Abstract
The present study investigated the effects of Vaccinium uliginosum L. (bilberry) on the learning and memory impairments induced by amyloid-β protein (AβP) 1-42. ICR Swiss mice were divided into 4 groups: the control (Aβ40-1A), control with 5% bilberry group (Aβ40-1B), amyloid β protein 1-42 treated group (Aβ1-42A), and Aβ1-42 with 5% bilberry group (Aβ1-42B). The control was treated with amyloid β-protein 40-1 for placebo effect, and Alzheimer’s disease (AD) group was treated with amyloid β-protein 1-42. Amyloid β-protein 1-42 was intracerebroventricular (ICV) micro injected into the hippocampus in 35% acetonitrile and 0.1% trifluoroacetic acid. Although bilberry added groups tended to decrease the finding time of hidden platform, no statistical significance was found. On the other hand, escape latencies of AβP injected mice were extended compared to that of Aβ40-1. In the Probe test, bilberry added Aβ1-42B group showed a significant (P<0.05) increase of probe crossing frequency compared to Aβ1-42A. Administration of amyloid protein (Aβ1-42) decreased working memory compared to Aβ40-1 control group. In passive avoidance test, bilberry significantly (P<0.05) increased the time of staying in the lighted area compared to AD control. The results suggest that bilberry may help to improve memory and learning capability in chemically induced Alzheimer’s disease in experimental animal models.
Keywords: Vaccinium uliginosum L., Alzheimer’s disease, hidden platform, passive avoidance, hippocampus
INTRODUCTION
The improvement of living standards with the development of medical technology has increased life expectancy and the elderly population in the world (1). In addition, degenerative brain disease and senile diseases are also increasing rapidly. Alzheimer’s disease (AD) is the most common cause of dementia accounting for 50 to 75% of all cases (2,3). As a result, short-term memory is often damaged, and judgment, language skills, and learning ability also tend to fall remarkably. AD is a neuropathological disorder characterized by deposition of amyloid β-protein (AβP) containing plaques and intracellular neurofibrillary tangles, and loss of neurons in the brain along with progressive cognitive impairment (4). Behaviorally, many articles have reported that AβP injection or infusion can lead to deficits in a number of learning and memory paradigms including passive avoidance test, radial arm maze spatial modification, and spatial reference memory test conducted in the water maze (5,6).
Vaccinium uliginosum L. (bilberry) is a wild plant, which mainly grows in North Korea’s alpine plateau Baekdu, Mount Seorak, and highlands in South Korea (7), and in some other countries, including China, Japan, Canada, and Finland. Bilberry contains at least 15 different anthocyanosides (myrtllin, malvidin, cyaniding, delphinidine, and others), catechine, tannins (2~10%), and carbohydrates including invertose, organic acid, pectin and alkaloids. Some of these compounds are also antioxidants that appear to enhance collagen cross-linkages, promote collagen synthesis, and inhibit collagen degradation, thereby stabilizing connective tissues, which are stable in boiling water and dry heat for many days (8). Bilberry has antioxidant capacity similar to extracts of green tea, grape seeds and pine bark, as revealed in vivo experiments (9). However, memory-related diseases of bilberry research are still scarce.
Therefore, this study was performed to investigate the effects of bilberry on the learning and memory impairment of chemically induced Alzheimer’s disease.
MATERIALS AND METHODS
Materials
Bilberry (Vaccinium uliginosum L.) produced in North Korea was purchased from Yonbiant (Yonbian, China). Fresh bilberry was freeze-dried at −60°C, powdered and mixed with other diet ingredients.
The moisture content of Vaccinium uliginosum L. (bilberry) was 94% and the rest is composed of carbohydrate, crude protein, lipids, and ash. Contents of Vaccinium uliginosum L. were 4.4, 0.2, 1.25, and 0.2%, respectively. Bilberry contains at least 15 different anthocyanosides (myrtllin, malvidin, cyaniding, delphinidine, and others), catechine, tannins (2~10%), and other components such as invertose, organic acid, pectin, and alkaloids (10,11).
