Abstract
Hesperetin has been shown to possess a potential anti-angiogenic effect, including vascular formation by endothelial cells. However, the mechanisms underlying the potential anti-angiogenic activity of hesperetin are not fully understood. In the present study, we evaluated whether hesperetin has anti-angiogenic effects in human umbilical vascular endothelial cells (HUVECs). HUVECs were treated with 50 ng/mL vascular endothelial growth factor (VEGF) to induce proliferation as well as vascular formation, followed by treatment with several doses of hesperetin (25, 50, and 100 μM) for 24 h. Cell proliferation and vascular formation were analyzed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and tube formation assay, respectively. In addition, cell signaling related to cell proliferation and vascular formation was analyzed by western blot. Furthermore, a mouse aorta ring assay was performed to confirm the effect of hesperetin on vascular formation. Hesperetin treatment did not cause differences in HUVECs proliferation. However, hesperetin significantly inhibited VEGF-induced cell migration and tube formation of HUVECs (P<0.05). Moreover, hesperetin suppressed the expression of ERK, p38 MAPK, and PI3K/AKT in the VEGF-induced HUVECs. In an ex vivo model, hesperetin also suppressed microvessel sprouting of mouse aortic rings. Taken together, the findings suggest that hesperetin inhibited vascular formation by endothelial cells via the inhibition of the PI3K/AKT, ERK and p38 MAPK signaling.
Keywords: hesperetin, vascular formation, PI3K/AKT, HUVECs
INTRODUCTION
During vessel development, angiogenesis and vasculogenesis (1) are highly regulated processes that involve the activation, growth, and migration of endothelial cells and capillary morphogenesis (2,3). Angiogenesis is also an important event in the development of solid tumors because tumors cannot grow beyond a size of 2~3 mm3 owing to a lack of oxygen and other essential nutrients (4). Thus, inhibition of vascular formation presents a promising strategy for cancer treatment (5). Therefore, the identification of anti-angiogenic agents with novel mechanisms of action is an attractive strategy for studying angiogenic processes and could provide potential lead candidates for the development of new drugs associated with vascular formation. Vascular endothelial growth factor (VEGF) is significantly involved in the proliferation, migration, and invasion of endothelial cells (6) through the activation of several signaling pathways such as ERK (7), JNK (8), AKT (9), and p38 MAPK (10). In particular, the PI3K/AKT signaling pathways play important roles in the regulation of many cellular processes, including cell growth and proliferation, differentiation, and apoptosis (11).
Hesperetin (3′,5,7-trihydroxy-4′-methoxyflavanone), a member of the flavanone subclass of flavonoids, is found in fruit sources including various citrus species (12). Reports based on the in vitro action of flavonoids in cancer cells have found various anticancer effects such as the inhibition of cell proliferation and kinase activity, and the induction of apoptosis (13–15). Hesperetin has also shown potential activity as a novel antiangiogenic agent for human umbilical vascular endothelial cells HUVECs (16). However, the mechanisms underlying the potential antiangiogenic activity of hesperetin are not fully understood.
We previously demonstrated that hesperetin inhibited tube formation in mouse embryonic stem cells (17). Therefore, the objectives of the present study were to analyze the effects of hesperetin on the proliferation and vascular formation of HUVECs. The PI3K/AKT, ERK, and p38 MAPK signaling in hesperetin-treated HUVECs and microvessel sprouting using mouse aortic rings assay were analyzed.
MATERIALS AND METHODS
Reagent
Hesperetin (Fig. 1) was purchased from Sigma-Aldrich (St. Louis, MO, USA). The compound was dissolved in 100% dimethyl sulfoxide (DMSO). A 100 mmol/L stock solution of hesperetin was prepared and stored as small aliquots at −20°C until needed. We purchased 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), DMSO, gelatin, and horseradish peroxidase (HRP)-conjugated anti-mouse and anti-rabbit antibodies from Sigma-Aldrich. Recombinant human VEGF (VEGF165) was obtained from R&D Systems (Minneapolis, MN, USA). Growth factor-reduced Matrigel was purchased from BD Biosciences (San Jose, CA, USA). The antibodies p-p38 (Thr180/Tyr182), p-JNK (Thr183/Tyr185), JNK, p-PI3K (Tyr458), PI3K and p-AKT (Ser473), AKT were purchased from Cell Signaling Technology (Danvers, MA, USA). The HRP-conjugated β-actin, ERK, p38α and p-ERK (Thr202/Tyr204) antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA).
Fig. 1.
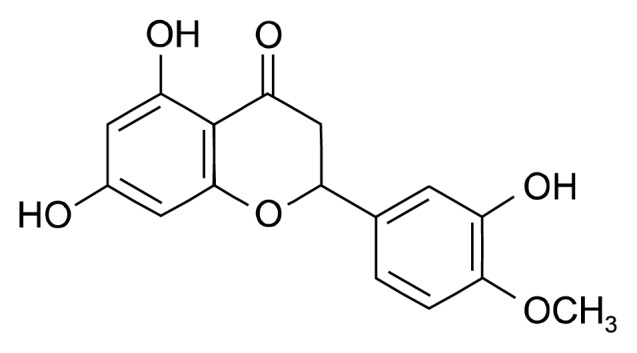
Chemical structure of hesperetin.
