Abstract
The induction of detoxification enzymes by benzyl isothiocyanate (BITC) and its synthetic N-acetyl-L-cysteine (NAC) conjugate (NAC-BITC) was examined in Hepa1c1c7 murine hepatoma cells. BITC and NAC-BITC inhibited Hepa1c1c7 cell growth in a dose-dependent manner. Cell growth was 4.5~57.2% lower in Hepa1c1c7 cells treated with 0.1~10 μM BITC than in control-treated Hepa1c1c7 cells. The NAC-BITC treatment had a similar inhibitory pattern on Hepa1c1c7 cell growth; 0.5 μM and 10 μM NAC-BITC decreased cell growth by 13.6% and 47.4%, respectively. Treatment of Hepa1c1c7 cells with 0.1~2.0 μM BITC also elicited a dose-response effect on the induction of quinone reductase quinone reductase (QR) activity and QR mRNA expression. Treatment with 1 μM and 2 μM BITC caused 1.8- and 2.8-fold inductions of QR mRNA, respectively. By comparison, treatment with 1 μM and 2 μM NAC-BITC caused 1.6- and 1.9-fold inductions of QR mRNA, respectively. Cytochrome P450 (CYP) 1A1 and CYP2E1 induction were lower in 0.1~2 μM BITC-treated cells than in control-treated cells. CYP2E1 activity was 1.2-fold greater in 0.1 μM NAC-BITC-treated cells than in control-treated cells. However, the CYP2E1 activity of cells treated with higher concentrations (i.e., 1~2 μM) of NAC-BITC was similar to the activity of control-treated cells. Considering the potential of isothiocyanatesto prevent cancer, these results provide support for the use of BITC and NAC-BITC conjugates as chemopreventive agents.
Keywords: cruciferous vegetables, benzyl isothiocyanate, N-acetyl cysteine, detoxification enzyme, chemoprevention
INTRODUCTION
Cruciferous vegetables such as broccoli, cabbage, and cauliflower contain unique secondary metabolites called glucosinolates. Glucosinolates have no biological activity themselves, but they are hydrolyzed by plant myrosinase (thioglucoside glucohydrolase; EC 3.2.3.1) when plant cells are cut, ground, or chewed. Glucosinolates release biologically active isothiocyanates (ITCs) (1,2). A major metabolic endpoint of ITCs in rodents and humans is glutathione (GSH) conjugation (3,4). More than half of the ITCs consumed by humans are excreted within 12 h as N-acetyl-L-cysteine (NAC) conjugates in the urine (3). NAC and L-cysteine (L-Cys) conjugates are degradation products of ITC via the mercapturic acid pathway.
Glucotropaeolin, one of the major glucosinolates of cruciferous vegetables, undergoes myrosinase-dependent hydrolysis to yield benzyl isothiocyanate (BITC) and several minor products (5). Once absorbed, the primary metabolic pathway of BITC is conjugation with GSH, followed by conversion via the mercapturic acid pathway to an NAC conjugate, which is excreted into the urine (6). Thus, the presence of the NAC-BITC conjugate in the urine indicates BITC uptake and metabolism (7–9).
ITCs play important roles in modulating the activation and detoxification of carcinogens (10,11). ITCs have been studied for their chemopreventive potential, which is primarily attributed to their activity as inducers of phase II enzymes involved in the detoxification and elimination of carcinogens (12,13).
Quinone reductase (QR), a cytosolic flavoprotein, is best known for protecting cells against the toxicities of quinones and their metabolic precursors by catalyzing the obligatory two-electron reduction of these compounds (14,15). Many studies have shown that elevation of QR activity correlates with protection against chemical-induced carcinogenesis in animal models (16,17).
Cytochrome P450 (CYP) superfamily gene products convert procarcinogenes into DNA-reactive electrophilic forms by phase I reactions (18). CYP enzyme variants are involved in the metabolism of many different potential carcinogens and drugs. The CYP1 family of enzymes is highly expressed in myeloblastic and lymphoid cell lines. In addition, the CYP1A1 and CYP2E1 enzymes are responsible for the bioactivation of some chemicals [e.g., polycyclic aromatic hydrocarbons (PAHs), ethanol] in peripheral blood lymphocytes (18,19).
The present study compared the effects of BITC and its NAC compound, a major metabolite of BITC, on cell growth and the induction of protein and mRNA expression of QR, CYP1A1, and CYP2E1 in Hepa1c1c7 murine hepatoma cells.
