolation.
Analysis of Antigen-Specific CD8+ T Cell Responses by Flow Cytometry
Staining of the cell surface was performed on freshly isolated PBMCs after red blood lysis. Cells were stained in staining buffer for 30 min with allophycocyanin-labelled tetramer-OVA257-264 (TM-SIINFEKL) and fluorescently labelled antibodies specific for mouse CD3 (BD Biosciences), CD4, CD8 and the killer cell lectin-like receptor G1 (KLRG1) (eBiosciences). 7AAD (Life Technologies) was used for the exclusion of dead cells.
Overnight intracellular cytokine analysis of PBMCs was performed after incubating the cells with 2 μM of minimal CTL OVA257-264 SIINFEKL (OVA8) in the presence of brefeldin A (7.5 μg/mL) (BD Biosciences, Breda, the Netherlands). On the next day, after cell surface staining with fluorescently labelled antibodies to mouse CD3 and CD8, cells were fixed with Cytofix/Cytoperm solution (BD Biosciences, Breda, the Netherlands) and permeabilised with Perm/Wash solution. Subsequently, cells were stained with fluorescently labelled antibodies against IFN-γ and TNF-α at 4°C for 30 min. Finally, data acquisition of samples was done with a BD LSR II flow cytometer and data were analysed by using the FlowJo software (Tree Star).
In Vivo Cytotoxicity Assay
Splenocytes from naive congenic CD45.1+ mice were counted and split into two equal parts, after lysis of erythrocytes. Cells were labelled with CFSE for 1 h at 37°C, with a final concentration of either 5 μM (target population) or 0.5 μM (control). The CFSE staining reaction was blocked with 10% (v/v) foetal calf serum, and the cells of the target population were pulsed for 1 h with OVA8 in complete culture medium at 37°C. Next, the cells were washed four times with PBS before the two fractions (target population-control) were mixed in a 1:1 number ratio and adoptively transferred intravenously in recipient previously immunised C57BL/6 mice in a volume of 200 μL in PBS. Two days after the adoptive transfer of the target cells (day 24 of vaccination schedule), mice were sacrificed, spleens were isolated and single-cell suspensions were analysed by flow cytometry for specific killing following the equation below:
Statistical Analysis
The significance of differences in the in vitro and in vivo assays testing the properties of the induced CD8+ T cells was evaluated by GraphPad Prism 5 (GraphPad) software, using an analysis of variance (ANOVA) at a 0.05 significance level, followed by Tukey’s and non-parametric Mann-Whitney post-test.
RESULTS
Peptide and Adjuvant Successfully Loaded in Cationic Liposomes
Following the film dehydration-rehydration method, cationic DOTAP-based liposomal formulation was formed (29) loaded with the ovalbumin-derived SLP (OVA24), containing the well-defined CTL epitope SIINFEKL. The obtained OVA24-loaded liposomes had an average size of about 140 nm, a low PDI (<0.2) and a positive zeta-potential. Measurement of the extracted SLP from concentrated liposomes revealed a final peptide loading efficiency of about 40% (Table I).
Table I.
Physicochemical Properties of Liposomal Formulations
| Z ave diameter (nm) | PDI | ZP (mV) | OVA24 LE (%) | Poly(I:C) LE (%) | |
|---|---|---|---|---|---|
| Empty liposomes | 142 ± 5 | 0.15 ± 0.02 | 34 ± 4 | NA | NA |
| OVA24-loaded Liposomes | 130 ± 5* | 0.12 ± 0.02 | 25 ± 3* | 40 ± 8 | NA |
| OVA24/poly(I:C)-loaded liposomes | 148 ± 8 | 0.16 ± 0.05 | 28 ± 3 | 38 ± 5 | 50 ± 4 |
Data are averages ± SD of at least five independent batches
Z ave average particle diameter in nanometres, PDI polydispersity index, ZP zeta-potential, LE loading efficiency, NA not applicable
*p < 0.05 (size and ZP are significantly different from empty liposomes, evaluated by one-way ANOVA with Tukey’s test)
The co-encapsulation of poly(I:C) did not substantially influence the liposome characteristics. The zeta-potential of the adjuvanted liposomes was comparable to that of the non-adjuvanted ones, while the average size was only slightly larger (ca. 150 nm, PDI <0.2). This could be expected, because the initial molar ratio of negatively charged poly(I:C) subunits (MW = 335.5 g/mol) and DOTAP (MW = 698.5 g/mol) was only about 0.06 and most of the poly(I:C) was likely localised inside the liposomes. Furthermore, the loading efficiency of OVA24 was very similar in the poly(I:C)-loaded liposomes and that of poly(I:C) was about 50% (Table I), yielding a final formulation containing about 500 μg/mL OVA24 peptide and 100 μg/mL poly(I:C). Our data show that both poly(I:C)-adjuvanted and non-adjuvanted OVA24-liposomes can be prepared in a reproducible fashion, despite potential competition between these two negatively charged compounds regarding their association with the cationic liposomes.
