Abstract
Purpose
This first-in-human dose-escalation trial evaluated the safety, tolerability, maximal tolerated dose (MTD), dose limiting toxicities (DLTs), pharmacokinetics, pharmacodynamics and preliminary clinical activity of pictilisib (GDC-0941), an oral, potent and selective inhibitor of the Class I phosphatidylinositol-3-kinases (PI3K).
Patients and Methods
Sixty patients with solid tumors received pictilisib at 14 dose levels from 15 to 450mg once-daily, initially on days 1-21 every 28 days and later, utilizing continuous dosing for selected dose levels. Pharmacodynamic studies incorporated 18F-FDG-PET, and assessment of phosphorylated AKT and S6 ribosomal protein in platelet-rich plasma and tumor tissue.
Results
Pictilisib was well-tolerated. The most common toxicities were grade 1-2 nausea, rash and fatigue while the DLT was grade 3 maculopapular rash (450mg, 2 of 3 patients; 330mg, 1 of 7 patients). The pharmacokinetic profile was dose-proportional and supported once-daily dosing. Levels of phosphorylated serine-473 AKT were suppressed >90% in platelet rich plasma at 3 hours post-dose at the MTD and in tumor at pictilisib doses associated with AUC >20uM.hr. Significant increase in plasma insulin and glucose levels, and >25% decrease in 18F-FDG uptake by PET in 7 of 32 evaluable patients confirmed target modulation. A patient with V600E BRAF mutant melanoma and another with platinum-refractory epithelial ovarian cancer exhibiting PTEN loss and PIK3CA amplification demonstrated partial response by RECIST and GCIG-CA125 criteria, respectively.
Conclusion
Pictilisib was safely administered with a dose-proportional pharmacokinetic profile, on-target pharmacodynamic activity at dose levels ≥100mg and signs of antitumor activity. The recommended Phase II dose was continuous dosing at 330mg once-daily.
INTRODUCTION
Phosphatidylinositol-3-kinase (PI3K) regulates processes involved in the hallmark traits of cancer, such as cell growth, survival, metabolism, invasion and metastases.1 Multiple isoforms of PI3K exist in mammalian cells and these isoforms are subdivided into three classes based on structural features and lipid substrate preferences.1 The Class IA isoforms (p110α, β and δ) are responsible for the production of the second messenger phosphatidyl-inositol-3,4,5 triphosphate (PIP3).2,3 PI3K activation initiates a signal transduction cascade, of which the major effectors are the kinases AKT and mTORC1.4 PTEN is a tumor suppressor gene which functions as a phosphatase, and is the primary negative regulator of PI3K, through hydrolysis of PIP3.5 Deregulation of the PI3K pathway has been frequently implicated in a wide range of malignancies, including glioma, prostate, breast, ovarian and endometrial cancer.6 Alteration of the pathway commonly occurs through mutation or amplification of PIK3CA which encodes the p110α catalytic subunit, loss of function of PTEN (through deletion, mutation or reduced expression), alterations in the INPP4B and PHLPP phosphatases, mutations of the PI3K regulatory subunits encoded by PIK3R1 and PIK3R3, or through activation of upstream receptor tyrosine kinases or crosstalk with the RAS pathway.3,6,7
Pictilisib (GDC-0941; Genentech Inc., South San Francisco, CA) is an oral, potent, selective pan-inhibitor of class I PI3K (IC50 against purified recombinant human PI3K isoforms: p110α=3 nM, p110β=33 nM, p110δ=3 nM, p110γ=75 nM) with 193-fold less activity against mTOR compared to p110α.8 Antitumor activity was demonstrated in human tumor xenograft murine models; at an oral dose of 150mg/kg, pictilisib achieved 98% and 80% growth inhibition in PI3K pathway-activated U87MG glioblastoma and IGROV1 ovarian cancer xenografts, respectively.9 At this dose level, pictilisib achieved plasma concentrations in tumor tissue >GI50-antiproliferative concentrations for >8 hours and demonstrated downstream PI3K pathway biomarker changes such as time- and dose-dependent inhibition of phosphorylation of AKT (Ser473) and P70S6 kinase (Thr421/Ser424).9 These data led us to conduct a first-in-human Phase I dose-escalation study of pictilisib in patients with advanced solid tumors to evaluate safety and tolerability, define dose-limiting toxicities (DLTs) and the maximum tolerated dose (MTD) of pictilisib administered orally. Secondary objectives included characterization of pharmacokinetics, assessment of pharmacodynamics including changes in tumor 18F-fluorodeoxyglucose (18F-FDG) uptake (by positron emission tomography [PET] studies), tumor expression of phosphorylated (Ser235/Ser236) S6 ribosomal protein (phospho-S6) in mandatory pre- and on-treatment biopsies and phosphorylated (Ser473) AKT (phospho-AKT) in platelet-rich plasma (PRP), and preliminary evaluation of antitumor activity.
PATIENTS AND METHODS
This single-center trial was conducted in accordance with the Declaration of Helsinki at The Royal Marsden NHS Foundation Trust (London, UK) after approval by local institutional review boards. Informed consent from all patients was obtained.
Eligibility
Patients aged ≥18 years with histologically confirmed solid tumors and no conventional treatment option were eligible. Other inclusion criteria included: fasting serum glucose ≤120mg/dl, HbA1c ≤upper limit of normality, prior chemotherapy or radiotherapy completed ≥4 weeks previously, toxicity from prior therapy resolved to grade ≤1, an Eastern Cooperative Oncology Group performance status ≤1, expected life expectancy ≥12 weeks and adequate organ functions. Significant exclusion criteria included diabetes mellitus requiring medication, significant respiratory disease (requiring supplemental oxygen or predicted diffusion capacity of carbon monoxide [DLCO] ≤50%), and use of anticoagulation or chronic corticosteroid.
Study Design
This was an open-label, single-centre, Phase I study utilizing a modified 3+3 dose-escalation design. During dose escalation, the dose of pictilisib was doubled until drug-related toxicity of grade ≥2 was observed. Dose escalations from this point were limited to ≤50% of the previous dose (if grade ≤2) or 33% in the event of grade ≥3 toxicities.
Pictilisib was administered on day 1, followed by a 1-week washout to evaluate single-dose pharmcokinetics and pharmacodynamics. Dosing was once-daily for 21 or 28 days every 28 days (21/28 or 28/28 schedule, respectively). The recommended starting dose in humans of 15mg once-daily was chosen based on the no observed adverse effect level and MTD in 28-day rodent and dog species studies. The 21/28 starting schedule was chosen to implement a drug-free period to allow recovery from acute toxicities and limit cumulative toxicities to maximize the administered dose of pictilisib. A continuous dosing schedule (28/28) was implemented to further explore safety and pharmacodynamics of dose levels 330-400mg.