MATERIALS AND METHODS
Materials
BITC, NAC, pyridine, bovine serum albumin (BSA), β-naphthoflavone (BNF), β-nicotinamide adenine dinucleotide phosphate, and flavin adenine dinucleotide (FAD) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All other chemicals were reagent grade and obtained from commercial sources. The organic solvents used for NAC-BITC synthesis were reagent grade.
Synthesis of the NAC conjugate of BITC
The NAC conjugate of BITC was synthesized using published methods (9) and was confirmed by mass spectrometry-fast atom bombardment analysis (MS-FAB).
Cell culture
Hepa1c1c7 mouse hepatoma cells were obtained from the Korean Cell Line Bank (Seoul, Korea). The cells were grown in RPMI medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 units/mL penicillin, and 100 μg/mL streptomycin (Synovis Life Technologies, St. Paul, MN, USA) for 24 h. The cells were then treated with medium containing BITC or NAC-BITC dissolved in dimethyl sulfoxide (DMSO; 0.1% v/v final concentration) or DMSO alone for an additional 24 h. Next, the cells were trypsinized, centrifuged at 4,000 rpm, washed with phosphate-buffered saline (PBS), centrifuged at 4,000 rpm, lysed in 0.05% deoxycholate solution, and stored at −80°C until QR and protein analysis.
Cell proliferation
Cell proliferation was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cells (1,000 cells/well) were cultured for 24 h at 37°C in 96-well plates containing fresh FBS-free medium with various amounts of each sample. Each well was then incubated with MTT for 4 h. The liquid was removed and DMSO was added to dissolve the solid residue. A microplate reader (Molecular Devices, Sunnyvale, CA, USA) was used to determine the optical density of each well at 570 nm.
Quinone reductase activity
Hepa1c1c7 cells were seeded into 96-well plates (10,000 cells/well) with α-minimum essential medium supplemented with 10% FBS, 100 units/mL penicillin, and 100 μg/mL streptomycin and incubated for 24 h. Then, the medium was replaced with medium containing BITC or NAC-BITC, and the cells were incubated for an additional 24 h. The medium was decanted, and the cells were incubated in a 0.8% digitonin and 2 mM EDTA solution (pH 7.8) at 37°C for 10 min. The plates were then agitated on an orbital shaker (100 rpm) at room temperature for 10 min, and 200 μL of reaction mixture (BSA, MTT, 25 mM Tris-HCl, 0.01% Tween 20, 5 μM FAD, 1 mM glucose-6-phosphate, 30 μM nicotinamide adenine dinucleotide phosphate, glucose-6-phosphate dehydrogenase, and 50 μM menadione) was added to each well. After 5 min, the plates were scanned at 595 nm. Specific QR activity was determined by measuring NADPH-dependent menadiol-mediated reduction of MTT to blue formazan. QR activity induction was calculated by comparing the specific QR activity of sample-treated cells with that of solvent-treated cells. Cells grown with 1 μM BNF were used as a positive control for each experiment.
Isolation of total RNA
Hepa1c1c7 cells were plated in 100-mm culture dishes at a density of 3×106 cells per 100 mL medium. After pre-incubation for 24 h, each plate was filled with fresh FBS-free medium that contained various concentrations of BITC or NAC-BITC. The cells were then incubated for an additional 24 h at 37°C. Total RNA was isolated with TRIzol® reagent (Life Technologies, Boone, NC, USA). The homogenized samples were incubated at room temperature for 5 min to permit the complete dissociation of nucleoprotein complexes. After the addition of 0.2 volumes of chloroform, samples were shaken vigorously for 15 s, incubated for 2~3 min, and centrifuged at 12,000 g for 15 min at 4°C. The total RNA remaining in the upper aqueous phase was precipitated by mixing with an equal volume of isopropanol. The mixture was incubated for 10 min at 4°C and centrifuged at 12,000 g for 10 min at 4°C. The total RNA pellet was washed with 70% ethanol, dried, and dissolved in RNase-free water. The concentration and purity of total RNA were calculated by measuring the absorbance of the final solution at 260 and 280 nm.
