Abstract
Tumor metabolism and bioenergetics have become important topics for cancer research and are promising targets for anticancer therapy. Although glucose serves as the main source of energy, proline, an alternative substrate, is important, especially during nutrient stress. Proline oxidase (POX), catalyzing the first step in proline catabolism, is induced by p53 and can regulate cell survival as well as mediate programmed cell death. In a mouse xenograft tumor model, we found that POX greatly reduced tumor formation by causing G2 cell cycle arrest. Furthermore, immunohistochemical staining showed decreased POX expression in tumor tissues. Importantly, HIF-1α signaling was impaired with POX expression due to the increased production of α-ketoglutarate, a critical substrate for prolyl hydroxylation and degradation of HIF-1α. Combined with previous in vitro findings and reported clinical genetic associations, these new findings lead us to propose POX as a mitochondrial tumor suppressor and a potential target for cancer therapy.
Keywords: proline oxidase, tumor suppressor, hypoxia inducible factor, mouse model, metabolism
Introduction
Though controversial over the years, a molecular basis for the “Warburg effect” is emerging from genetic and pharmacological studies which demonstrate that specific oncogene and tumor suppressor mutations directly regulate glycolysis, oxidative phosphorylation, and other energy/nutrient supply processes (1–5). The combination of these mutations and the hypoxic conditions in many tumor types is likely to control synergistically the overall metabolic state and the fate of individual tumors. There are several mitochondrial tumor suppressors that have been identified (6). The critical ways they exert their anti-tumor effect are through hypoxia inducible factor (HIF), a key player in tumor formation, and affects the tricarboxylic acid (TCA) cycle. Our previous and current findings indicate proline oxidase plays a similar role and is a mitochondrial tumor suppressor.
Proline oxidase (POX), also called proline dehydrogenase (PRODH), is a mitochondrial inner-membrane enzyme involved in the degradation of the secondary amino acid proline. The function of this flavoenzyme is to catalyze the rate-limiting two-electron oxidation of proline to Δ1-pyrroline-5-carboxylate (P5C) and the subsequent transfer of reducing equivalents from the reduced flavin cofactor to the mitochondrial electron transport chain (7). Together with P5C reductase, which converts P5C back to proline, POX mediates the proline cycle to shuttle redox equivalents between mitochondria and cytosol. P5C is the intermediate providing a direct carbon bridge connecting the TCA cycle through glutamate and α-ketoglutarate (α-KG) and to the urea cycle through ornithine (8). Another important feature about proline is that together with hydroxyproline, it constitutes over 25 % of incorporated residues of collagen, which is the most abundant protein in the extracellular matrix and, in fact, in the entire human body. When degraded during inflammation and tumor progression, collagen releases a large quantity of proline after digestion by matrix metalloproteinases and prolidase. The contribution of these products to a variety of cellular processes has recently been recognized (9).
Recent research has revealed the response of POX to energy and nutrient stress. After it was found to be one of genes markedly induced by p53 (10), its role in cell proliferation, apoptotic cell death, particularly in cancer cells has been intensively investigated. POX negatively regulates the growth of various types of cancer cells, which prompted us to investigate further its potential role in human cancer development. In this study, we employed multiple approaches to define the role of POX in tumor suppression and characterized mediating mechanisms. In addition to inducing apoptotic cell death, POX also caused cell cycle arrest at G2 phase. Further, we found that POX inhibited tumor formation in a mouse xenograft model. Importantly, in human tissue samples, POX expression levels were much lower in tumor tissues than in their normal tissue counterparts. Finally, HIF signaling was reduced in POX-expressing cells. These findings, combined with published data and genetic information, suggest that POX is a mitochondrial tumor suppressor protein.
Materials and Methods
Cell culture
The DLD-1 human colon cancer cells are from America Type Culture Collection (ATCC; Manassas, VA). The generation, characterization and maintenance of the DLD-1 Tet-Off POX and DLD-1 Tet-Off Vector cell lines have been previously described (Donald, Liu). Dimethyloxalylglycine (DMOG) was purchased from Frontier Scientific (Logan, UT). Doxycycline (DOX) was purchased from Sigma (St. Louis, MO).
Multiple protein sequence alignments
These sequence alignments were done using the CLUSTALW2 program at http://align.genome.jp/. The amino acid sequences were aligned and compared among the species of homo sapiens, mus musculus, rattus norvegicus, danio rerio and drosophila melanogaster.
Cell growth assays
The CellTiter 96™ aqueous non-radioactive cell proliferation assay (MTS assay; Promega, Madison, WI), performed according to the manufacturer's protocol, was described in our previous studies (11). Each data point was performed in triplicate, and the results were reported as mean absorption ± standard error.
Western blot analysis
The methods for cell lysate preparation, protein concentration determination and electrophoresis have been described previously (11). The primary antibodies used were GADD45α (Chemicon) GADDβ, -γ, -34, -153 (Santa Cruz Co. Santa Cruz, CA), HIF-1α, VEGF, geminin, cyclin B1, p21, cdc25c, cdc2, p-cdc2 (tyr-15), p-cdc2 (thr-161) (Cell Signaling, Beverly, MA), (Calbiochem, San Diego, CA), and actin (Sigma, St. Louis, MO). The anti-POX polyclonal antibody was prepared and tested in the lab. Blots were developed using the chemiluminescence (ECL) procedure (Amersham, Piscataway, NJ). All Western blots shown are representative of at least two independent experiments.
Flow cytometry
The methods have been reported previously (11). DLD-1 Tet-Off POX cells were cultured in the medium with various concentration of DOX. Propidium iodide-stained cells were analyzed with an EPICS-XL-MCL flow cytometer (Beckman Coulter, Inc., Miami, FL). Each data point was performed in triplicate, and the results were reported as the mean ± standard error.
Semi-quantitative RT-PCR analysis
Total RNA was prepared from DLD-1-POX cells using Qiagen RNeasy kits (Qiagen). RT-PCR reactions were performed using Ready-To-Go RT-PCR Beads (Amersham). The RT reaction was carried out using 0.5µg RNA at 42°C for 30 min, followed by 5 minutes at 95°C to inactivate the enzyme. Then, 2µM specific primers were added per reaction and subjected to 23–28 cycles of amplification at: 94°C 30s/60°C 30s/72°C 45s. RT-PCR to actin was performed as control. All PCR shown are representative of at least two independent experiments. The specific sequences of the primers used have been reported elsewhere (12–15).