Definitions of DLT and MTD
DLTs were based on toxicities observed in the first cycle and assessed by the investigator as possibly related to pictilisib. A DLT was defined as grade 4 neutropenia for >5 days or accompanied by fever >38.5°C, grade 4 thrombocytopenia or grade 3 non-hematologic toxicity of any duration with the exception of alopecia. Grade 3-4 nausea, vomiting and diarrhea were only considered DLTs if they occurred despite optimal medical management. Grade ≥3 total bilirubin, AST, ALT were considered DLTs except when pre-existing Grade 1 values and were <7.5× ULN due to known liver metastases. Decreases in DLco ≥20% were also considered DLTs. The MTD was defined as the highest dose at which ≤1 of 6 DLTs (<33%) patients experienced DLT at that dose level.
Safety and Efficacy
Clinical and laboratory assessments were conducted at baseline and weekly thereafter. Safety assessments included medical history, physical examination, electrocardiogram, hematology, biochemistry (including fasting glucose and HbA1C,), and assessment of pulmonary toxicity with pulse oximetry, DLCO, and high resolution CT (HRCT) of the chest. Adverse events were graded using CTCAE (Common Toxicity Criteria for Adverse Events) version 3.0. Tumor assessments were performed at the end of cycles 1 and 2, and then every 2 cycles using RECIST guidelines.10
Pharmacokinetics
Plasma levels of pictilisib were determined from samples collected on day 1: pre-dose, and 0.5, 1, 2, 3, 4, 8, 24, 48 and 72 hours post-dose; and on day 15: pre-dose, and 0.5,1,2,3,4,8, and 24 hrs post-dose. Pharmacokinetic samples for pictilisib were analyzed using a validated HPLC/MS/MS method. Standard pharmacokinetic parameters were determined using a non-compartmental method (WinNonlin version 5.2.1; Pharsight Corporation, Mountain View, CA)
Pharmacodynamics
Platelet-rich plasma was obtained from patients pre-dose, and at 1, 3, 8, 24 hours post-dose on days 1 and 15 of cycle 1 for analysis of phospho-AKT (Ser473) using an electrochemiluminescense assay (Luminex xMAP, Luminex Corp, Austin, TX). Wherever feasible, patients underwent tumor biopsies pre-treatment and 1-4 hours post-dose on day 15; samples were fixed, sectioned and stained with haematoxylin and eosin, and for phospho-S6 using anti-phospho-S6 (Ser235/236) (Cell Signaling Technology Inc., Beverly, MA), or anti-phospho-AKT (Serine 473) clone D9E (Cell Signaling Technology). Plasma was obtained pre-dose and at 1 hour post-dose on cycle 1 day 1 for analysis of glucose and insulin levels at dose levels ≥100mg. Whole body 18F-FDG-PET scans were performed at baseline, 1-4 hours post-dose between day 22 and the end of cycle 1 as well as between day 50 and the end of cycle 2.
PI3K pathway alteration biomarkers
PIK3CA mutations were identified in circulating tumor plasma DNA (ctDNA) using a site-specific molecular characterization protocol.11 Archival and fresh tumor samples were analyzed using the SEQUENOM® OncoCarta Panel (Sequenom Inc., San Diego, CA). PIK3CA amplification was assessed by fluorescence in-situ hybridization (FISH) and PTEN status by immunohistochemistry.12
RESULTS
Patient characteristics
Sixty patients with confirmed progressive cancer at study entry were enrolled, most of whom were heavily pre-treated (median of 3 prior systemic therapies [range 0-16)] (Table 1). All patients were included in the safety analysis.
Table 1. Demographics and clinical characteristics of all treated patients.
| Characteristic | All patients |
|
|---|---|---|
| No. of patients | % | |
|
| ||
| Sex | ||
| Male | 30 | 50 |
| Female | 30 | 50 |
|
| ||
| Age, years | ||
| Median | 59 | |
| Range | 27-77 | |
|
| ||
| ECOG performance status at screening | ||
| 0 | 28 | 47 |
| 1 | 32 | 53 |
|
| ||
| Primary cancer diagnosis | ||
| Colorectal | 16 | 27 |
| Breast | 9 | 15 |
| Soft tissue sarcoma | 7 | 12 |
| Melanoma | 5 | 8 |
| Ovarian | 3 | 5 |
| Gastric | 2 | 3 |
| Prostate | 2 | 3 |
| Others | 16 | 27 |
|
| ||
| No. of prior lines of systemic therapies | ||
| Median | 3 | |
| Range | 0 - 16 | |
Abbreviation: ECOG: Eastern Cooperative Oncology Group
Treatment & dose escalation
Sixty patients were treated in 14 dose-schedules (Table 2). Dose escalation on the 21/28 schedule proceeded through 11 dose levels from 15mg. At 450mg, 2 of 3 patients experienced DLTs. The dose level of 330mg was then evaluated with 1 DLT observed in 7 patients treated at this dose level on a 21/28 schedule. Subsequently, the dose levels of 330mg, 340mg and 400mg on the 28/28 schedule were assessed.
Table 2. Summary of adverse events (AEs).
AEs possibly or likely treatment-related that occurred in ≥10% of patients according to maximum grade for each patient by dose level and grade. AEs grades ≥3 are presented in separate columns for each dose-schedule only when observed.
| Dose level (mg) | 15 | 30 | 45 | 60 | 80 | 100 | 130 | 180 | 245 | 330 | 450 | 330 | 340 | 400 | All | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||||||
| Schedule | 21/28 | 21/28 | 21/28 | 21/28 | 21/28 | 21/28 | 21/28 | 21/28 | 21/28 | 21/28 | 21/28 | 28/28 | 28/28 | 28/28 | 21 or 28/28 | ||||||
|
| |||||||||||||||||||||
| No. of patients | 4 | 3 | 4 | 4 | 3 | 5 | 3 | 3 | 3 | 7 | 3 | 10 | 7 | 1 | 60 | ||||||
|
| |||||||||||||||||||||
| Toxicity grade | All | All | All | All | All | All | ≥3 | All | All | All | ≥3 | All | ≥3 | All | ≥3 | All | ≥3 | All | All | ≥3 | All |
|
| |||||||||||||||||||||
| Any adverse event | 2 | 1 | 2 | 2 | 3 | 3 | 1 | 1 | 2 | 3 | 2 | 7 | 2 | 3 | 2 | 10 | 2 | 6 | 1 | 9 | 46 |
| Gastrointestinal | |||||||||||||||||||||
| Nausea | 1 | 0 | 0 | 2 | 1 | 3 | 0 | 0 | 0 | 1 | 0 | 4 | 0 | 2 | 0 | 9 | 0 | 4 | 1 | 0 | 28 |
| Diarrhea | 0 | 0 | 2 | 1 | 1 | 1 | 0 | 0 | 1 | 2 | 0 | 3 | 0 | 0 | 1 | 7 | 0 | 1 | 0 | 1 | 19 |
| Vomiting | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 2 | 0 | 3 | 0 | 4 | 0 | 0 | 15 |
| Decreased appetite | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 0 | 1 | 0 | 6 | 0 | 3 | 0 | 0 | 14 |
| Dysgeusia | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 5 | 0 | 2 | 0 | 0 | 13 |
| Skin & mucosa | |||||||||||||||||||||
| Rash * | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 4 | 2 | 3 | 2 | 4 | 0 | 1 | 1 | 5 | 14 |
| Stomatitis ** | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 2 | 1 | 0 | 7 |
| Xeroderma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 1 | 1 | 4 | 0 | 0 | 0 | 1 | 8 |
| Constitutional | |||||||||||||||||||||
| Fatigue | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 1 | 0 | 4 | 0 | 2 | 0 | 6 | 0 | 3 | 1 | 0 | 21 |
Including preferred terms of rash, rash maculo-papular, rash macular, rash pruritic, rash erythematous, and rash papular.