Endothelial cell culture
Human umbilical vascular endothelial cells (HUVECs) were obtained from ATCC (Rockville, MD, USA) and cultured in endothelial growth medium (EGM)-2 (Lonza, Walkersville, MD, USA) supplemented with 10% FBS at 37°C in a 5% CO2 atmosphere. HUVECs at passages three to five were used in the experiments. The commercially available vascular endothelial cell-specific supplement EGM™-2MV BulletKit™ (Lonza) was used (18).
Cell viability assay
Cell viability was assessed by an MTT assay. HUVECs (5×103 cells/well) were seeded into a 96-well plate with EGM-2 supplemented with 10% FBS. After allowing, the culture medium was removed, and the cells were rinsed twice with phosphate buffered saline (PBS) and then incubated with serum-free medium for 12 h. Following serum starvation, the cells were cultured in fresh 2% FBS medium containing various concentrations of hesperetin at 37°C for 24 h in the presence or absence of VEGF (50 ng/mL). After the incubation, an MTT solution was added, and the plate was incubated for an additional 4 h. The resulting formazan deposit was dissolved with DMSO, and the absorbance was detected at 570 nm with a VersaMax ELISA microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Cell cycle analysis
HUVECs were plated in 100mm diameter culture dishes and then incubated. The next day, the cells were treated with various concentrations (0 to 100 μM) of hesperetin for 24 h. The cells were harvested (trypsinization and centrifugation) and fixed with 70% ethanol overnight at 4°C. After washing, the cells were subsequently stained with 50 μg/mL of PI and 50 μg/mL of RNase A for 1 h in the dark and then subjected to flow cytometry analysis in order to determine the percentage of cells at specific cell cycle phase. Flow cytometry analysis was performed using a BD FACSCalibur flow cytometer (BD Biosciences) equipped with a 488-nm argon laser. Events were evaluated for each sample and the cell cycle distribution was analyzed using BD CellQuest software (BD Biosciences). The results were presented as the number of cells versus the amount of DNA as indicated by fluorescence signal intensity. All the experiments were conducted three times.
Flow cytometry analysis of apoptosis
To determine the level of apoptosis following hesperetin exposure for 24 h in HUVECs, the Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit (BD Pharmingen™) was used. In this assay, Annexin V-FITC binds to phosphatidylserine, which translocates to the outer leaflet of the plasma membrane during the early stages of cell apoptosis. Therefore, the apoptotic cells were specifically stained with Annexin V-FITC, whereas the necrotic cells were doubly stained with both Annexin V-FITC and PI. The cells were suspended in binding buffer at a final cell concentration of 1×105 cells/mL and incubated with both annexin V-FITC and PI for 25 min in the dark. The DNA contents of the stained cells were analyzed using CellQuest Software and a FACS Vantage SE flow cytometer (BD Biosciences).
Scratch-wound migration assay
HUVECs were allowed to grow to full confluence in 6-well plates pre-coated with 0.1% gelatin and then incubated with 10 mg/mL mitomycin C (Sigma-Aldrich) at 37°C in a 5% CO2 atmosphere for 2 h to inactivate the HUVECs. Monolayers HUVECs were wounded by scratching with a 0.2-mL pipette tip. Fresh medium containing various concentrations of hesperetin was added. Images were taken with an inverted phase contrast light microscope (Olympus Optical Co. Ltd., Tokyo, Japan) after 24 h incubation. The migrated cells were then counted from three randomly selected fields under an optical microscope at 200× magnification. The migrated cells were quantified by manual counting (DMC advanced Adobe Photoshop software, Adobe Systems Software Ireland Ltd., San Jose, CA, USA), and the inhibition was calculated as a percentage relative to control.
Transwell migration assay
The chemotactic motility of the HUVECs was determined using a Transwell migration assay kit (Corning incorporated, Corning, NY, USA) with an 8-μm pore size as described elsewhere (19). Briefly, the inserts of the transwell plate were coated with 0.2% gelatin for 30 min. After the Transwell was washed three times with PBS, fresh EBM supplemented with 50 ng/mL VEGF was placed in the lower chamber and the HUVEC (4×104 cells/well) were seeded in the top chamber. Then, the cells were treated with hesperetin for 8 h at 37°C in a 5% CO2 atmosphere. After the incubation, the non-migrated cells on the top surface of the membrane were gently scraped away with a cotton swab. The membrane containing the migrated cells was fixed with 4% paraformaldehyde for 10 min and stained with hematoxylin. Images were recorded using an Olympus inverted microscope, and the migrated cells were quantified by manual counting. The percentage of migrated cells inhibited by hesperetin was normalized to the untreated control cell migration.
