Abstract
Cadmium (Cd) is a toxic metal, targeting the lung, liver, kidney, and testes following acute intoxication, and causing nephrotoxicity, immunotoxicity, osteotoxicity and tumors after prolonged exposures. Reactive oxygen species (ROS) are often implicated in Cd toxicology. This minireview focused on direct evidence for the generation of free radicals in intact animals following acute Cd overload and discussed the association of ROS in chronic Cd toxicity and carcinogenesis. Cd-generated superoxide anion, hydrogen peroxide, and hydroxyl radicals in vivo have been detected by the electron spin resonance spectra, which are often accompanied by activation of redox sensitive transcription factors (e.g., NF-κB, AP-1 and Nrf2) and alteration of ROS-related gene expression. It is generally agreed upon that oxidative stress plays important roles in acute Cd poisoning. However, following long-term Cd exposure at environmentally-relevant low levels, direct evidence for oxidative stress is often obscure. Alterations in ROS-related gene expression during chronic exposures are also less significant compared to acute Cd poisoning. This is probably due to induced adaption mechanisms (e.g, metallothionein and glutathione) following chronic Cd exposures, which in turn diminish Cd-induced oxidative stress. In chronic Cd-transformed cells, less ROS signals are detected with fluorescence probes. Acquired apoptotic tolerance renders damaged cells to proliferate with inherent oxidative DNA lesions, potentially leading to tumorigenesis. Thus, ROS are generated following acute Cd overload and plays important roles in tissue damage. Adaptation to chronic Cd exposure reduces ROS production, but acquired Cd tolerance with aberrant gene expression plays important roles in chronic Cd toxicity and carcinogenesis.
Keywords: Cadmium, reactive oxygen species, spin-trapping technique, chronic toxicity, adaptation, malignant transformation
Introduction
Cadmium (Cd) is a toxic heavy metal. In humans, acute Cd exposure via inhalation results in pulmonary edema and respiratory tract irritation, while chronic exposure often leads to renal dysfunction, anemia, osteoporosis, and bone fractures (Friberg et al., 1986). Cd is also a potent carcinogen in a number of tissues, and is classified by IARC as a human carcinogen (Waalkes et al., 2003).
Reactive oxygen species (ROS) are often implicated in Cd toxicology, either in a variety of cell culture systems (Liu et al., 1990; Hart et al., 1999; Liu and Jan, 2000; He et al., 2008), or in intact animals through all routes of exposure (Manca et al., 1994; Kayama et al., 1995; Yamano et al., 2000; Amara et al., 2008). It has been suggested that the mechanisms of acute Cd toxicity involve the depletion of glutathione and protein-bound sulfhydryl groups, resulting in enhanced production of ROS such as superoxide ion, hydrogen peroxide, and hydroxyl radicals (Manaca et al., 1994; Bagchi et al., 1997; Liu and Jan, 2000). Cd-increased ROS in turn produces lipid peroxidation, and results in DNA damage. However, little is known about direct evidence and mechanism for Cd-generated radicals until recently (Liu et al., 2008).
In contrast to acute toxicity, the roles of ROS in chronic Cd toxicity and carcinogenesis have been controversial depending on experimental conditions. For example, ROS has been implicated in chronic Cd nephrotoxicity (Shaikh et al., 1999), immunotoxicity (Ramirez and Gimenez, 2003), and carcinogenesis (Waisberg et al., 2003). Cd-induced ROS could overwhelm the antioxidant defenses, leading to increased lipid peroxidation and oxidative DNA damage (Bagchi et al., 1997; Waisberg et al., 2003). On the other hand, administration of Cd to animals at low levels for one year increases hepatic and renal glutathione levels, without elevations in tissue lipid peroxidation levels (Kamiyama et al., 1995). Cd-induced lipid peroxidation and increases in hepatic iron are evident 24 hr after a single oral dose, but these changes diminish after repeated Cd exposures (Djukic-Dosic et al., 2008). A biphasic ROS response to Cd exposure through the drinking water has also been proposed (Thijssen et al., 2007). ROS and ROS-related gene expression occur right after Cd exposure, but return to normal levels after 8 weeks of exposure (Thijssen et al., 2007). Similarly, ROS appears to play a minimal role in chronic Cd-induced malignant transformation in rat liver cells (Qu et al., 2005).
This minireview will first focus on studies using the spin-trapping technique in conjunction with electron spin resonance (ESR) for ROS detection following acute Cd overload, and discuss the roles of ROS in acute and chronic Cd toxicity and carcinogenesis, emphasizing Cd adaptation mechanisms.