In Vitro Antigen Cross-Presentation
The efficiency of the OVA24-loaded liposomes to activate DCs and induce the uptake and processing of the peptide for MHC class I cross-presentation and subsequent activation of CD8+ antigen-specific T cells was assessed in in vitro assays. DCs were incubated with the liposomal formulations and the controls, followed by washing steps to remove the excess of the formulations. Subsequently, the DCs were co-cultured with the OVA-specific B3Z hybridoma to sensitively detect MHC class I presented SIINFEKL epitopes. B3Z is a CD8+ T cell line specific for the H-2 Kb-restricted SIINFEKL CTL epitope of ovalbumin; it contains the lacZ reporter gene under the regulation of NF-AT element of the IL-2 promoter (27). Therefore, after ligation of the T cell receptor (TCR), the β-galactosidase protein is expressed, driven by the IL-2 promoter, allowing quantitative measurement of T cell activation by this reporter enzyme in a colourimetric assay.
Incubation of DCs with OVA24-loaded liposomes led to an improved activation of OVA-specific CD8+ T cells in vitro, in comparison with sOVA24, indicating a more efficient antigen presentation by the DCs (Fig. 1). All SLP-loaded liposome formulations showed a comparable efficacy regardless of the inclusion of the poly(I:C) in the formulation. The latter was expected, since antigen presentation to the B3Z reporter T cell hybridoma is independent of B7-ligation signalling.
Fig. 1.
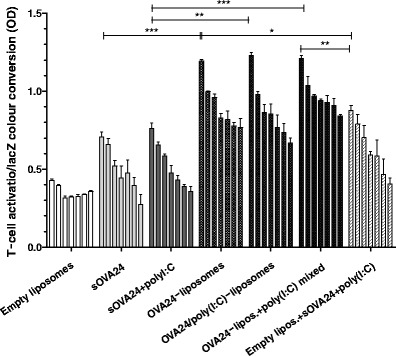
Activation of SIINFEKL-specific B3Z CD8+ T cells after culturing overnight with DCs pulsed for 2.5 h with titrated amounts (4.0–0.063 μM) of OVA24 formulations (empty liposomes were diluted to lipid concentrations corresponding to those of OVA24-loaded liposomes). Graphs depict T cell activation based on the OD at 590 nm value measured upon colour conversion of cell lysates after addition of CPRG (lacZ substrate). Data were analysed with one-way ANOVA with Tukey’s multiple comparison test, and p value was calculated with non-parametric Mann-Whitney test. ***p < 0.0005, **p < 0.005 and *p < 0.05 (significant data; results represented as mean ± SD, n = 3). The plus symbol denotes liposomes and poly(I:C) that are mixed, and the solidus symbol denotes co-encapsulated compounds
DC Maturation
DCs are the most potent APCs and express several sensing receptors including TLR3, the receptor for the immunostimulant poly(I:C). Signalling through the TLRs leads to the activation and maturation of DCs, as can be measured by enhanced expression of the co-stimulatory marker CD80 and the production of the effector cytokine IL-12. These are essential signals for an effective DC-T cell interaction and the initiation of an adaptive immune response. Figure 2a shows that all poly(I:C)-adjuvanted formulations induced enhanced expression of the DC surface marker CD80 in vitro, comparable to stimulation with LPS, a well-known TLR4 and a commonly studied TLR ligand that induces robust DC maturation (positive control). Additionally, the elevated levels of IL-12 that were detected in the culture medium of the activated cells (Fig. 2b) indicate that poly(I:C) maintains its bioactivity after encapsulation or co-delivery (mixed) with the liposomes, albeit slightly reduced compared to free poly(I:C) (Fig. 2a).
Fig. 2.

DC activation in vitro as measured by the expression of CD80 activation marker on the DC surface after overnight incubation of DCs at 37°C with titrated amounts of OVA24 formulations. The cells were stained with fluorescently labelled anti-CD80 antibody and analysed by flow cytometry. Graphs depict the percentages of activated DC cells according to the expression of CD80 protein (a) and IL-12 cytokine concentration in culture medium after overnight incubation of DCs at 37°C with titrated amounts of OVA24 formulations. Cells incubated with LPS (control) produced 3.0 ng/mL IL-12, while poly(I:C) in solution followed the same concentration trend as sOVA24 + poly(I:C) sample (b). Data were analysed with one-way ANOVA with Tukey’s multiple comparison test, and p was value calculated with one-tailed Mann-Whitney test. *p ≤ 0.05 (significant data; results represented as mean ± SD, n = 3). The plus symbol denotes liposomes and poly(I:C) that are mixed, and the solidus symbol denotes co-encapsulated compounds
OVA24-Specific CD8+ T Cell Responses In Vivo
To investigate the efficacy of OVA24-loaded liposomal vaccines to induce T cell mediated immunity in vivo, a series of vaccination studies were performed. Naive wild-type BL6 mice were injected with different SLP formulations; the strength of OVA-specific CD8+ T cell responses was assessed by detecting antigen-specific CD8+ T cell frequencies in blood and spleens with MHC Kb-tetramer-OVA257-264 (TM-SIINFEKL) allophycocyanin-labelled conjugates.