Tube formation assay with HUVECs on Matrigel
Matrigel (70 μL/well) was added to a 96-well plate and polymerized for 30 min at 37°C. The HUVEC (3×104 cells) were seeded onto each well of the Matrigel-coated 96-well plate and then incubated in 2% FBS-EBM-2 with various concentrations of hesperetin in the presence of VEGF (50 ng/mL). After 8 h of incubation, the formation of endothelial cell tubular structure was visualized under an inverted microscope and photographed at 40× magnification. Furthermore, tube formation was quantified by calculating the tube length and was expressed as a percentage by normalization with untreated control cells.
Western blot analysis
Cells were treated with hesperetin for 24 h. Harvested cells were lysed in protein extraction solution (Intron Biotechnology, Inc., Seongnam, Korea) containing protease inhibitors and phosphatase inhibitors for 10 min at 4°C. The total protein concentration in the supernatants was measured by the Bradford assay. After heating at 95°C for 5 min, total protein samples (40 μg) were subjected to 6~15% SDS-PAGE. The proteins were transferred onto PVDF membranes (Millipore Corporation, Bedford, MA) at 100 V for 60~100 min. The membranes were incubated with 5% BSA in TBST (TBS with 0.05% Tween 20) for 30 min at room temperature and then with primary antibodies diluted (1:200~1:1,000) in 5% BSA in TBST overnight at 4°C. The membranes were washed three times with TBST and incubated with the corresponding secondary antibodies. Protein bands were detected using an enhanced chemiluminescence detection kit (Intron Biotechnology, Inc.) and an LAS-1000 Imager (Fuji Film Corp., Tokyo, Japan).
Mouse aortic ring assay
The mouse aortic ring assay was performed as previously described (20). Forty-eight-well plates were covered with 150 μL of Matrigel and then incubated at 37°C and 5% CO2 for 30 min. The aortas isolated from mice (Central Laboratory Animal Inc., Seoul, Korea) were cleaned of periadventitial fat and connective tissues and cut into 1~1.5 mm long rings. After rinsing with PBS, the aortas were placed in the Matrigel-covered wells and covered with an additional 200 μL of Matrigel. The artery rings were cultured in 1 mL of EGM without serum for 24 h, and then the medium was replaced with 1 mL of EGM containing supplements with vehicle or hesperetin (25, 50, or 100 μM). The medium was replaced every 2 days with medium that had the same composition as described above. After 7 days, the microvessel growth was measured by taking photographs with the Olympus inverted microscope (40× objectives). The length of the capillary was estimated using a phase-contrast microscope by measuring the distance from the cut end of the aortic segment to the approximate middle point of the capillary. The length of the capillary was measured using DMC advanced Adobe Photoshop software (Adobe Systems Software Ireland Ltd.). Each value represents the average of 3~4 culture samples.
Statistical analysis
The results are expressed as the mean±SD. Statistical significance was determined using a one-way analysis of variance (ANOVA) and Student’s t-test for paired data. A P value of <0.05 was considered statistically significant. The calculations were performed using SPSS for windows version 10.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
The effect of hesperetin on the proliferation of VEGF-induced HUVECs
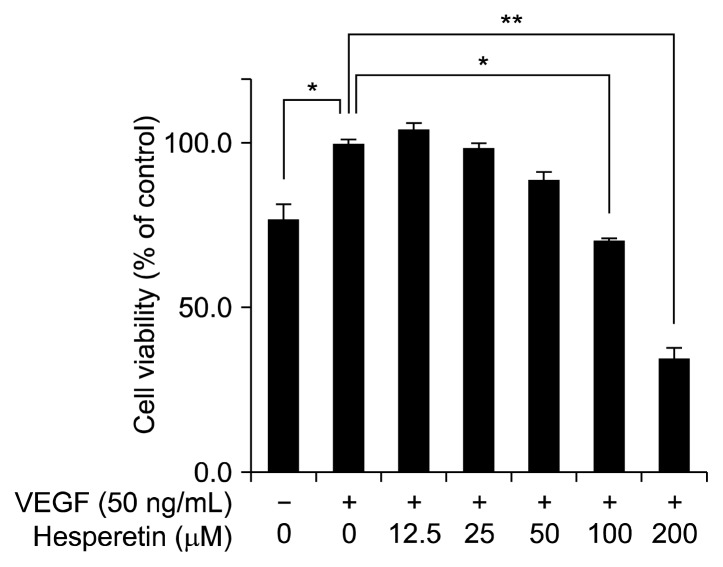
In general, angiogenesis and vasculogenesis inhibitors suppress endothelial cell proliferation. To determine the anti-angiogenic activity of hesperetin, we first evaluated whether hesperetin inhibits the proliferation of VEGF-induced HUVECs. To determine the non-cytotoxic concentration of hesperetin against HUVECs, the cells were initially treated with hesperetin (0~200 μM) for 24 h. Subsequently, cell viability was evaluated with the MTT assay. When treated with VEGF (50 ng/mL) for 24 h, the proliferation of HUVECs was significantly increased by approximately 25% compared with that of the control cells (without VEGF; P<0.05). Although the proliferation of VEGF-stimulated HUVECs was decreased by treatment with 100 μM hesperetin, this was not significant compared with that of the control cells (Fig. 2). Therefore, all further analyses of the biological activities of hesperetin in HUVECs were performed using <100 μM hesperetin.