Oxidative stress and acute Cd overload
ESR evidence for Cd-induced free radical generation
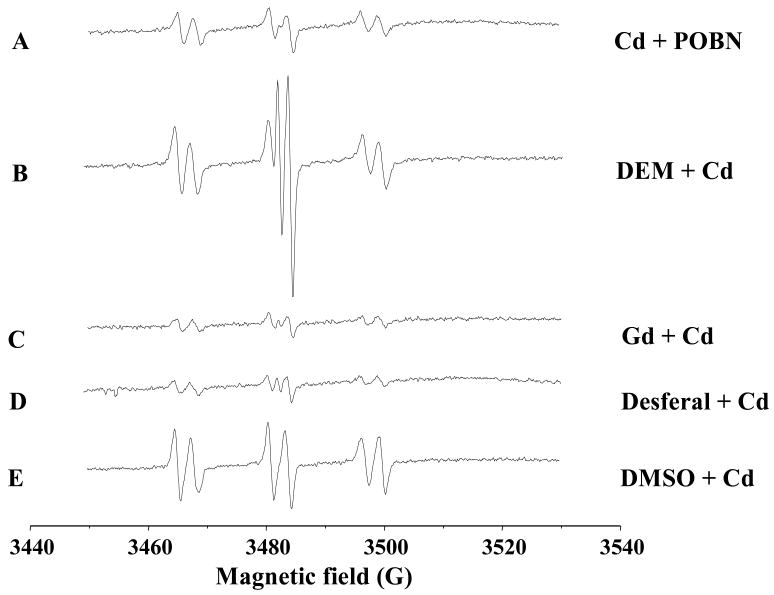
The spin-trapping technique involves a reaction of a short-lived radical with a paramagnetic compound (spin trap) to form a relatively long-lived free radical product (spin adduct), which can then be detected using conventional ESR. The intensity of the ESR signal is used to measure the radicals trapped by the spin-trapping compound, and the hypertine couplings of the spin adduct are generally used to characterize the originally trapped radicals. Liver is the major target of Cd, and the trapped Cd-radical adducts can be excreted into the bile. Thus, biliary analysis of trapped radicals reflects the Cd-generated radicals in the liver (Kadiiska and Mason, 2000). The spin-trapping agent α-(4-pyridyl-1-oxide)-N-t-butylnitrone (POBN) is given immediately after Cd injection into bile-duct cannulated rats, and the POBN-radical adducts can be detected in the bile. Cd overload increases the POBN-trapped radical adducts (Fig. 1), including the generation of superoxide anion (O2•−) and hydrogen peroxide (H2O2). Using 13C DMSO as an isotope substitution technique, the formation of POBN/methyl radical adduct (POBN/•13CH3) and POBN/lipid peroxides (POBN/•L) can also be identified in the bile of Cd overload animals (Liu et al., 2008).
Figure 1.
Representative ESR spin-trapping evidence for cadmium-induced POBN radical adduct in bile duct-cannulated rats. After anesthesia, surgery was performed to cannulate bile duct. After the bile flow is table, rats were given CdCl2 (40 umol/kg, ip), followed by POBN (1 g/kg, ip). Bile samples were collected from 40–60 min after Cd administration and anaylyzed by ESR. (A) Cd + POBN; (B–E) Cd + POBN in rats pretreated with diethyl maleate DEM (0.85 ml/kg, 2 hr); gadolinium chloride (10 mg/kg, iv, 24 hr); Desferal (50 mg/kg, ip, 1 hr); and DMSO (2 ml/kg, ip 2 hr). Instrument settings of Burker EMX spectrometer; microwave power, 20 mW, modulation amplitude, 1G, scan time 660 s; and time constant, 1.3 s; and a significant scan 80G.
The Cd-generated radical adducts can be detected in tissue extracts, using N-tert-butyl-alpha-phenylnitrone (PBN) as the spin-trapping agent (Kadiiska and Mason, 2002). Simultaneously injection of Cd with and PBN significantly increases the ESR signals of PBN-trapped radical adducts in the liver of mice (Liu et al., 2002). The extraction of PBN-trapped radicals from the liver in the presence of 2,2′-dipyridyl can prevent ex vivo radical formation with trace transition metals (Kadiiska and Mason, 2002). Cd-generated radicals can also be detected with the spin trap 5,5-dimethyl-1-pyrroline N-oxide (DMPO) in cultured cells (Yang et al., 2007), in isolated mitochondria (Wang et al., 2004), or in the cell-free system in the presence of metallothionein (O’Brien and Salacinski, 1998).
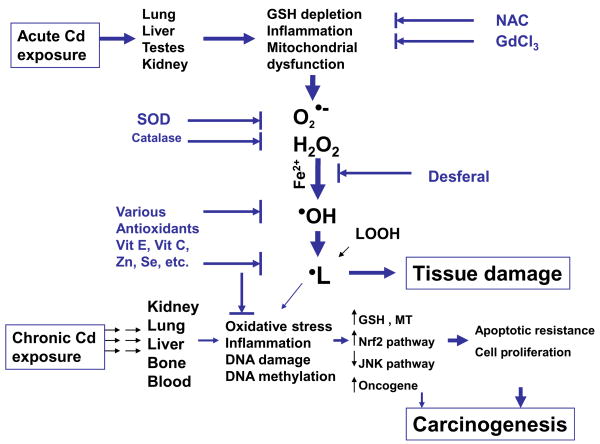
Oxidative stress has been implicated in Cd toxicology, but the majority of studies used indirect measures for ROS. The ESR studies provide direct evidence to identify Cd-generated free radicals in vitro and in vivo as superoxide anion (O2•−), hydrogen peroxide (H2O2), hydoxy radical (•OH) and lipid peroxides (•L), as illustrated in Fig. 3.
Figure 3.
Proposed pathways for ROS in Cd toxicology and carcinogenesis following acute and chronic exposures
Factors modulating Cd-induced free radical generation trapped in vivo
Cd is a redox-stable metal; therefore, radical production by Cd must be mediated through some indirect mechanisms. One proposed mechanism by which Cd may generate free radicals is the disruption of the cellular antioxidant systems. Glutathione (GSH) is abundant in the liver and is thought to be the first line of defense against Cd hepatotoxicity as Cd binds tightly to thiol groups, and depletion of hepatic GSH by diethyl maleate significantly enhances Cd-induced hepatotoxicity (Dudley and Klaassen, 1984). Depletion of hepatic GSH by diethyl maleate also dramatically enhances Cd-generated POBN-radical adduct signals in the bile (Fig. 1B), suggesting that disruption of the cellular GSH system is a key element for Cd-induced oxidative stress in the liver.
Cd-induced inflammation in the liver is another important mechanism for Cd-induced oxidative stress (Kayama et al., 1995; Yamano et al., 2000). The activation of Kupffer cells, the resident macrophages of the liver, is an important source for Cd-induced inflammatory mediators such as IL-1β, TNF-α, IL-6, and IL-8 (Kayama et al., 1995; Yamano et al., 2000). Inhibition of Kupffer cell function with gadolinium chloride has been shown to decrease Cd hepatotoxicity in rats (Sauer et al., 1997) or in mice (Harstad and Klaassen, 2002). Pretreatment of rats with gadolinium chloride abolishes Cd-increased POBN-adduct signals (Fig. 1C), suggesting that Kupffer cells could release inflammatory cytokines in response to Cd overload, which in turn contribute to Cd-generated free radicals in the liver.