Animal Studies
The animal protocol was approved by the National Cancer Institute-Frederick Animal Care and Use committee. Sixty-two Balb/C athymic mice were randomized to four groups. Two groups with nine mice in each were injected with 2x106 of DLD-1 Tet-off Vector cells fed water with or without 100 µg/ml of DOX. The other two groups, with 22 mice in each, were given same number of DLD-1 Tet-off POX cells, also in (+) DOX or (−) DOX conditions. For mice that did not develop tumors in (−) DOX group, half of them (8 mice) were switched to receive drinking water with DOX while the other half were kept in (−) DOX. The tumor development in these two new groups was monitored. The mice were observed for tumor development three times a week and tumors were measured twice a week after they developed.
Immunohistochemical staining
The immunostaining for POX was performed by the Pathology/Histotechnology Laboratory, NCI-Frederick. The human tissue arrays, which contained paired cancer/normal tissues from the same patient, were purchased from Cybridi, Co (Frederick, MD). Two independent pathologists read and graded the expression as 5 levels: 0, +, ++, +++, and ++++. The expression of POX in tumor and normal tissues from the same patients were then compared, and changes (up-regulation, no change, or down-regulation) were determined. Data were statistically analyzed using the Z-test. The xenograft tumors were collected from athymic mice and fixed in 10 % formaldehyde and then embedded in paraffin. Tissue sections were then mounted on slides and processed for either hematoxylin & eosin staining or immunostaining for POX, BrdU, or for TUNEL assay.
Determination of the a-keto acids
α-Keto acids were determined by HPLC as their fluorescent quinoxalinol derivatives as per the method of Pailla (16). Analysis was carried out by HPLC on a C18 reversed phase column with fluorescence detection using an excitation λ of 350 nm and an emission λ of 410 nm. Concentration of the keto acids were calculated from linear calibration plots using the internal standard method.
Determination of the organic acids: lactic acid, succinic acid, and fumaric acid
Organic acids were determined by Gas Chromatography/Mass Spectrometry (GC/MS) as per the method of Hagen (17). The following characteristic fragment ions were monitored; 261 m/z (lactic acid), 289 m/z (succinic acid), 287 m/z (fumaric acid), and 179 m/z (benzoic acid). Concentrations of the organic acids were calculated from linear calibration plots using the internal standard method.
Soft agar colony formation assay
DLD-1 Tet-Off POX cells were seeded at 1 × 103 per well in six-well dishes with a bottom layer of 0.7% agar in DMEM and a top layer of 0.35% agar in DMEM, while both layers contained DOX in concentrations of 20, 1.0, 0.2, and 0.0 ng/ml, respectively. Colonies (>0.2 mm in diameter) were counted after 10 to 14 days. At least two independent assays were performed in triplicate.
Statistical analysis
Z-test was used for immunohistochemical staining data. Null hypothesis of proportion of decrease is equal to 50%. For other data, Fisher’s exact test and Student's t-test were used.
Results
POX is highly conserved during evolution
Using ClustalW2 alignment software, we compared amino acid sequences of POX protein from several species. We found homologues of POX in Homo sapiens, Mus musculus, Rattus norvegicus, Danio rerio, and Drosophila melanogaster (Fig. S1). This finding indicates that this gene is highly conserved among various species during evolution, and suggests an important role for POX in eukaryotic cells.
POX overexpression inhibited tumor development in a mouse xenograft model
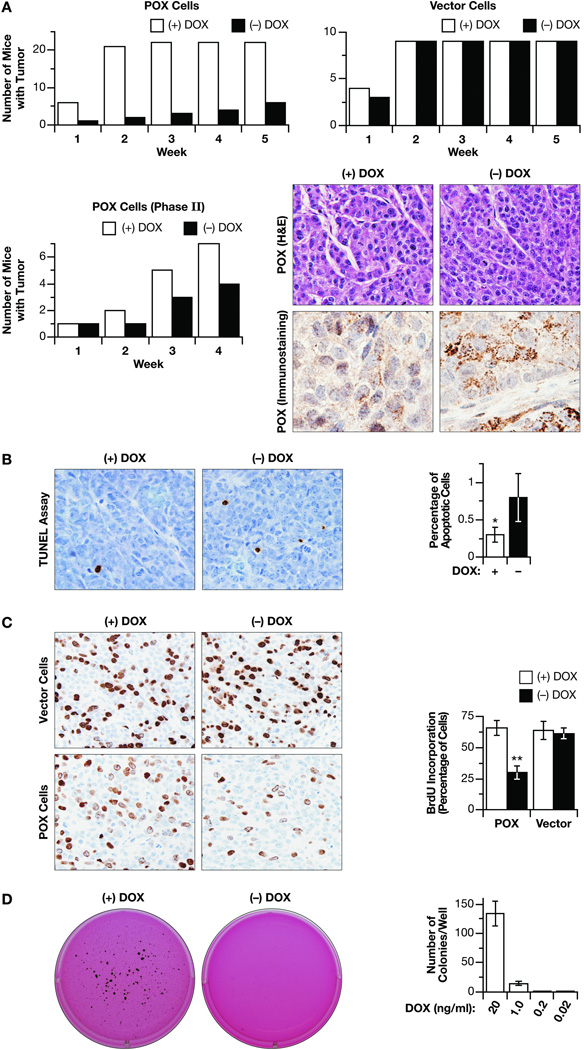
We and others have shown that POX inhibited cell growth in a variety of cultured cancer cells (11, 18–21). To extend this cancer suppressing effect to in vivo studies, we developed a mouse xenograft model by injecting DLD-1 Tet-off POX cells and DLD-1 Tet-off Vector cells into athymic mice. In mice receiving vector control cells, tumors developed quickly. The addition of DOX to the drinking water made no difference for tumor development (Fig. 1A). For mice injected with DLD-1-POX cells, in the (+) DOX group, as was the case with vector controls, tumors developed quickly. By contrast, tumor development was greatly reduced in mice in the (−) DOX group (Fig. 1A). To extend this experiment further, we switched half (8) of the mice in the (−) DOX group which were tumor-free by week 5 to receive drinking water with DOX to suppress POX expression, and then compared the tumor development in these two new groups (Phase II). Data again showed that the (−) DOX group had fewer mice with tumors (Fig. 1A). The induced-expression of POX by removal of DOX in drinking water was demonstrated by immunostaining for POX, which was mainly found in the cell cytoplasm consistent with its mitochondrial location (Fig. 1A).
