Abstract
Black rice contains many biologically active compounds. The aim of this study was to investigate the protective effects of black rice extracts (whole grain extract, WGE and rice bran extract, RBE) on tert-butyl hydroperoxide (TBHP)-induced oxidative injury in HepG2 cells. Cellular reactive oxygen species (ROS), antioxidant enzyme activities, malondialdehyde (MDA) and glutathione (GSH) concentrations were evaluated as biomarkers of cellular oxidative status. Cells pretreated with 50 and 100 μg/mL of WGE or RBE were more resistant to oxidative stress in a dose-dependent manner. The highest WGE and BRE concentrations enhanced GSH concentrations and modulated antioxidant enzyme activities (glutathione reductase, glutathione-S-transferase, catalase, and superoxide dismutase) compared to TBHP-treated cells. Cells treated with RBE showed higher protective effect compared to cells treated with WGE against oxidative insult. Black rice extracts attenuated oxidative insult by inhibiting cellular ROS and MDA increase and by modulating antioxidant enzyme activities in HepG2 cells.
Keywords: black rice, oxidative stress, antioxidant enzyme, glutathione, HepG2 cell
INTRODUCTION
Oxidative stress, which can induce cell aging and chronic degenerative diseases, is caused by high levels of reactive oxygen species (ROS) (1). Cells have non-enzymatic and enzymatic defense mechanisms to protect against oxidative stress (2). However, antioxidant systems cannot completely prevent the deleterious effects of ROS (3).
Antioxidant compounds protect against oxidative damage caused by oxidative stress (4). Consumption of natural antioxidants has recently increased due to safety concerns associated with synthetic antioxidants and chemical pharmaceuticals. Among common dietary sources of natural antioxidants, dark-colored plants are richest in phenolic compounds, vitamins, and other phytochemicals (5). Black rice (Oryza sativa L.), which has dark purple grains, is an anthocyanin-rich rice that may improve health. Several studies have reported that black rice reduces oxidative stress and improves atherosclerotic lesions in animal models (6–8).
The objective of this study was to investigate the protective effects of black rice extracts on tert-butyl hydroperoxide (TBHP)-induced oxidative injury in HepG2 cells. Generation of cellular ROS, malondialdehyde and glutathione concentrations, and antioxidant enzyme activities were measured as biomarkers of cellular oxidative status.
MATERIALS AND METHODS
Materials
HepG2 cells were obtained from the Korean Collection for Type Cultures (Daejeon, Korea). Thiobarbituric acid (TBA), 2′,7′-dichlorofluorescin diacetate (DCFH-DA), tert-butyl hydroperoxide (TBHP), trichloroacetic acid, reduced glutathione (GSH), oxidized glutathione, glutathione reductase (GR), peroxidase, 1-chloro-2,4-dinitrobenzene (CDNB), hydrogen peroxide (H2O2), ethylenediaminetetra-acetic acid (EDTA), 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), xanthine, xanthine oxidase, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). The WST-1 (4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate sodium salt) reagent was obtained from Roche Diagnostics Deutschland GmbH (Mannheim, Germany), and fetal bovine serum (FBS), Dulbecco’s modified Eagle’s medium (DMEM), trypsin-EDTA, and penicillin-streptomycin were obtained from Gibco BRL (Gaithersburg, MD, USA). All other reagents and solvents used were of analytical and HPLC grade.
Preparation of black rice extracts
Black rice was obtained from the Rural Development Administration, Korea. Whole black rice was milled into rice bran using the gradual milling system (85% milling degree). Both whole grain rice and rice bran were extracted with methanol by shaking overnight at room temperature. After centrifugation (10,000 g, 5 min), the supernatant was filtered and evaporated under vacuum at 40°C. Whole grain extract (WGE) and rice bran extract (RBE) were dissolved in dimethyl sulfoxide (DMSO) and filtered through sterile 0.2 μm cellulose acetate syringe filters. WGE and RBE were diluted with FBS-free DMEM to obtain the needed sample concentrations.
Cell culture
HepG2 cells were grown in DMEM supplemented with 10% FBS and 100 U/mL each of penicillin and streptomycin. Cells were maintained in a humidified atmosphere of 5% CO2 and 95% air at 37°C.