Including preferred terms of stomatitis, mucosal inflammation, mouth ulceration.
DLTs and MTD
The MTD was exceeded at 450mg once-daily (21/28 schedule) with a DLT of grade 3 rash in 2 patients. This was a maculopapular rash covering 70-80% of the body surface area that presented approximately 2 weeks after commencement of daily pictilisib dosing and resolved spontaneously 2 weeks after treatment discontinuation. At 330mg once-daily (21/28 schedule), the grade 3 maculopapular rash observed in 1 of 7 patients had a similar temporal pattern of onset and resolution; this was also declared as a DLT. On the 28/28 schedule, no DLT was observed.
Safety and tolerability
Pictilisib was well-tolerated up to 330mg (21/28 schedule); most adverse events were mild to moderate in severity with no treatment-related deaths (Table 2). At the assessed dose levels, there did not appear to be a significant difference in the toxicity profile between the 21/28 and 28/28 schedules. Treatment-related adverse events that occurred in ≥10% of patients included: nausea, diarrhea, vomiting, fatigue, dysgeusia, decreased appetite and rash. In addition to the 2 DLTs of grade 3 rash at the 450mg dose level, the third patient at this dose level experienced grade 2 rash; nonetheless, this patient received 8 months of pictilisib with concomitant use of oral antihistamines and skin emollients. Of 10 patients treated with 330mg once-daily(28/28 schedule), grade 1 or 2 rash was observed in 2 patients, and grade 3 rash (occurring after the DLT-defining window) in 2 patients; these similarly resolved with the introduction of drug holidays and supportive medications including emollients and corticosteroids.
Other clinically-relevant drug-related adverse events ≥grade 3 were grade 4 hyperglycemia (n=1, 130mg) and grade 3 pneumonitis (n=1, 340mg). The grade 4 hyperglycemia was transient, unaccompanied by clinically significant symptoms, signs or acidosis, and occurred in a patient with cholangiocarcinoma and previous pancreatico-duodenectomy who started the use of low-dose prednisolone 2 days prior to the event. Grade 3 pneumonitis was observed at the end of cycle 1 in a breast cancer patient previously treated with chest radiotherapy who developed grade 1 dyspnea, reduced DLCO and a ground glass appearance on HRCT; these resolved following 2 weeks of drug interruption and concomitant use of prednisolone. When pictilisib was reintroduced at 240mg, the dyspnea and HRCT changes recurred; these subsequently resolved following permanent discontinuation of pictilisib due to disease progression.
Pharmacokinetics
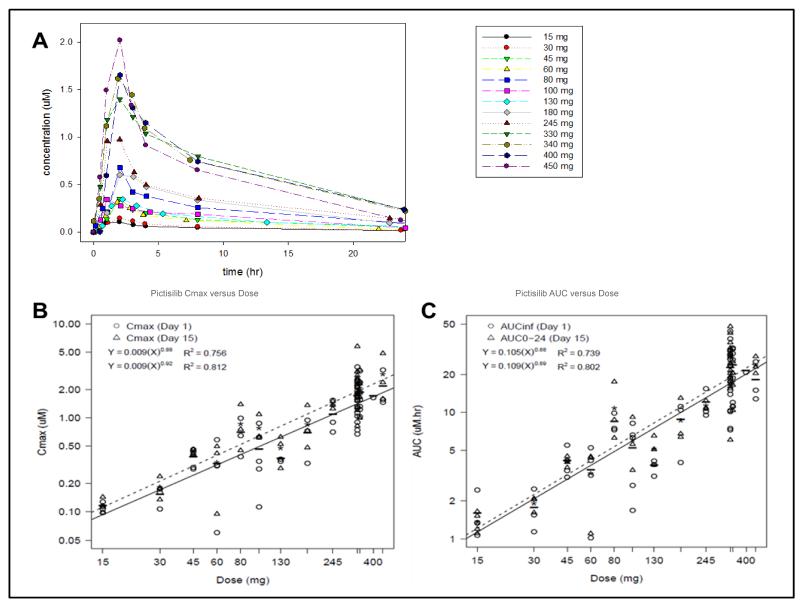
Pharmacokinetic parameters of pictilisib were estimated for all dose cohorts and are summarized in Table 3 and Supplementary Table 1. Under fasting conditions, pictilisib was rapidly absorbed after oral administration (median Tmax of 2 hours [range 0.5-8]); this was independent of dose and was unchanged after multiple doses. Terminal plasma elimination half-life (T1/2) on day 1 ranged between 13.1 and 24.1 hours. Dose-proportional increases in exposure (Cmax and AUC0-24) was observed across the dose levels studied (Figure 1). Similar pharmacokinetic characteristics were seen on day 15. The accumulation index (AUCDay15/AUCDay1) ranged from 1.2 to 2.2, suggesting modest accumulation following multiple doses.
Table 3. Key pharmacokinetic parameters of pictilisib on days 1 and 15.