The Fenton reaction driven with iron is an important source for free radical generation. The iron chelator Desferal has been shown to decrease the POBN-radical ESR signals in the bile of rats treated with formate (Dikalova et al., 2001) or aflatoxin B1 (Towner et al., 2003), presumably by inhibition of iron-driven Fenton reaction in the liver. Desferal can also abolish the Cd-generated POBN-radical adducts in the bile (Fig. 1D), clearly indicating the involvement of endogenous iron-dependent hydroxyl radical generation as a mechanism of Cd-induced oxidative stress.
Dimethyl sulfoxide (DMSO) has both pro-oxidant and antidote effects, depending on the dose and chemicals under investigation. For example, in plasmid pBR322 DNA, DMSO protects against Cd- and H2O2-induced DNA strands break (Badisa et al., 2007). DMSO treatment protects rats against the hepatotoxicity of acetaminophen, bromobenzene, and thioacetamide, but it fails to protect carbon tetrachloride-induced hepatotoxicity (Siegers, 1978), and even potentiates carbon tetrachloride-induced liver injury (Freston and Bouchier, 1967). DMSO administration decreases the formate-induced POBN-radical adducts in the rat bile (Dikalova et al., 2001), but increases Cd-induced POBN-radical adducts by 5-fold (Fig. 1E), and the formation of POBN-methyl radical adduct can be identified by ESR.
Mitochondrion is an important target of metal toxicity including Cd (Belyaeva et al., 2008). It has been proposed that Cd initially binds to protein thiols in mitochondrial membrane, affects mitochondrial permeability transition, inhibits respiratory chain reaction, and then generates ROS (Dorta et al., 2003). Cd is shown to inhibit mitochondrial complex III, resulting in accumulation of semiubiquinones at the Q0 sites. The unstable semiubiquinones are prone to transfer one electron to molecular oxygen to form superoxide anion (Wang et al., 2004). Cd effects on mitochondrial electron transfer are the major origin for Cd generated ROS not only in mammalian cells but also in plants (Heyno et al., 2008). Selenium, an essential trace element in glutathione peroxidase, is shown to protect against Cd-induced apoptosis from ROS generated through mitochondrial dysfunction (Zhou et al., 2008).
Biological evidence for Cd-induced free radical generation
Lipid peroxidation is a major consequence of Cd-induced oxidative stress (Valko et al., 2006), and is correlated with ESR evidence in both in vivo (Liu et al., 2002) and in vitro (Yang et al., 2007). Consistent with Cd-induced free radical adduct formation and lipid peroxidation, a cascade of biological changes are evoked to overcome oxidative stress. The association of these biological changes further supports the ESR evidence for Cd-generated radical adducts.
The transcription factors such as AP-1 and NF-κB are sensitive to oxidative stress in the cell. The activation of these transcription factors by Cd has been shown in intact animals and in cultured cells (Hart et al., 1999; Liu et al., 2002; Qu et al., 2005; Yang et al., 2007). However, under different experimental conditions, a decrease in NF-κB has also been reported (Xie and Shaikh, 2006). The ROS sensitive nuclear factor E2-related factor 2 (Nrf2) is also activated by Cd-generated ROS (He et al., 2008), in an attempt to combat oxidative stress in the cell.
The mitogen-activated protein kinases (MAPK) pathways are important for signal transduction in response to oxidative stress. Mammals express at least three distinct groups of MAPKs, including extracellular signal-regulated protein kinase 1/2 (Erk1/2), c-Jun N-terminal kinase (JNK), and p38 MAPK. Cadmium is known to activate the MAPK pathway via generation of ROS (Qu et al., 2007, Chen et al., 2008). The activation of MAPKs by Cd is associated ROS production in intact animals (Liu et al., 2002; Regunathan et al., 2003) and in cultured cells (Qu et al., 2006; Chen et al., 2008), which in turn plays important role in Cd-induced apoptosis to eliminate oxidative damaged cells (Qu et al., 2007).
The expression of ROS-related genes in response to Cd-induced oxidative stress is often increased. In microarray analyses, the expression of heme oxygenase-1, oxidative stress protein A170, heat-shock proteins, and oxidative DNA damage responsive GADD45, GADD153, are increased following Cd overload; while the expression of genes encoding metabolism are generally suppressed in an attempt to switch the cellular energy to overcome oxidative stress (Liu et al., 2002; Regunathan et al., 2003; Zhou et al., 2004; Croute et al., 2005). In the cultured cells, Cd increases the expression of genes related to cellular redox status, such as heme oxygenase-1, GSH S-transferases, metallothionein (MT), NAD(P)H:quinone oxidoreductase, and other stress genes (Hart et al., 1999; Yang et al., 2007, He et al., 2008). It should be noted that MT-null mice are more sensitive than wild-type mice to Cd-induced ROS-related gene expression (Liu et al., 2002; Regunathan et al., 2003). Thus, oxidative stress defense mechanisms are activated in response to Cd overload in mammalian cells.
Oxidative stress and long-term, low-dose Cd exposure
ROS may not necessarily associate with chronic Cd exposure
Contrary to direct ESR evidence for ROS generation following acute Cd overload, ESR evidence for free radical generation following long-term, low-dose Cd exposure is often obscure. Mice are given a diet containing 100 ppm CdCl2 for 6 months, followed by injection of POBN, and liver and kidneys are removed for extraction of POBN-trapped radicals. Chronic Cd exposure does not enhance the POBN-trapped radicals in the liver and kidneys, nor increase the hepatic and renal lipid peroxidation levels (data not shown). Thus, it is important to discuss the involvement of ROS production during the long-term exposure to Cd at environmentally-relevant low levels.