Semi-quantitative RT-PCR
First-strand cDNA was synthesized with 1 μg of total RNA and 1 μM of oligo(dT15) primer using Omniscript Reverse Transcriptase (Qiagen, Valencia, CA, USA). The primers used in this study are shown in Table 1. The PCR protocol consisted of denaturation at 94°C for 3 min, 3-step cycling (30 cycles) at 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min, and extension at 72°C for 10 min. The amplified PCR products were loaded into a 1.0% agarose gel, stained with ethidium bromide, illuminated with a UV transilluminator, and photographed with a Polaroid camera (Kodak, Needham, MA, USA). The densities of bands were measured by the ImageJ software program, version 1.34 (Bethesda, MD, USA).
Table 1.
Sequences of primers used in this study
| Target gene | Primer | Sequence (5′→3′) |
|---|---|---|
| CYP1A1 | Sense | TCC CTA AGT ATC CTC CGT GA |
| Antisense | GTA ATC GAA GCG TTT GTT GA | |
| CYP2E1 | Sense | TGA CCT CCG TTA CCT GCC TA |
| Antisense | GTG TCA AAC CCA GCT CCA AA | |
| QR | Sense | TCG GAG AAC TTT CAG TAC CC |
| Antisense | TGC AGA GAG TAC ATG GAG CC | |
| GAPDH | Sense | GAC CCC TTC ATT GAC CTC AAC |
| Antisense | CAT ACC AGG AAA TGA GCT TG |
Statistical analysis
Statistical analyses were performed using the Statistical Analysis System (SAS Institute, Cary, NC, USA). One-way analysis of variance was used to determine differences between treatments; P<0.05 was considered significant.
RESULTS AND DISCUSSION
Characterization of the synthetic NAC-BITC conjugate
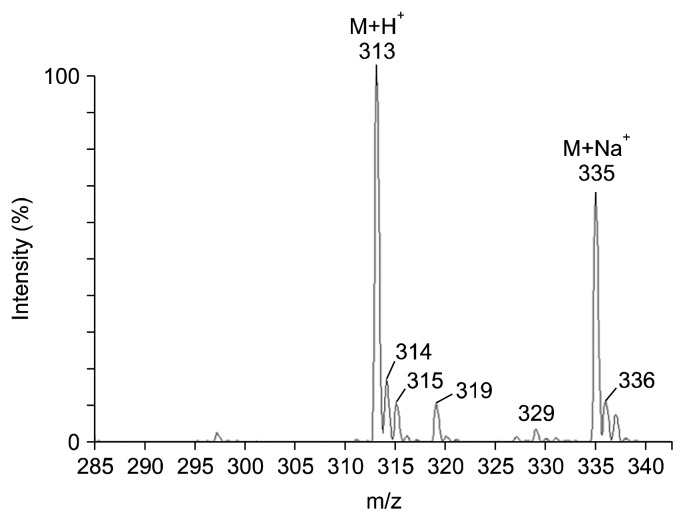
The NAC conjugate of BITC was synthesized using our published method (9). MS-FAB revealed a mass spectrum with a strong molecular ion (M+) as the major peak at 313, which is the molecular weight of the NAC conjugate of BITC plus one (Fig. 1). The synthetic sample had a distinct M+Na+ peak at 335. The discrepancy between the major peaks is believed to have resulted from the addition of sodium chloride to the synthetic sample, which was added to enhance the molecular ion.
Fig. 1.
Mass spectrum of the synthetic N-acetylcysteine conjugate of BITC.
Cell proliferation assay
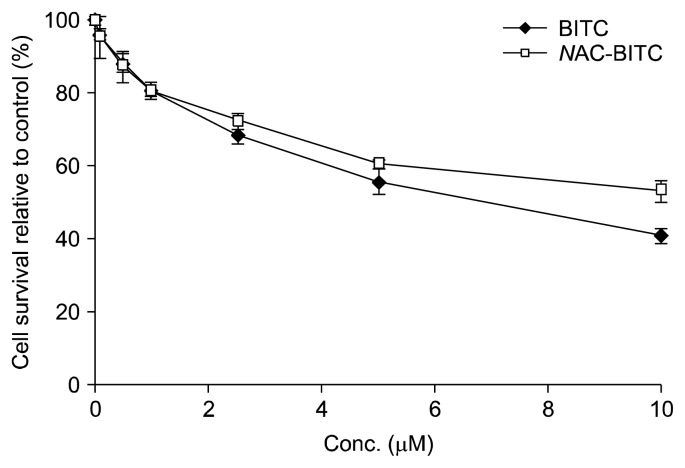
A series of MTT assays was used to determine the dose-response effect of NAC-BITC on the growth and proliferation of Hepa1c1c7 cells. Fig. 2 illustrates the effects of 0.1~10 μM BITC and NAC-BITC on the growth of Hepa1c1c7cells. Twenty-four hours of incubation with BITC inhibited the proliferation of Hepa1c1c7 cells in a dose-dependent fashion (n=4). Hepa1c1c7 cell numbers were significantly lower with the 0.1~10 μM BITC treatment than with the control treatment. A similar inhibitory pattern was observed with the NAC-BITC treatment. The growth of Hepa1c1c7 cells treated with 0.5 μM and 10 μM NAC-BITC was 13.6% and 47.4% lower than that of control-treated cells, respectively.