Cell viability and protective effect of WGE and RBE
HepG2 cells were seeded into 96-well plates at a density of 1×104 cells/well. After 24 h, the growing medium was exchanged with FBS-free DMEM containing different concentrations of WGE and RBE (50 and 100 μg/mL). To evaluate the protective effect of black rice after 12 h, cells were treated with 1 mM TBHP for 2 h. The protective effect against oxidative stress was measured using the MTT assay. MTT was added to all wells and the plates were incubated at 37°C for 4 h. The medium was removed and formazan crystals produced in the wells were dissolved in DMSO. Absorbance was read at 550 nm using a spectrophotometer (Beckman Instruments Inc., Brea, CA, USA).
Determination of ROS
Cellular ROS levels were measured using a DCFH-DA fluorescent probe (9). HepG2 cells were seeded into 96-well black plates at a density of 5×104 cells/well. After sample treatment, 25 μM DCFH was added to the wells for 2 h. Cells were washed twice with FBS-free medium before the addition of 1 mM TBHP. The fluorescence intensity was measured with a fluorescence spectrophotometer (Perkin-Elmer, Norwalk, CT, USA) for 90 min at an excitation wavelength of 485 nm and an emission wavelength of 530 nm.
Determination of lipid peroxidation
Cells were seeded into 6-well plates at a density of 1×106 cells/well. After 48 h, the culture medium was replaced with FBS-free medium containing 50 and 100 μg/mL of WGE or RBE. After 12 h, the cells were treated for 2 h with 1 mM TBHP to induce oxidative stress. After treatment, the cells were harvested and lysed for 10 s using a Vibra-Cell VCX 750 sonicator (Sonics & Materials, Inc., Newtown, CT, USA). The lysates were centrifuged at 10,000 g for 5 min at 4°C. The supernatants were used immediately for protein determination and lipid peroxidation assays. Lipid peroxidation (estimated as MDA content) was determined using the thiobarbituric acid reacting substances (TBARS) assay (10). The supernatants were added to an equal volume of TBA solution (15% trichloroacetic acid, 0.25 N HCl, and 0.375% TBA) and heated for 15 min in a boiling water bath. After centrifugation at 10,000 g for 5 min, the absorbance was measured at 535 nm. The results were expressed as nmol/mg of protein using a molar extinction coefficient of 1.56×105 M−1cm−1.
Determination of glutathione and antioxidant enzyme activities
Total intracellular GSH was determined by the DTNB-GSSG reductase recycling method described by Baker et al. (11). The concentration of GSH in cell lysate was expressed as nmol GSH/mg of protein. Glutathione-S-transferase (GST) activity was assayed at 340 nm using the catalytic reaction of reduced GSH and CDNB as the substrates producing a dinitrophenyl thioether chromphere. Results were expressed as nmol/min/mg protein (12). CAT activity was determined by measuring the reduction rate of H2O2 using a spectrophotometer at 240 nm and was expressed as μmol/min/mg protein (13). GR activity was determined by measuring the decrease of absorbance at 340 nm due to the consumption of NADPH in the reduction of oxidized glutathione and was expressed as nmol/min/mg protein (14). SOD activity was measured at 450 nm using WST-1 that produces a water-soluble formazan dye. The formazan was formed by the reaction between WST-1 and superoxide anion to detect the superoxide anion generated by xanthine and xanthine oxidase. The results were expressed as U/mg protein (15).
Statistics
The results were expressed as mean±standard deviation (SD). Statistical analysis of data was done by employing unpaired two-tailed student t-test and one-way analysis of variance (ANOVA) using SAS version 9.1 (SAS Institute, Cary, NC, USA). A value of P<0.05 was considered significant.
