Abstract
As a part of ongoing research to elucidate and characterize antioxidant and anti-inflammatory nutraceuticals, solvent-partitioned fractions from Spergularia marina were tested for their ability to scavenge radicals and suppress inflammation. The results of the 2′,7′-dichlorofluorescein diacetate assay indicate that solvent-partitioned fractions from S. marina scavenged intracellular radicals in H2O2-stimulated mouse macrophages. The tested fractions decreased the generation of nitric oxide (NO) and the expression of inflammation mediators, namely, inducible nitric oxide synthase (iNOS) and interleukin (IL)-6, by lipopolysaccharide (LPS)-induced mouse macrophages, indicating that S. marina decreases inflammation. Among all tested fractions [i.e., H2O, n-buthanol (n-BuOH), 85% aqueous methanol (aq. MeOH), and n-hexane], the 85% aq. MeOH fraction showed the strongest antioxidant and anti-inflammatory response. The 85% aq. MeOH fraction scavenged 80% of the free radicals produced by H2O2-induced control cells. In addition, NO production was 98% lower in 85% aq. MeOH fraction-treated cells compared to LPS-induced control cells. The mRNA expression of iNOS and IL-6 was also suppressed in 85% aq. MeOH fraction-treated cells. The results of the current study suggest that the phenolic compound components of S. marina are responsible for its antioxidant and anti-inflammatory effects.
Keywords: antioxidant, anti-inflammatory, solvent fraction, halophyte, Spergularia marina
INTRODUCTION
Currently, elevated oxidative stress is thought to be responsible for various chronic diseases and complications such as aging, diabetes, and cancer (1,2). Recent studies have shown that there is a close interaction between the oxidative stress and inflammation pathways that underline chronic diseases (3–5). Reactive oxygen species (ROS) such as superoxide, hydroxyl, and peroxyl radicals are generated in situations of oxidative stress. Although the body has its own cellular mechanisms to pacify these radicals (i.e., antioxidant enzymes and antioxidants), excessive oxidative stimulation can overpower these inherent defense mechanisms (6). Untreated exposure to free radicals causes vital cell damage that can lead to inflammation and clinical disease (7). Therefore, functional treatments should be developed to treat this underlying cause of clinical disease. In this regard, researchers have turned their attention to attenuating severe inflammation instead of suppressing its symptoms. Likewise, several recent studies have explored the use of natural antioxidant and anti-inflammatory agents as biochemical mechanisms to counter common oxidative stress and inflammation pathways (8–10).
Modern medicine and folk medicine-derived treatments, especially plant materials are being used worldwide to treat oxidative stress and inflammation (11–13). Among the bioactive material sources that have been studied, halophytes have garnered increasing attention throughout the past decade. Halophytes are known for their resistance against the harsh environmental conditions of high salinity waters, mangrove swamps, and marshes (14). Halophytes are praised by researchers for their ability to resist and suppress excessive ROS because of their strong antioxidant capacities (15,16). One such halophyte, Salicornia herbacea, is reported to have antioxidant and anti-inflammatory effects (17).
Spergularia, a halophyte, is a common plant that is widely distributed throughout subtropical areas. Spergularia sp. are a source of therapeutic agents such as flavonoids and saponins (18,19). Several species of Spergularia have been experimented with and appear to have beneficial effects on human health. These beneficial effects include anti-diabetic (20), hypoglycemic (21), diuretic (22), and cholesterol-lowering (23) effects. A prominent research trend to develop novel nutraceutical substances from natural plants has spurred interest in Spergularia sp., but the mechanism of action of Spergularia sp. remains unknown. For decades, Spergularia marina, a local food preference in South Korea, has been regarded as a nutritious source of amino acids, vitamins, and minerals, but the bioactive nutraceuticals present in S. marina Griseb have remained unknown. In the present study, the antioxidant and anti-inflammatory activities of the solvent fractions of S. marina Griseb extract were measured in cell-based in vitro models.
MATERIALS AND METHODS
Plant materials and fractionation
The S. marina Griseb was purchased from Yaerak village greenhouse, Munnaemyeon, Haenam in Jeollanam-do, Korea in February, 2012. The sample was air-dried under shade, ground to a powder, and extracted three times with EtOH. The extracts were concentrated under reduced pressure.