Fig. 2.
Effects of BITC and NAC-BITC on Hepa1c1c7 cell proliferation, as measured by the MTT assay.
Tan et al. (20) demonstrated that 48 h of exposure to 0.5~20 μM BITC, phenethyl isothiocyanate (PEITC), and sulforaphane induced a dose-dependent inhibition of human bronchial epithelial cell proliferation. Zhang et al. (21) reported that the growth of several cancer cell lines, including the MCF-7 breast cancer cell line, the HepG2 liver cancer cell line, the HT-29 colon cancer cell line, and the HaCaT skin cancer cell line, was inhibited by 3 h and 72 h treatments with allyl isothiocyanate (AITC) BITC, and PEITC. Zhang et al. (21) also determined the effects of the major in vivo metabolites of AITC and BITC (i.e., the GSH and Cys conjugates of AITC and BITC) in HL60/S blood cancer cells. They found that 3 h and 72 h treatments with the conjugates and their parent ITC compounds had similar inhibitory effects on cell growth. This indicates that ITC metabolites may retain the anticancer activity of their parent compounds.
Detoxification enzyme activity
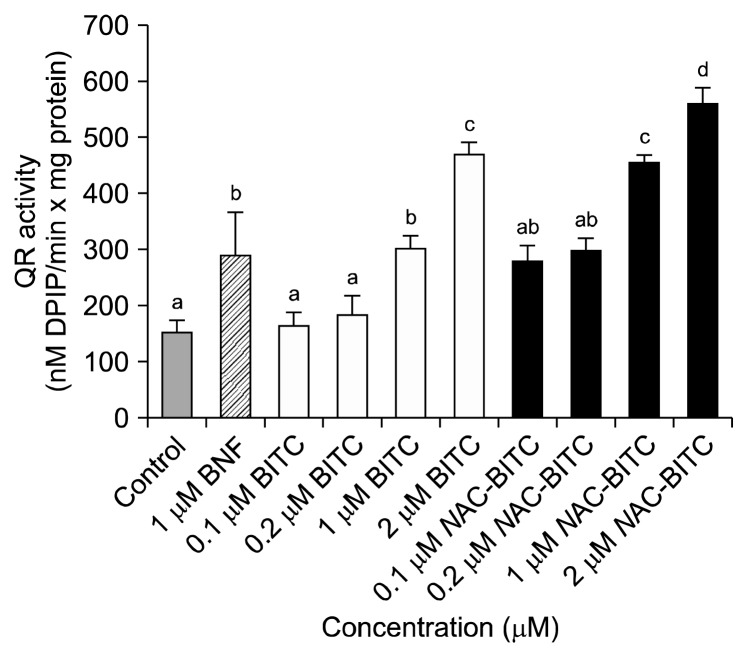
The effect of BITC and NAC-BITC on the activation of QR was examined in this study. BITC and NAC-BITC significantly induced QR activity at all concentrations tested (Fig. 3). The QR activity was normalized to the protein content of each treatment. The QR activity of the positive control (i.e., 1 μM BNF) was 281.4 nM 2,6-dichlorophenolindophenol (DPIP)/min, which was about 1.8-fold greater than the QR activity of the negative control (154.2 nM DPIP/min). A dose-dependent induction of QR was observed from 1 μM to 2 μM BITC; QR activity was increased 1.7- and 3.1-fold at the 1 μM and 2 μM BITC doses, respectively. A similar induction of QR activity was observed with NAC-BITC; treatment with 1 μM and 2 μM NAC-BITC was associated with a 1.9- and 3.1-fold induction of QR activity. The 0.1 μM BITC and 0.1 μM NAC-BITC treatments did not significantly induce QR activity.