The potency of our SLP-liposomal vaccine was firstly investigated in a dose-response in vivo setting. Using SLP doses ranging from 0.2 to 25 nmol, mice were immunised intradermally with either OVA24/poly(I:C)-loaded liposomes or sOVA24 + poly(I:C) mixed. The antigen-specific CD8+ T cell responses were monitored in blood (day 21) and spleens (day 28) ex vivo. Our adjuvanted liposome vaccines were capable of inducing strong T cell responses which were clearly detectable ex vivo without the need of additional re-stimulation in vitro (Fig. 3 and Supplemental figure 4). Irrespective of the dose used, the liposomal sOVA24/poly(I:C) formulations proved their superiority to the soluble ones with regard to the induction of antigen-specific T cells. The systemic CD8+ T cell frequencies in blood 1 week after the booster injection (Fig. 3a) that were induced by the three highest doses of liposomal vaccines were comparably high, showing that the peptide liposomal-loaded vaccine is significantly (at least 25-fold) more effective than the SLP mixed with poly(I:C) and delivered in solution. At day 28 (2 weeks after the booster injection), the CD8+ T cell response in blood has declined to lower levels, but the superiority of the liposome vaccine is still evident (Supplemental figure 1). At this time, mice were sacrificed and their spleens were analysed, again showing significantly higher frequencies of OVA-specific CD8+ T cells induced by the liposomal vaccine in a dose-dependent fashion (Fig. 3b).
Fig. 3.
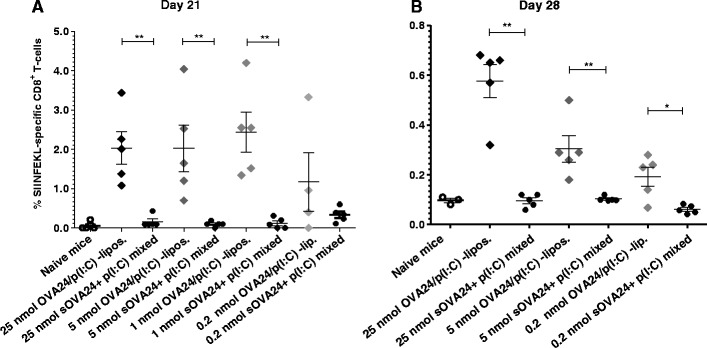
Antigen-specific CD8+ T cell responses following intradermal vaccination of C57BL/6 mice on day 0 and day 14 with different SLP doses of poly(I:C)-adjuvanted formulations, as monitored in blood on day 21 (a) and in spleens on day 28 (b). p value was calculated with one-tailed Mann-Whitney test. **p < 0.005 and *p < 0.05 (significant data). Each dot represents the response of an individual mouse (results represented as mean ± SEM, n = 5). Control mice and groups of mice immunised with 1 nmol of vaccine that are missing from panel B were sacrificed 1 day later for the need of the assay presented in Supplemental figure 2). The plus symbol denotes soluble OVA24 and poly(I:C) that are mixed, and the solidus symbol denotes co-encapsulated in liposomes compounds
Using the intermediate dose of 5 nmol, a number of SLP formulations were tested in vivo following the same immunisation schedule as previously for the dose-response experiment. Six days after the prime immunisation, the poly(I:C)-adjuvanted OVA24-loaded liposomes showed detectable OVA-specific T cell responses, which further improved especially 21 days after prime (i.e., 7 days after boost) immunisation, while the soluble peptide mixed with poly(I:C) did not elicit any significant response within this timeline (Fig. 4), similar to our previous observation (Fig. 3).
Fig. 4.
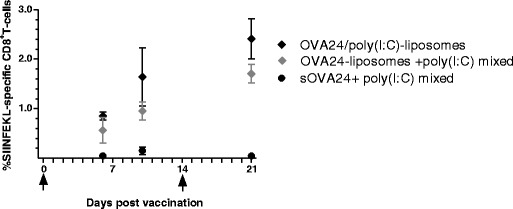
Induced T cell response within the immunisation schedule: Ova24/poly(I:C)-loaded liposomes induce high frequencies of OVA-specific CD8+ T cells in C57BL/6 mice that were vaccinated i.d. with formulations containing 5 nmol of OVA24, on days 0 and 14 (indicated by arrows). The graph depicts the percentages of OVA-tetramer (TM-SIINFEKL) in blood (results represented as mean ± SEM, n = 5) as a function of time. The plus symbol denotes liposomes and poly(I:C) that are mixed, and the solidus symbol denotes co-encapsulated compounds
On day 21, the OVA-specific CD8+ T cell responses in blood in mice immunised with OVA24-liposomal formulations, OVA24/poly(I:C)-liposomes and OVA24-liposomes + poly(I:C) mixed, were significantly enhanced, leading to a T cell induction frequency of about 2% (Fig. 5a), irrespective of the administration route that was used (see Supplemental figure 2 for the responses induced by the subcutaneous administration route). A similar trend was observed 28 days post-vaccination in splenocytes, although here the differences between groups were not significant (Fig. 5b).
Fig. 5.