The crude extracts (25 g) were suspended in a mixture of CH2Cl2 and water. The organic layer was further partitioned in a mixture of 85% aqueous methanol (aq. MeOH) and n-hexane and the aqueous layer was fractioned in a mixture of n-buthanol (n-BuOH) and H2O. This process yielded the following solvent fractions of S. marina Griseb extract: 85% aq. MeOH (1.6 g) and n-hexane (3.5 g) fractions from the organic layer and n-BuOH (1.2 g) and water (18.9 g) fractions from the aqueous layer.
Cell culture and cytotoxicity
Murine RAW 264.7 cells were grown as monolayers in a 5% CO2 and 37°C humidified atmosphere using Dulbecco’s modified Eagle’s medium (DMEM) (Gibco-BRL, Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, and 100 μg/mL penicillin-streptomycin (Gibco-BRL). The medium was changed two or three times per week.
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, which is based on the conversion of MTT to MTT-formazan by mitochondrial enzymes, was used to determine the cytotoxic effects of the solvent-partitioned fractions from S. marina on cultured cells. The cells were plated in 96-well plates at a density of 5×103 cells/well. After 24 h, the cells were washed with fresh medium and were treated with control medium or medium supplemented with S. marina Griseb. After incubation for 24 h, cells were rewashed, 100 μL of MTT solution (1 mg/mL) was added, and the cells were incubated for an additional 4 h. Finally, 100 μL of dimethyl sulfoxide (DMSO) was added to solubilize the formazan crystals. The amount of formazan present in each well was determined by measuring the absorbance of each well at 540 nm with a GENios® microplate reader (Tecan Austria GmbH, Grödig, Austria). Relative cell viability was determined by the measuring the amount of MTT converted into formazan crystals. The viability of the RAW 264.7 cells and dose response curves are shown as a percentage of the viability of the control-treated RAW 264.7 cells.
Determination of intracellular formation of ROS using 2′,7′-dichlorofluorescin diacetate (DCF-DA) labeling
The intracellular formation of ROS was assessed using DCF-DA, an oxidation sensitive dye, as a substrate. RAW 264.7 cells growing in fluorescence microtiter 96-well plates were loaded with 20 μM DCF-DA in Hank’s buffered salt solution (HBSS) and incubated for 20 min in the dark. The nonfluorescent DCF-DA dye freely penetrated into cells, where it was hydrolyzed by intracellular esterases to 2′,7′-dichlorodihydrofluororescein (DCFH), which was trapped inside the cells. The cells were then treated with different concentrations of each test compound and incubated for 1 h. The cells were washed with PBS three times, and then 500 μM H2O2 in HBSS was added to the cells. Every 30 min, a GENios® microplate reader (Tecan Austria GmbH) was used to measure the formation of 2′,7′-dichlorofluorescein (DCF) due to the oxidation of DCFH in the presence of various ROS at an excitation wavelength (Ex) of 490 nm and an emission wavelength (Em) of 620 nm. The dose-dependent and time-dependent effects of each treatment were plotted and compared with the intensity of the fluorescence of the control and blank groups.
Measurement of nitric oxide production
RAW 264.7 cells (2×105 cells/well) were seeded onto 96-well plates with DMEM without phenol red. The cells were allowed to adhere overnight and then were pretreated with the S. marina Griseb extracts for 1 h. Cellular nitric oxide (NO) production was stimulated by adding 1 μg/mL (final concentration) of lipopolysaccharide (LPS). LPS-stimulated cells were incubated for 24 h and 48 h. After incubation, Griess reagent [1% sulfanilamide, 2% phosphoric acid, and 0.1% N-(1-naphthyl)ethylenediamine dihydrochloride] was used to determine NO production. Briefly, 50 mL of culture supernatant was mixed with an equal volume of Griess reagent. After 15 min of incubation at room temperature, the absorbance was measured at 550 nm with a GENios® microplate reader (Tecan Austria GmbH). Nitrite concentrations were calculated by regression analysis. Serial dilutions of sodium nitrite were used as a standard.