RESULTS AND DISCUSSION
Protective effect of WGE and RBE on TBHP-induced cytotoxicity
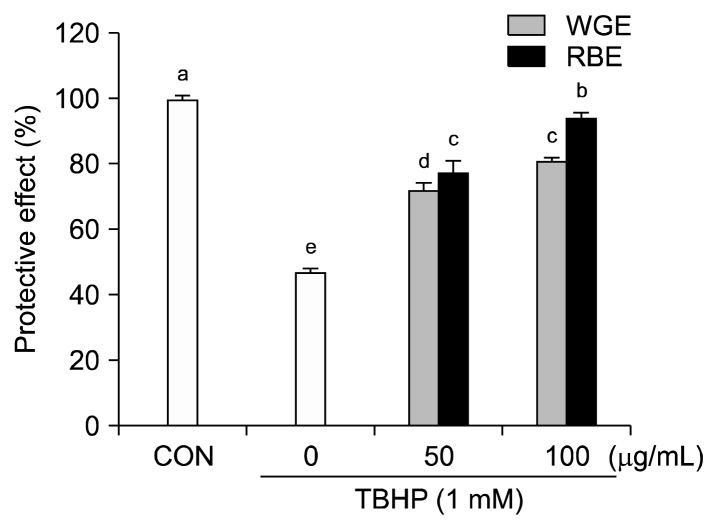
Before determining the cytoprotective effect of WGE and RBE, it was necessary to ensure that there was no cytotoxicity. Exposure to 50 and 100 μg/mL WGE and RBE over a 12 h incubation period did not alter cell viability compared to the control (data not shown). The cytoprotective effect of WGE and RBE against TBHP-induced oxidative damage was evaluated. HepG2 cells were treated with different concentrations of WGE and RBE (50 and 100 μg/mL) for 12 h and the cells were exposed to TBHP (1 mM) for 2 h. Exposure to TBHP induced a 50% decrease in cell viability (Fig. 1). However, pretreatment with 50 and 100 μg/mL WGE and RBE for 12 h significantly increased cell viability in a dose-dependent manner, indicating that pretreated cells were protected against oxidative injury. Black rice derives its name from its anthocyanin compounds, including cyaniding 3-glucoside and peonidin 3-glucoside, which possess anti-oxidant and anti-inflammatory activities (16). Black rice contains other beneficial components, including polyphenolics, flavonoids, vitamin E, phytic acid, and γ-oryzanol. These antioxidants are present in much higher concentrations in the bran than in the endosperm (17). In this study, the cytoprotective effect of RBE was significantly higher than that of WGE.
Fig. 1.
Protective effect of black rice extracts on TBHP-induced cytotoxicity in HepG2 cells. Values are mean±SD of at least three separate experiments. Different letters above bars indicate significant differences (by ANOVA and Duncan’s test, P<0.05).
Effect of black rice extracts on ROS generation and lipid peroxidation
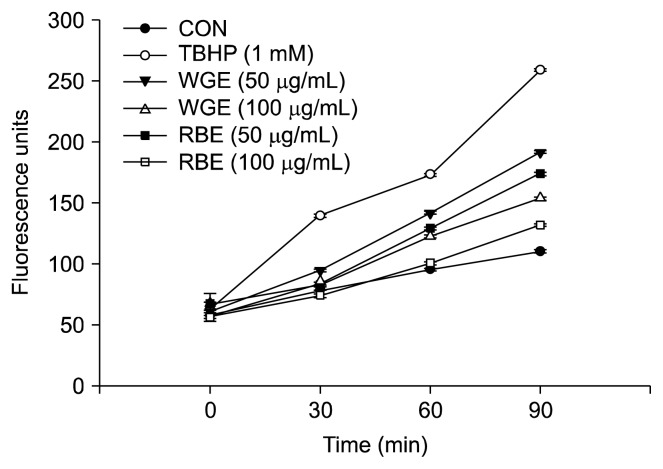
To evaluate the cellular oxidative stress generated from TBHP, the intracellular ROS production was measured for 90 min. Cells treated with TBHP showed a significant increase in ROS generation over time compared to untreated controls (Fig. 2). However, pretreatment of HepG2 cells with 50 and 100 μg/mL of WGE and RBE significantly reduced ROS generation in the presence of TBHP. Remarkably, pretreatment with 100 μg/mL of RBE significantly reduced ROS generation to near control values. Evaluation of ROS levels is a good indicator of oxidative insult to living cells (9).
Fig. 2.
Effect of black rice extracts on intracellular reactive oxygen species (ROS) generation in HepG2 cells. Values are mean± SD of at least three separate experiments.