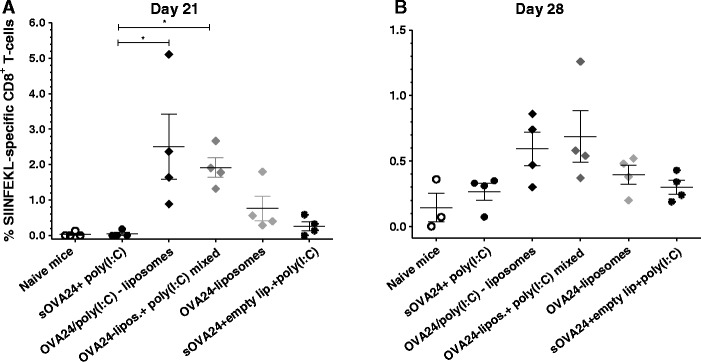
Antigen-specific CD8+ T cell responses in blood on day 21 (a) and in splenocytes on day 28 (b) following intradermal vaccination with 5 nmol of OVA24 formulations on day 0 and day 14. The experiment was performed three times with comparable results. Data were analysed with Kruskal-Wallis with Dunn’s multiple comparison post-test, and p value was calculated with non-parametric Mann-Whitney test. *p < 0.05 (significant data; results represented as mean ± SEM, n = 4). The plus symbol denotes liposomes and poly(I:C) that are mixed, and the solidus symbol denotes co-encapsulated compounds
From the above results, we can conclude that for an efficient antigen-specific CD8+ T cell immune response, incorporation of the SLP peptide in the liposomal delivery system appears crucial (Figs. 4 and 5): unlike liposomal OVA24, sOVA24 mixed with poly(I:C) or with poly(I:C) and empty liposomes did not induce any significant measurable CD8+ T cell response. In contrast, poly(I:C) significantly enhanced the induced T cell response elicited by the OVA24-loaded liposomes, no matter whether the TLR3 ligand was incorporated or mixed with the liposomes, as measured in blood (Figs. 4 and 5a) and in splenocytes (Fig. 5b). Altogether, the above results suggest that the efficacy of poly(I:C) adjuvanted OVA24-loaded liposomes to induce T cell immunity is clearly superior to that of sOVA24 mixed with poly(I:C).
While monitoring of T cell responses with TM-SIINFEKL allowed us to assess the capability of the formulations to expand antigen-specific CD8+ T cells, it does not give insight into their functionality. Therefore, flow cytometric analysis was used to also determine the KLRG1 expression on the surface of the induced CD8+ T cells as a measure of potential effector functionality (30,31). KLRG1 is expressed on antigen-specific memory T cells that are fully capable of responding with effector functions to antigen encounter (30,31). The percentages of the effector-memory-induced cells defined by the expression of KLRG1, within the antigen-specific T cell population, were in all SLP dosages significantly higher for the liposomal formulations (no obvious dose-dependent effect was observed) compared to the soluble SLP poly(I:C)-adjuvanted formulations, which did not induce detectable KLRG1 expression (Fig. 6 and Supplemental figure 5). These data demonstrate the functional effectiveness of adjuvanted liposomal OVA24 formulations.
Fig. 6.

Induction of KLRG1+ effector-memory CD8+ T cells within the population of SIINFEKL+-specific CD8+ T cells (depicted in Fig. 3a). The bar graphs depict the mean percentages (+SEM) at day 21. p values calculated with one-tailed Mann-Whitney test. *p < 0.05 (significant data, n = 5). The plus symbol denotes soluble OVA24 and poly(I:C) that are mixed, and the solidus symbol denotes co-encapsulated in liposomes compounds
Induction of CD8+ Cytotoxic T Cells In Vivo
An in vivo cytotoxicity assay was performed in order to assess target cell-specific killing potency of the induced CD8+ T cells. Intradermal immunisation of mice with 5 nmol OVA24-liposomes combined with poly(I:C) led to a strong cytotoxic activity (Fig. 7), in contrast to vaccination with the mixture of sOVA24 and poly(I:C) (Fig. 7). In detail, immunisation with OVA24/poly(I:C)-liposomes led to a killing capacity up to 80% when delivered intradermally, while the same formulation delivered subcutaneously showed a less effective killing activity, up to 40%. Moreover, intradermally administered OVA24-liposomes mixed with poly(I:C) led to a weaker cytotoxicity efficiency of around 50%. In summary, these results demonstrate that there is an administration route effect with regard to the killing capacity of our vaccine and that the poly(I:C) adjuvanticity depends on the way it is delivered with the liposomes. Interestingly, the observed cytotoxic activity of the induced T cells was maintained for at least 2 weeks after the boost immunisation (day 29) in mice receiving an even lower dose (1 nmol SLP) of the liposomal vaccine (see Supplemental figure 3), confirming the efficacy of the SLP liposomal vaccine based on the observed frequencies of SIINFEKL-specific CD8+ T cells at day 28 (Fig. 5).
Fig. 7.