Fig. 2.
The inhibitory effects of hesperetin on cell viability of HUVECs. HUVECs were cultured with hesperetin (0~200 μM) in the presence of VEGF (50 ng/mL) for 24 h. Cell proliferation is expressed as the percentage of viable cells cultured in the absence of hesperetin and is expressed as the mean±SD. *P<0.05, **P<0.01 compared to control.
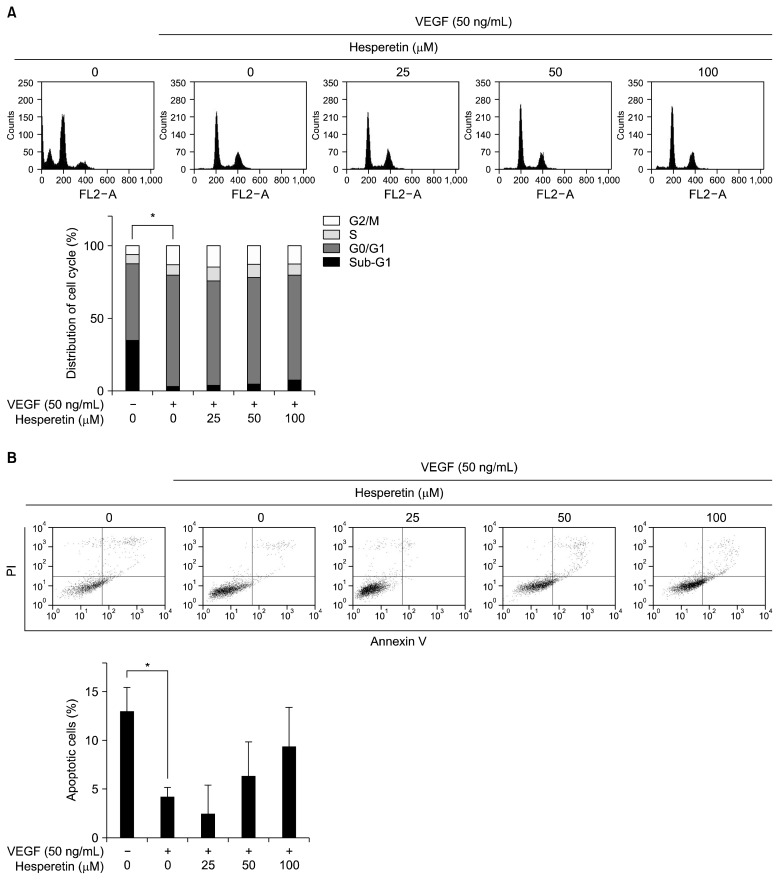
The cell cycle of HUVECs in the presence of hesperetin was measured using flow cytometry. The cells were harvested 24 h after treatment with hesperetin at various concentrations and analyzed for their cell cycle distributions (sub-G1, G0/G1, S, and G2/M). As shown in Fig. 3A, the sub-G1 phase which is indicative of apoptotic cell death of HUVECs was dramatically decreased by VEGF treatment (P<0.05). However, no differences were found between the doses used. Although the Annexin V analysis showed that VEGF treatment decreased apoptosis of HUVECs (P<0.05), no other apoptotic effect on HUVECs was found, except for the fact that the number of apoptotic cells were slightly increased by hesperetin in a concentration-dependent manner (Fig. 3B). These findings suggest that hesperetin at the highest concentration used did not induce apoptosis of HUVECs.
Fig. 3.
Effects of hesperetin on cell cycle and apoptosis in VEGF-induced HUVECs. (A) HUVECs were treated with hesperetin (0~100 μM) for 24 h, stained with propidium iodide (PI) and then analyzed on a FACSCalibur flow cytometer. Quantitation of the PI staining data is presented as the percentages of cell cycle distribution. (B) HUVECs were treated with hesperetin (0~100 μM) for 24 h, stained with Annexin V and PI and analyzed on a FACS Vantage SE flow cytometer. The expressed as the mean±SD. *P<0.05 compared to control.