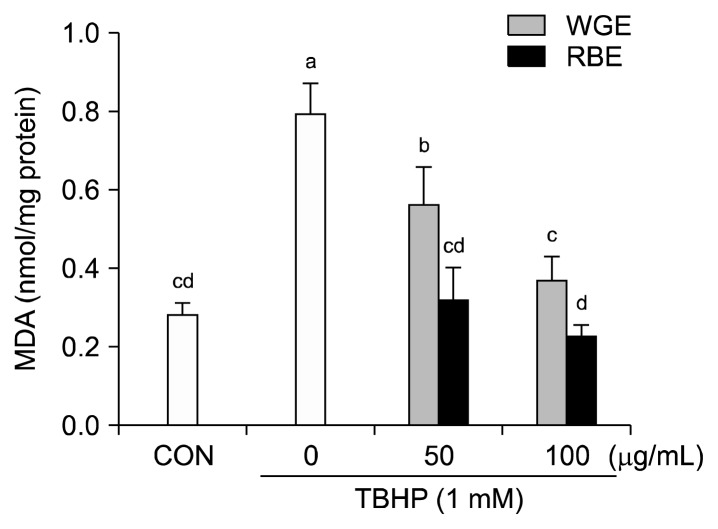
Intracellular MDA concentration is a direct result of membrane unsaturated fatty acid peroxidation and was used as a marker for TBHP-induced oxidative insult in HepG2 cells. Treatment of HepG2 cells with 1 mM TBHP for 3 h resulted in a significant increase in MDA concentration. However, a 6 h pretreatment with the samples prevented the increase in MDA concentration (Fig. 3). Pretreatment with WGE (100 μg/mL) and RBE (50 and 100 μg/mL) completely inhibited lipid peroxidation. Accumulation of lipid peroxides in cell membranes is associated with increased oxidative stress (18). Hou et al. (19) reported that black rice extracts had a protective effect on alcohol-induced hepatic lipid peroxidation in rat liver.
Fig. 3.
Effect of black rice extracts on TBHP-induced lipid peroxidation. Values are mean±SD of at least three separate experiments. Different letters above bars indicate significant differences (by ANOVA and Duncan’s test, P<0.05).
Effect of black rice extract on GSH depletion and anti-oxidant enzyme activities
GSH is widely used as an index of intracellular non-enzymatic antioxidant defense. Therefore, the effect of 100 μg/mL of WGE and RBE on GSH levels in the HepG2 cells was evaluated (Table 1). Treatment with 1 mM TBHP induced a remarkable decrease in GSH levels compared to the control (47.02 and 27.75 μmol/mg protein, respectively). However, pretreatment with WGE and RBE for 6 h increased GSH levels. Pretreatment with 100 μg/mL of WGE prevented GSH depletion to near control levels. Pretreatment with 100 μg/mL of RBE resulted in significantly higher GSH levels. Whether WGE and RBE prevented TBHP-induced damage by modifying antioxidant enzyme activity was determined. Cells were pretreated with 100 μg/mL of WGE and RBE for 12 h before inducing TBHP oxidative stress (Table 1). In control cells, treatment with TBHP for 3 h induced a significant increase in the enzyme activities of GST, CAT, and SOD except GR. However, when cells were pre-treated for 12 h with 100 μg/mL of WGE and RBE, the TBHP-induced increase in enzyme activities of GST, CAT, and SOD was suppressed.
Table 1.
Effect of black rice extracts on reduced GSH and antioxidant enzyme activities in HepG2 cell
| Treatment | GSH | GR | GST | CAT | SOD |
|---|---|---|---|---|---|
| Control | 47.02±9.06b | 2.72±0.19a | 4.44±0.20b | 1.49±0.22b | 15.35±3.74b |
| TBHP | 27.75±0.76c | 4.38±0.92a | 12.89±2.58a | 2.84±0.72a | 26.87±2.79a |
| Whole grain 100+TBHP | 51.55±3.96b | 3.59±1.62a | 6.15±0.92b | 1.27±0.37b | 15.71±3.39b |
| Rice bran 100+TBHP | 71.85±8.01a | 3.20±0.29a | 5.71±0.68b | 1.16±0.38b | 5.11±0.15c |
Glutathione, GSH (μmol/mg protein); glutathione reductase, GR (nmol/min/mg protein); glutathione S-transferase, GST (nmol/min/mg protein); catalase, CAT (μmol/min/mg protein); superoxide dismutase, SOD (U/mg protein). Values are mean±SD of at least three separate experiments. Different letters in the same column indicate significant differences (by ANOVA and Duncan’s test, P<0.05).