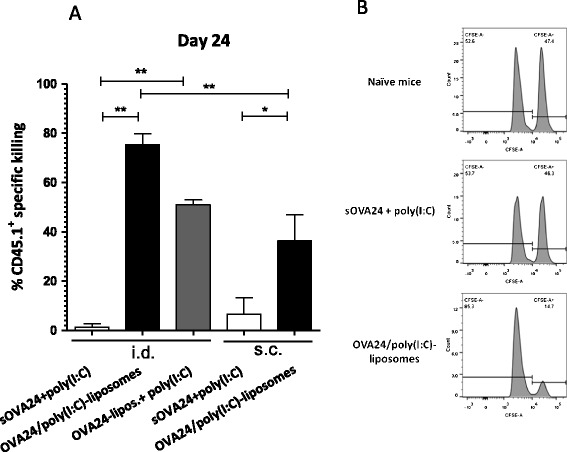
In vivo cytotoxicity assay. The normalised mean percentages (±SEM) of the killing activity are depicted based on the percentages of transferred CD45.1+ that could be detected against the control naive mice. Mice immunised with 5 nmol of OVA24 vaccine intradermally (i.d.) or subcutaneously (s.c.) (the last two bars) was monitored 1 week after the boost immunisation. The bar graphs depict the mean percentages (+SEM) at day 22 (a). Data were analysed with one-way ANOVA with Tukey’s multiple comparison test, and p value was calculated with one-tailed Mann-Whitney test. **p < 0.005, and *p < 0.05 (significant data, n = 5). Representative histograms of CFSE-labelled positive target cells (right peak = peptide pulsed and left peak = control) (b). The plus symbol denotes liposomes and poly(I:C) that are mixed, and the solidus symbol denotes co-encapsulated compounds
The capability of the induced CD8+ T cells to produce effector cytokines, such as IFN-γ and TNF-α is an indication of their functional effectiveness. IFN-γ is vital for protection and therapy against intracellular pathogens as well as tumours. Mice vaccinated with liposomal formulations of the peptide with poly(I:C) showed a higher efficiency to induce CD8+ T cells that produce IFN-γ compared to the adjuvanted sOVA24 in PBS (Fig. 8a). A fraction of these IFN-γ producing T cells were also positive for TNF-α (Fig. 8b). This simultaneous production of both IFN-γ and TNF-α cytokines shows the functional capacity of the SLP-liposomal formulations regarding the induction of antigen-specific effector CD8+ T cells.
Fig. 8.

Intracellular cytokine analysis. Blood of immunised mice was stimulated overnight ex vivo with SIINFEKL minimal peptide (OVA8) so that cytokine production will be amplified at detectable levels. CD8+ T cells producing interferon-γ (a) and CD8+ T cells producing both interferon-γ and TNF-α (b) Data were analysed with one-way ANOVA with Tukey’s multiple comparison test, and p value was calculated with one-tailed Mann-Whitney test. **p < 0.005 and *p < 0.05 (significant data, n = 4). The plus symbol denotes liposomes and poly(I:C) that are mixed, and the solidus symbol denotes co-encapsulated compounds
DISCUSSION
The design of safe and effective therapeutic cancer vaccines for human use remains an urgent but unmet medical need. Liposomes can be a promising delivery system, able to promote the uptake of the loaded antigen by DCs and elicit an anti-tumour immunity after vaccination. In this study, we showed that poly(I:C) adjuvanted-DOTAP-based liposomes loaded with a SLP can be a successful vaccine candidate for the induction of a functional CD8+ CTL response that is required for tumour eradication.
The superior immunogenicity of our OVA24-loaded DOPC/DOTAP liposome formulation can be attributed to the adjuvant nature of cationic liposomes. It has been reported that DOTAP or other cationic liposomes promote a vaccine-induced anti-tumour immune response, more efficiently than anionic or neutral liposomes (32), since they can enhance DC activation and maturation and the subsequent T cell priming. This ability is due to their lipid structure: cationic lipids, such as DOTAP with a quaternary ammonium group, are more potent than lipids with a tertiary ammonium group (33). Priming of DCs by antigen-loaded DOTAP-based liposomes induces the generation of reactive oxygen species (ROS), which in turn leads to the trigger of various signalling pathways, such as extracellular signal-regulated kinase (ERK) and p38, and consequently the production of cytokines and chemokines and expression of co-stimulatory molecules (34).
It has been reported that liposomes consisting only of DOTAP were more efficient for the delivery of an HPV E7-derived peptide and that incorporation of an inert lipid could reduce the anti-tumour activity of the liposomes (33). However, in most cases, the role of a “helper” neutral lipid has been shown to be essential for the stability of the formulation and the antigen delivery (35). The unsaturated zwitterionic lipid DOPC is known to possess such a role, contributing to the stability of the DOTAP-based loaded formulation by the physical interactions of the two phospholipids (salt bridges formation) and alterations on the membrane fluidity that improves the stability as well as the encapsulation efficiency (35).
The high surface charge density in cationic liposomal vaccines, apart from the improved adjuvant effect, can also be crucial for the loading and entrapment of the therapeutic agent, in our case the OVA24 peptide. OVA24 loading in the liposomes proved to be challenging mainly due to the peptide’s nature: it is neither very hydrophilic nor very lipophilic; it does not easily dissolve in aqueous solutions and is insoluble in many organic solvents, such as chloroform. Preparation and encapsulation procedure (mechanism of SLP loading) had to be optimised, in order to achieve a high peptide loading efficiency. In an attempt to find the most suitable formulation, liposomes of various lipid compositions were prepared, most of them yielding low loading efficiencies, especially when liposomes consisted of only neutral lipids or positively charged lipids in a low molar ratio (results not shown). This suggests that the peptide entrapment in the DOPC/DOTAP formulation is highly dependent on electrostatic interactions between the positive charged liposomes and the negatively charged peptide (at physiological pH). Indeed, decreasing the pH below OVA24’s isoelectric point (pI 4.3) led to a substantial drop in loading efficiency, and the loading efficiency of the SLP in neutral or negatively charged liposomes was very low (unpublished data).