In chronic studies, the lack of ROS production following long-term Cd exposure is not uncommon, especially following oral Cd administration. For example, a prolonged Cd exposure (100 ppm, 23 weeks) through the drinking water does not produce overt changes in cellular redox status and lipid peroxidation levels (Thijssen et al., 2007). Dietary Cd exposure (up to 80 ppm) for one year even decreases lipid peroxidation levels in the liver and kidney of the bank vole (Wlostowski et al., 2000). A single oral dose of Cd (20 mg/kg) initially increases hepatic lipid peroxidation levels and iron concentrations 5 hr after Cd administration in mice, but repeated oral doses (10 mg/kg, daily for 14 days) produces no change or a slightly decrease in hepatic lipid peroxidation levels (Djukic-Dosic et al., 2008). ROS tolerance is also seen with a long-term (one year) injection of Cd at low levels (0.3 mg/kg, 3 days/week), without increases in tissue lipid peroxidation levels (Kamiyama et al., 1995). In rats given chronic Cd injections (0.6 mg/kg for 12 weeks), kidney injury is evident with dramatic increase in expression of kidney injury molecule-1 and MT (Prozialeck et al., 2007), but the changes in the expressions of ROS-related genes and oxidative DNA damage genes are not appreciable.
Chronic Cd exposure, however, does increase ROS production under different experimental conditions. For example, oral administration of Cd to Sprague-Dawley rats at a daily dose of 4.4 mg/kg in drinking water increases lipid peroxidation (as determined by the formation of thiobarbituric acid reactive substances) in liver mitochondria and microsomes 15 days after administration, reaching peak between 60 and 75 days of treatment, with increased urinary excretion of lipid metabolites (Bagchi et al., 1997). Chronic injection of Cd (≈ 0.6 mg/kg, 5 days/week for 22 weeks) to Sprague-Dawley rats increases hepatic and renal lipid peroxidation levels, along with increases in tissue glutathione levels (Shaikh et al., 1999). Co-administration of antioxidants N-acetylcysteine or vitamin E prevents Cd-induced lipid peroxidation and protects animals from Cd toxicity to the liver and kidney (Shaikh et al., 1999). Many experimental variables could influence Cd-induced ROS production under chronic exposure conditions. It is hypothesized that during chronic Cd exposure, adaptation mechanisms are induced to offset Cd-induced ROS and oxidative stress. When excess ROS generation overwhelms the capability of the antioxidant defense system, lipid peroxidation and oxidative damage ensure.
Minimal role of ROS in Cd-induced malignant transformation
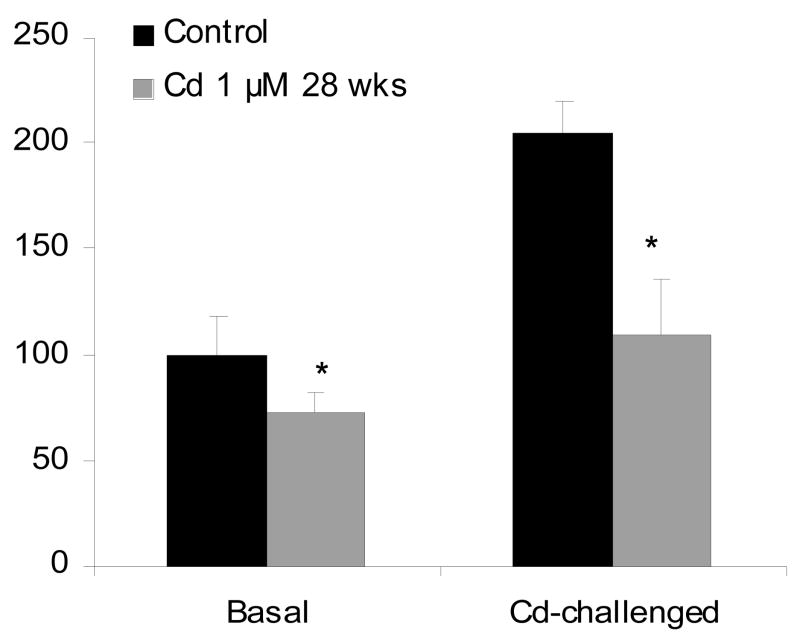
Cd-induced ROS is thought to be important in Cd carcinogenesis (Waisberg et al., 2003; Valko et al., 2006), either through oxidative stress or through the inhibition of oxidative DNA damage repair (Waisberg et al., 2003). However, little is known about direct evidence for ROS to be associated with Cd-induced cancers or Cd-induced malignant transformation. Exposure of liver cells to a low-dose (1.0 μM) of Cd that does not produce ROS, produces malignant transformation after 28 weeks of continuous exposure, as evidenced by forming highly aggressive tumors upon inoculation of cells into nude mice (Qu et al., 2005). To examine the role of ROS in Cd carcinogenesis, cellular ROS levels in control and Cd-transformed cells are determined using fluorescence probes, dihydroethidium for superoxide anion and 2,7′-dichlorofluorescin diacetate for hydrogen peroxide (Qu et al., 2005). Acute challenge exposure of liver cells to higher doses of Cd, ROS production is evident at doses above 10 μM Cd, and at 50 μM Cd, the fluorescence intensity is dramatically increased. In Cd-transformed cells, the intracellular levels of superoxide anion are significantly lower at basal conditions as compared to passage-matched control cells (Fig. 2), and these transformed cells were also highly tolerant to high dose of Cd (50 μM) induced ROS production, as evidenced by much weak fluorescence signals generated from the oxidation of dihydroethidium or 2,7′-dichlorofluorescin diacetate (Fig. 2). These data clearly indicate the minimal role of ROS in Cd-induced malignant transformation in rat liver cells, a target for Cd carcinogenesis (Waalkes, 2003). These Cd transformed cells also showed acquired apoptotic tolerance, with marked reduction of redox-sensitive SAPK/JNK signal transduction pathways (Qu et al., 2006; 2007). Chronic exposure of human urothelial UROtsa cells to the low concentration (1.0 μM) of Cd produces malignant transformation, but not at higher concentrations (5 μM or 9 μM) which kill the cell (Somji et al., 2006). These Cd transformed cells showed increased resistance to Cd toxicity and oxidative DNA damage, notably, resistance to Cd-induced apoptosis (Somji et al., 2006). Similarly, acquired apoptotic resistance was also evident in chronic Cd-transformed human prostate epithelial cells, not only to Cd itself, but also to a number of anticancer agents such as etoposide and cisplatin (Achanzar et al., 2002). Thus, Cd at low, environmentally-relevant concentrations, can induce malignant transformation in rodent and human cells, and this effect is not necessarily associated with increased ROS levels in the cells. In fact, all these Cd transformed cells are tolerant to oxidative stress and show a high rate of cell proliferation (Achanzar et al., 2002; Qu et al., 2005; 2007; Somji et al., 2006). It is likely that Cd-induced apoptotic resistance is very important for Cd-induced malignancy, as the DNA damaged cells could escape from elimination through apoptosis, and proliferate with inherent DNA lesions, eventually progressing to malignant phenotype.