The cellular antioxidant enzyme system plays an important role in the defense against oxidative stress, and changes in the activity of antioxidant enzymes can be used as biomarkers for the antioxidant response (20). Various dietary phytochemicals have been studied, and significant changes in antioxidant enzyme activities have been reported. For example, a tocotrienol-rich fraction from grape seeds protected against cell damage by preventing increased activities of antioxidant enzymes induced by TBHP (21). Further, Kim et al. (22) reported that procyanidin fractions from defatted grape seeds prevented TBHP-induced increase in antioxidant enzyme activities, including glutathione peroxidase, CAT, and SOD. The changes in GR, GST, CAT, and SOD activities by pretreatment with black rice extracts (WGE and RBE) compared to treatment with TBHP alone, clearly indicate the ability of the cell defense system to respond to an oxidative insult. Although major bioactive components of black rice were not measured in this study, previous studies demonstrated the presence of anthocyanins, in particular, cyanidin-3-O-glucoside and peonidin-3-O-glycoside (16,23,24). These are most abundant in the aleurone layer of black rice. Park et al. (25) reported that anthocyanins in black rice included cyanidin-3-O-glucoside (95%) and peonidin-3-O-glycoside (5%) and had strong antioxidant activities in vitro. Therefore, we hypothesized that anthocyanins such as cyanidin-3-O-glycosid and peonidin-3-O-glycoside might be the major components responsible for the protective effects in the present study. However, it cannot be excluded that flavonoids or phenolic acids in the extracts may partially contribute to the protective effects observed in this study.
In conclusion, our data showed that treatments with black rice extracts have a protective effect against oxidative stress by modulating ROS production, GSH level, MDA generation, and antioxidant enzyme activities in HepG2 cells. Black rice may be used as a bioactive ingredient in the production and development of functional foods.
ACKNOWLEDGEMENTS
This study was supported by a grant (PJ00959305) from The Rural Development Administration, Korea.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Willcox K, Ash SL, Catignani GL. Antioxidants and prevention of chronic disease. Crit Rev Food Sci Nutr. 2004;44:275–295. doi: 10.1080/10408690490468489. [DOI] [PubMed] [Google Scholar]
- 2.Gaté L, Paul J, Ba GN, Tew KD, Tapiero H. Oxidative stress induced in pathologies; the role of antioxidants. Biomed Pharmacother. 1999;53:169–180. doi: 10.1016/S0753-3322(99)80086-9. [DOI] [PubMed] [Google Scholar]
- 3.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Bramley PM. Is lycopene beneficial to human health? Phytochemistry. 2000;54:233–236. doi: 10.1016/S0031-9422(00)00103-5. [DOI] [PubMed] [Google Scholar]
- 5.Clifford MN. Anthocyanins–nature, occurrence and dietary burden. J Sci Food Agric. 2000;80:1063–1072. doi: 10.1002/(SICI)1097-0010(20000515)80:7<1063::AID-JSFA605>3.0.CO;2-Q. [DOI] [Google Scholar]
- 6.Ling WH, Wang LL, Ma J. Supplementation of the black rice outer layer fraction to rabbits decreases atherosclerotic plaque formation and increases antioxidant status. J Nutr. 2002;132:20–26. doi: 10.1093/jn/132.1.20. [DOI] [PubMed] [Google Scholar]
- 7.Xia M, Ling WH, Ma J, Kitts DD, Zawistowski J. Supplementation of diets with the black rice pigment fraction attenuates atherosclerotic plaque formation in apolipoprotein E deficient mice. J Nutr. 2003;133:744–751. doi: 10.1093/jn/133.3.744. [DOI] [PubMed] [Google Scholar]