Experimental animals and diet
In this study, forty male ICR Swiss mice weighing about 25 g were purchased from Daehan Biolink Inc. (Seoul, Korea) and randomly divided into 4 groups (10 animals per group): the control (Aβ40-1A), control with 5% bilberry group (Aβ40-1B), amyloid β protein 1-42 treated group (Aβ1-42A) and Aβ1-42 with 5% bilberry group (Aβ1-42B) (Table 1). Five percent of powdered bilberry was included in the experimental diet. Mice were housed in a temperature (22±2°C) and light-controlled room (12-h light cycle starting at 8:00 am) and were fed ad libitum with a powered mixed diet based on AIN-76 formula. Amyloid β protein 1-42 (Sigma Chemical Co., St. Louis, MO, USA) was dissolved in 35% acetonitrile containing 0.1% trifluoroacetic acid and 1.8 μL of the solution was intracerebroventricular (ICV) micro injected into the hippocampus (12). Amyloid β protein 40-1 (Sigma Chemical Co.) was treated in the same way as the control. The animal study was conducted in accordance with the guidelines and approval of the Institutional Animal Care and Use Committees (IACUC) of Hallym University (Hallym-2010-78).
Table 1.
Experimental animal groups and diet
| Group1) | Diet and treatment |
|---|---|
| Aβ40-1A | Basal diet2)+Aβ40-1 |
| Aβ40-1B | Basal diet+Aβ40-1+5% Bilberry |
| Aβ1-42A | Basal diet+Aβ1-42 |
| Aβ1-42B | Basal diet+Aβ1-42+5% Bilberry |
Aβ40-1A, Alzheimer’s disease control group treated with amyloid-β protein 40-1 for placebo effect (1.8 μL ICV microinjection to hippocampus); Aβ40-1B, bilberry added control group; Aβ1-42A, Alzheimer’s disease (AD) group induced by amyloid-β protein1-42 (1.8 μL ICV microinjection to hippocampus); Aβ1-42B, bilberry added AD group.
Mice were allowed to have free access to diet and provided powered mixed diet based on AIN-76 formula including AIN-76 mineral and vitamin mixtures.
Hidden platform test
Morris water maze test (13), with some modification (14), was carried out from days 9 to 16 after the micro injection of AβP. The experimental apparatus consisted of a circular water tank with 140 cm in diameter and 45 cm high. A transparent platform (10 cm in diameter and 25 cm high) was set inside the tank, which was filled to a height of 27 cm with water at 23°C. The surface of the platform was 2 cm below the surface of the water. The pool was located in a large test room, in which there were many clues attached external to the maze (13,15–17). These clues were visible from the pool and could be used by the mice for spatial orientation. The position of the clues remained unchanged throughout the test. The hidden platform was located in a constant position throughout the test period in the middle of one quadrant, equidistant from the center and edge of the pool. In each training session, the latency to escape onto the hidden platform was recorded. If the mice found the platform, they were allowed to remain there for 15 s and were then returned to their home cage. If the mice were unable to find the platform within 90 s, they were put on the platform for 15 s and then the training was terminated. This training was conducted for 5 consecutive days, twice a day, from days 9~13 after the AβP injection.
Probe test
A probe trial in order to assess memory consolidation was performed 24 h after the water maze tests. In this trial, the platform was removed from the tank, and the mice were allowed to swim freely. For these tests, percentage time in the target quadrant and target site crossings within 120 s was recorded. The time spent in the target quadrant is taken to indicate the degree of memory consolidation that has taken place after learning. Swimming pattern of each mouse was monitored by a camera above the center of the pool connected to a SMART-LD program (13). For the probe trials, an annulus-crossing index was calculated to represent the number of passes over the platform site, minus the mean of passes over alternative sites in other quadrants. The index expresses the spatial place preference and controls for alternative search strategies without place preferences, such as circular search paths (18,19).
Working memory (repeated acquisition) test
The working memory test was similar to the standard training of water maze except that the platform location was changed in each session. As the platform was changed daily, this test could evaluate the working memory component (20). For each trial, the mouse was put into the pool at the starting position. The first trial of each session was an informative sample trial in which the mouse was allowed to swim to the platform in its new location and to remain there for 15 s. The mouse was then placed in the home cage for an intertrial interval of 1 min. Spatial working memory was assessed as the mean performance in the second trial of 3 consecutive days, 14 to 16 days after the start of AβP injection.