Fig. 1.
in vivo tumor growth inhibition. DLD-1 Tet-off POX cells and vector control cells were injected into Balb/C nude mice. (A). Numbers of mice which developed tumors after injection of cells when mice were given water with or without DOX. The left panel shows mice injected with POX cells; the right panel shows mice injected with vector cells. For those mice injected with POX cells in the (−) DOX group that didn’t develop tumors, half (8 mice) was switched to receive water with DOX. The numbers of mice developed tumors were recorded. H&E staining and immunohistochemical staining for POX are shown to demonstrate the induced expression and location of POX. (B) TUNEL assay showed relatively high apoptosis induction in POX-expressing cells. The right panel shows quantitation of at least 3 slide preparations. Mean ± SD are shown. (C). Immunohistochemical staining shows the reduced BrdU incorporation by POX. Right panel shows quantification from at least 3 slides. Mean ± SD are shown. (D). Soft agar assay showed reduced clonogenic ability of DLD-1 cells by POX. Right panel is a quantitation of clones per well and represent data ± of at least 4 determinations. The asterisks indicated statistically significant difference (* p < 0.05, ** p < 0.01). Magnification for C-E was×40 or×100.
To understand the mechanism by which POX inhibited in vivo tumor development, we assessed proliferation and apoptosis using the mouse tumor tissues. In TUNEL assays, consistent with in vitro data, we found higher levels of apoptosis in (−) DOX condition than in (+) DOX (Fig. 1B). We also performed BrdU incorporation assay to assess cell proliferation. The incorporation rate was greatly reduced in POX cells in (−) DOX (29.4 %) compared with that in (+) DOX (65.6 %) or vector cells in either condition (Fig. 1C). Because the apoptotic rate was low we suspected that cell cycle blockade/inhibition of proliferation was the major reason for POX’s inhibition of tumor development and growth.
To characterize the effects on growth further, we performed soft agar colony formation assays. When cells were kept in (+) DOX condition (20 ng/ml), they readily formed clones. When in (−) DOX, the cell’s cloning ability was totally blocked. DOX at a concentration as low as 1.0 ng/ml markedly reduced clone formation (Fig. 1D).
POX also caused G2 cell cycle arrest to inhibit cell growth
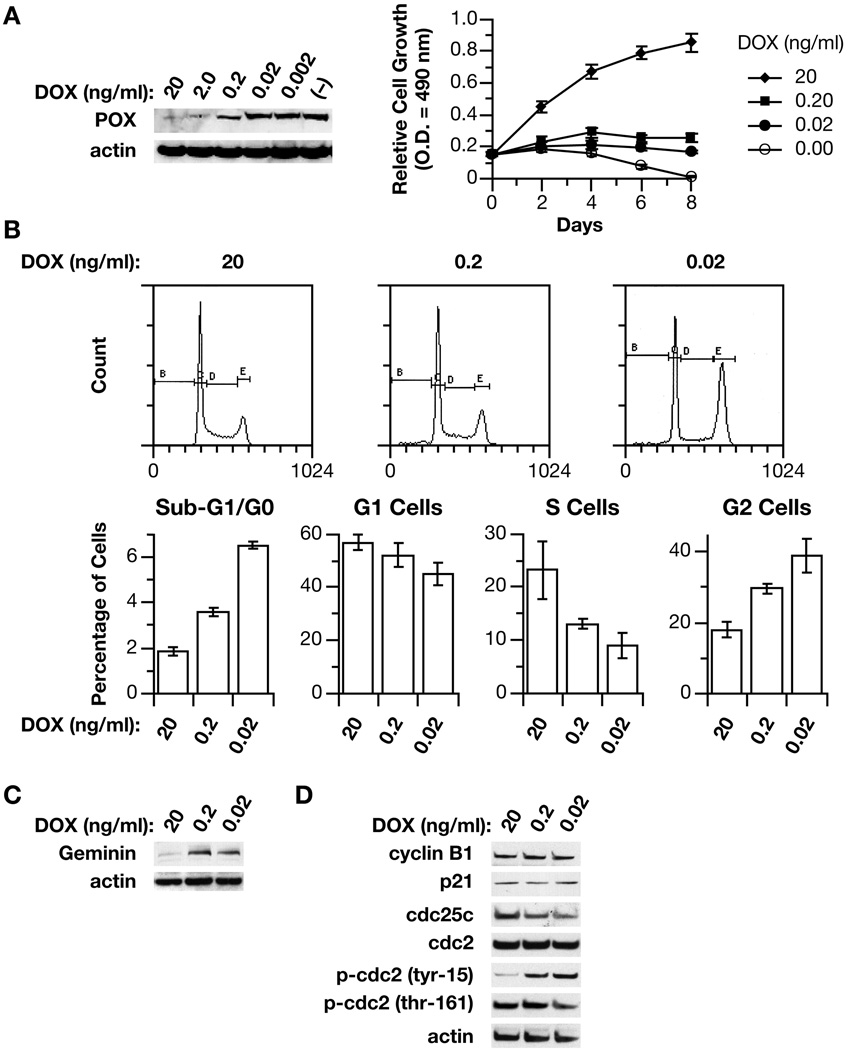
A tumor suppressor protein exerts its inhibitory function through either induction of apoptosis or blockade of cell cycle, or both. The published data from this and other laboratories clearly demonstrated the role of POX in the induction of apoptosis, and the mechanism and signaling pathways involved have been suggested (11, 18–23). However, our animal work indicated that POX might also regulate progression of cells in the cell cycle. Since apoptosis and not cell cycle distribution was the prominent feature of cells when POX was highly expressed in previously published studies (11, 18–20), we suspected that expression levels of POX might be critical for its roles. Thus, taking advantage of the Tet expression system, which allows the introduction of target genes dose-dependently by DOX, we determined the effects of POX at different levels on cell growth and the cell cycle. First we confirmed that POX was indeed suppressed by DOX in a dose-dependent fashion in DLD-1 Tet-off POX cells (Fig. 2A). Then we turned on the expression of POX at different levels by varying DOX concentrations and monitored its effects on cell growth. Consistent with our previous data, cells grew normally with DOX (20 ng/ml) while cells died when in (−) DOX. Importantly we found that cells neither grew nor died when cultured in medium with reduced DOX (0.2 and 0.02 ng/ml), suggesting a possible blockade in cell cycle (Fig. 2A).