The effect of hesperetin on the migration, invasion, and tube formation of VEGF-induced HUVECs
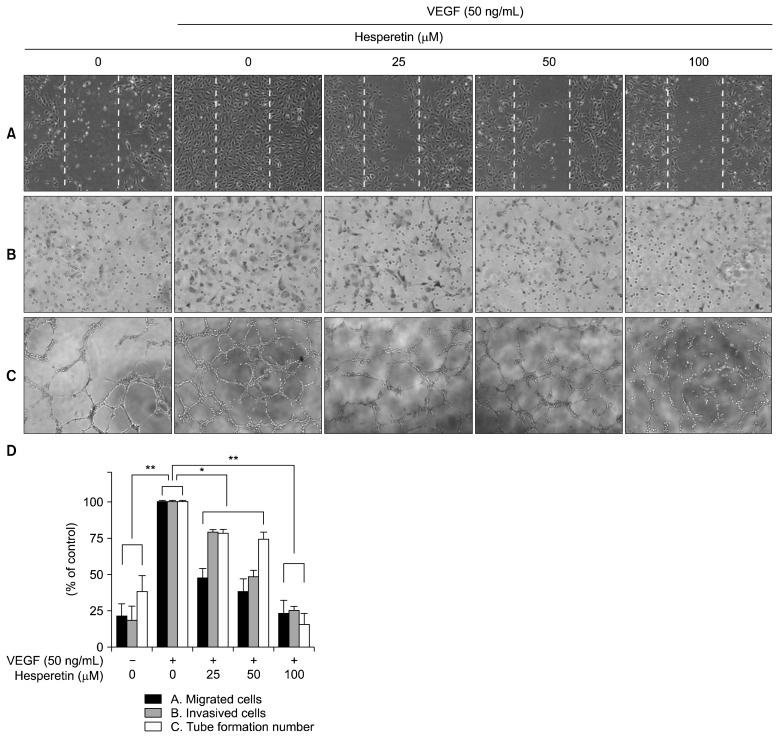
Endothelial cell migration and tube formation are essential steps in angiogenesis. We therefore determined the effects of hesperetin on endothelial cell migration using both wound healing and Transwell migration assays in vitro. Hesperetin suppressed the migration of VEGF-stimulated HUVECs in a concentration-dependent manner (P<0.05, Fig. 4A and 4D). Hesperetin also inhibited the migration of HUVECs in the Matrigel-coated Transwell migration assay (P<0.05, Fig. 4B and 4D). To further investigate the effect of hesperetin on endothelial cells, we examined the VEGF-stimulated tube formation of HUVECs in the Matrigel (P<0.05, Fig. 4C and 4D). Furthermore, it is well known that endothelial cells are able to spontaneously form capillary-like networks in a Matrigel in vitro (21). As shown in Fig. 4C, HUVECs spontaneously formed a capillary-like tube structure after 4~8 h of incubation in the Matrigel. However, VEGF-stimulated HUVECs formed tube structures that were more prominent, with more stable and longer networks. Hesperetin treatment, however, remarkably inhibited the vascular formation of VEGF-stimulated HUVECs, resulting in less elongated, broken, and foreshortened tubes. These data suggest that hesperetin inhibited the capillary-like tube formation of VEGF-induced HUVECs.
Fig. 4.
The effects of hesperetin on the migration and capillary structure formation of VEGF-induced HUVECs. (A) Hesperetin inhibited HUVECs migration. Cells were grown to confluency in six-well plates, wounded, and treated with the indicated concentrations of hesperetin and VEGF (50 ng/mL). (B) Hesperetin inhibited endothelial cell migration using Transwell migration assay. HUVECs treated with several doses of hesperetin were seeded in the upper chamber, and the bottom chamber was filled with EBM medium containing VEGF (50 ng/mL). The cells with an irregular shape in the images are cells that migrated into the lower chamber. (C) Hesperetin inhibited the tube formation of VEGF-induced HUVECs. Cells were placed in 96-well plates coated with Matrigel. After 4~8 h in the absence and presence of hesperetin, the tubular structures were photographed. The migrated cells were quantified by manual counting. (D) Calculation of cell number for migrated, invasive, and tube formations in VEGF-induced HUVECs depend on hesperetin treatment. The results are reported as the mean±SD. *P<0.05, **P<0.01 versus VEGF-stimulated cells.
The effect of hesperetin on the proliferation and vascular formation-related signaling pathway in VEGF-stimulated HUVECs
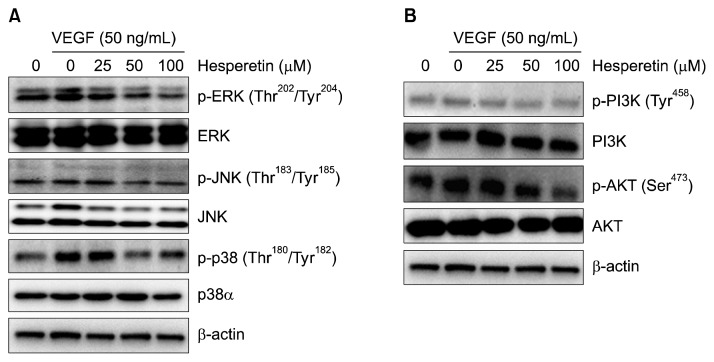
To further understand the molecular basis of the hesperetin-mediated anti-angiogenic activity, we investigated cellular signaling pathways in VEGF-stimulated HUVECs. As shown in Fig. 5A and 5B, VEGF treatment (50 ng/mL) of HUVECs induced the activation of all MAPK signaling pathways including that of ERK, JNK, and p38. Hesperetin treatment markedly suppressed the phosphorylation of ERK and p38 MAPK in these VEGF-induced HUVECs. In contrast, the phosphorylation of JNK was marginally affected by hesperetin. These data suggest a potential inhibition of the migration, proliferation, and tube formation of the VEGF-induced HUVECs by hesperetin.
Fig. 5.
Effect of hesperetin on angiogenic signaling. Hesperetin inhibited the phosphorylation of MAPK (A) and PI3K/AKT (B) in VEGF-induced HUVECs. After starvation in EGM without serum overnight, HUVECs were washed twice with PBS and then incubated in the presence of VEGF containing (50 ng/mL) medium with hesperetin for 24 h. β-Actin was used as an internal control.