Fig. 3.
Effects of 24 h of BITC and NAC-BITC treatment on the catalytic activity of quinone reductase in Hepa1c1c7 cells. Within each treatment, different letters (a–d) indicate significant differences. Values are mean±SD (n=4, P<0.05).
In our previous research (22), we studied the effect of AITC and its NAC conjugate on QR induction. We found that AITC and NAC-AITC caused dose-related cell growth inhibition and QR induction. Treatments with 1 μM and 2 μM AITC resulted in 2.0- and 3.1-fold inductions of QR activity, respectively. Treatment with 1 μM and 2 μM NAC-AITC was associated with a 2.9- and 3.7-fold induction of QR activity, respectively.
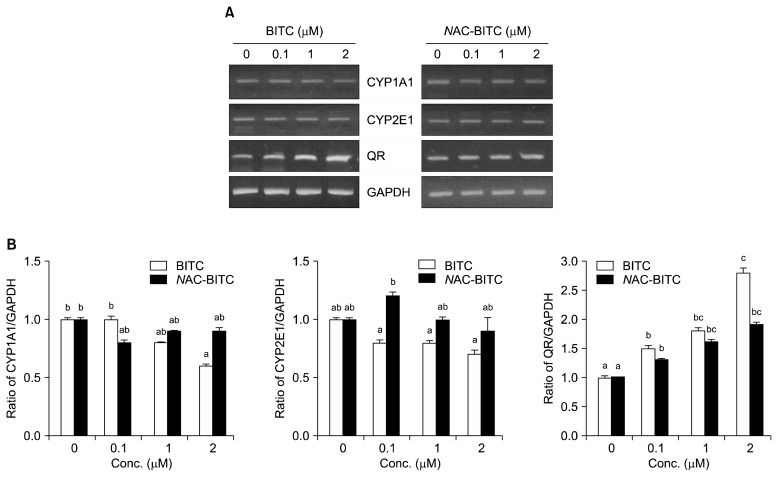
Fig. 4 depicts QR mRNA levels in Hepa1c1c7 cells after 24 h of exposure to BITC or NAC-BITC. QR mRNA levels were quantified by RT-PCR, and GAPDH was used as an endogenous standard. BITC had a dose-dependent effect on Hepa1c1c7 cell QR mRNA expression; the 1 μM and 2 μM BITC doses resulted in 1.8- and 2.8-fold increases in QR mRNA expression, respectively. Spectrophotometric and RT-PCR analyses yielded very similar QR expression patterns. Treatment with NAC-BITC also resulted in a dose-dependent increase in QR expression; the 1 μM and 2 μM NAC-BITC treatments led to 1.6- and 1.9-fold inductions in QR mRNA expression, respectively. CYP1A1 and CYP2E1 induction were lower in cells treated with 0.1~2 μM BITC than in control-treated cells, indicating that BITC is an efficient CYP1A1 and CYP2E1 inactivator. CYP2E1 activity was 1.2-fold lower in cells treated with a 0.1 μM dose of NAC-BITC than in control-treated cells. However, the CYP2E1 activity of cells treated with 1~2 μM NAC-BITC was similar to that of controls.
Fig. 4.
QR, CYP1A1, and CYP2E1 mRNA expression in Hepa1c1c7 cells treated with varying concentrations of BITC and NAC-BITC for 24 h. The RT-PCR experiments were repeated three times with similar results. (A) Electrophoresis gel photo of PCR products. (B) The ratio of the densitometry values of QR/GAPDH, CYP1A1/GAPDH, and CYP2E1/GAPDH in cells treated with different concentrations of BITC and NAC-BITC. Within each treatment, different letters (a–c) indicate significant differences. Values are mean±SD (n=3, P<0.05).
Goosen et al. (23) studied the effect of BITC on rat and human cytochrome P450 and showed that BITC was a mechanism-based inactivator of rat P450s 1A1, 1A2, 2B1, and 2E1 and of human P450s 2B6 and 2D6. Silverman (24) reported that the reactive intermediate responsible for the inactivation of P450 1A2 may be released more readily from the active site of the enzyme, thereby increasing the rate of inactivation.