Reverse transcription-polymerase chain reaction (RT-PCR) and real-time PCR analyses
TRIzol® reagent (Invitrogen Co., Carlsbad, CA, USA) was used to isolate the total RNA from RAW 264.7 macrophages treated with/without the solvent-partitioned fractions from S. marina. To synthesize cDNA, the total RNA (2 μg) was added to RNase-free water and oligo(dT). The mixture was denatured at 70°C for 5 min and then immediately cooled. RNA was reverse transcribed in a master mix containing 1× RT buffer, 1 mM dNTPs, 500 ng oligo(dT), 140 U M-MLV reserve transcriptase, and 40 U RNase inhibitor at 42°C for 60 min and then at 72°C for 5 min using an automatic T100 Thermal Cycler (Bio-Rad, Hemel Hempstead, Hertfordshire, UK). The target cDNA was amplified using the following sense and antisense primers: forward 5′-TTC-CAG-AAT-CCC-TGG-ACA-AG-3′ and reverse 5′-TGG-TCA-AAC-TCT-TGG-GGT-TC-3′ for inducible nitric oxide synthase (iNOS); forward 5′-AGT-TGC-CTT-CTT-GGG-ACT-GA-3′ and reverse 5′-CAG-AAT-TGC-CAT-TGC-ACA-AC-3′ for interleukin (IL)-6; and forward 5′-CCA-CAG-CTG-AGA-GGG-AAA-TC-3′ and reverse 5′-AAG-GAA-GGC-TGG-AAA-AGA-GC-3′ for β-actin. The cDNA was amplified with 30 cycles of 95°C for 45 s, 60°C for 1 min, and 72°C for 45 s. After amplification, the PCR products were separated by electrophoresis on a 1.5% agarose gel for 30 min at 100 V. The gels were then stained with 1 mg/mL ethidium bromide and visualized by UV light using a Davinch-Chemi imager™ (CAS-400SM, Wako Co., Osaka, Japan).
Gene expression was measured by real-time PCR in a Thermal Cycler Dice® Real Time System TP800 (Takara Bio Inc., Ohtsu, Japan) following the manufacturer’s protocol. Briefly, 1.0 μL of DNA sample was mixed with 12.5 μL of Maxima® SYBR Green qPCR Master Mix (Thermo Scientific, Inc., Waltham, MA, USA), which contained Taq DNA polymerase, dNTPs, and reaction buffer. The target cDNA was amplified using the same primers described for the RT-PCR analysis plus the following sense and antisense primers: forward 5′-AGA-AGG-AAA-TGG-CTG-CAG-AA-3′ and reverse 5′-GCT-CGG-CTT-CCA-GTA-TTG-AG-3′ for cyclooxygenase (COX)-2; forward 5′-AGC-CCC-CAG-TCT-GTC-TCC-TT-3′ and reverse 5′-CAT-TCG-AGG-CTC-CAG-TGA-AT-3′ for tumor necrosis factor (TNF)-α; and forward 5′-GGG-CCT-CAA-AGG-AAA-GAA-TC-3′ and reverse 5′-TAC-CAG-TTG-GGG-AAC-TCT-GC-3′ for IL-1β. The mixture was denatured at 95°C for 10 min, and then underwent 40 PCR cycles of 95°C for 15 s and 60°C for 60 s. Relative quantification was calculated using the 2−ΔΔCT method. β-actin was used as the internal control.
Determination of total polyphenol content
The total polyphenol content of S. marina fractions was determined by the Folin-Ciocalteu method. In brief, 20 μL of each extract was mixed with 100 μL of 1:10 Folin-Ciocalteu reagent in a microplate. Then, 80 μL of 7.5% Na2CO3 was added to each well and the microplate was incubated in the dark at room temperature for 2 h. After incubation, the absorbance at 600 nm was recorded for each well. Gallic acid was used as the standard reference. Polyphenol content was expressed as mg gallic acid equivalents per gram of sample (mg GAE/g).
Statistical analysis
The data are presented as mean±SD. Differences between the means of the individual groups were analyzed with the analysis of variance (ANOVA) procedure of Statistical Analysis System SAS v9.1 (SAS Institute, Cary, NC, USA) with Duncan’s multiple range tests. The significance of differences was determined at the P<0.05 level.
RESULTS AND DISCUSSION
Effect of solvent-partitioned fractions from S. marina on free radical scavenging activity
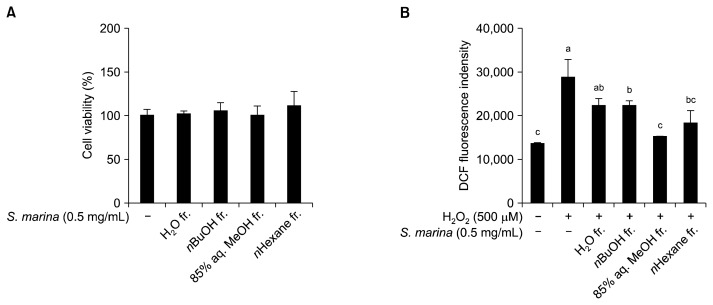
Prior to performing the RAW 264.7 mouse macrophage-based in vitro oxidative stress assay, the cytotoxicity of the solvent-partitioned fractions from S. marina was evaluated by MTT assay. For all solvent fractions tested, an S. marina concentration of 0.5 mg/mL did not affect the viability of RAW 264.7 mouse macrophages (Fig. 1A). Hence, this S. marina concentration was used for further in vitro assays.