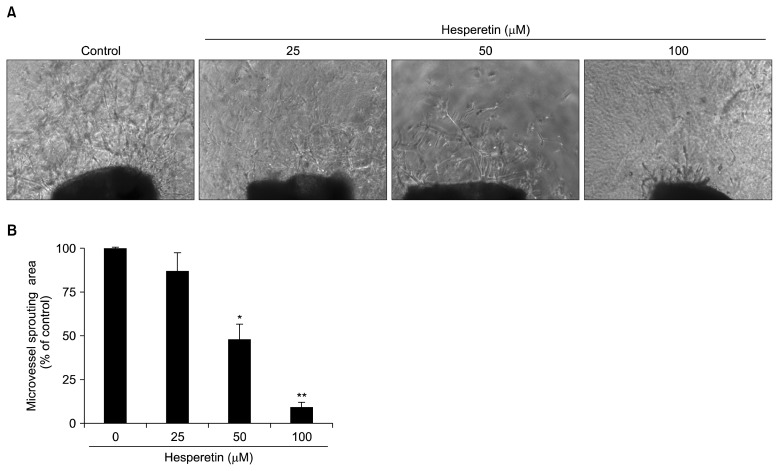
The effect of hesperetin on capillary spouting in the mouse aortic ring assay
The mouse aortic ring assay was used to investigate the effect of hesperetin on capillary spouting/vascular formation. Compared with that of the control group, vascular formation by mouse aortic rings was suppressed by hesperetin in a concentration-dependent manner, especially at a concentration of 50 and 100 μM (P<0.05, Fig. 6).
Fig. 6.
Effect of hesperetin on microvessel outgrowth arising from mouse aortic rings. Aortic rings isolated from mice were embedded in Matrigel in 48-well plates and then fed medium containing various concentrations of hesperetin for 7 days. Representative photographs of three independent experiments are shown. The microvessel length was measured on day 7 of culture. The values are the means±SD (n=3), and *P<0.05, **P<0.01 versus control cells.
DISCUSSION
In the present study, we demonstrated that hesperetin suppressed vascular formation in VEGF-induced HUVECs both in vitro and in an ex vivo system through PI3K/AKT signaling. These findings suggest that hesperetin is a novel anti-angiogenic compound by attenuating the activation of ERK/p38 MAP kinase and AKT kinase.
Modern pharmaceutical discovery programs owe much to natural products. Indeed, pharmacologically active compounds from plants, microbes, and marine animals represent an important pipeline for new investigational drugs (22). Phytochemical-mediated anti-angiogenic intervention is an upcoming area of research that promises an effective cancer prevention strategy. In a previous report, we demonstrated that honokiol and magnolol showed potential activity as a novel antiangiogenic agent in mouse embryonic stem cells-derived endothelial cells (23,24). Several phytochemicals have been shown to target tumor angiogenesis using in vitro and in vivo model systems (25–28). The mouse aortic ring assay is an ex vivo organ culture assay commonly used in angiogenesis research (29). This assay system is widely used as an effective tool for evaluating the anti-angiogenic activity of test compounds in a complex system in which endothelial cells, fibroblasts, pericytes, and smooth muscle cells interact (30). Angiogenesis is the formation of new blood vessels from the endothelium of the existing vasculature; the inhibition of angiogenesis is associated with a significant delay in tumor growth (31). Angiogenesis also plays essential roles in tumor invasion and metastasis (32). Thus, anti-angiogenic therapy is currently one of the most promising and effective therapies against cancer (33).
Hesperetin possesses notable anti-proliferative activity and induces apoptosis (14,15,34). The present study demonstrated that hesperetin exerted anti-angiogenic activity through the suppression of tube formation, cell migration, and endothelial cell proliferation in VEGF-induced HUVECs (Fig. 4). Further investigation revealed that hesperetin functioned as an angiogenesis inhibitor via the suppression of the VEGF receptor 2 (R2)-mediated signaling pathway PI3K/AKT (Fig. 5). MAPK signaling is considered one of the critical molecular events in the growth, survival, and migration of vascular endothelial cells in VEGF-induced angiogenesis. VEGF activates three MAPKs, namely ERK, JNK, and p38 MAPK (8,10). ERK activation results in increased proliferation of endothelial cells (7,8), whereas p38 MAPK activation triggers actin-based cell motility (10). Especially, the activation of the PI3K/AKT pathway contributes to the VEGF-mediated stimulation of the proliferation and migration of endothelial cells (35). Our data suggest that all MAPKs mediate VEGF-induced tube formation. However, the inhibitory effects of hesperetin were closely associated with the suppression of p38 MAPK activation as well as that of PI3K/AKT in VEGF-induced HUVECs (Fig. 5). Shiojima et al. have shown that the activation of PI3K/AKT promoted cell survival, migration, and cytoskeletal rearrangement (36). These findings are in line with our data. In addition, we confirmed that hesperetin suppressed the phosphorylation of AKT in VEGF-induced HUVECs (Fig. 5B). This effect may be a possible mechanism underlying the anti-angiogenic activity of hesperetin in VEGF-induced HUVECs.