Passive avoidance test
The passive avoidance test is also widely accepted as a simple and rapid test method for measuring memory capacity (21). Four days after Aβ (1-42) injection, mice were evaluated by one trial passive avoidance test using Gemini avoidance system (San Diego Instruments, San Diego, CA, USA). The passive avoidance box consisted of two compartments (53×53×32 cm high), one illuminated, and one dark, both equipped with a grid floor. On the acquisition trial, each mouse was placed in the illuminated compartment. After the 120 s of habituation period, the guillotine floor was opened, and the initial latency to enter the dark compartment was recorded. The mice were placed in the illuminated compartment facing away from the dark compartment. After 30 min, when the mice moved completely into the dark compartment, they received an electric shock (0.25 mÅ, 3 s duration). Then, the mice were returned to their home case.
One day later, the mice were placed in the illuminated compartment, and the latency period to enter the dark compartment defined as “retention” was measured (22). The time when the mice entered into the dark compartment was recorded and described as step-through latency. The retention trials were set at a limit of 300 s of cut-off time.
Statistics
Data from individual experiments were expressed as the means±standard error of means (SEM). Statistical analysis (one-way ANOVA) was performed by statistical analysis system program (SAS Institute, Cary, NC, USA) and Duncan’s multiple range test at P<0.05.
RESULTS AND DISCUSSION
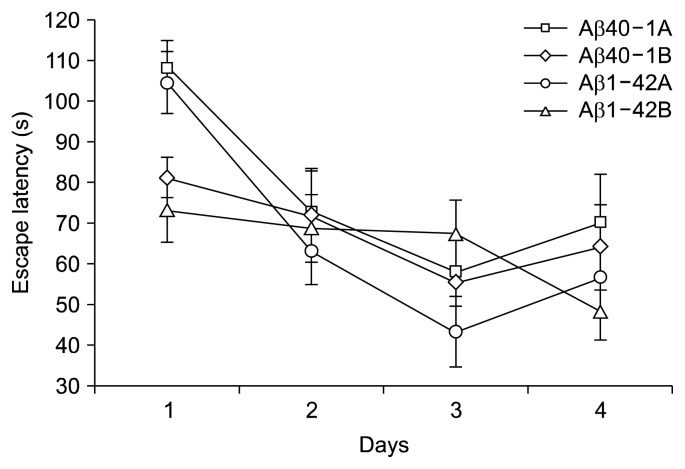
Hidden platform test with modification of Morris maze test was performed to evaluate hippocampus-dependent memory changes in escape latency onto the hidden platform (Fig. 1). The test was performed on 4 consecutive days, 5 times a day. On the first day, platform finding time of all the groups was not different from each other. On the 2nd day, Aβ40-1B showed 8.2 s faster than the control 79.9 s. On the 3rd and 4th day, bilberry added groups tended to decrease the finding time, but no statistical significance was found. On the other hand, escape latencies of AβP injected mice were extended compared to that of Aβ40-1. However, bilberry tended to shorten the time of finding the platform compared to the control of Aβ40-1 and Aβ1-42. Jin et al. (23) observed increasing neuronal survival and enhancing memory in Alzheimer’s disease model rats when Cordyceps militaris L. extracts were administered (24).
Fig. 1.
Effect of the bilberry on the escape latency in the hidden platform test of amyloid β-protein 1-42 induced memory impaired mice. Data represent means±SEM (n=10). No statistical difference was found.
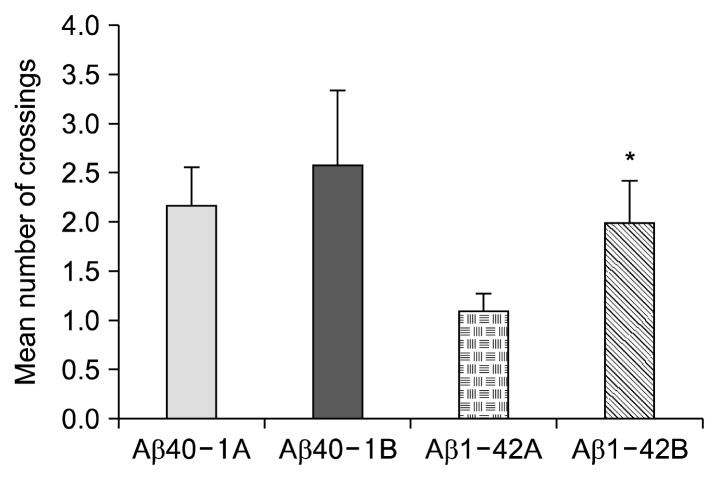
In addition to hidden platform test, probe test was performed to assess long-term memory. Finding the hidden platform of the mice was averaged 1.7 times during 120 s in the control Aβ40-1 group while it was averaged 2.6 times in the bilberry administered Aβ40-1B group, which was a 52.9% increase (Fig. 2).