Mechanisms for protecting cells from cancer initiation include decreasing the expression of metabolic enzymes responsible for generating reactive species (i.e., phase I enzymes) and increasing the expression of enzymes that can deactivate radicals and electrophiles known to intercede in normal cellular processes (i.e., phase II enzymes). Reduction of electrophilic quinones by QR is an important detoxification pathway that converts quinones to hydroquinones and reduces oxidative cycling.
Dietary ITCs have been studied for their ability to inhibit phase I metabolic activation of procarcinogens and enhance the phase II conjugation and elimination of carcinogens. They are prospective chemopreventive agents, although some concerns have recently been raised about their ability to promote carcinogenesis. The antiproliferative and antitumor activities of dietary ITCs and their conjugates are being studied further. A vegetable-rich diet may reduce the incidence of cancer. The results of the present study are important because they indicate that ITC conjugates may be involved in the mechanism by which ITCs inhibit cancer and they raise the possibility that conjugates themselves inhibit cancer.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Fenwick GR, Heaney RK. Glucosinolates and their breakdown products in cruciferous crops, foods and feeding-stuffs. Food Chem. 1983;11:249–271. doi: 10.1016/0308-8146(83)90074-2. [DOI] [Google Scholar]
- 2.Lampe JW, Peterson S. Brassica, biotransformation and cancer risk: genetic polymorphisms alter the preventive effects of cruciferous vegetables. J Nutr. 2002;132:2991–2994. doi: 10.1093/jn/131.10.2991. [DOI] [PubMed] [Google Scholar]
- 3.Hwang ES, Jeffery EH. Induction of quinone reductase by the sulforaphane and N-acetylcysteine conjugate of sulforaphane in murine hepatoma cells. J Med Food. 2005;8:198–203. doi: 10.1089/jmf.2005.8.198. [DOI] [PubMed] [Google Scholar]
- 4.Cramer JM, Jeffery EH. Sulforaphane absorption and excretion following ingestion of a semi-purified broccoli powder rich in glucoraphanin and broccoli sprouts in healthy men. Nutr Cancer. 2011;63:196–201. doi: 10.1080/01635581.2011.523495. [DOI] [PubMed] [Google Scholar]
- 5.Masuda H, Harada Y, Tanaka K, Nakajima M, Tateba H. Biotechnology for Improved Foods and Flavors. American Chemical Society; Washington, DC, USA: 1996. Characteristic odorants of Wasabi, Japanese horseradish, in comparison with those of horseradish (Armoracia rusticana) pp. 67–78. (ACS Symposium Series 637). [DOI] [Google Scholar]
- 6.Mennicke WH, Görler K, Krumbiegel G. Metabolism of some naturally occurring isothiocyanates in the rat. Xenobiotica. 1983;13:203–207. doi: 10.3109/00498258309052256. [DOI] [PubMed] [Google Scholar]
- 7.Conaway CC, Yang YM, Chung FL. Isothiocyanates as cancer chemopreventive agents: their biological activities and metabolism in rodents and humans. Curr Drug Metab. 2002;3:233–255. doi: 10.2174/1389200023337496. [DOI] [PubMed] [Google Scholar]
- 8.Chung FL, Jiao D, Getahun SM, Yu MC. A urinary biomarker for uptake of dietary isothiocyanates in humans. Cancer Epidemiol Biomarkers Prev. 1998;7:103–108. [PubMed] [Google Scholar]
- 9.Hwang ES, Jeffery EH. Evaluation of urinary N-acetyl cysteinyl allyl isothiocyanate as a biomarker for intake and bioactivity of Brussels sprouts. Food Chem Toxicol. 2003;41:1817–1825. doi: 10.1016/S0278-6915(03)00235-7. [DOI] [PubMed] [Google Scholar]
- 10.Kassie F, Laky B, Gminski R, Mersch-Sundermann V, Scharf G, Lhoste E, Kansmüller S. Effects of garden and water cress juices and their constituents, benzyl and phenethyl isothiocyanates, towards benzo(a)pyrene-induced DNA damage: a model study with the single cell gel electrophoresis/Hep G2 assay. Chem Biol Interact. 2003;142:285–296. doi: 10.1016/S0009-2797(02)00123-0. [DOI] [PubMed] [Google Scholar]