Fig. 2.
POX caused G2 cell cycle arrest. (A). POX was dose-dependently repressed by increasing DOX concentrations in DLD-1 Tet-Off POX cells. Cell growth assay showed increasing DOX concentrations released DLD-1 cells from POX-mediated inhibition of growth. (B). Flow cytometry assay showed that increasing POX expression with decreasing DOX concentration induced G2 cell cycle arrest. (C). Western blot showed the induction of Geminin, by increasing POX expression with decreasing DOX. (D). The effects of POX expression with decreasing DOX concentrations on several important regulators of G2 phase determined by Western blots.
To investigate cell cycle distribution further, we performed flow cytometry. Based on the dose-dependent effect of DOX on POX expression, cells were treated with DOX at 100- and 1000-fold dilutions of the usual concentration for the (+) DOX condition (0.2 and 0.02 ng/ml) for three days to induce POX expression at relatively low levels, with cells in (+) DOX (20 ng/ml) as control. We found that the percentages of cells in sub-G1/G0 phase were low and increased only slightly (from 2% to 4–6%); the significant changes were in S phase, which decreased from 23% to 8–13%, and in G2 phase, which increased from 18 % to 30–39%, indicating a G2 cell cycle arrest by POX when it was expressed at moderate levels (Fig. 2B). Geminin is a nuclear protein that inhibits DNA replication, and has been used as a marker for G2 phase (24). Its expression was also upregulated by POX in DLD-1 cells (Fig. 2C).
To investigate further the molecular regulation of G2 phase and G2/M transition, we examined CDC2, the cyclin-dependent kinase that normally drives cells into mitosis and is the ultimate target of pathways that mediate rapid G2 arrest in response to DNA damage (25). We found that although total CDC2 did not change, the phosphorylated CDC2 at tyrosine 15 increased while phosphorylation at theronine 161 decreased with POX expression (Fig. 2D). These changes indicated that CDC2 was in an inactive status. CDC25C, the phosphatase that removes the inhibitory phosphates from CDC2 and activates cyclinB-CDC2, was found to be downregulated (Fig. 2E). It is likely that these changes were directly responsible for POX-induced G2 cell cycle arrest.
GADDs may play a role in POX-induced G2 cell cycle arrest
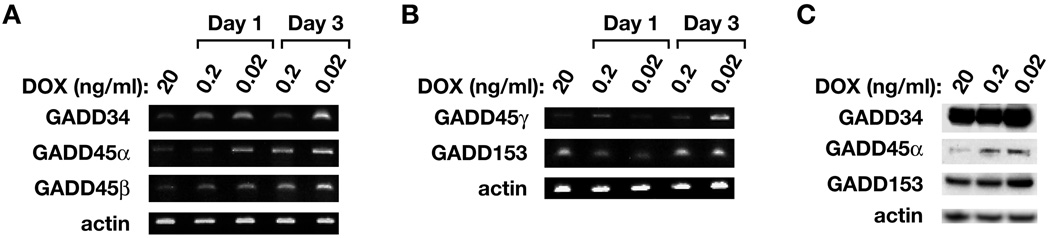
The finding that POX arrested cells in G2 phase prompted us to identify pathways and genes whose expression might be responsible for this arrest, as well as for POX-induced apoptosis and other changes. First we turned to cDNA microarray (Affymatrix U133A 2.0), which covers 14,500 well-characterized human genes and transcripts. We found that the expression of 814 genes had been changed more than 2 fold when POX is over expressed (manuscript in preparation). Among them, the increased expression of the GADD (growth arrest and DNA damage inducible gene) family was of interest, because the most important role of this family is to cause G2 cell cycle arrest, and GADD45α, the main member of this family, is a downstream gene of p53; it directly interacts with p21 and PCNA, both important direct regulators of the cell cycle. It also plays a role in apoptotic induction in certain situations (26). Interestingly, most genes in this family were upregulated with POX overexpression. The array data revealed that GADD45α was induced by 3.2 fold, GADDβ by 6.3 fold, GADDγ by 3.8 fold, and GADD34 by 4.4 fold. To confirm this, we performed semi-quantitive RT-PCR and Western blots for these members. The POX expression of DLD-1 cells was induced at different levels for 1 or 3 days. Elevation of GADD34, GADD45α, -β, -γ at the RNA level and GADD34, GADD45α and GADD153 at the protein level was observed (Fig. 3). The detailed role of GADDs in POX’s inhibition of cell growth is still under investigation. But, certainly, the upregulation of GADDs may play a role in POX-induced G2 cell cycle arrest, as well as apoptosis.
Fig. 3.
The involvement of GADD family proteins. The DLD-1 Tet-off POX cells were cultured in medium with 20 ng/ml [(+) DOX], or 100 and 1000 dilution (0.2 ng/ml, 0.02 ng/ml) for 1 or 3 days. The cells were harvested and RNA and cell lysates were prepared and then semi-quantitative RT-PCR (A & B) and western blots (C) for GADD members were performed. Actin was used as control.