Figure 2.
Cadmium-induced ROS generation in control and Cd-transformed rat liver cells. Cells exposed to 1.0 μM Cd for 28 weeks and passage matched control cells were treated with 50 μM Cd for 60 min in the presence of 3 μM dihydroethidium for the production of superoxide anion O2 for 20 min. The fluorescence images were captured with a confocal microscope. Data represent mean ± SEM of three determinations. *Significantly different from controls, p<0.05.
Cd-induced oxidative DNA damage is important for progression to neoplasia (Waalkes, 2003; Valko et al., 2006). As an epigenetic mechanism for Cd carcinogenesis, it should be noted that Cd can also induce alterations in DNA methylation status, including initially DNA hypomethylation following acute exposure (Takiguchi et al., 2003; Huang et al., 2008), and subsequently DNA hypermethylation following the long-term exposure at low doses/concentrations (Takiguchi et al., 2003; Benbrahim-Tallaa et al., 2007; Jiang et al., 2008). Cd-induced DNA hypermethylation in Cd-transformed prostate cells is associated with increases in DNA methyltransferases activity (Benbrahim-Tallaa et al., 2007) and decreases in oxidative stress and the redox-sensitive signal transduction pathways such as the JNK pathway, resulting in apoptotic resistance (Qu et al., 2007). Cd-induced DNA hypermethylation decreases the expression of tumor suppressor genes (e.g., p16, RASSF1A), which could be important in Cd-induced malignant transformation in human prostate cells (Benbrahim-Tallaa et al., 2007).
Adaptive mechanisms after chronic, low-dose Cd exposure
A number of cellular defense mechanisms are known to be activated in response to low dose of Cd exposures, among them are induction of MT, increase in cellular glutathione, activation of antioxidant transcription factor Nrf2 and other antioxidant components. These mechanisms are not mutually exclusive, but may function together in an integrated way to confer resistance to Cd-induced oxidative stress.
MT is a cysteine-rich, Cd-binding protein playing an important role in protecting Cd toxicity and in combating Cd-induced ROS through Cd sequestration or through its free radical scavenging activity (Dudley and Klaassen, 1984; Klaassen et al., 1999). Following chronic Cd exposure, tissue MT is markedly increased (Wlostowski et al., 2000; Prozialeck et al., 2007; Thijssen et al., 2007). Increased MT binds Cd in the cytosol, reducing Cd available amount to critical organelles. MT is also rich in sulfhydryl groups to trap Cd generated ROS in the cells (Klaassen et al., 1999). During chronic Cd carcinogenesis, marked elevations in MT is responsible for resistance to apoptosis and ROS in Cd transformed rat liver cells (Qu et al., 2006), in Cd-tranformed human urothelial UROtsa35 cells (Somiji et al., 2006), or in Cd-transformed human prostate epithelial cells (Qu et al., 2007).
GSH is thought to be the first line of defense against Cd toxicity. In contrast to sharply depletion of GSH during acute Cd exposure (Dudley and Klaassen, 1984; Liu et al., 1990), chronic Cd exposure often result in elevations of tissue GSH levels (Kamiyama et al., 1995; Shaikh et al., 1999; Waisberg et al., 2003), which in turn will diminish oxidative damage from the metal. Differences in intracellular GSH level regulation between acute and chronic Cd exposure could be a major reason for differences in ROS accumulation in the cells.
Studies using supplement with N-acetyl cysteine, vitamin E, vitamin C, and selenium to enhance body anti-oxidant machinery have been shown to decrease Cd-induced oxidative stress in kidney, liver, and testes, with improved cellular and tissue functions in many in vitro and in vivo studies (Shaikh et al., 1999; Sen Gupta et al., 2004; Zhou et al., 2008). Thus, the adaptive mechanisms for Cd-induced oxidative stress would also likely involve the up-regulation of both enzymatic and non-enzymatic antioxidants in the liver to overcome Cd insults.
Nuclear factor erythroid 2-related factor 2 (Nrf2) has emerged as a key transcription factor in the transcriptional regulation of ARE-dependent gene expression in response to oxidative stimuli and a spectrum of toxic signals. Cd dramatically increased ROS production in Nrf2-null MEF cells, as evidenced by increased dihydroethium fluorescence intensity at as low as 2 μM, as compared to 50–100 μM in the wild-type cells, corresponding to increased cell death (He et al., 2008). The Nrf2-regulated genes, such as heme oxygenase-1 and NAD(P)H:quinone oxidoreductase 1, are markedly induced in wild-type cells, but not in Nrf2-null cells. Thus the activation of Nrf2 and related antioxidant components is another important adaptation mechanism in protecting against chronic Cd toxicity. In addition to the Nrf2 pathways, Cd activation of AP-1 and NF-κB pathways (Qu et al., 2005; Liu et al., 2002; Yang et al., 2007), as well as other cellular components, would contribute to adaptive response to oxidative stress.