- 8.Chiang AN, Wu HL, Yeh HI, Chu CS, Lin HC, Lee WC. Antioxidant effects of black rice extract through the induction of superoxide dismutase and catalase activities. Lipids. 2006;41:797–803. doi: 10.1007/s11745-006-5033-6. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999;27:612–616. doi: 10.1016/S0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- 10.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/S0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 11.Baker MA, Cerniglia GJ, Zaman A. Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Anal Biochem. 1990;190:360–365. doi: 10.1016/0003-2697(90)90208-Q. [DOI] [PubMed] [Google Scholar]
- 12.Habig WH, Pabst MJ, Jakoby WB. Glutathione-S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 13.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 14.Smith IK, Vierheller TL, Thorne CA. Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis(2-nitrobenzoic acid) Anal Biochem. 1988;408:408–413. doi: 10.1016/0003-2697(88)90564-7. [DOI] [PubMed] [Google Scholar]
- 15.Ukeda H, Kawana D, Maeda S, Sawamura M. Spectrophotometric assay for superoxide dismutase based on the reduction of highly water-soluble tetrazolium salts by xanthine-xanthine oxidase. Biosci Biotechnol Biochem. 1999;63:485–488. doi: 10.1271/bbb.63.485. [DOI] [PubMed] [Google Scholar]
- 16.Hu C, Zawistowski J, Ling W, Kitt DD. Black rice (Oryza sativa L. indica) pigmented fraction suppresses both reactive oxygen species and nitric oxide in chemical and biological model systems. J Agric Food Chem. 2003;51:5271–5277. doi: 10.1021/jf034466n. [DOI] [PubMed] [Google Scholar]
- 17.Kong S, Lee J. Antioxidants in milling fractions of black rice cultivars. Food Chem. 2010;120:278–281. doi: 10.1016/j.foodchem.2009.09.089. [DOI] [Google Scholar]
- 18.Lieber CS. Alcohol and the liver: metabolism of alcohol and its role in hepatic and extrahepatic diseases. Mt Sinai J Med. 2000;67:84–94. [PubMed] [Google Scholar]
- 19.Hou Z, Qin P, Ren G. Effect of anthocyanin-rich extract from black rice (Oryza sativa L. Japonica) on chronically alcohol-induced liver damage in rats. J Agric Food Chem. 2010;58:3191–3196. doi: 10.1021/jf904407x. [DOI] [PubMed] [Google Scholar]
- 20.Alía M, Ramos S, Mateos R, Granado-Serrano AB, Bravo L, Goya L. Quercetin protects human hepatoma HepG2 against oxidative stress induced by tert-butyl hydroperoxide. Toxicol Appl Pharmacol. 2006;212:110–118. doi: 10.1016/j.taap.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Choi Y, Lee SM, Kim Y, Yoon J, Jeong HS, Lee J. A tocotrienol-rich fraction from grape seeds inhibits oxidative stress induced by tert-butyl hydroperoxide in HepG2 cells. J Med Food. 2010;13:1240–1246. doi: 10.1089/jmf.2009.1342. [DOI] [PubMed] [Google Scholar]
- 22.Kim Y, Choi Y, Ham H, Jeong HS, Lee J. Protective effects of oligomeric and polymeric procyanidin fractions from defatted grape seeds on tert-butyl hydroperoxide induced oxidative damage in HepG2 cells. Food Chem. 2013;137:136–141. doi: 10.1016/j.foodchem.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Ryu SN, Park SZ, Ho CT. High performance liquid chromatographic determination of anthocyanin pigments in some varieties of black rice. J Food Drug Anal. 1998;6:729–736. [Google Scholar]
- 24.Lee JH. Identification and quantification of anthocyanins from the grains of black rice (Oryza sativa L.) varieties. Food Sci Biotechnol. 2010;19:391–397. doi: 10.1007/s10068-010-0055-5. [DOI] [Google Scholar]
- 25.Park YS, Kim SJ, Chang HI. Isolation of anthocyanin from black rice (Heugjinjubyeo) and screening of its antioxidant activities. Kor J Microbiol Biotechnol. 2008;36:55–60. [Google Scholar]