Fig. 2.
Effect of bilberry in the probe test. Probe crossing frequency was significantly (P<0.05) increased by bilberry administration in AD group. Amyloid β-protein 1-42 induced memory deficit mice showed dramatic decrease of the probe crossing frequency compared to Aβ40-1A. Data represent means±SEM (n=10). *P<0.05 as compared to the control group.
Bilberry administered AD mice found an average of 2.0 times during 120 seconds while the control Aβ1-42 found only an average of 1.1 times, which was a significant increase (P<0.05) of 81.8%. Amyloid β-protein 1-42 induced memory deficit mice showed a dramatic decrease in the probe crossing frequency compared to Aβ40-1A. The result suggests that bilberry has an effect on the improvement of long-term memory in mice.
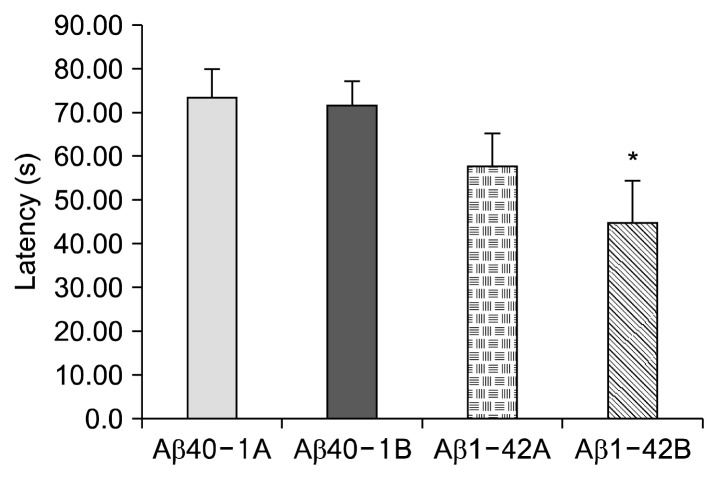
The results of the working memory test are shown in Fig. 3. The average platform finding time was 57.5 s in the Aβ40-1B control group, while the bilberry group showed 44.9 s, which was 22% lower than the control. Although bilberry shortened the time of finding the platform, there was no statistical significance. AD mice were low in working memory compared to the control Aβ40-1A mice. Similar results were reported by Joseph et al. (25) that when bilberry-kinds of blueberries were administered, a significant improvement of working memory was observed. According to Ramirez et al. (26), short-term memory was significantly improved by bilberry (Vaccinium bilberry) administration in rats, but long-term memory was not influenced.
Fig. 3.
Effect of the bilberry on amyloid β-protein 1-42 induced memory deficits in the working memory test in mice. Data represent means±SEM (n=10). *P<0.05 as compared to the control group.
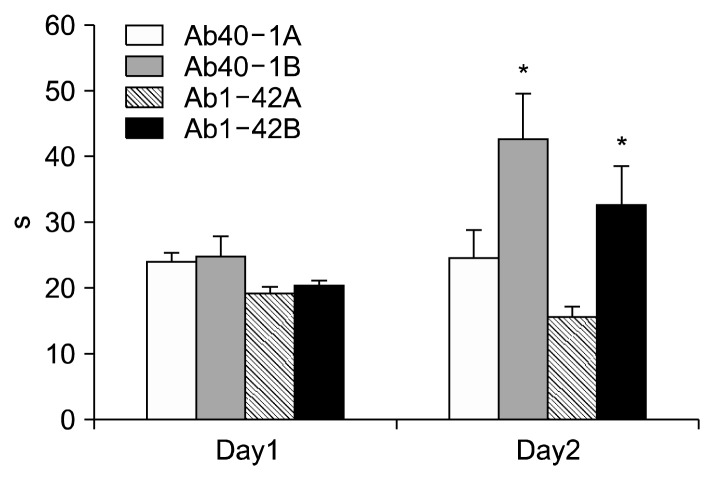
The effects of damaging the memory by the injection of Aβ1-42 were also confirmed by passive avoidance test (Fig. 4). AβP injection in the group of Aβ1-42 revealed a significant (P<0.05) decrease on the time of entering the dark room compared to Aβ40-1. On the first day, the difference between each group was not significant. However, on the second day, dark room entering time of the control was averaged at 24.6 s and that of Aβ40-1B, Aβ1-42A, and Aβ1-42B was 42.6, 15.6, and 32.5 s, respectively.