Summary
As illustrated in Figure 3, acute Cd overload can generated free radicals, including superoxide anion (O2•−), hydrogen peroxide (H2O2), hydoxy radical (•OH) and lipid peroxides (•L). Cd is not a redox active metal, and free radical generation by Cd must be mediated through indirect mechanisms, including glutathione depletion, Kupffer cell activation, inflammation, and involvement of iron for the Fenton reaction. However, during long-term exposure to Cd at low doses, ROS accumulation is not necessarily associated with Cd-induced chronic toxicity and carcinogenesis. Adaptive mechanisms including induction of MT, GSH, and cellular antioxidants could diminish Cd-induced oxidative stress. Initial oxidative DNA damage and subsequent apoptotic resistance, epigenetic DNA methylation status changes, and aberrant gene expressions, all of which could play integrated roles in Cd carcinogenesis.
Acknowledgments
The authors thank Drs. Chikara Kojima and Larry Keefer for their critical review of this manuscript. This review was supported, in part, by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, and the National Institute of Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achanzar WE, Webber MM, Waalkes MP. Altered apoptotic gene expression and acquired apoptotic resistance in cadmium-transformed human prostate epithelial cells. Prostate. 2002;52:236–244. doi: 10.1002/pros.10106. [DOI] [PubMed] [Google Scholar]
- Amara S, Abdelmelek H, Garrel C, Guiraud P, Douki T, Ravanat JL, Favier A, Sakly M, Ben Rhouma K. Preventive effect of zinc against cadmium-induced oxidative stress in the rat testis. J Reprod Dev. 2008;54:129–134. doi: 10.1262/jrd.18110. [DOI] [PubMed] [Google Scholar]
- Badisa VL, Latinwo LM, Odewumi CO, Ikediobi CO, Badisa RB, Ayuk-Takem LT, Nwoga J, West J. Mechanism of DNA damage by cadmium and interplay of antioxidant enzymes and agents. Environ Toxicol. 2007;22:144–151. doi: 10.1002/tox.20248. [DOI] [PubMed] [Google Scholar]
- Belyaeva EA, Dymkowska D, Wieckowski MR, Wojtczak L. Mitochondria as an important target in heavy metal toxicity in rat hepatoma AS-30D cells. Toxicol Appl Pharmacol. 2008;231:34–42. doi: 10.1016/j.taap.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L, Waterland RA, Dill AL, Webber MM, Waalkes MP. Tumor suppressor gene inactivation during cadmium-induced malignant transformation of human prostate cells correlates with overexpression of de novo DNA methyltransferase. Environ Health Perspect. 2007;115:1454–1459. doi: 10.1289/ehp.10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi D, Vuchetich PJ, Bagchi M, Hassoun EA, Tran MX, Tang L, Stohs SJ. Induction of oxidative stress by chronic administration of sodium dichromate and cadmium chloride to rats. Free Radic Biol Med. 1997;22:471–478. doi: 10.1016/s0891-5849(96)00352-8. [DOI] [PubMed] [Google Scholar]
- Chen L, Liu L, Huang S. Cadmium activates the mitogen-activated protein kinase (MAPK) pathway via induction of reactive oxygen species and inhibition of protein phosphatases 2A and 5. Free Radic Biol Med. 2008;45:1035–1044. doi: 10.1016/j.freeradbiomed.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Croute F, Beau B, Murat JC, Vincent C, Komatsu H, Obata F, Soleilhavoup JP. Expression of stress-related genes in a cadmium-resistant A549 human cell line. J Toxicol Environ Health A. 2005;68:703–718. doi: 10.1080/15287390590925447. [DOI] [PubMed] [Google Scholar]
- Dikalova AE, Kadiiska MB, Mason RP. An in vivo ESR spin-trapping study: free radical generation in rats from formate intoxication--role of the Fenton reaction. Proc Natl Acad Sci USA. 2001;98:13549–13553. doi: 10.1073/pnas.251091098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djukić-Cosić D, Curcić Jovanović M, Plamenac Bulat Z, Ninković M, Malicević Z, Matović V. Relation between lipid peroxidation and iron concentration in mouse liver after acute and subacute cadmium intoxication. J Trace Elem Med Biol. 2008;22:66–72. doi: 10.1016/j.jtemb.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Dorta DJ, Leite S, DeMarco KC, Prado IM, Rodrigues T, Mingatto FE, Uyemura SA, Santos AC, Curti C. A proposed sequence of events for cadmium-induced mitochondrial impairment. J Inorg Biochem. 2003;97:251–257. doi: 10.1016/s0162-0134(03)00314-3. [DOI] [PubMed] [Google Scholar]
- Dudley RE, Klaassen CD. Changes in hepatic glutathione concentration modify cadmium-induced hepatotoxicity. Toxicol Appl Pharmacol. 1984;72:530–538. doi: 10.1016/0041-008x(84)90130-3. [DOI] [PubMed] [Google Scholar]
- Friberg L, Elinder CG, Kjellstrom T, Nordberg GF. Cadmium and health: a toxicological and epidemiological appraisal. Boca Raton, FL: CRC Press; 1986. [Google Scholar]
- Freston JW, Bouchier IA. Potentiation of carbon tetrachloride toxicity by dimethyl sulphoxide. Nature. 1967;214:734–5. doi: 10.1038/214734a0. [DOI] [PubMed] [Google Scholar]