| Elimination half-life (hr) | Tmax (hr) | Cmax (μM) | AUC 0-24 (hr*μM) | |||
|---|---|---|---|---|---|---|
| Day 1 | No. of patients | Geometric mean | Median | Range | Geometric mean | Geometric mean |
| 15 mg QD | 4 | 19.2 | 2 | 1-2 | 0.112 | 0.978 |
| 30 mg QD | 3 | 24.1 | 2 | 1-2 | 0.144 | 1.18 |
| 45 mg QD | 4 | 19.5 | 2 | 2 | 0.362 | 2.77 |
| 60 mg QD | 4 | 18.8 | 2 | 1-2 | 0.242 | 2.02 |
| 80 mg QD | 3 | 20.1 | 2 | 1-2 | 0.632 | 5.45 |
| 100 mg QD | 5 | 14.6 | 2 | 1-8 | 0.362 | 3.19 |
| 130 mg QD | 3 | 13.1 | 2 | 1-2.5 | 0.359 | 2.99 |
| 180 mg QD | 3 | 17.1 | 2 | 2-3 | 0.611 | 5.95 |
| 245 mg QD | 3 | 16.8 | 2 | 1-2 | 0.994 | 8.18 |
| 330 mg QD | 17 | 15.7 | 2 | 0.5-8 | 1.52 | 14.4 |
| 340 mg QD | 7 | 20.9 | 2 | 1-4 | 1.68 | 14.9 |
| 400 mg QD | 1 | 14.8 | 2 | 2 | 1.65 | 15.6 |
| 450 mg QD | 3 | 15.0 | 2 | 1-2 | 1.95 | 13.9 |
| Day 15 | ||||||
| 15 mg QD | 4 | 1.5 | 1-4 | 0.116 | 1.35 | |
| 30 mg QD | 3 | 1 | 0.5-4 | 0.179 | 1.90 | |
| 45 mg QD | 4 | 2 | 2 | 0.436 | 4.09 | |
| 60 mg QD | 3 | 3 | 2-4 | 0.27 | 2.78 | |
| 80 mg QD | 3 | 3 | 3-4 | 0.779 | 9.86 | |
| 100 mg QD | 3 | 2 | 1-3 | 0.751 | 5.64 | |
| 130 mg QD | 3 | 1 | 1-2 | 0.457 | 5.03 | |
| 180 mg QD | 3 | 1 | 1-3 | 0.783 | 8.25 | |
| 245 mg QD | 3 | 1 | 1-3 | 1.37 | 11.3 | |
| 330 mg QD | 17 | 3 | 0-8 | 2.07 | 22.8 | |
| 340 mg QD | 6 | 1 | 1-4 | 1.95 | 16.5 | |
| 450 mg QD | 3 | 2 | 1-3 | 2.64 | 23.5 | |
Figure 1. Pharmacokinetic profile of pictilisib.
(A) Plasma concentration versus time profile for all dose levels.
(B) Maximum plasma concentration (Cmax) versus dose
(C) area under the curve (AUC) versus dose. Solid and dashed lines represent the fitted regression lines for days 1 and 15, respectively
Pharmacodynamics
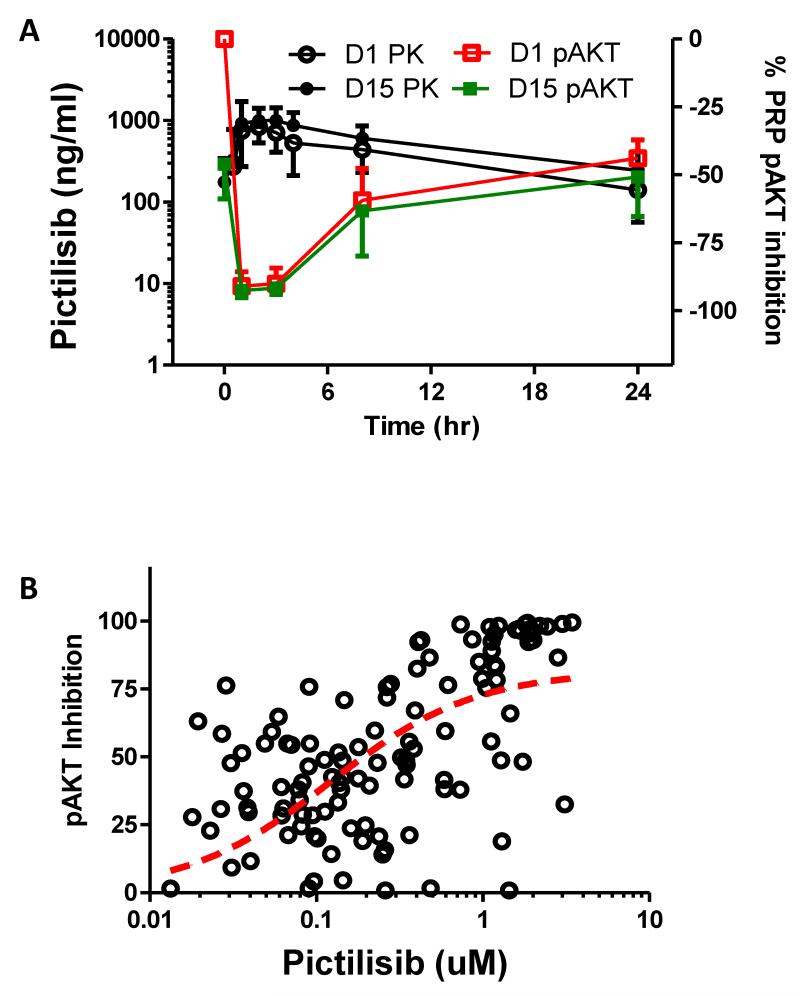
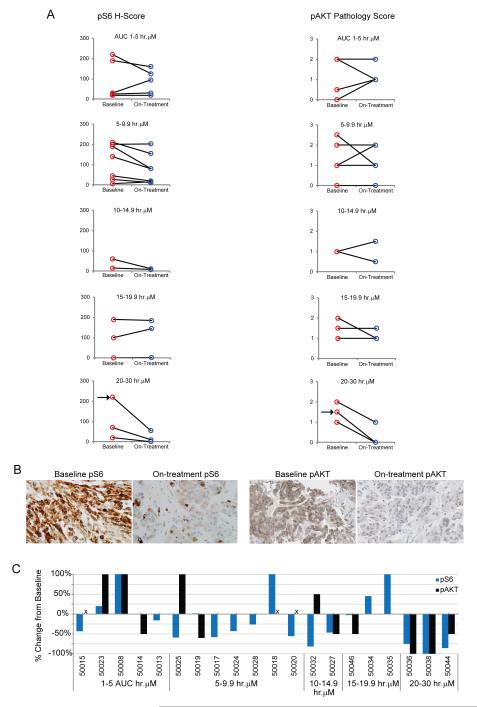
We observed a dose- and concentration-dependent decrease in phospho-AKT in platelet rich plasma on days 1 and 15 (Figure 2). Inhibition up to 90% of phospho-AKT level in platelet rich plasma was demonstrated 1 to 3 hours post-dose at the RP2D; this effect was sustained (50% of baseline) at 24 hours post-dose. Reductions in S6 and AKT phosphorylation in tumor biopsies was greatest at the highest exposure level of pictilisib (AUC >20uM.hr on day 15), with all three patients in this cohort showing ≥75% decrease in S6 phosphorylation and 2 patients also exhibiting a 100% decrease in AKT phosphorylation (Figure 3). Following pictilisib treatment at 330mg on cycle 1 day 1, there was a statistically significant increase in plasma insulin and glucose levels at 1 hour from baseline with mean fold-change (i.e. ratio of post-over pre-treatment level) in plasma insulin and glucose levels of 3.58 (95%CI: 2.33-5.49) and 1.18 (95%CI: 1.10-1.26), respectively (Supplementary figure 1. 18F-FDG-PET imaging showed a reduction from baseline of 18F-FDG-tracer uptake at dose levels ≥45mg with an overall median change in maximum standardized uptake value (SUVmax) of −13% (range −47 to 22) (Supplementary figure 2). Seven of 32 evaluable patients (22%) achieved a metabolic partial response by EORTC-PET criteria (>25% reduction in mean SUVmax without new lesions) on 18F-FDG-PET.10
Figure 2.