Fig. 4.
Effect of the bilberry on amyloid β-protein 1-42 induced memory deficits in the passive avoidance test in mice. Data represent means±SEM (n=10). *P<0.05 as compared to the control group.
Bilberry has significantly increased (P<0.05) the time of staying in the lighted room compared to Aβ40-1 and Aβ1-42. Bilberry in AD group particularly has shown to enhance hippocampus-dependent memory by 108.3% (P<0.05).
The results indicate that bilberry may improve long term memory of electric shock and learning capability. These findings suggest that the brain mechanisms involved in spatial learning may be different from those in passive avoidance, and that bilberry may play a role as one of the neurotransmitters related to the impairment of ICR Swiss mice in the passive avoidance test (27–29). In conclusion, the overall results in this study suggest that bilberry may help to protect from the risk of chemically induced memory impairments in experimental animal models. Further studies are needed to evaluate the efficacy of bilberry for human applications in the impairment of memory related diseases.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Heomz A. Alzheimer disease: a status report for 2002. American Council on Science and Health; New York, NY, USA: 2002. [Google Scholar]
- 2.Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 3.Ferri CP, Sousa R, Albanese E, Ribeirom WS, Honyashiki M. In: World Alzheimer report 2009–executive summary. Prince M, Jackson J, editors. Alzheimer’s Disease International; London, UK: 2009. pp. 1–22. [Google Scholar]
- 4.Hardy J. A hundred years of Alzheimer’s disease research. Neuron. 2006;52:3–13. doi: 10.1016/j.neuron.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura S, Murayama N, Noshita T, Annoura H, Ohno T. Progressive brain dysfunction following intracerebroventricular infusion of beta1-42-amyloid peptide. Brain Res. 2001;912:128–136. doi: 10.1016/S0006-8993(01)02704-4. [DOI] [PubMed] [Google Scholar]
- 6.Tang G, Zhang M, Jiang S, Wu X, Jin T, Gu N, Lin S, Qian Y, Wang D, Wang H. Presinilin 1 gene polymorphism and Alzheimer’s disease in Chinese. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 1999;16:1–4. [PubMed] [Google Scholar]
- 7.Chung YH, Hyun JO. Monographic study of the endemic plants in Korea XI. Taxonomy and interspecific relationships of the genus Vaccinium. Korean J Environ Biol. 1989;7:1–17. [Google Scholar]
- 8.Jonadet M, Meunier MT, Bastide J, Bastide P. Anthocyanosides extracted from Vitis vinifera, Vaccinium myrtillus and Pinus maritimus. I. Elastase-inhibiting activities in vitro. II. Compared angioprotective activities in vivo. J Pharm Belg. 1983;38:41–46. [PubMed] [Google Scholar]
- 9.Pietta P, Simonetti P, Mauri P. Antioxidant activity of selected medicinal plant. J Agric Food Chem. 1998;46:4487–4490. doi: 10.1021/jf980310p. [DOI] [Google Scholar]
- 10.Foster S. Bilberry: food and medicine. The Herb Companion. 1997:68–69. [Google Scholar]
- 11.Häkkinen SH, Kärenlampi SO, Heinonen IM, Mykkänen HM, Törrönen AR. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J Agric Food Chem. 1999;47:2274–2279. doi: 10.1021/jf9811065. [DOI] [PubMed] [Google Scholar]
- 12.Laursen SE, Belknap JK. Intracerebroventricular injections in mice. Some methodological refinements. J Pharmacol Methods. 1986;16:355–357. doi: 10.1016/0160-5402(86)90038-0. [DOI] [PubMed] [Google Scholar]
- 13.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 14.Nitta A, Itoh A, Hasegawa T, Nabeshima T. β-Amyloid protein-induced Alzheimer’s disease animal model. Neurosci Lett. 1994;170:63–66. doi: 10.1016/0304-3940(94)90239-9. [DOI] [PubMed] [Google Scholar]