The reduced expression of POX in human cancer tissues
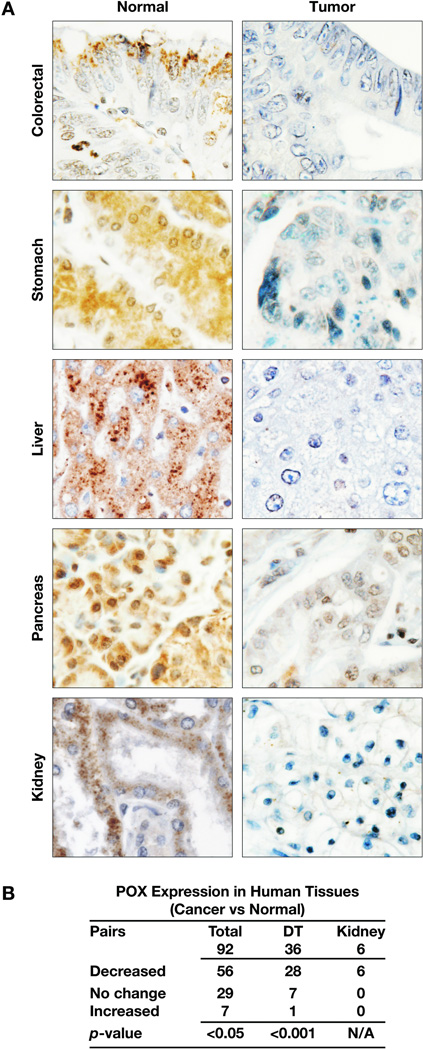
To demonstrate the role/function of a gene product in cancer development and progression, it is important to determine its differential expression levels in human tumors. Taking advantage of the availability of human tissue arrays that provide a large number and variety of cancer tissues along with normal tissue counterparts from the same patient as control, we performed immunohistochemical staining for the expression of POX. In total, we had 92 pairs of tumor/normal tissues from kidney, bladder, stomach, colon and rectum, liver, pancreas, breast, prostate, ovary, brain, lung, skin, etc. Strikingly, POX levels were much lower in tumor tissues when compared to normal tissues (Fig. 4). Statistical analysis showed the difference significant (p<0.05). This difference was more significant in kidney, where all 6 pairs showed lower POX expression in tumors, and digestive tract tissues, including those from colon, rectum, stomach, liver and pancreas (Fig. 4B). These findings indicate that POX may be related to cancer development and progression in humans, particularly in gastrointestinal tissues and kidney.
Fig. 4.
The reduced expression of POX in human tumor tissues. 92 pairs of human cancer and normal tissues from same patient, including 36 pairs from gastrointestinal system and 6 pairs from kidney were immunohistochemically stained for POX. (A). The representative images were shown from tissues of colon, stomach, liver, pancreas and kidney. Magnification,×100. (B). Z-test was used to do statistical analysis (Null hypothesis of proportion of decrease is equal to 50 %). DT: digestive tract tissues.
A potential mitochondrial tumor suppressor
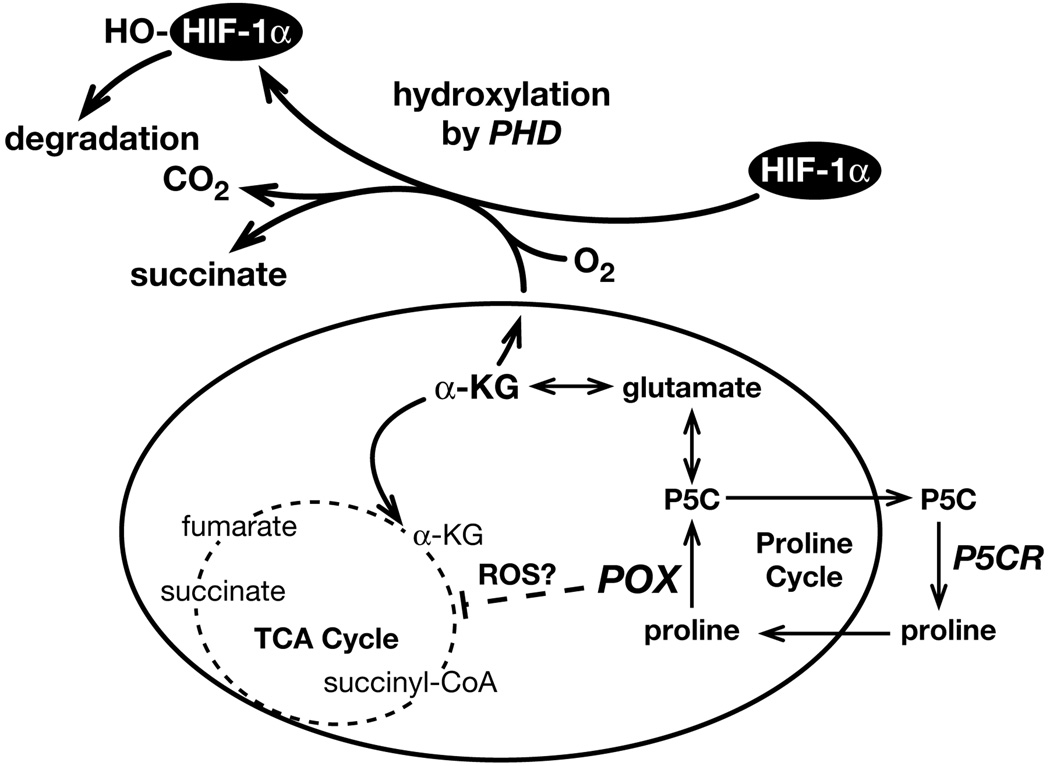
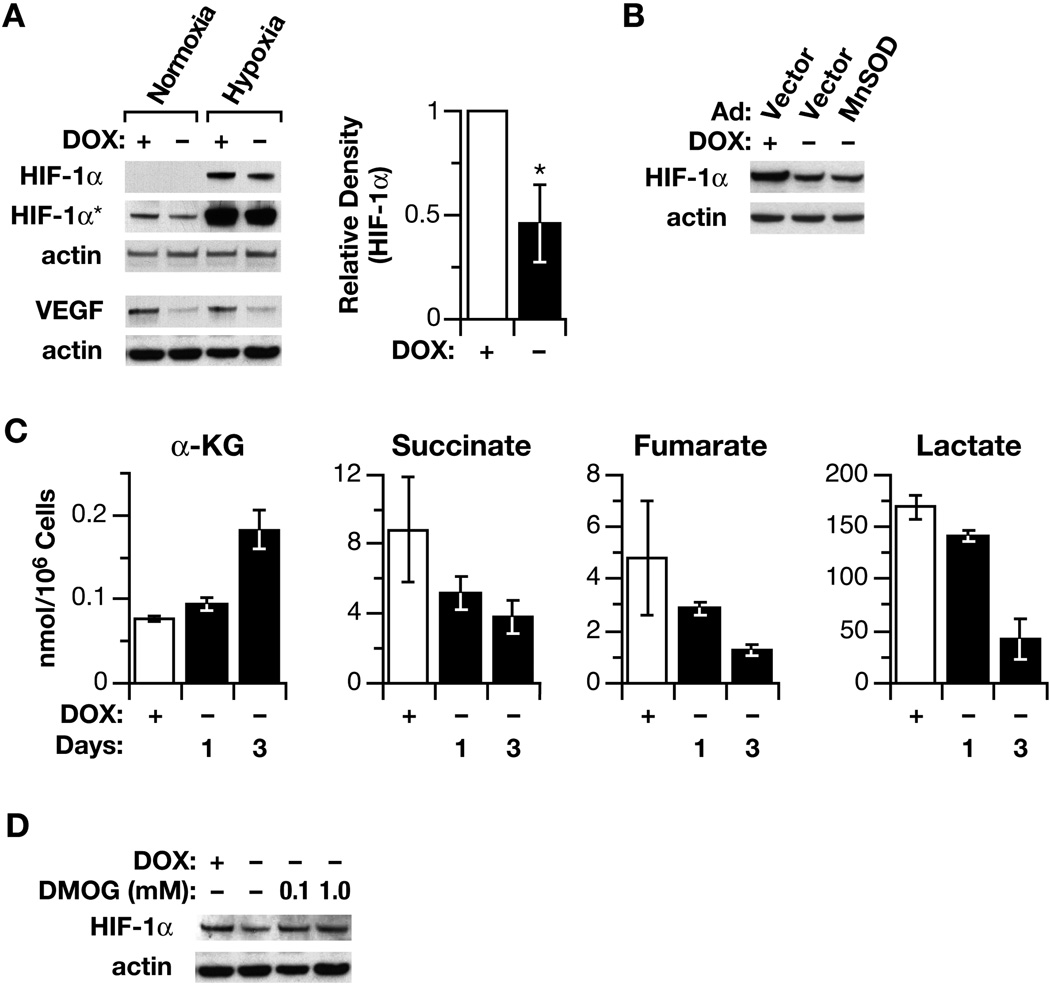
POX protein is located in the mitochondrial inner membrane, and has an anaplerotic role through glutamate and α-KG for the TCA cycle (8) ( Fig. 6). Due to this special location and function, we considered that POX may regulate HIF signaling, as has been suggested for other mitochondrial tumor suppressors (6). Thus, we investigated HIF signaling. We found that HIF-1α levels were greatly reduced by POX in both normoxic and hypoxic conditions (Fig. 5A). As expected, the expression of its downstream gene, VEGF, was also downregulated by POX (Fig. 5A). Further, we tried to determine the mechanism by which HIF signaling was inhibited. As the most important oxygen sensor, the stability and transcriptional activity of HIF-1α are regulated through post-translational hydroxylation of specific prolyl and asparaginal residues in its α-subunits. These oxygen-sensitive modifications are catalyzed by members of the 2-oxoglutarate (α-ketoglutarate) dioxygenase family (27). Recent studies have revealed that several TCA cycle intermediates and glycolytic metabolites, including succinate and fumarate, inhibit PHD activity and stabilize HIF signaling (27–31). We suspected that as an important link between proline metabolism and TCA cycle, α-KG, a substrate of PHD, might play a critical role in the downregulation of HIF by POX (Fig. 6). Thus, we determined the effect of POX on the cellular levels of α-KG by HPLC analysis and found increased levels of α-KG with overexpression of POX (Fig. 5C). Further, the levels of several TCA cycle intermediates were measured by GC/MS and succinate, fumarate and lactate all were found to be decreased with POX expression (Fig. 5C). To directly decide whether α-KG played a role in the inhibition of HIF signaling by POX, we employed the widely used, cell-permeating α-KG analogue DMOG. The results demonstrated that DMOG blocked POX’s inhibition of HIF (Fig. 5D), further supporting the important role of α-KG in the regulation of HIF signaling. This finding further assures the important connection between proline cycle and TCA cycle.
Fig. 6.
Proposed mechanisms by which POX inhibit HIF signaling. The interconversion between proline and P5C, catalyzed by POX and P5CR, respectively, forms the proline cycle. When POX expression is high, P5C, glutamate and α-KG are sequentially produced, forming an important link between proline and the TCA cycle. As an important substrate of prolyl hydroxylase, α-KG increases the hydroxylation of HIF-1α, which will be degradated through ubiquitinal and proteosomal degradation systems. This is probably the principal mechanism by which POX expression decreases HIF signaling. On the other hand, the TCA cycle is affected, as demonstrated by decreased cellular levels of fumarate, succinate, and lactate. The decreased levels of these TCA cycle intermediates may also contribute to impair HIF signaling. In fact, inhibition of HIF could be another mechanism, along with those previously identified, by which POX exerts its tumor suppressing role.
Fig. 5.
The affect of POX on HIF signaling and TCA cycle intermediates. (A). The inhibition of HIF-1α and its downstream gene VEGF was demonstrated by Western blots in both normoxic and hypoxic conditions (1 % of O2). The relative average densitometric data of four Western blots to HIF-1α in normoxic condition were also shown (B). To determine the effect of ROS/superoxide on HIF signaling, MnSOD was introduced by an adenovirus vector, which did not reverse the POX effect on the expression of HIF-1α. (C). DLD-POX cells were plated with doxycycline. After 18 h, fresh medium with or without doxycycline was substituted for the plating medium. Levels of α-KG were determined by high-pressure liquid chromatography. Levels of succinate, fumerate, and lactate were determined by GC/MS. Data are expressed as nmol/106 cells and represent the mean ± SD of at least 3 determinations. (D) The effect of DMOG on HIF-1α levels was demonstrated by Western blots. The asterisks indicated statistically significant difference (**p < 0.01. HIF-1α* indicated longer exposure).
As a critical mediator of POX-mediated signaling and induction of apoptosis, the role of ROS/superoxides in HIF inhibition with POX expression was also examined. The data showed that after the removal of DOX from the culture medium and the induction of POX, HIF expression was decreased, but unlike our previously reported effects of POX on other signaling pathways, the effect on HIF-1α was not reversed by MnSOD, suggesting ROS/superoxides were not a mediator for HIF inhibition (Fig. 5B). Since α-KG, a critical substrate for PHD-catalyzed hydroxylation of HIF-1α and degradation, was markedly elevated with POX overexpression, it seems likely that it is the mediator for POX-dependent inhibition of HIF signaling.