(A) Percentage change in levels of phosphorylated AKT (pAKT) in platelet-rich plasma (PRP) and plasma level of pictilisib up to 24 hours following administration of pictilisib (at RP2D of 330mg) on days 1 and 15 of cycle 1 (n=8).
(B) Pictilisib concentration-dependent inhibition of phosphorylated AKT (pAKT) in platelet-rich plasma (PRP) following systemic exposure to pictilisib. Data represent available concentration-matched PRP pAKT inhibition from duration of Phase I investigation
Figure 3. Pharmacodynamic analysis in tumor biopsies.
(A) Phospho-S6 (pS6) and phospho-AKT (pAKT) levels in baseline and on-treatment paired tumor biopsies grouped according to AUC0-24 (hr*uM) on day 15. pS6 staining levels were measured using a standard H-Score method, and pAKT staining levels were measured using a validated, qualitative scoring method (represented on y axis). Pre- and on-treatment samples from the same patient were stained in the same experiment and examined blind. Arrows indicate patient 50036.
(B) Immunohistochemistry images of pS6 and pAKT from patient 50036.
(C) Percent change from baseline of pS6 and pAKT levels. Tumor biopsies for three patients were not evaluable for phospho-AKT and are indicated with an “x”. All patients received doses of >60mg of pictilisib with the exception of patient 50008 who received 45 mg.
Antitumor Activity
One patient with BRAF V600E-mutated metastatic melanoma, but no detected PI3K pathway deregulation, achieved a confirmed RECIST-partial response; she received pictilisib at 330mg once-daily (21/28 schedule) for 9.5 months. She had previously been treated sequentially with paclitaxel and dacarbazine, but had not received a BRAF or MEK inhibitor. A heavily-pretreated, platinum-refractory advanced epithelial ovarian cancer patient with PIK3CA amplification (with high polysomy and >60% of tumor cells harboring 4 copies of PIK3CA) and loss of PTEN achieved radiological stable disease for 4 months with GCIG-CA125 partial response13 (Supplementary Figure 3) associated with 36% reduction in SUV on 18max F-FDG-PET and 56% reduction in tumor phospho-S6 expression. A patient with cKIT exon 9 mutant gastrointestinal stromal tumor but no evidence of PI3K pathway deregulation achieved stable disease for 7.5 months on pictilisib 450mg once-daily, and this was associated with pharmacodynamic changes of 47% reduction in SUV on 18max F-FDG-PET and 75% reduction in tumor phospho-S6 expression.
Of 60 patients, 12 (20%) remained on study for >3 months and 2 (3%) for >6 months. Supplementary Table 2 shows the pharmacokinetic-pharmacodynamic-clinical relationship of patients who demonstrated a partial response by RECIST, GCIG-CA125 or 18F-FDG-PET EORTC criteria.
DISCUSSION
In this first-in-human dose-escalation Phase I study, we confirm the feasibility of safely inhibiting Class I PI3K in patients with solid tumors. Pictilisib was well-tolerated at doses shown here to modulate PI3K signaling in normal and tumor tissues, and demonstrated dose-proportional pharmacokinetics. The most common drug-related toxicities included grade 1-2 nausea, fatigue, diarrhea, vomiting, dysgeusia and reduced appetite. The RP2D of oral pictilisib was 330mg continuous once-daily dosing. Dose limiting toxicity was grade 3 maculopapular rash occurring in 2 of 3 patients treated at 450mg/day and 1 of 7 patients at 330mg/day. Our data indicate that the severity of the rash was not clearly related to higher exposures of pictilisib (Supplementary Figure 4) and resolved with discontinuation of pictilisib. The rash was maculopapular but not acneiform or blistering, did not show a predilection for sun-exposed areas, and was similar to the rash observed with other PI3K, AKT and mTOR inhibitors, thereby pointing to a mechanism-based toxicity.14-16 Although this toxicity could be a potential pharmacodynamic biomarker of pictilisib, its underlying pathophysiology remains unclear and warrants further evaluation.
Our study design incorporated the use of the ‘Pharmacological Audit Trail’ guidelines using appropriate pharmacokinetic-pharmacodynamic biomarkers.17,18 Our study design incorporated the use of the pharmacological audit trail guidelines using appropriate pharmacokinetic-pharmacodynamic biomarkers. The audit trail has been used by both academia19 and industry20 as a guideline to help answer critical ‘go/no-go’ decisions based on scientifically measurable and rigorous end points. This audit trail has utility in providing broad guidance of the drug development process, even though the same precise effects in preclinical models may not be exactly reproduced in patients, as demonstrated in this trial. The favorable preclinical PK properties of pictilisib were reproduced in this Phase I trial.9 Preclinical pharmacokinetic-pharmacodynamic modeling based on MDA-MB-361, a human breast cancer xenograft with HER2 amplification and PIK3CA 1633G>A mutation, predicted the minimal target plasma AUC to be 6uM*hr (3000 ng*hr/ml) in order to achieve ≥90% tumor growth inhibition; this target AUC was achieved in most patients treated at doses >80mg following one week of consecutive dosing and support the view that pictilisib is associated with biologically meaningful activity at these dose levels and exposures, especially at the RP2D. Based on these preclinical data, we additionally postulated that ≥90% inhibition of AKT phosphorylation is needed to inhibit cancer cell proliferation.9,21 We demonstrate here a dose-response relationship between drug exposure and target modulation in normal tissue, with up to 90% decrease in AKT phosphorylation in platelet rich plasma at up to 3 hours post-dose at the RP2D. Whilst a direct correlation between pictilisib exposure and decrease in S6 and AKT phosphorylation in tumor is less clear, at the highest level of drug exposure (AUC >20uM/hr) there was clear evidence of pharmacodynamic effect, with >75% decrease in S6 phosphorylation and 100% reduction in AKT phosphorylation in 2 of 3 patients. The lack of consistent target modulation in tumor at lower drug exposures contrasts with the consistent dose-response relationship in platelet-rich plasma and is possibly related to different assay conditions which likely favour greater inhibition in the latter, together with disparities in drug concentrations between normal and tumor tissues due to likely differences in tissue architecture and haemodynamics. Such differences highlight the complexity of accurately defining the pharmacokinetic-pharmacodynamic relationship of molecularly targeted drugs, along with additional important issues including extrapolation of preclinical models to predict effects in patients, inter-patient variation due to host pharmacogenomic variation, intra-and inter-patient tumour heterogeneity, and differences in analytical sensitivity/methodology in platelet-rich plasma versus tumour biopsies. All of these are likely to be important, highlighting the importance of evaluating different pharmacodynamic biomarkers to increase confidence in the overall pharmacological effects of a drug within the audit trail framework, as we have done here.
To this end, at the RP2D of 330mg we demonstrate multimodal pharmacodynamic evidence of target modulation including the reduction of 18F-FDG-PET tracer uptake, inhibition of phospho-AKT in platelet-rich plasma and phospho-S6 in tumor tissue as well as increase in plasma glucose and insulin levels. These pharmacodynamic changes were observed regardless of molecular genetic status and from dose levels ≥100mg.