- 15.Kee N, Teixeira CM, Wang AH, Frankland PW. Imaging activation of adult-generated granule cells in spatial memory. Nat Protoc. 2007;2:3033–3044. doi: 10.1038/nprot.2007.415. [DOI] [PubMed] [Google Scholar]
- 16.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolfer DP, Stagljar-Bozicevic M, Errington ML, Lipp HP. Spatial memory and learning in transgenic mice: fact or artifact? News Physiol Sci. 1998;13:118–123. doi: 10.1152/physiologyonline.1998.13.3.118. [DOI] [PubMed] [Google Scholar]
- 18.Gass P, Wolfer DP, Balschun D, Rudolph D, Frey U, Lipp HP, Schütz G. Deficits in memory tasks of mice with creb mutations depend on gene dosage. Learn Mem. 1998;5:274–288. [PMC free article] [PubMed] [Google Scholar]
- 19.Wehner JM, Sleight S, Upchurch M. Hippocampal protein kinase C activity is reduced in poor spatial learners. Brain Res. 1990;523:181–187. doi: 10.1016/0006-8993(90)91485-Y. [DOI] [PubMed] [Google Scholar]
- 20.Morris RG, Schenk F, Tweedie F, Jarrard LE. Ibotenate lesions of hippocampus and/or subiculum: dissociating components of allocentric spatial learning. Eur J Neurosci. 1990;2:1016–1028. doi: 10.1111/j.1460-9568.1990.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 21.LeDoux JE. Emotional memory systems in the brain. Behav Brain Res. 1993;58:69–79. doi: 10.1016/0166-4328(93)90091-4. [DOI] [PubMed] [Google Scholar]
- 22.LeDoux JE. Emotional memory: in search of systems and synapses. Ann NY Acad Sci. 1993;702:149–157. doi: 10.1111/j.1749-6632.1993.tb17246.x. [DOI] [PubMed] [Google Scholar]
- 23.Jin DQ, Park BC, Lee JS, Choi HD, Lee YS, Yang JH, Kim JA. Mycelial extract of Cordyceps ophioglossoides prevents neuronal cell death and ameliorates β-amyloid peptide-induced memory deficits in rats. Biol Pharm Bull. 2004;27:1126–1129. doi: 10.1248/bpb.27.1126. [DOI] [PubMed] [Google Scholar]
- 24.Lee BB, Park JB, Park JH, Shin HJ, Kwon SO, Yeom MJ, Sur BJ, Kim SH, Kim MH, Lee HJ, Yoon SH, Hahm DH. Cordyceps militaris improves neurite outgrowth in Neuro2A cells and reverses memory impairment in rats. Food Sci Biotechnol. 2011;20:1599–1608. doi: 10.1007/s10068-011-0221-4. [DOI] [Google Scholar]
- 25.Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, Bickford PC. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci. 1999;19:8114–8121. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramirez MR, Izquierdo I, do Carmo Bassols Raseira M, Zuanazzi JA, Barros D, Henriques AT. Effect of lyophilised Vaccinium berries on memory, anxiety and locomotion in adult rats. Pharmacol Res. 2005;52:457–462. doi: 10.1016/j.phrs.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Ikegami S, Shumiya S, Kawamura H. Age-related changes in radial-arm maze learning and basal forebrain cholinergic systems in senescence accelerated mice (SAM) Behav Brain Res. 1992;51:15–22. doi: 10.1016/S0166-4328(05)80307-9. [DOI] [PubMed] [Google Scholar]
- 28.Meguro K, Yanai K, Yokoyama H, Sakurai A, Maeyama K, Watanabe T, Matsuzawa T, Yanai K, Yokoyama H, Sakurai A, Maeyama K, Watanabe T, Matsuzawa T. Neurochemical studies on central histaminergic neuron system of senescence accelerated mice. Biogenic Amines. 1992;8:299–307. [Google Scholar]
- 29.Yamaguchi Y, Kawashima S. Effects of amyloid-β-(25–35) on passive avoidance, radial-arm maze learning and choline acetyltransferase activity in the rat. Eur J Pharmacol. 2001;412:265–272. doi: 10.1016/S0014-2999(01)00730-0. [DOI] [PubMed] [Google Scholar]