In this study, we showed that a cationic liposomal formulation loaded with a SLP and combined with poly(I:C) is more potent than the soluble equivalent vaccine for the induction of a CD8+ T cellular immune response. In addition, the induced T cell responses that were observed upon administration of the liposomes were much higher compared to other vaccine systems that have been used for SLP delivery, such as Montanide, even when the dose was reduced more than 25 times. SLP-loaded liposomes can promote the entry of the peptide into the cytoplasm, their fragmentation by the proteasome and the access of the peptide fragments to the MHC class I proteins that will eventually result in a CTL response (36). The liposomal formulations and controls of OVA24 and TLR ligand were delivered intradermally. Yet, subcutaneous injection was used as a control as the most commonly used administration route. It is known that the administration route can influence the nature of the induced T cell response (37,38) and the targeted APC subsets (39). After intradermal injection, liposomes can form an antigen depot at the injection site, which facilitates a prolonged antigen loading of cross-presenting specialised DCs (such as CD8α+) almost exclusively in the local draining lymph nodes (40), whereas upon subcutaneous immunisation, the antigen more likely is taken up by DCs at the injection site and subsequently transported to lymph nodes, leading to a less efficient T cell activation. Interestingly, OVA24/poly(I:C)-loaded liposomes seem to have a significant efficacy to induce a specific T cell immune response after 21 days, regardless of the administration route used. However, intradermal administration appeared to be superior to the subcutaneous route regarding the effectiveness of the SLP-specific induced T cells, as indicated by cytokine production and killing capacity. It is known that activated T cells amplify their reactions via the secretion of cytokines, such as IFN-γ that plays a key role in the induction of cellular immunity. Additionally, the detection of high and simultaneous production of IFN-γ and TNF-α by CD8+ T cells in mice immunised intradermally with the poly(I:C)-adjuvanted OVA24-liposomes indicates the functional properties of the induced immune response: The simultaneous production of IFN-γ and TNF-α is a functional capacity of effective vaccine-induced CD8+ T cells (41–43). Furthermore, the efficacy of our liposomal vaccine has also been proven via its capacity to induce cytotoxic specific CD8+ T cells capable of killing target cells in vivo. Cancer vaccines loaded with peptides and co-delivered with poly(I:C) have been reported to elicit tumour antigen-specific cytotoxic T cell responses in vivo or even suppress the tumour growth (44). While poly(I:C) in solution with OVA24 (with or without empty liposomes) did not enhance the peptide’s immunogenicity, when combined with loaded liposomes, the adjuvant effect was pronounced. Although the SLP-specific CD8+ T cell priming was improved in mice immunised with the adjuvanted liposomes irrespective of the way the poly(I:C) was delivered (encapsulated or mixed), we observed a significant difference between the OVA24/poly(I:C)-loaded liposomes and the OVA24-liposomes + poly(I:C) mixed formulation, regarding the functionality of the induced T cells. The OVA24/poly(I:C) co-encapsulated liposomal formulation appeared to be more effective regarding the production of IFN-γ and TNF-α cytokines from the activated T cells and more effective in inducing killing capacity. This finding is likely due to the enhanced poly(I:C) internalisation by the liposomes and the promoted interaction between the TLR3 and its ligand in the endosomes (45,46). We therefore favour the co-encapsulation of both the SLP antigen and TLR ligand adjuvant in the same liposome.
CONCLUSION
Therapeutic vaccination with overlapping SLPs has been proven superior to vaccination with minimal peptide epitopes for the induction of long-term effector CD8+ T cell immune responses. The features of cationic liposomes, including safety, storage stability and versatility with regard to the accommodation of antigens as well as adjuvants with variable physicochemical properties, make them promising for vaccine delivery. Our data clearly show the superior efficacy of our cationic SLP-loaded liposomal formulation adjuvanted with poly(I:C) compared to soluble SLP and its potential to be used as a effective therapeutic vaccine against cancer.
Electronic supplementary material
(DOCX 448 kb)
Contributor Information
Ferry Ossendorp, Phone: +31 71 526 3843, Email: f.a.ossendorp@lumc.nl.
Wim Jiskoot, Phone: +31 71 527 4314, Email: w.jiskoot@lacdr.leidenuniv.nl.