PI3K signaling regulates insulin sensitivity, and hyperglycemia has been predicted to be a hallmark toxicity of PI3K inhibition.6,22,23 However, pictilisib-related hyperglycemia was limited to grade 1-2 elevations in this study with grade ≥3 hyperglycemia being observed in only 1 patient with an extrahepatic cholangiocarcinoma who had undergone a previous pancreatic resection and was commenced on a corticosteroid 2 days prior to the adverse event. We confirmed target and pathway modulation at the RP2D with observations of convincing pharmacodynamic changes in phospho-S6 and 18FDG-PET in tumor, and phospho-AKT, glucose and insulin in plasma. Nonetheless, it is possible that homeostatic mechanisms via negative feedback loops may cause drug resistance and account for the lack of more significant hyperglycemia.
The toxicity profile of pictilisib is in contrast to BKM120 (another pan-Class I PI3K inhibitor) where rash, hyperglycemia and mood alterations were the observed DLTs.15 Apart from rash, the latter two were not significant toxicities of pictilisib. One would not have expected such differences if these DLTs are mechanism-based toxicities, especially when the observed in vitro potency of pictilisib is higher than that of BKM120.24 Pictilisib has a lower central nervous system (CNS) penetration than BKM120 while the targeted disruption of insulin signaling in the brain has been shown to lead to a diabetes mellitus phenotype.15,25-28 It is likely that the marked hyperglycemia observed with BKM120 is due to the synergistic inhibition of PI3K signaling in peripheral tissues (e.g. muscle and adipose tissues) with non-canonical insulin-targeted tissues (including the brain), and the lack of CNS penetration may have enhanced the clinical therapeutic index of pictilisib relative to BKM120. The other pan-Class I PI3K inhibitors which have undergone Phase I clinical evaluation include SAR245408 and the irreversible wortmanin derivative PX-866.29,30 Both drugs were associated with minimal hyperglycaemia but differences were observed in the frequency of rash, which occurred in 26% of patients treated with SAR245048 (all grades) and in none of the patients treated with PX-866. The importance of the therapeutic window of the pan-Class I inhibitors with regards to their pharmacodynamic effect is critical, and in this respect our hypothesis that ≥90% inhibition of AKT phosphorylation is needed to inhibit cancer cell proliferation reveals potentially important differences between pictilisb and the other drugs in this class, with SAR245048 reporting a 40-80% reduction in tumor AKT phosphorylation, in comparison to pictilisib achieving 100% AKT inhibition in twp patients at the MTD. There is currently no validated predictive biomarker for PI3K pathway inhibitors. Somatic mutational sequencing and assessment of PTEN expression status were therefore undertaken. Our results highlight ongoing difficulties in the attempt to identify predictive biomarkers for pan-Class I PI3K inhibitors, with no clear relationship between PI3K mutation/amplification or PTEN expression status and response to pictilisib.3 In this trial, PI3K pathway alterations were identified in 9 of 60 patients (15%), comprising 3 PTEN negative; 1 PTEN negative and PIK3CA amplification by FISH; and 5 PIK3CA mutations. Of these, none achieved a RECIST-based response and only 1 achieved a response by GCIG-CA125 criteria. This suggests the prediction of sensitivity may require more complex biomarker signatures rather than single mutational events.3,21 Additionally, an association between 18F-FDG-PET changes and RECIST response was not detected, in keeping with results from an earlier study of mTOR inhibitors.31
We note the modest response rates in this trial and therefore, whilst we postulate that ~90% inhibition of AKT phosphorylation for several hours is needed to inhibit cancer cell proliferation based on our preclinical tumor phosphorylation data9 the exact duration and magnitude to which the pathway should be inhibited remains unclear and more work is required to relate the extent of downstream phosphoprotein biomarker modulation to efficacy in patients. We also believe that important distinctions should be made between the utility of reduction in AKT phosphorylation as a pharmacodynamic and predictive biomarker of response. The best RECIST-responder in this study bore a BRAF V600E mutant melanoma with no demonstrable PI3K mutation; she achieved a radiological partial response by RECIST and remained on pictilisib for 9.5 months (she was not previously treated with a BRAF inhibitor and this was not therefore a withdrawal response). Responses to PI3K pathway inhibitors have been reported in BRAF mutated cancer cell lines, including those without any known PI3K pathway aberration.32 Although these cancer cell lines are primarily dependent on RAS-RAF-MEK-ERK signalling for their proliferation, they are likely to have a degree of co-dependence on the PI3K-AKT pathway for their proliferation and survival, possibly explaining the response to pictilisib in this patient. In addition, there may be other activating effects in the PI3K pathway that we have been unable to assess that predispose to sensitivity including potential immunomodulatory effects given that T cell receptor signalling involves the PI3K pathway.33
An increasing understanding of potential resistance mechanisms21 and promising preclinical data from combinatorial use of pictilisib34-36 has led to clinical trials of pictilisib in combination with drugs including erlotinib, fulvestrant, trastuzumab emtansine (Kadcyla®), GDC-0973 (a MEK inhibitor), paclitaxel, and chemotherapy regimens comprising paclitaxel/bevacizumab, carboplatin/paclitaxel/bevacizumab, and cisplatin/pemetrexed. Results from these ongoing studies are awaited.
In summary, pictilisib was safely tolerated at doses up to the RP2D which is 330mg once-daily administered continuously. Displaying favorable pharmacokinetic properties, the plasma exposure levels achieved with ≥80mg once-daily dosing were consistent with those associated with effective target modulation and antitumor activity predicted by preclinical in vivo pharmacokinetic-pharmacodynamic modeling. Analysis of pharmacodynamic biomarkers in peripheral blood and tumor tissue crucially provided evidence of significant PI3K pathway modulation at the RP2D. There is also evidence of clinical antitumor activity. Overall, these data provide strong support for the continued clinical evaluation of pictilisib.
Supplementary Material
Plots of fold change (i.e. ratios of 1 hour-post- over pre-treatment) in plasma levels of insulin (left panel) and glucose (right panel) on cycle 1 day 1 following treatment with different doses of pictilisib (Represented for each dose level are the geometric mean and the associated 95% confidence intervals of the ratios).
Following pictilisib treatment, there was a 56% reduction in tumoral phospho-S6 levels (A), 30% reduction in mean SUVmax in her subcapsular liver metastases [indicated in blue arrows] (B), a decrease in serum CA125 from 1214 U/ml to 400 U/ml (C) and stable disease by RECIST for 4 months. Biomarker analyses revealed the presence of PIK3CA amplification by fluorescence in-situ hybridization (with high polysomy and >60% of tumor cells having 4 copies of PIK3CA) and loss of PTEN by immunohistochemistry.
TRANSLATIONAL RELEVANCE.