Fig. 1.
Cytotoxicity (A) and cellular radical scavenging activity (B) of the solvent-partitioned fractions from S. marina in RAW 264.7 cells. (A) Cytotoxicity was determined by MTT assay. (B) After preincubation in 20 μM DCF-DA, RAW 264.7 cells were treated with the solvent fractions for 120 min. Following the addition of 500 μM H2O2, DCF fluorescence was measured at λexcitation 490 nm and λemission 620 nm. Means with different letters (a–c) are significantly different (P<0.05) by Duncan’s multiple range test.
The DCF-DA assay was used to determine the anti-oxidant potential of S. marina solvent fractions in RAW 264.7 cells. ROS scavenging ability was determined by measuring the change in fluorescent intensity accompanied by the oxidation of DCFH to DCF in H2O2-exposed RAW 264.7 cells. For all fractions tested, the intracellular oxidative stress of H2O2-induced RAW 264.7 cells treated with 0.5 mg/mL of S. marina was lower than the intracellular oxidative stress of untreated, H2O2-induced control cells (Fig. 1B). DCFH oxidation was lowest in cells treated with the 85% aq. MeOH fraction, indicating that this fraction significantly decreased H2O2-induced free radical scavenging. The oxidative stress level of H2O2-stimulated cells (i.e., control cells) was approximately 80% greater than the oxidative stress level of cells that were not exposed to H2O2 (i.e., blank cells). The free radical scavenging effects of the H2O, n-BuOH, and n-hexane fractions were 41%, 40%, and 63%, respectively. Several published reports have indicated that MeOH fractions contain phenolic substances, especially flavonoids that can act as strong antioxidants (24). Halophytes have also been reported to contain such compounds (25). This suggests that the phenolic and/or flavonoid content of S. marina may be responsible for its strong antioxidant properties.
Anti-inflammatory effects of solvent-partitioned fractions from S. marina
Strong antioxidant molecules are considered to show anti-inflammatory effect due to close relation between inflammatory and oxidative stress pathways (8). Therefore, the anti-inflammatory activity of solvent-partitioned fractions from S. marina on LPS-stimulated RAW 264.7 mouse macrophages was evaluated in this study. When activated with pathogenic substances (e.g., LPS), macrophages initiate and regulate inflammatory responses through a broad range of inflammatory mediators. LPS-stimulated macrophages produce inflammatory mediators such as free radicals, NO, iNOS, and IL-6 (26).
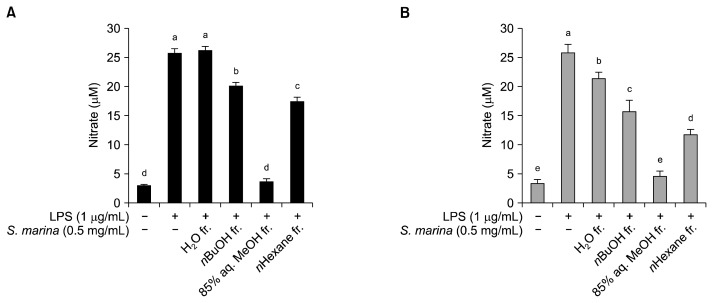
After confirming the anti-oxidative effect of the S. marina solvent fractions, the anti-inflammatory potential of these fractions was evaluated by assessing NO production by LPS-stimulated RAW 264.7 cells in the presence and absence of the solvent-partitioned S. marina fractions. The presence of LPS activated the RAW 264.7 mouse macrophages, leading to an elevation in NO production from 3.4 μM to 26 μM after a 24 h-incubation (Fig. 2A) and from 3.6 μM to 26 μM after a 48 h-incubation (Fig. 2B). In accordance with its strong anti-oxidant effect, the 85% aq. MeOH fraction lowered the NO production the most. Treatment with the 0.5 mg/mL concentration of the 85% aq. MeOH fraction decreased the amount of NO produced under LPS-stimulation to approximately 3.9 μM and 4.6 μM for the 24 h and 48 h incubation periods, respectively. The other S. marina fractions were not as effective as the 85% aq. MeOH fraction at decreasing NO production by LPS-stimulated RAW 264.7 cells, especially during the 24 h incubation trial. After 24 h of incubation, the n-BuOH and n-hexane fractions decreased NO production to 20 μM and 17 μM, respectively, while the H2O fraction did not decrease NO production by LPS-stimulated cells. However, after 48 h of incubation, the H2O, n-BuOH, and n-hexane fractions decreased NO production to 23 μM, 16 μM, and 12 μM, respectively.