- Hart BA, Lee CH, Shukla GS, Osier A, Eneman JD, Chiu JF. Characterization of cadmium-induced apoptosis in rat lung epithelial cells: evidence for the participation of oxidant stress. Toxicology. 1999;133:43–58. doi: 10.1016/s0300-483x(99)00013-x. [DOI] [PubMed] [Google Scholar]
- Harstad EB, Klaassen CD. Gadolinium chloride pretreatment prevents cadmium chloride-induced liver damage in both wild-type and MT-null mice. Toxicol Appl Pharmacol. 2002;180:178–185. doi: 10.1006/taap.2002.9385. [DOI] [PubMed] [Google Scholar]
- He X, Chen MG, Ma Q. Activation of Nrf2 in defense against cadmium-induced oxidative stress. Chem Res Toxicol. 2008;21:1375–1383. doi: 10.1021/tx800019a. [DOI] [PubMed] [Google Scholar]
- Heyno E, Klose C, Krieger-Liszkay A. Origin of cadmium-induced reactive oxygen species production: mitochondrial electron transfer versus plasma membrane NADPH oxidase. New Phytol. 2008;179:687–699. doi: 10.1111/j.1469-8137.2008.02512.x. [DOI] [PubMed] [Google Scholar]
- Huang D, Zhang Y, Qi Y, Chen C, Ji W. Global DNA hypomethylation, rather than reactive oxygen species (ROS), a potential facilitator of cadmium-stimulated K562 cell proliferation. Toxicol Lett. 2008;179:43–47. doi: 10.1016/j.toxlet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Jiang G, Xu L, Song S, Zhu C, Wu Q, Zhang L, Wu L. Effects of long-term low-dose cadmium exposure on genomic DNA methylation in human embryo lung fibroblast cells. Toxicology. 2008;244:49–55. doi: 10.1016/j.tox.2007.10.028. [DOI] [PubMed] [Google Scholar]
- Kadiiska MB, Mason RP. Ethylene glycol generates free radical metabolites in rats: an ESR in vivo spin trapping investigation. Chem Res Toxicol. 2000;13:1187–1191. doi: 10.1021/tx9901940. [DOI] [PubMed] [Google Scholar]
- Kadiiska MB, Mason RP. In vivo copper-mediated free radical production: an ESR spin-trapping study. Spectrochim Acta A Mol Biomol Spectrosc. 2002;58:1227–1239. doi: 10.1016/s1386-1425(01)00713-2. [DOI] [PubMed] [Google Scholar]
- Kayama F, Yoshida T, Elwell MR, Luster MI. Role of tumor necrosis factor-a in cadmium-induced hepatotoxicity. Toxicol Appl Pharmacol. 1995;131:224–234. doi: 10.1006/taap.1995.1065. [DOI] [PubMed] [Google Scholar]
- Kamiyama T, Miyakawa H, Li JP, Akiba T, Liu JH, Liu J, Marumo F, Sato C. Effects of one-year cadmium exposure on livers and kidneys and their relation to glutathione levels. Res Commun Mol Pathol Pharmacol. 1995;88:177–186. [PubMed] [Google Scholar]
- Klaassen CD, Liu J, Choudhuri S. Metallothionein: an intracellular protein to protect against cadmium toxicity. Annu Rev Pharmacol Toxicol. 1999;39:267–294. doi: 10.1146/annurev.pharmtox.39.1.267. [DOI] [PubMed] [Google Scholar]
- Liu F, Jan KY. DNA damage in arsenite- and cadmium-treated bovine aortic endothelial cells. Free Radic Biol Med. 2000;28:55–63. doi: 10.1016/s0891-5849(99)00196-3. [DOI] [PubMed] [Google Scholar]
- Liu J, Kershaw WC, Klaassen CD. Rat primary hepatocyte cultures are a good model for examining metallothionein-induced tolerance to cadmium toxicity. In Vitro Cell Dev Biol. 1990;26:75–79. doi: 10.1007/BF02624158. [DOI] [PubMed] [Google Scholar]
- Liu J, Kadiiska MB, Corton JC, Qu W, Waalkes MP, Mason RP, Liu Y, Klaassen CD. Acute cadmium exposure induces stress-related gene expression in wild-type and metallothionein-I/II null mice. Free Radic Biol Med. 2002;32:525–535. doi: 10.1016/s0891-5849(01)00826-7. [DOI] [PubMed] [Google Scholar]
- Liu J, Qian SY, Guo Q, Jiang J, Waalkes MP, Mason RP, Kadiiska KB. Cadmium generates reactive oxygen- and carbon-centered radical species in rats: Insights from in vivo spin-trapping studies. Free Radic Biol Med. 2008;45:475–481. doi: 10.1016/j.freeradbiomed.2008.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manca D, Richard AC, van Tra H, Chevalier G. Relation between lipid peroxidation and inflammation in the pulmonary toxicity of cadmium. Arch Toxicol. 1994;68:364–369. doi: 10.1007/s002040050083. [DOI] [PubMed] [Google Scholar]
- O’Brien P, Salacinski HJ. Evidence that the reactions of cadmium in the presence of metallothionein can produce hydroxyl radicals. Arch Toxicol. 1998;72:690–700. doi: 10.1007/s002040050562. [DOI] [PubMed] [Google Scholar]
- Prozialeck WC, Vaidya VS, Liu J, Waalkes MP, Edwards JR, Lamar PC, Bernard AM, Dumont X, Bonventre JV. Kidney injury molecule-1 is an early biomarker of cadmium nephrotoxicity. Kidney Int. 2007;72:985–993. doi: 10.1038/sj.ki.5002467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu W, Diwan BA, Reece JM, Bortner CD, Pi J, Liu J, Waalkes MP. Cadmium-induced malignant transformation in rat liver cells: role of aberrant oncogene expression and minimal role of oxidative stress. Int J Cancer. 2005;114:346–355. doi: 10.1002/ijc.20736. [DOI] [PubMed] [Google Scholar]
- Qu W, Fuquay R, Sakurai T, Waalkes MP. Acquisition of apoptotic resistance in cadmium-induced malignant transformation: specific perturbation of JNK signal transduction pathway and associated metallothionein overexpression. Mol Carcinog. 2006;45:561–571. doi: 10.1002/mc.20185. [DOI] [PubMed] [Google Scholar]