References
- 1.Melief CJ, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer. 2008;8(5):351–60. doi: 10.1038/nrc2373. [DOI] [PubMed] [Google Scholar]
- 2.Shanker A, Marincola FM. Cooperativity of adaptive and innate immunity: implications for cancer therapy. Cancer Immunol Immunother. 2011;60(8):1061–74. doi: 10.1007/s00262-011-1053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinman R, Inaba K. Immunogenicity: role of dendritic cells. Bioessays. 1989;10(5):145–52. doi: 10.1002/bies.950100503. [DOI] [PubMed] [Google Scholar]
- 4.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 5.Appay V, Douek DC, Price DA. CD8+ T cell efficacy in vaccination and disease. Nat Med. 2008;14(6):623–8. doi: 10.1038/nm.f.1774. [DOI] [PubMed] [Google Scholar]
- 6.Vulink A, Radford KJ, Melief C, Hart DN. Dendritic cells in cancer immunotherapy. Adv Cancer Res. 2008;99:363–407. doi: 10.1016/S0065-230X(07)99006-5. [DOI] [PubMed] [Google Scholar]
- 7.Inaba K, Young JW, Steinman RM. Direct activation of CD8+ cytotoxic T lymphocytes by dendritic cells. J Exp Med. 1987;166(1):182–94. doi: 10.1084/jem.166.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008;29(3):372–83. doi: 10.1016/j.immuni.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Rosalia RA, Quakkelaar ED, Redeker A, Khan S, Camps M, Drijfhout JW, et al. Dendritic cells process synthetic long peptides better than whole protein, improving antigen presentation and T-cell activation. Eur J Immunol. [Research Support, Non-U.S. Gov’t] 2013;43(10):2554–65. doi: 10.1002/eji.201343324. [DOI] [PubMed] [Google Scholar]
- 10.Bijker MS, van den Eeden SJ, Franken KL, Melief CJ, van der Burg SH, Offringa R. Superior induction of anti-tumor CTL immunity by extended peptide vaccines involves prolonged, DC-focused antigen presentation. Eur J Immunol. 2008;38(4):1033–42. doi: 10.1002/eji.200737995. [DOI] [PubMed] [Google Scholar]
- 11.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ. Berends-van der Meer DM, Vloon AP, et al. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin Cancer Res. 2008;14(1):169–77. doi: 10.1158/1078-0432.CCR-07-1881. [DOI] [PubMed] [Google Scholar]
- 12.Aucouturier J, Ascarateil S, Dupuis L. The use of oil adjuvants in therapeutic vaccines. Vaccine. 2006;24(2):S2-44–5. doi: 10.1016/j.vaccine.2005.01.116. [DOI] [PubMed] [Google Scholar]
- 13.Cai S, Yang Q, Bagby TR, Forrest ML. Lymphatic drug delivery using engineered liposomes and solid lipid nanoparticles. Adv Drug Deliv Rev. 2011;63(10–11):901–8. doi: 10.1016/j.addr.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaks K, Jordan M, Guth A, Sellins K, Kedl R, Izzo A, et al. Efficient immunization and cross-priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J Immunol. 2006;176(12):7335–45. doi: 10.4049/jimmunol.176.12.7335. [DOI] [PubMed] [Google Scholar]
- 15.Nordly P, Rose F, Christensen D, Nielsen HM, Andersen P, Agger EM, et al. Immunity by formulation design: induction of high CD8+ T-cell responses by poly(I:C) incorporated into the CAF01 adjuvant via a double emulsion method. J Control Release. 2011;150(3):307–17. doi: 10.1016/j.jconrel.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 16.Hansen J, Lindenstrom T, Lindberg-Levin J, Aagaard C, Andersen P, Agger EM. CAF05: cationic liposomes that incorporate synthetic cord factor and poly(I:C) induce CTL immunity and reduce tumor burden in mice. Cancer Immunol Immunother. 2012;61(6):893–903. doi: 10.1007/s00262-011-1156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregoriadis G. The immunological adjuvant and vaccine carrier properties of liposomes. J Drug Target. 1994;2(5):351–6. doi: 10.3109/10611869408996809. [DOI] [PubMed] [Google Scholar]
- 18.Al-Jamal WT, Kostarelos K. Liposomes: from a clinically established drug delivery system to a nanoparticle platform for theranostic nanomedicine. Acc Chem Res. 2011;44(10):1094–104. doi: 10.1021/ar200105p. [DOI] [PubMed] [Google Scholar]
- 19.Hubbell JA, Thomas SN, Swartz MA. Materials engineering for immunomodulation. Nature. 2009;462(7272):449–60. doi: 10.1038/nature08604. [DOI] [PubMed] [Google Scholar]
- 20.Watson DS, Endsley AN, Huang L. Design considerations for liposomal vaccines: influence of formulation parameters on antibody and cell-mediated immune responses to liposome associated antigens. Vaccine. 2012;30(13):2256–72. doi: 10.1016/j.vaccine.2012.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10(11):787–96. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 22.Joshi MD, Unger WJ, Storm G, van Kooyk Y, Mastrobattista E. Targeting tumor antigens to dendritic cells using particulate carriers. J Control Release. 2012;161(1):25–37. doi: 10.1016/j.jconrel.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Ossendorp F, Mengede E, Camps M, Filius R, Melief CJ. Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors. J Exp Med. 1998;187(5):693–702. doi: 10.1084/jem.187.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gregoriadis G, Bacon A, Caparros-Wanderley W, McCormack B. Plasmid DNA vaccines: entrapment into liposomes by dehydration-rehydration. Methods Enzymol. 2003;367:70–80. doi: 10.1016/S0076-6879(03)67006-0. [DOI] [PubMed] [Google Scholar]