Fig. 2.
Effect of the solvent-partitioned fractions from S. marina on intracellular NO levels in LPS-stimulated RAW 264.7 cells. The cells were pretreated with LPS (1 μg/mL), followed by treatment with the solvent fractions for 24 h (A) and 48 h (B). The nitrite content of the culture media was analyzed. Means with different letters (a–e) are significantly different (P<0.05) by Duncan’s multiple range test.
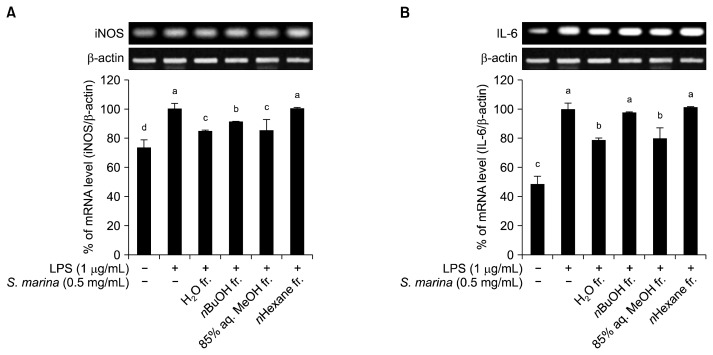
In order to evaluate the anti-inflammatory effect of solvent-partitioned fractions from S. marina in detail, the expression of key indicators of the inflammatory response were measured by RT-PCR and quantitative real-time PCR. The inflammatory response of macrophages is accompanied by the induction of inflammatory gene expression. This induction of the inflammatory response is mediated by the generation of transcriptional factors by inducible iNOS and cytokines such as IL-6 (27).
Various reports have suggested that elevated iNOS expression is closely related to the pathogenesis of inflammation and diseases such as cancer and Alzheimer’s disease (28,29). Therefore, the effect of S. marina fractions on the gene expression of the inflammation mediator enzyme iNOS was observed in LPS-stimulated mouse macrophages. As shown in Fig. 3A, iNOS mRNA expression was elevated by the inflammatory response to LPS and slightly suppressed by treatment with the H2O, n-BuOH, and 85% aq. MeOH fractions of S. marina. However, treatment with n-hexane fraction did not affect LPS-stimulated iNOS expression. Among all fractions tested, the 85% aq. MeOH and H2O fractions were the most effective at suppressing iNOS mRNA expression.
Fig. 3.
Effect of the solvent-partitioned fractions from S. marina on iNOS (A) and IL-6 (B) gene expression in LPS-stimulated RAW 264.7 cells. The cells were treated with various concentrations of the solvent fractions for 1 h prior to the addition of LPS. Following LPS-stimulation, the cells were further incubated for 24 h. Means with different letters (a–d) are significantly different (P<0.05) by Duncan’s multiple range test.
The effect of the S. marina solvent fractions on the gene expression of IL-6, an inflammatory cytokine, was also evaluated. As expected, treatment with the H2O and 85% aq. MeOH fractions of S. marina decreased the expression of IL-6, while the n-BuOH and n-hexane fractions did not significantly change the expression levels (Fig. 3B).