The Phosphatidylinositol-3-kinase (PI3K) pathway is one of the most commonly deregulated in cancer and is currently a major focus for anticancer drug development. This manuscript describes the first-in-man phase I trial of pictilisib (GDC-0941), one of the very first pan-class I selective PI3K inhibitors evaluated in patients with advanced cancer. Pictilisib demonstrated a favorable pharmacokinetic profile and was well tolerated at biologically active doses. Comprehensive pharmacodynamic biomarker evaluation showed suppression of AKT and S6 phosphorylation in platelet rich plasma and tumor, together with significant changes in plasma insulin and glucose levels, and decreases in 18F-FDG uptake by PET. In addition, anti-tumor activity is reported in patients with PI3K pathway deregulation. These results provide the basis for further evaluation of pictilisib in rationally designed monotherapy or combination trials. In addition, this phase I trial of pictilisib exemplifies the use of ‘Pharmacological Audit Trail’ guidelines for molecularly targeted therapy in cancer.
ACKNOWLEDGEMENTS
The authors would like to thank the patients who participated in this study and their families
GRANT SUPPORT: This study was supported by Genentech Inc. The Drug Development Unit, The Royal Marsden NHS Foundation Trust, and The Institute of Cancer Research (London) is supported in part by programme grants from Cancer Research UK. Support was also provided by Experimental Cancer Medicine Center grants (to The Institute of Cancer Research and the Cancer Research UK Center), the National Institute for Health Research Biomedical Research Center (jointly to The Royal Marsden NHS Foundation Trust and The Institute of Cancer Research) and the Wellcome Trust (grant 090952/Z/09/Z to Dr. Ang). Paul Workman is a Cancer Research UK Life Fellow.
Footnotes
DISCLOSURES:
The following authors have no conflicts of interest to declare: Debashis Sarker, Jooern Ang, Rebecca Kristeleit, Krunal Shah, Victor Moreno, Gallia Levy, Jenny Wu, Stan Kaye.
The following authors have relevant conflicts of interest to declare:
Richard Baird has received honoraria and research funding from Genentech Inc.
Paul Clarke has received royalties from The Institute of Cancer Research ‘Rewards to Inventors’ scheme for development of PI3K inhibitors.
Florence Raynaud has received royalties from The Institute of Cancer Research ‘Rewards to Inventors’ scheme for development of PI3K inhibitors, and is a consultant for ELARA pharmaceuticals.
Johann De-Bono has served on advisory boards for Genentech, GSK, Merck, Pfizer, AstraZeneca, Millenium, Chugai.
Joseph Ware, Ray Lin, Jill Fredrickson, Mark Lackner, Yibing Yan, Lori Friedman and Mika Derynck are all currently employed by Genentech Inc, and have stock ownership in Roche.
Jill Spoerke is currently employed by Genentech Inc.
Kathryn Mazina was a prior employee at Roche and has stock ownership in Roche Mika Derynck has the following relevant patent to disclose: Methods of treating mesothelioma with a PI3K Compound, patent no. P4665R1.
Paul Workman was a founder of, consultant to, and Scientific Advisory Board member of Piramed Pharma (acquired by Roche); has been a founder of, consultant to and Scientific Advisory Board and Main Board member of Chroma Therapeutics; is on the Medical Advisory Board of WILEX; is a Member of the Scientific Advisory Board of Nextech Invest and Nuevolution; and was formerly an employee of AstraZeneca.
Paul Workman, Paul Clarke, Florence Raynaud, Johann de Bono are current employees of The Institute of Cancer Research, which has a commercial interest in the development of PI3K inhibitors, including pictilisib (GDC-0941), and operates a rewards-to-inventors scheme. Paul Workman, Paul Clarke and Florence Raynaud have previously been involved in a commercial collaboration with Yamanouchi (now Astellas Pharma) and with Piramed Pharma and intellectual property arising from the program has been licensed to Genentech. Debashis Sarker, Joo Ern Ang, Richard Baird, Rebecca Kristeleit, Krunal Shah, Victor Moreno and Stan Kaye are previous employees of The Institute of Cancer Research and are not part of the rewards-to-inventors scheme for pictilisib.
CLINICALTRIALS.GOV IDENTIFIER: NCT00876122
PRESENTED IN PART AT: 20th European Organization for the Research and Treatment of Cancer (EORTC) - National Cancer Institute (NCI) - American Association for Cancer Research (AACR) symposium on “Molecular targets and Cancer Therapeutics”, Geneva, Switzerland, 21-24 October 2008; 45th Annual Meeting of the American Society of Clinical Oncology (ASCO), May 29-June 2, 2009, Orlando, FL; Joint 15th Congress of the European CanCer Organization (ECCO) and 34th Congress of the European Society for Medical Oncology (ESMO) 20-24 September 2009, Berlin, Germany; 46th Annual Meeting of ASCO, June 4-8, 2010, Chicago, IL; and 47th Annual Meeting of ASCO, June 3-7, 2010, Chicago, IL; Joint 16th Multi-disciplinary Congress of ECCO and 36th Congress of ESMO, 23-27th September 2011, Stockholm, Sweden
REFERENCES
- 1.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges M. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11:329–41. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 2.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–62. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 3.Clarke PA, Workman P. Phosphatidylinositide-3-kinase inhibitors: addressing questions of isoform selectivity and pharmacodynamic/predictive biomarkers in early clinical trials. J Clin Oncol. 2012;30:331–3. doi: 10.1200/JCO.2011.38.7167. [DOI] [PubMed] [Google Scholar]
- 4.Huang J, Manning BD. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem Soc Trans. 2009;37:217–22. doi: 10.1042/BST0370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22:2954–63. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 6.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28:1075–83. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–44. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Folkes AJ, Ahmadi K, Alderton WK, Alix S, Baker SJ, Box G, et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-t hieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem. 2008;51:5522–32. doi: 10.1021/jm800295d. [DOI] [PubMed] [Google Scholar]
- 9.Raynaud FI, Eccles SA, Patel S, Alix S, Box G, et al. Biological properties of potent inhibitors of class I phosphatidylinositide 3-kinases: from PI-103 through PI-540, PI-620 to the oral agent GDC-0941. Mol Cancer Ther. 2009;8:1725–38. doi: 10.1158/1535-7163.MCT-08-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 11.Chanock SJ, Burdett L, Yeager M, Llaca V, Langerod A, Presswalla S, et al. Somatic sequence alterations in twenty-one genes selected by expression profile analysis of breast carcinomas. Breast Cancer Res. 2007;9:R5. doi: 10.1186/bcr1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reid AH, Attard G, Brewer D, Miranda S, Riisnaes R, Clark J, et al. Novel, gross chromosomal alterations involving PTEN cooperate with allelic loss in prostate cancer. Mod Pathol. 2012;25:902–10. doi: 10.1038/modpathol.2011.207. [DOI] [PubMed] [Google Scholar]