- 11.Perocco P, Bronzetti G, Canistro D, Valgimigli L, Sapone A, Affatato A, Pedulli GF, Pozzetti L, Broccoli M, Iori R, Barillari J, Sblendorio V, Legator MS, Paolini M, Abdel-Rahman SZ. Glucoraphanin, the bioprecursor of the widely extolled chemopreventive agent sulforaphane found in broccoli, induces Phase-I xenobiotic metabolizing enzymes and increases free radical generation in rat liver. Mutat Res. 2006;595:125–136. doi: 10.1016/j.mrfmmm.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Wallig MA, Kingston S, Staack R, Jefferey EH. Induction of rat pancreatic glutathione S-transferase and quinone reductase activities by a mixture of glucosinolate breakdown derivatives found in brussels sprouts. Food Chem Toxicol. 1998;36:365–373. doi: 10.1016/S0278-6915(97)00156-7. [DOI] [PubMed] [Google Scholar]
- 13.Steck SE, Gammon MD, Hebert JR, Wall DE, Zeisel SH. GSTM1, GSTT1, GSTP1, and GSTA1 polymorphisms and urinary isothiocyanate metabolites following broccoli consumption in humans. J Nutr. 2007;137:904–909. doi: 10.1093/jn/137.4.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinkova-Kostova AT, Talalay P. Persuasive evidence that quinone reductase type 1 (DT diaphorase) protects cells against the toxicity of electrophiles and reactive forms of oxygen. Free Radic Biol Med. 2000;29:231–240. doi: 10.1016/S0891-5849(00)00300-2. [DOI] [PubMed] [Google Scholar]
- 15.Nioi P, Hayes JD. Contribution of NAD(P)H: quinone oxidoreductase 1 to protection against carcinogenesis, and regulation of its gene by the Nrf2 basic-region leucine zipper and the arylhydrocarbon receptor basic helix-loop-helix transcription factors. Mutat Res. 2004;555:149–171. doi: 10.1016/j.mrfmmm.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 16.Boone CW, Steele VE, Kelloff GJ. Screening for chemopreventive (anticarcinogenic) compounds in rodents. Mutat Res. 1992;267:251–255. doi: 10.1016/0027-5107(92)90069-E. [DOI] [PubMed] [Google Scholar]
- 17.Song LL, Kosmeder JW, 2nd, Lee SK, Gerhäuser C, Lantvit D, Moon RC, Moriarty RM, Pezzuto JM. Cancer chemopreventive activity mediated by 4′-bromoflavone, a potent inducer of phase II detoxification enzyme. Cancer Res. 1999;59:578–585. [PubMed] [Google Scholar]
- 18.Sasaki JC, Fellers RS, Colvin ME. Metabolic oxidation of carcinogenic arylamines by P450 monooxygenases: theoretical support for the one-electron transfer mechanism. Mutat Res. 2002;506–507:79–89. doi: 10.1016/S0027-5107(02)00154-9. [DOI] [PubMed] [Google Scholar]
- 19.Syed K, Doddapaneni H, Subramanian V, Lam YW, Yadav JS. Genome-to-function characterization of novel fungal P450 monooxygenases oxidizing polycyclic aromatic hydrocarbons (PAHs) Biochem Biophys Res Commun. 2010;399:492–497. doi: 10.1016/j.bbrc.2010.07.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan XL, Shi M, Tang H, Han W, Spivack SD. Candidate dietary phytochemicals modulate expression of phase II enzymes GSTP1 and NQO1 in human lung cells. J Nutr. 2010;140:1404–1410. doi: 10.3945/jn.110.121905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Tang L, Gonzalez V. Selected isothiocyanates rapidly induce growth inhibition of cancer cells. Mol Cancer Ther. 2003;2:1045–1052. [PubMed] [Google Scholar]
- 22.Hwang ES, Lee HJ. Induction of quinone reductase by allylisothiocyanate (AITC) and the N-acetylcysteine conjugate of AITC in Hepa1c1c7 mouse hepatoma cells. Biofactors. 2006;26:7–15. doi: 10.1002/biof.5520260102. [DOI] [PubMed] [Google Scholar]
- 23.Goosen TC, Mills DE, Hollenberg PF. Effects of benzyl isothiocyanate on rat and human cytochromes P450: identification of metabolites formed by P450 2B1. J Pharmacol Exp Ther. 2001;296:198–206. [PubMed] [Google Scholar]
- 24.Silverman RB. Mechanism-based enzyme inactivators. In: Purich DL, editor. Contemporary Enzyme Kinetics and Mechanisms. Academic Press; San Diego, CA, USA: 1996. pp. 291–335. [Google Scholar]