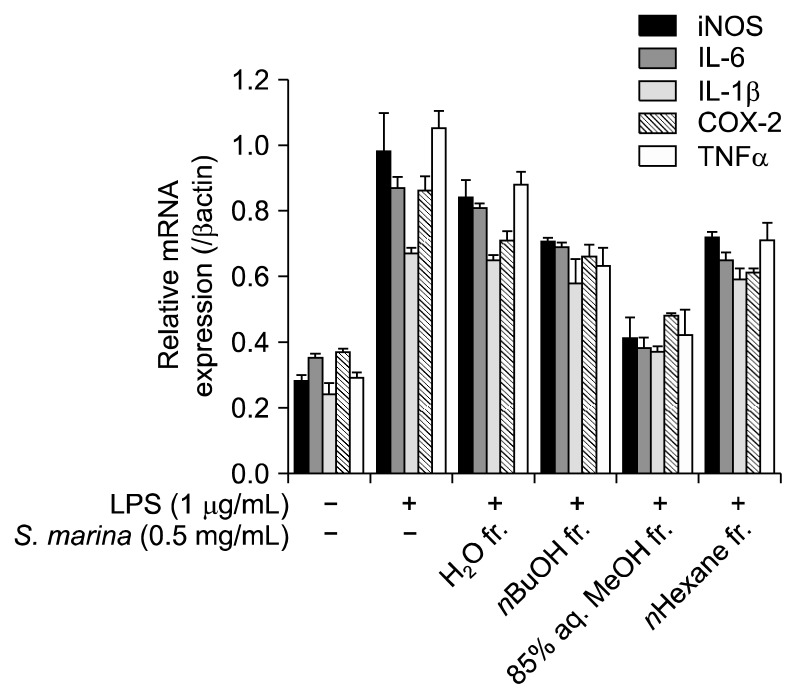
To further confirm the fractions’ anti-inflammatory effects, the expression of key markers related to inflammation was evaluated by quantitative real-time PCR. The data obtained were in accordance with our previous results. The MeOH fraction lowered the expression of key markers, namely iNOS, IL-6, COX-2, IL-1β, and TNF-α (Fig. 4). While all fractions were effective to an extent, the MeOH fraction was the most effective inhibitor of anti-inflammatory marker expression. In the presence of the MeOH fraction of S. marina, the LPS-stimulated expression of inflammation markers was significantly lowered to the level of unstimulated, blank cells.
Fig. 4.
Effect of the solvent-partitioned fractions from S. marina on the mRNA expression of key inflammation markers in LPS-stimulated RAW 264.7 cells. mRNA expression was measured by real-time PCR assay.
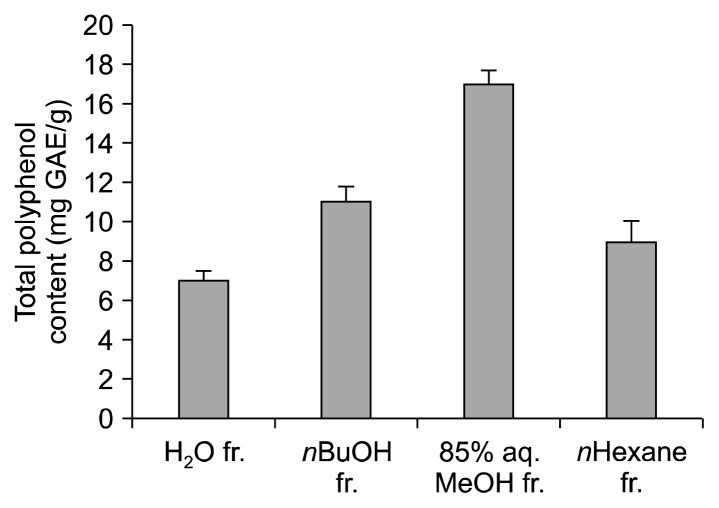
Collectively, S. marina was shown to possess antioxidant and anti-inflammatory effects in vitro. Comparison of the solvent-partitioned fractions from S. marina also presented valuable insights for future activity-based compound isolation. The strong effect of the 85% aq. MeOH fraction suggested that this fraction contained more bioactive phenolic compounds with notable anti-oxidant and anti-inflammatory effects than the other fractions tested. Reports have already described that the MeOH fractions of marine plants contain various bioactive substances, including tannins and flavonoids (24). Because previous works indicate that polyphenols may be one class of bioactive materials present in S. marina, we also measured the polyphenol content of the S. marina fractions. A notably greater polyphenol content was observed in the MeOH fraction of S. marina than in the H2O, n-BuOH, or n-hexane fractions of S. marina (Fig. 5). Previous work also indicates that antioxidant tannins and flavonoids possess anti-inflammatory activities (30). S. marina fractions were shown to contain antioxidant and anti-inflammatory substances, 85% aq. MeOH being the most active fraction among others. Coupled with the high phenolic content of 85% aq. MeOH fraction, anti-oxidant and anti-inflammatory materials of S. marina were suggested to be phenol derivatives.