DISCUSSION
The POX gene is evolutionarily conserved with a high degree of homology existing in drosophila, zebrafish, mouse, rat, human, etc., suggesting a fundamental biological role. In this present study, in keeping with previously published data from this and other laboratories, we established the tumor suppressor effect of POX in cancer by showing its effects on the regulation of several important signaling pathways: the induction of apoptosis, the blockade of the cell cycle and inhibition of tumor growth/development in vitro and in vivo. Importantly, a significant number of human tumors had reduced expression of POX. Additionally, the data revealed that POX could be a mitochondrial tumor suppressor by affecting HIF signaling and TCA cycle.
Tumor suppressors may lose their normal functions through various mechanisms; this loss would facilitate malignant transformation and cell proliferation (32). We sought genetic and epigenetic variations in the POX gene and its promoter using human tumors and normal tissues, but no definitive changes have been found thus far (occasional polymorphisms but no mutations or hypermethylations). Nevertheless, the literature indicates genetic changes in POX in several human cancers. The POX gene is located at chromosome 22q11.2, a common breakpoint and a region subject to translocation. Deletions at this site has been associated with a variety of human cancers (33, 34). The linkage of POX alterations with cancer development is also supported by the observation that there is much higher frequency of malignancy in patients with chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome) (34). Two tumor suppressors have been identified in chromosome 22q11, hSNF5 and NF2 (35, 36). Some studies suggest the presence of another tumor suppressor gene whose alteration is responsible for the development of several types of cancer (37–39). One genetic study described ependymomas with 22q11 loci loss of heterozygosity, but neither NF2 nor hSNF5 gene was involved (40, 41). Thus, the existence of other tumor suppressor genes at this locus was suggested. It is tempting to speculate that POX may be this putative suppressor,
As a mediator of these cancer suppressive effects, we found a novel POX-mediated mechanism in its downregulation of HIF-1 signaling. In DLD-1 POX cells, overexpression of POX increased the cellular level of α-KG, an important substrate for the hydroxylation of HIF-1α by PHDs. As a substrate for PHD, α-KG stimulates hydroxylation of HIF-1α, increases its binding to VHL protein and causes its decrease via rapid ubiquitinylation and subsequent proteasomal degradation. HIF is an anti-apoptotic factor; thus, the POX-mediated decrease in HIF signaling may contribute to POX-induced apoptosis and inhibition of cell and tumor growth.
We and others previously have shown the critical role of ROS/superoxides in POX-induced apoptosis (11, 18, 19, 21). Actually, the effects of ROS on HIF could be either positive or negative, depending on ambient oxygen levels, etc. (27). As our data showed that MnSOD had no effect on POX-mediated inhibition of HIF-1α (Fig. 6D), the regulation of HIF signaling is most likely not due directly to POX-mediated generation of superoxides. In fact, α-KG is the likely mediator of the decrease in HIF-1α by POX, and this decrease occurs in spite of the POX-mediated increase in ROS and the known effect of ROS in stabilizing HIF-1α.
Thus, there are two important biochemical mechanisms related to POX-mediated stress responses. Dysregulation or loss of these responses contributes to tumor development and/or uncontrolled cell growth. One is redox stress resulting from increased levels of ROS. Another is metabolic signaling mediated by increased levels of α-KG. Both may produce their effects through activation/inhibition of well-recognized signaling pathways. Actually, these two functions may be linked and share a common denominator. For example, α-KG dehydrogenase, the enzyme converting α-KG to succinyl-CoA, is an important target of ROS, and is itself a generator of oxidative stress (42).
Mitochondrial dysfunction has long been linked to cancer, and a number of mechanisms have been suggested (3–5). It is, therefore, tempting to speculate on possible consequences in cells with POX dysregulation. POX may be upregulated under stress conditions to retard the cell cycle and augment apoptosis on the one hand, or alternatively, to provide TCA cycle intermediates and to provide redox coupling of the proline cycle to alternative energy pathways (43). The loss of the cancer suppressive functions of POX decreases POX-mediated apoptosis, removes the POX-mediated suppression of HIF signaling and augments the conversion to aerobic glycolysis, features of the Warburg effect.
It may be pointed out that increased cancer risk in patients with inherited deficiency of the POX has not been described. A possible explanation is the redundant function of hydroxyproline oxidase in apoptosis (44). Furthermore, patients with this rare inborn error are usually identified in childhood, and careful follow-up into the age range with increased cancer risk is not available
Finally, genetic or epigenetic mechanisms for the decrease or loss of POX deserve further study. Although well-recognized tumor suppressors such as BRCA1/2 and APC underlie familial cancer syndromes, with sporadic tumors, a substantial number of low-penetrance alleles remain to be identified. Loss of these alleles in combination may lead to malignant transformation. It is possible that POX dysregulation, along with other carcinogenic factors, can stimulate uncontrolled cell growth and eventually tumor formation. Thus, POX as a target for cancer therapeutics, in combinational metabolic interventions in particular, remains an attractive possibility.
Acknowledgments
This research is supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. This project also has been funded in part with Federal funds from the National Cancer Institute, National Institutes of Health under Contract No. HHSN261 200800001. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. NCI-Frederick is accredited by AAALAC International and follows the Public Health Service Policy for the Care and Use of Laboratory Animals. Animal care was provided in accordance with the procedures outlined in the “Guide for Care and Use of Laboratory Animals” (National Research Council; 1996; National Academy Press; Washington, D.C.).