- 13.Rustin GJ, Vergote I, Eisenhauer E, Pujade-Lauraine E, Quinn M, Thigpen T, et al. Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG) Int J Gynecol Cancer. 2011;21:419–23. doi: 10.1097/IGC.0b013e3182070f17. [DOI] [PubMed] [Google Scholar]
- 14.Rodon J, Dienstmann R, Serra V, Tabernero J. Development of PI3K inhibitors: lessons learned from early clinical trials. Nat Rev Clin Oncol. 2013;10:143–53. doi: 10.1038/nrclinonc.2013.10. [DOI] [PubMed] [Google Scholar]
- 15.Bendell JC, Rodon J, Burris HA, de Jonge M, Verweij J, Birle D, et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30:282–90. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 16.Yap TA, Yan L, Patnaik A, Fearen I, Olmos D, Papadopoulos K, et al. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol. 2011;29:4688–95. doi: 10.1200/JCO.2011.35.5263. [DOI] [PubMed] [Google Scholar]
- 17.Workman P. How much gets there and what does it do?: The need for better pharmacokinetic and pharmacodynamic endpoints in contemporary drug discovery and development. Curr Pharm Des. 2003;9:891–902. doi: 10.2174/1381612033455279. [DOI] [PubMed] [Google Scholar]
- 18.Yap TA, Sandhu SK, Workman P, de Bono JS. Envisioning the future of early anticancer drug development. Nat Rev Cancer. 2010;10:514–23. doi: 10.1038/nrc2870. [DOI] [PubMed] [Google Scholar]
- 19.Dean E, Greystoke A, Ranson M, Dive C. Biomarkers of cell death applicable to early clinical trials. Exp Cell Res. 2012;318:1252–9. doi: 10.1016/j.yexcr.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 20.Hait WN. Forty years of Translational Cancer Research. Cancer Discovery. 2011;1:383–390. doi: 10.1158/2159-8290.CD-11-0196. [DOI] [PubMed] [Google Scholar]
- 21.Workman P, Clarke PA, Raynaud FI, van Montfort RL. Drugging the PI3 kinome: from chemical tools to drugs in the clinic. Cancer Res. 2010;70:2146–57. doi: 10.1158/0008-5472.CAN-09-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powis G, Ihle N, Kirkpatrick DL. Practicalities of drugging the phosphatidylinositol-3-kinase/Akt cell survival signaling pathway. Clin Cancer Res. 2006;12:2964–6. doi: 10.1158/1078-0432.CCR-06-0617. [DOI] [PubMed] [Google Scholar]
- 23.Foukas LC, Claret M, Pearce W, Okkenhaug K, Meek S, Peskett E, et al. Critical role for the p110alpha phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature. 2006;441:366–70. doi: 10.1038/nature04694. [DOI] [PubMed] [Google Scholar]
- 24.Brachmann SM, Kleylein-Sohn J, Gaulis S, Kauffmann A, Blommers MJ, Kazic-Legeux M, et al. Characterization of the mechanism of action of the pan class I PI3K inhibitor NVP-BKM120 across a broad range of concentrations. Mol Cancer Ther. 2012;11:1747–57. doi: 10.1158/1535-7163.MCT-11-1021. [DOI] [PubMed] [Google Scholar]
- 25.Salphati L, Lee LB, Pang J, Plise EG, Zhang X. Role of P-glycoprotein and breast cancer resistance protein-1 in the brain penetration and brain pharmacodynamic activity of the novel phosphatidylinositol 3-kinase inhibitor GDC-0941. Drug Metab Dispos. 2010;38:1422–6. doi: 10.1124/dmd.110.034256. [DOI] [PubMed] [Google Scholar]
- 26.Bruning JC, Gautam D, Burks DJ, Gilette J, Schubert M, Orban PC, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–5. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 27.Okamoto H, Nakae J, Kitamura T, Park BC, Dragastis I, Accili D. Transgenic rescue of insulin receptor-deficient mice. J Clin Invest. 114:214–23. doi: 10.1172/JCI21645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8:1376–82. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro GI, Rodon J, Bedell C, Kwak EL, Baselga J, Brana I, et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of SAR245408 (XL147), an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2014;20:233–45. doi: 10.1158/1078-0432.CCR-13-1777. [DOI] [PubMed] [Google Scholar]
- 30.Hong DS, Bowles DW, Falchook GS, Messersmith WA, George GC, O’Bryant CL, et al. A multicenter phase I trial of PX-866, an oral irreversible phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2012;18:4173–82. doi: 10.1158/1078-0432.CCR-12-0714. [DOI] [PubMed] [Google Scholar]
- 31.Ma WW, Jacene H, Song D, Vilardelli F, Messersmith WA, Laheru D, et al. [18F]fluorodeoxyglucose positron emission tomography correlates with Akt pathway activity but is not predictive of clinical outcome during mTOR inhibitor therapy. J Clin Oncol. 2009;27:2697–704. doi: 10.1200/JCO.2008.18.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lassen A, Atefi M, Robert L, Wong DJ, Cerniglia M, Comin-Anduix B, et al. Effects of AKT inhibitor therapy in response and resistance to BRAF inhibition in melanoma. Mol Cancer. 2014;13:83. doi: 10.1186/1476-4598-13-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ali K, Soond DR, Piñeiro R, Hagemann T, Pearce W, Lim EL, Bouabe H, et al. Inactivation of PI(3)K p110δ breaks regulatory T-cell-mediated immune tolerance to cancer. Nature. 2014;510:407–11. doi: 10.1038/nature13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15:429–40. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 35.Hoeflich KP, O’Brien C, Boyd Z, Cavet G, Guerroero S, Jung K, et al. In vivo antitumor activity of MEK and phosphatidylinositol 3-kinase inhibitors in basal-like breast cancer models. Clin Cancer Res. 2009;15:4649–64. doi: 10.1158/1078-0432.CCR-09-0317. [DOI] [PubMed] [Google Scholar]
- 36.Yao E, Zhou W, Lee-Hoeflich ST, Truong T, Haverty PM, Eastham-Anderson J, et al. Suppression of HER2/HER3-mediated growth of breast cancer cells with combinations of GDC-0941 PI3K inhibitor, trastuzumab, and pertuzumab. Clin Cancer Res. 2009;15:4147–56. doi: 10.1158/1078-0432.CCR-08-2814. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plots of fold change (i.e. ratios of 1 hour-post- over pre-treatment) in plasma levels of insulin (left panel) and glucose (right panel) on cycle 1 day 1 following treatment with different doses of pictilisib (Represented for each dose level are the geometric mean and the associated 95% confidence intervals of the ratios).
Following pictilisib treatment, there was a 56% reduction in tumoral phospho-S6 levels (A), 30% reduction in mean SUVmax in her subcapsular liver metastases [indicated in blue arrows] (B), a decrease in serum CA125 from 1214 U/ml to 400 U/ml (C) and stable disease by RECIST for 4 months. Biomarker analyses revealed the presence of PIK3CA amplification by fluorescence in-situ hybridization (with high polysomy and >60% of tumor cells having 4 copies of PIK3CA) and loss of PTEN by immunohistochemistry.