- 25.Silva AL, Rosalia RA, Sazak A, Carstens MG, Ossendorp F, Oostendorp J, et al. Optimization of encapsulation of a synthetic long peptide in PLGA nanoparticles: low-burst release is crucial for efficient CD8+ T cell activation. Eur J Pharm Biopharm. 2013;83(3):338–45. doi: 10.1016/j.ejpb.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Winzler C, Rovere P, Rescigno M, Granucci F, Penna G, Adorini L, et al. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J Exp Med. 1997;185(2):317–28. doi: 10.1084/jem.185.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanderson S, Shastri N. LacZ inducible, antigen/MHC-specific T cell hybrids. Int Immunol. 1994;6(3):369–76. doi: 10.1093/intimm/6.3.369. [DOI] [PubMed] [Google Scholar]
- 28.Bal SM, Hortensius S, Ding Z, Jiskoot W, Bouwstra JA. Co-encapsulation of antigen and Toll-like receptor ligand in cationic liposomes affects the quality of the immune response in mice after intradermal vaccination. Vaccine. 2011;29(5):1045–52. doi: 10.1016/j.vaccine.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 29.Colonna C, Conti B, Genta I, Alpar OH. Non-viral dried powders for respiratory gene delivery prepared by cationic and chitosan loaded liposomes. Int J Pharm. 2008;364(1):108–18. doi: 10.1016/j.ijpharm.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 30.Voehringer D, Koschella M, Pircher H. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1) Blood. 2002;100(10):3698–702. doi: 10.1182/blood-2002-02-0657. [DOI] [PubMed] [Google Scholar]
- 31.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 32.Ma Y, Zhuang Y, Xie X, Wang C, Wang F, Zhou D, et al. The role of surface charge density in cationic liposome-promoted dendritic cell maturation and vaccine-induced immune responses. Nanoscale. 2011;3(5):2307–14. doi: 10.1039/c1nr10166h. [DOI] [PubMed] [Google Scholar]
- 33.Chen W, Yan W, Huang L. A simple but effective cancer vaccine consisting of an antigen and a cationic lipid. Cancer Immunol Immunother. 2008;57(4):517–30. doi: 10.1007/s00262-007-0390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan W, Chen W, Huang L. Reactive oxygen species play a central role in the activity of cationic liposome based cancer vaccine. J Control Release. 2008;130(1):22–8. doi: 10.1016/j.jconrel.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Campbell RB, Balasubramanian SV, Straubinger RM. Phospholipid-cationic lipid interactions: influences on membrane and vesicle properties. Biochim Biophys Acta. 2001;1512(1):27–39. doi: 10.1016/S0005-2736(01)00290-5. [DOI] [PubMed] [Google Scholar]
- 36.de Jong S, Chikh G, Sekirov L, Raney S, Semple S, Klimuk S, et al. Encapsulation in liposomal nanoparticles enhances the immunostimulatory, adjuvant and anti-tumor activity of subcutaneously administered CpG ODN. Cancer Immunol Immunother. 2007;56(8):1251–64. doi: 10.1007/s00262-006-0276-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lesterhuis WJ, de Vries IJ, Schreibelt G, Lambeck AJ, Aarntzen EH, Jacobs JF, et al. Route of administration modulates the induction of dendritic cell vaccine-induced antigen-specific T cells in advanced melanoma patients. Clin Cancer Res. 2011;17(17):5725–35. doi: 10.1158/1078-0432.CCR-11-1261. [DOI] [PubMed] [Google Scholar]
- 38.Korsholm KS, Hansen J, Karlsen K, Filskov J, Mikkelsen M, Lindenstrom T, et al. Induction of CD8+ T-cell responses against subunit antigens by the novel cationic liposomal CAF09 adjuvant. Vaccine. 2014;32(31):3927–35. doi: 10.1016/j.vaccine.2014.05.050. [DOI] [PubMed] [Google Scholar]
- 39.Aguilar JC, Rodriguez EG. Vaccine adjuvants revisited. Vaccine. 2007;25(19):3752–62. doi: 10.1016/j.vaccine.2007.01.111. [DOI] [PubMed] [Google Scholar]
- 40.Ludewig B, Barchiesi F, Pericin M, Zinkernagel RM, Hengartner H, Schwendener RA. In vivo antigen loading and activation of dendritic cells via a liposomal peptide vaccine mediates protective antiviral and anti-tumour immunity. Vaccine. 2000;19(1):23–32. doi: 10.1016/S0264-410X(00)00163-8. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J, Tian H, Li C, Cheng L, Zhang S, Zhang X, et al. Antitumor effects obtained by autologous Lewis lung cancer cell vaccine engineered to secrete mouse interleukin 27 by means of cationic liposome. Mol Immunol. 2013;55(3–4):264–74. doi: 10.1016/j.molimm.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 42.van Duikeren S, Fransen MF, Redeker A, Wieles B, Platenburg G, Krebber WJ, et al. Vaccine-induced effector-memory CD8+ T cell responses predict therapeutic efficacy against tumors. J Immunol. 2012;189(7):3397–403. doi: 10.4049/jimmunol.1201540. [DOI] [PubMed] [Google Scholar]
- 43.van Duikeren S, Arens R. Predicting the efficacy of cancer vaccines by evaluating T-cell responses. Oncoimmunology. 2013;2(1):e22616. doi: 10.4161/onci.22616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang C, Zhuang Y, Zhang Y, Luo Z, Gao N, Li P, et al. Toll-like receptor 3 agonist complexed with cationic liposome augments vaccine-elicited antitumor immunity by enhancing TLR3-IRF3 signaling and type I interferons in dendritic cells. Vaccine. 2012;30(32):4790–9. doi: 10.1016/j.vaccine.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 45.Reimer T, Brcic M, Schweizer M, Jungi TW. Poly(I:C) and LPS induce distinct IRF3 and NF-kappaB signaling during type-I IFN and TNF responses in human macrophages. J Leukoc Biol. 2008;83(5):1249–57. doi: 10.1189/jlb.0607412. [DOI] [PubMed] [Google Scholar]
- 46.Gitlin L, Barchet W, Gilfillan S, Cella M, Beutler B, Flavell RA, et al. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc Natl Acad Sci U S A. 2006;103(22):8459–64. doi: 10.1073/pnas.0603082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 448 kb)