- Qu W, Ke H, Pi J, Broderick D, French JE, Webber MM, Waalkes MP. Acquisition of apoptotic resistance in cadmium-transformed human prostate epithelial cells: Bcl-2 overexpression blocks the activation of JNK signal transduction pathway. Environ Health Perspect. 2007;115:1094–1100. doi: 10.1289/ehp.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regunathan A, Glesne DA, Wilson AK, Song J, Nicolae D, Flores T, Bhattacharyya MH. Microarray analysis of changes in bone cell gene expression early after cadmium gavage in mice. Toxicol Appl Pharmacol. 2003;191:272–293. doi: 10.1016/s0041-008x(03)00163-7. [DOI] [PubMed] [Google Scholar]
- Ramirez DC, Gimenez MS. Induction of redox changes, inducible nitric oxide synthase and cyclooxygenase-2 by chronic cadmium exposure in mouse peritoneal macrophages. Toxicol Lett. 2003;145:121–132. doi: 10.1016/s0378-4274(03)00237-6. [DOI] [PubMed] [Google Scholar]
- Sauer JM, Waalkes MP, Hooser SB, Kuester RK, McQueen CA, Sipe IG. Suppression of Kupffer cell function prevents cadmium induced hepatocellular necrosis in the male Sprague-Dawley rat. Toxicology. 1997;121:155–164. doi: 10.1016/s0300-483x(97)00062-0. [DOI] [PubMed] [Google Scholar]
- Sen Gupta R, Sen Gupta E, Dhakal BK, Thakur AR, Ahnn I. Vitamin C and vitamin E protect the rat testes from cadmium-induced reactive oxygen species. Mol Cells. 2004;17:132–9. [PubMed] [Google Scholar]
- Shaikh ZA, Vu TT, Zaman K. Oxidative stress as a mechanism of chronic cadmium-induced hepatotoxicity and renal toxicity and protection by antioxidants. Toxicol Appl Pharmacol. 1999;154:256–263. doi: 10.1006/taap.1998.8586. [DOI] [PubMed] [Google Scholar]
- Siegers CP. Antidotal effects of dimethyl sulphoxide against paracetamol-, bromobenzene-, and thioacetamide-induced hepatotoxicity. J Pharm Pharmacol. 1978;30:375–377. doi: 10.1111/j.2042-7158.1978.tb13260.x. [DOI] [PubMed] [Google Scholar]
- Somji S, Zhou XD, Garrett SH, Sens MA, Sens DA. Urothelial cells malignantly transformed by exposure to cadmium (Cd(+2)) and arsenite (As(+3)) have increased resistance to Cd(+2) and As(+3)-induced cell death. Toxicol Sci. 2006;94:293–301. doi: 10.1093/toxsci/kfl108. [DOI] [PubMed] [Google Scholar]
- Takiguchi M, Achanzar WE, Qu W, Li G, Waalkes MP. Effects of cadmium on DNA-(Cytosine-5) methyltransferase activity and DNA methylation status during cadmium-induced cellular transformation. Exp Cell Res. 2003;286:355–365. doi: 10.1016/s0014-4827(03)00062-4. [DOI] [PubMed] [Google Scholar]
- Thijssen S, Cuypers A, Maringwa J, Smeets K, Horemans N, Lambrichts I, Van Kerkhove E. Low cadmium exposure triggers a biphasic oxidative stress response in mice kidneys. Toxicology. 2007;236:29–41. doi: 10.1016/j.tox.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Towner RA, Qian SY, Kadiiska MB, Mason RP. In vivo identification of aflatoxin-induced free radicals in rat bile. Free Radic Biol Med. 2003;35:1330–1340. doi: 10.1016/j.freeradbiomed.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Waalkes MP. Cadmium carcinogenesis. Mutat Res. 2003;533:107–120. doi: 10.1016/j.mrfmmm.2003.07.011. [DOI] [PubMed] [Google Scholar]
- Waisberg M, Joseph P, Hale B, Beyersmann D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology. 2003;192:95–117. doi: 10.1016/s0300-483x(03)00305-6. [DOI] [PubMed] [Google Scholar]
- Wang Y, Fang J, Leonard SS, Rao KM. Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic Biol Med. 2004;36:1434–1443. doi: 10.1016/j.freeradbiomed.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Wlostowski T, Krasowska A, Godlewska-Zylkiewicz B. Dietary cadmium decreases lipid peroxidation in the liver and kidneys of the bank vole (Clethrionomys glareolus) J Trace Elem Med Biol. 2000;14:76–80. doi: 10.1016/S0946-672X(00)80033-4. [DOI] [PubMed] [Google Scholar]
- Xie J, Shaikh ZA. Cadmium-induced apoptosis in rat kidney epithelial cells involves decrease in nuclear factor-kappa B activity. Toxicol Sci. 2006;91:299–308. doi: 10.1093/toxsci/kfj131. [DOI] [PubMed] [Google Scholar]
- Yamano T, DeCicco LA, Rikans LE. Attenuation of cadmium-induced liver injury in senescent male fischer 344 rats: role of Kupffer cells and inflammatory cytokines. Toxicol Appl Pharmacol. 2000;162:68–75. doi: 10.1006/taap.1999.8833. [DOI] [PubMed] [Google Scholar]
- Yang Z, Yang S, Qian SY, Hong JS, Kadiiska MB, Tennant RW, Waalkes MP, Liu J. Cadmium-induced toxicity in rat primary mid-brain neuroglia cultures: role of oxidative stress from microglia. Toxicol Sci. 2007;98:488–494. doi: 10.1093/toxsci/kfm106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Jia X, Chapin RE, Maronpot RR, Harris MW, Liu J, Waalkes MP, Eddy EM. Cadmium at a non-toxic dose alters gene expression in mouse testes. Toxicol Lett. 2004;154:191–200. doi: 10.1016/j.toxlet.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Zhou YJ, Zhang SP, Liu CW, Cai YQ. Toxicol. 2008. 2008 Dec 24. The protection of selenium on ROS mediated-apoptosis by mitochondria dysfunction in cadmium-induced LLC-PK(1) cells. In Vitro. Epub ahead of print. [DOI] [PubMed] [Google Scholar]