In conclusion, the present findings demonstrate that hesperetin suppressed vascular formation via the inhibition of PI3K/AKT, ERK and p38 MAPK signaling in VEGF-induced HUVECs.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Patan S. Vasculogenesis and angiogenesis. Cancer Treat Res. 2004;117:3–32. doi: 10.1007/978-1-4419-8871-3_1. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holderfield MT, Hughes CC. Crosstalk between vascular endothelial growth factor, notch, and transforming growth factor-β in vascular morphogenesis. Circ Res. 2008;102:637–652. doi: 10.1161/CIRCRESAHA.107.167171. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 6.Cristi E, Perrone G, Toscano G, Verzì A, Nori S, Santini D, Tonini G, Vetrani A, Fabiano A, Rabitti C. Tumour proliferation, angiogenesis, and ploidy status in human colon cancer. J Clin Pathol. 2005;58:1170–1174. doi: 10.1136/jcp.2004.025536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanno S, Oda N, Abe M, Terai Y, Ito M, Shitara K, Tabayashi K, Shibuya M, Sato Y. Roles of two VEGF receptors, Flt-1 and KDR, in the signal transduction of VEGF effects in human vascular endothelial cells. Oncogene. 2000;19:2138–2146. doi: 10.1038/sj.onc.1203533. [DOI] [PubMed] [Google Scholar]
- 8.Meadows KN, Bryant P, Vincent PA, Pumiglia KM. Activated Ras induces a proangiogenic phenotype in primary endothelial cells. Oncogene. 2004;23:192–200. doi: 10.1038/sj.onc.1206921. [DOI] [PubMed] [Google Scholar]
- 9.Bai X, Cerimele F, Ushio-Fukai M, Waqas M, Campbell PM, Govindarajan B, Der CJ, Battle T, Frank DA, Ye K, Murad E, Dubiel W, Soff G, Arbiser JL. Honokiol, a small molecular weight natural product, inhibits angiogenesis in vitro and tumor growth in vivo. J Biol Chem. 2003;278:35501–35507. doi: 10.1074/jbc.M302967200. [DOI] [PubMed] [Google Scholar]
- 10.Wu G, Luo J, Rana JS, Laham R, Sellke FW, Li J. Involvement of COX-2 in VEGF-induced angiogenesis via P38 and JNK pathways in vascular endothelial cells. Cardiovasc Res. 2006;69:512–519. doi: 10.1016/j.cardiores.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Yap TA, Garrett MD, Walton MI, Raynaud F, de Bono JS, Workman P. Targeting the PI3K–AKT–mTOR pathway: progress, pitfalls, and promises. Curr Opin Pharmacol. 2008;8:393–412. doi: 10.1016/j.coph.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Garg A, Garg S, Zaneveld LJD, Singla AK. Chemistry and pharmacology of the citrus bioflavonoid hesperidin. Phytother Res. 2001;15:655–669. doi: 10.1002/ptr.1074. [DOI] [PubMed] [Google Scholar]
- 13.Zheng Q, Hirose Y, Yoshimi N, Murakami A, Koshimizu K, Ohigashi H, Sakata K, Matsumoto Y, Sayama Y, Mori H. Further investigation of the modifying effect of various chemopreventive agents on apoptosis and cell proliferation in human colon cancer cells. J Cancer Res Clin Oncol. 2002;128:539–546. doi: 10.1007/s00432-002-0373-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Binnerup SJ, Sørensen J. Nitrate and nitrite microgradients in barley rhizosphere as detected by a highly sensitive denitrification bioassay. Appl Environ Microbiol. 1992;58:2375–2380. doi: 10.1128/aem.58.8.2375-2380.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsuda H, Ohshima Y, Nomoto H, Fujita K, Matsuda E, Iigo M, Takasuka N, Moore MA. Cancer prevention by natural compounds. Drug Metab Pharmacokinet. 2004;19:245–263. doi: 10.2133/dmpk.19.245. [DOI] [PubMed] [Google Scholar]
- 16.Lam IK, Alex D, Wang YH, Liu P, Liu AL, Du GH, Lee SM. In vitro and in vivo structure and activity relationship analysis of polymethoxylated flavonoids: Identifying sinensetin as a novel antiangiogenesis agent. Mol Nutr Food Res. 2012;56:945–956. doi: 10.1002/mnfr.201100680. [DOI] [PubMed] [Google Scholar]