Fig. 5.
Total polyphenol content of the solvent-partitioned fractions from S. marina. Polyphenol content is expressed as mg gallic acid equivalents per gram of sample (mg GAE/g).
Taken together, the results of the present study suggest that S. marina could be a significant source of therapeutic agents. Further studies focused on the isolation and elucidation of active ingredients from the 85% aq. MeOH fraction of S. marina would pave the way for the efficient utilization of S. marina as a nutraceutical source. Nonetheless, the MeOH fraction of S. marina extract was exhibited to possess antioxidant and anti-inflammatory activities.
ACKNOWLEDGEMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), which is funded by the Ministry of Education, Science and Technology (NRF-2012R1A1A3014642).
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 2.Mayne ST. Antioxidant nutrients and chronic disease: use of biomarkers of exposure and oxidative stress status in epidemiologic research. J Nutr. 2003;133:933S–940S. doi: 10.1093/jn/133.3.933S. [DOI] [PubMed] [Google Scholar]
- 3.Holvoet P. Relations between metabolic syndrome, oxidative stress and inflammation and cardiovascular disease. Verh K Acad Geneeskd Belg. 2008;70:193–219. [PubMed] [Google Scholar]
- 4.Elmarakby AA, Sullivan JC. Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovasc Ther. 2012;30:49–59. doi: 10.1111/j.1755-5922.2010.00218.x. [DOI] [PubMed] [Google Scholar]
- 5.Khansari N, Shakiba Y, Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov. 2009;3:73–80. doi: 10.2174/187221309787158371. [DOI] [PubMed] [Google Scholar]
- 6.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29:222–230. doi: 10.1016/S0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 7.Conner EM, Grisham MB. Inflammation, free radicals, and antioxidants. Nutrition. 1996;12:274–277. doi: 10.1016/S0899-9007(96)00000-8. [DOI] [PubMed] [Google Scholar]
- 8.Geronikaki AA, Gavalas AM. Antioxidants and inflammatory disease: synthetic and natural antioxidants with anti-inflammatory activity. Comb Chem High Throughput Screen. 2006;9:425–442. doi: 10.2174/138620706777698481. [DOI] [PubMed] [Google Scholar]
- 9.Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302:71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fylaktakidou KC, Hadjipavlou-Litina DJ, Litinas KE, Nicolaides DN. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr Pharm Des. 2004;10:3813–3833. doi: 10.2174/1381612043382710. [DOI] [PubMed] [Google Scholar]
- 11.de las Heras B, Slowing K, Benedí J, Carretero E, Ortega T, Toledo C, Bermejo P, Iglesias I, Abad MJ, Gómez-Serranillos P, Liso PA, Villar A, Chiriboga X. Antiinflammatory and antioxidant activity of plants used in traditional medicine in Ecuador. J Ethnopharmacol. 1998;61:161–166. doi: 10.1016/S0378-8741(98)00029-4. [DOI] [PubMed] [Google Scholar]
- 12.Schinella GR, Tournier HA, Prieto JM, Mordujovich de Buschiazzo P, Ríos JL. Antioxidant activity of anti-inflammatory plant extracts. Life Sci. 2002;70:1023–1033. doi: 10.1016/S0024-3205(01)01482-5. [DOI] [PubMed] [Google Scholar]
- 13.Talhouk RS, Karam C, Fostok S, El-Jouni W, Barbour EK. Anti-inflammatory bioactivities in plant extracts. J Med Food. 2007;10:1–10. doi: 10.1089/jmf.2005.055. [DOI] [PubMed] [Google Scholar]
- 14.Flowers TJ, Colmer TD. Salinity tolerance in halophytes. New Phytol. 2008;179:945–963. doi: 10.1111/j.1469-8137.2008.02531.x. [DOI] [PubMed] [Google Scholar]
- 15.Amor NB, Jiménez A, Megdiche W, Lundqvist M, Sevilla F, Abdelly C. Response of antioxidant systems to NaCl stress in the halophyte Cakile maritima. Physiol Plant. 2006;126:446–457. doi: 10.1111/j.1399-3054.2006.00620.x. [DOI] [Google Scholar]