- 17.Choi EJ, Kim GD, Chee KM, Kim GH. Effects of hesperetin on vessel structure formation in mouse embryonic stem (mES) cells. Nutrition. 2006;22:947–951. doi: 10.1016/j.nut.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Kim GD, Kim GJ, Seok JH, Chung HM, Chee KM, Rhee GS. Differentiation of endothelial cells derived from mouse embryoid bodies: a possible in vitro vasculogenesis model. Toxicol Lett. 2008;180:166–173. doi: 10.1016/j.toxlet.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 19.Yi T, Yi Z, Cho SG, Luo J, Pandey MK, Aggarwal BB, Liu M. Gambogic acid inhibits angiogenesis and prostate tumor growth by suppressing VEGFR2 signaling. Cancer Res. 2008;68:1843–1850. doi: 10.1158/0008-5472.CAN-07-5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker M, Robinson SD, Lechertier T, Barber PR, Tavora B, D’Amico G, Jones DT, Vojnovic B, Hodivala-Dilke K. Use of the mouse aortic ring assay to study angiogenesis. Nat Protoc. 2011;7:89–104. doi: 10.1038/nprot.2011.435. [DOI] [PubMed] [Google Scholar]
- 21.Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nat Rev Cancer. 2005;5:423–435. doi: 10.1038/nrc1628. [DOI] [PubMed] [Google Scholar]
- 22.Newman DJ, Cragg GM. Marine natural products and related compounds in clinical and advanced preclinical trials. J Nat Prod. 2004;67:1216–1238. doi: 10.1021/np040031y. [DOI] [PubMed] [Google Scholar]
- 23.Kim GD, Bae SY, Park HJ, Bae K, Lee SK. Honokiol inhibits vascular vessel formation of mouse embryonic stem cell-derived endothelial cells via the suppression of PECAM and MAPK/mTOR signaling pathway. Cell Physiol Biochem. 2012;30:758–770. doi: 10.1159/000341455. [DOI] [PubMed] [Google Scholar]
- 24.Kim GD, Oh J, Park HJ, Bae K, Lee SK. Magnolol inhibits angiogenesis by regulating ROS-mediated apoptosis and the PI3K/AKT/mTOR signaling pathway in mES/EB-derived endothelial-like cells. Int J Oncol. 2013;43:600–610. doi: 10.3892/ijo.2013.1959. [DOI] [PubMed] [Google Scholar]
- 25.Fotsis T, Pepper MS, Aktas E, Breit S, Rasku S, Adlercreutz H, Wähälä K, Montesano R, Schweigerer L. Flavonoids, dietary-derived inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res. 1997;57:2916–2921. [PubMed] [Google Scholar]
- 26.Cao Y, Cao R, Bråkenhielm E. Antiangiogenic mechanisms of diet-derived polyphenols. J Nutr Biochem. 2002;13:380–390. doi: 10.1016/S0955-2863(02)00204-8. [DOI] [PubMed] [Google Scholar]
- 27.Tosetti F, Ferrari N, De Flora S, Albini A. ‘Angio-prevention’: angiogenesis is a common and key target for cancer chemopreventive agents. FASEB J. 2002;16:2–14. doi: 10.1096/fj.01-0300rev. [DOI] [PubMed] [Google Scholar]
- 28.Dorai T, Aggarwal BB. Role of chemopreventive agents in cancer therapy. Cancer Lett. 2004;215:129–140. doi: 10.1016/j.canlet.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 29.Auerbach R, Lewis R, Shinners B, Kubai L, Akhtar N. Angiogenesis assays: a critical overview. Clin Chem. 2003;49:32–40. doi: 10.1373/49.1.32. [DOI] [PubMed] [Google Scholar]
- 30.Kruger EA, Duray PH, Tsokos MG, Venzon DJ, Libutti SK, Dixon SC, Rudek MA, Pluda J, Allegra C, Figg WD. Endostatin inhibits microvessel formation in the ex vivo rat aortic ring angiogenesis assay. Biochem Biophys Res Commun. 2000;268:183–191. doi: 10.1006/bbrc.1999.2018. [DOI] [PubMed] [Google Scholar]
- 31.Tong YG, Zhang XW, Geng MY, Yue JM, Xin XL, Tian F, Shen X, Tong LJ, Li MH, Zhang C, Li WH, Lin LP, Ding J. Pseudolarix acid B, a new tubulin-binding agent, inhibits angiogenesis by interacting with a novel binding site on tubulin. Mol Pharmacol. 2006;69:1226–1233. doi: 10.1124/mol.105.020537. [DOI] [PubMed] [Google Scholar]
- 32.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 33.Chen SH, Murphy DA, Lassoued W, Thurston G, Feldman MD, Lee WM. Activated STAT3 is a mediator and biomarker of VEGF endothelial activation. Cancer Biol Ther. 2008;7:1994–2003. doi: 10.4161/cbt.7.12.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng Q, Hirose Y, Yoshimi N, Murakami A, Koshimizu K, Ohigashi H, Sakata K, Matsumoto Y, Sayama Y, Mori H. Further investigation of the modifying effect of various chemopreventive agents on apoptosis and cell proliferation in human colon cancer cells. J Cancer Res Clin Oncol. 2002;128:539–546. doi: 10.1007/s00432-002-0373-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cross MJ, Claesson-Welsh L. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci. 2001;22:201–207. doi: 10.1016/S0165-6147(00)01676-X. [DOI] [PubMed] [Google Scholar]
- 36.Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002;90:1243–1250. doi: 10.1161/01.RES.0000022200.71892.9F. [DOI] [PubMed] [Google Scholar]