- 16.Ksouri R, Megdiche W, Falleh H, Trabelsi N, Boulaaba M, Smaoui A, Abdelly C. Influence of biological, environmental and technical factors on phenolic content and anti-oxidant activities of Tunisian halophytes. C R Biol. 2008;331:865–873. doi: 10.1016/j.crvi.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 17.Kim YA, Kong CS, Um YR, Lim SY, Yea SS, Seo Y. Evaluation of Salicornia herbacea as a potential antioxidant and anti-inflammatory agent. J Med Food. 2009;12:661–668. doi: 10.1089/jmf.2008.1072. [DOI] [PubMed] [Google Scholar]
- 18.De Tommasi N, Piacente S, Gacs-Baitz E, De Simone F, Pizza C, Aquino R. Triterpenoid saponins from Spergularia ramosa. J Nat Prod. 1998;61:323–327. doi: 10.1021/np970398l. [DOI] [PubMed] [Google Scholar]
- 19.Jouad H, Lacaille-Dubois MA, Lyoussi B, Eddouks M. Effects of the flavonoids extracted from Spergularia purpurea Pers. on arterial blood pressure and renal function in normal and hypertensive rats. J Ethnopharmacol. 2001;76:159–163. doi: 10.1016/S0378-8741(01)00209-4. [DOI] [PubMed] [Google Scholar]
- 20.Vinholes J, Grosso C, Andrade PB, Gil-Izquierdo A, Valentão P, Pinho PGd, Ferreres F. In vitro studies to assess the antidiabetic, anti-cholinesterase and antioxidant potential of Spergularia rubra. Food Chem. 2011;129:454–462. doi: 10.1016/j.foodchem.2011.04.098. [DOI] [PubMed] [Google Scholar]
- 21.Eddouks M, Jouad H, Maghrani M, Lemhadri A, Burcelin R. Inhibition of endogenous glucose production accounts for hypoglycemic effect of Spergularia purpurea in streptozotocin mice. Phytomedicine. 2003;10:594–599. doi: 10.1078/094471103322331890. [DOI] [PubMed] [Google Scholar]
- 22.Jouad H, Lacaille-Dubois MA, Eddouks M. Chronic diuretic effect of the water extract of Spergularia purpurea in normal rats. J Ethnopharmacol. 2001;75:219–223. doi: 10.1016/S0378-8741(01)00193-3. [DOI] [PubMed] [Google Scholar]
- 23.Jouad H, Lemhadri A, Maghrani M, Zeggwagh NA, Eddouks M. Cholesterol-lowering activity of the aqueous extract of Spergularia purpurea in normal and recent-onset diabetic rats. J Ethnopharmacol. 2003;87:43–49. doi: 10.1016/S0378-8741(03)00102-8. [DOI] [PubMed] [Google Scholar]
- 24.Škerget M, Kotnik P, Hadolin M, Hraš AR, Simonič M, Knez Ž. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005;89:191–198. doi: 10.1016/j.foodchem.2004.02.025. [DOI] [Google Scholar]
- 25.Bertin RL, Gonzaga LV, Borges GSC, Azevedo MS, Maltez HF, Heller M, Micke GA, Tavares LBB, Fett R. Nutrient composition and, identification/quantification of major phenolic compounds in Sarcocornia ambigua (Amaranthaceae) using HPLC-ESI-MS/MS. Food Res Int. 2014;55:404–411. doi: 10.1016/j.foodres.2013.11.036. [DOI] [Google Scholar]
- 26.Meng F, Lowell CA. Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr, and Lyn. J Exp Med. 1997;185:1661–1670. doi: 10.1084/jem.185.9.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hori M, Kita M, Torihashi S, Miyamoto S, Won KJ, Sato K, Ozaki H, Karaki H. Upregulation of iNOS by COX-2 in muscularis resident macrophage of rat intestine stimulated with LPS. Am J Physiol Gastrointest Liver Physiol. 2001;280:G930–G938. doi: 10.1152/ajpgi.2001.280.5.G930. [DOI] [PubMed] [Google Scholar]
- 28.Pahl HL. Activators and target genes of Rel/NF-κB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto Y, Gaynor RB. Role of the NF-κB pathway in the pathogenesis of human disease states. Curr Mol Med. 2001;1:287–296. doi: 10.2174/1566524013363816. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Ravipati AS, Koyyalamudi SR, Jeong SC, Reddy N, Smith PT, Bartlett J, Shanmugam K, Münch G, Wu MJ. Antioxidant and anti-inflammatory activities of selected medicinal plants containing phenolic and flavonoid compounds. J Agric Food Chem. 2011;59:12361–12367. doi: 10.1021/jf203146e. [DOI] [PubMed] [Google Scholar]