REFERENCES
- 1.Bensaad K, Vousden KH. p53: new roles in metabolism. Trends Cell Biol. 2007;17(6):286–291. doi: 10.1016/j.tcb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Brahimi-Horn MC, Chiche J, Pouyssegur J. Hypoxia signalling controls metabolic demand. Curr Opin Cell Biol. 2007;19(2):223–229. doi: 10.1016/j.ceb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Hagland H, Nikolaisen J, Hodneland LI, Gjertsen BT, Bruserud O, Tronstad KJ. Targeting mitochondria in the treatment of human cancer: a coordinated attack against cancer cell energy metabolism and signalling. Exp Opin Ther Targets. 2007;11(8):1055–1069. doi: 10.1517/14728222.11.8.1055. [DOI] [PubMed] [Google Scholar]
- 4.Merida I, Avila-Flores A. Tumor metabolism: new opportunities for cancer therapy. Clin Transl Oncol. 2006;8(10):711–716. doi: 10.1007/s12094-006-0117-6. [DOI] [PubMed] [Google Scholar]
- 5.Ristow M. Oxidative metabolism in cancer growth. Curr Opin Clin Nut Metab Care. 2006;9(4):339–345. doi: 10.1097/01.mco.0000232892.43921.98. [DOI] [PubMed] [Google Scholar]
- 6.Gottlieb E, Tomlinson IP. Mitochondrial tumour suppressors: a genetic and biochemical update. Nat Rev Cancer. 2005;5(11):857–866. doi: 10.1038/nrc1737. [DOI] [PubMed] [Google Scholar]
- 7.Kowaloff EM, Phang JM, Granger AS, Downing SJ. Regulation of proline oxidase activity by lactate. Proc Natl Acad Sci U S A. 1977;74(12):5368–5371. doi: 10.1073/pnas.74.12.5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phang JM. The regulatory functions of proline and pyrroline-5-carboxylic acid. Curr Top Cell Reg. 1985;25:91–132. doi: 10.1016/b978-0-12-152825-6.50008-4. [DOI] [PubMed] [Google Scholar]
- 9.Phang JM, Pandhare J, Liu Y. The metabolism of proline as microenvironmental stress substrate. J Nut. 2008;138(10):2008S–2015S. doi: 10.1093/jn/138.10.2008S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B. A model for p53-induced apoptosis. Nature. 1997;389(6648):300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Borchert GL, Surazynski A, Phang JM. Proline oxidase, a p53-induced gene, targets COX-2/PGE2 signaling to induce apoptosis and inhibit tumor growth in colorectal cancers. Oncogene. 2008;27(53):6729–6737. doi: 10.1038/onc.2008.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fishel ML, Rabik CA, Bleibel WK, Li X, Moschel RC, Dolan ME. Role of GADD34 in modulation of cisplatin cytotoxicity. Bioch Pharmacol. 2006;71(3):239–247. doi: 10.1016/j.bcp.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 13.Yang J, Zhu H, Murphy TL, Ouyang W, Murphy KM. IL-18-stimulated GADD45 beta required in cytokine-induced, but not TCR-induced, IFN-gamma production. Nature immunology. 2001;2(2):157–164. doi: 10.1038/84264. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Sun H, Danila DC, et al. Loss of expression of GADD45 gamma, a growth inhibitory gene, in human pituitary adenomas: implications for tumorigenesis. J Clin Endocrinol Metab. 2002;87(3):1262–1267. doi: 10.1210/jcem.87.3.8315. [DOI] [PubMed] [Google Scholar]
- 15.Zou C, Guan Y, Zou C, et al. N-(4-hydroxyphenyl)retinamide (4-HPR) modulates GADD45 expression in radiosensitive bladder cancer cell lines. Cancer lett. 2002;180(2):131–137. doi: 10.1016/s0304-3835(01)00864-3. [DOI] [PubMed] [Google Scholar]
- 16.Pailla K, Blonde-Cynober F, Aussel C, De Bandt JP, Cynober L. Branched-chain keto-acids and pyruvate in blood: measurement by HPLC with fluorimetric detection and changes in older subjects. Clin Chem. 2000;46(6 Pt 1):848–853. [PubMed] [Google Scholar]
- 17.Hagen T, Korson MS, Sakamoto M, Evans JE. A GC/MS/MS screening method for multiple organic acidemias from urine specimens. Inter J Clin Chem. 1999;283(1–2):77–88. doi: 10.1016/s0009-8981(99)00037-6. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Borchert GL, Donald SP, et al. MnSOD inhibits proline oxidase-induced apoptosis in colorectal cancer cells. Carcinogenesis. 2005;26(8):1335–1342. doi: 10.1093/carcin/bgi083. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Borchert GL, Surazynski A, Hu CA, Phang JM. Proline oxidase activates both intrinsic and extrinsic pathways for apoptosis: the role of ROS/superoxides, NFAT and MEK/ERK signaling. Oncogene. 2006;25(41):5640–5647. doi: 10.1038/sj.onc.1209564. [DOI] [PubMed] [Google Scholar]
- 20.Maxwell SA, Rivera A. Proline oxidase induces apoptosis in tumor cells, and its expression is frequently absent or reduced in renal carcinomas. J Biol Chem. 2003;278(11):9784–9789. doi: 10.1074/jbc.M210012200. [DOI] [PubMed] [Google Scholar]
- 21.Rivera A, Maxwell SA. The p53-induced gene-6 (proline oxidase) mediates apoptosis through a calcineurin-dependent pathway. J Biol Chem. 2005;280(32):29346–29354. doi: 10.1074/jbc.M504852200. [DOI] [PubMed] [Google Scholar]
- 22.Donald SP, Sun XY, Hu CA, et al. Proline oxidase, encoded by p53-induced gene-6, catalyzes the generation of proline-dependent reactive oxygen species. Cancer Res. 2001;61(5):1810–1815. [PubMed] [Google Scholar]
- 23.Hu CA, Donald SP, Yu J, et al. Overexpression of proline oxidase induces proline-dependent and mitochondria-mediated apoptosis. Mol Cell Biochem. 2007;295(1–2):85–92. doi: 10.1007/s11010-006-9276-6. [DOI] [PubMed] [Google Scholar]
- 24.Zhu W, Chen Y, Dutta A. Rereplication by depletion of geminin is seen regardless of p53 status and activates a G2/M checkpoint. Mol Cell Biol. 2004;24(16):7140–7150. doi: 10.1128/MCB.24.16.7140-7150.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stark GR, Taylor WR. Control of the G2/M transition. Mol Biotechnol. 2006;32(3):227–248. doi: 10.1385/MB:32:3:227. [DOI] [PubMed] [Google Scholar]
- 26.Liebermann DA, Hoffman B. Gadd45 in stress signaling. J Mol Signal. 2008;3:15. doi: 10.1186/1750-2187-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verma A. Oxygen-sensing in tumors. Curr Opin Clin Nut Metab Care. 2006;9(4):366–378. doi: 10.1097/01.mco.0000232895.28674.79. [DOI] [PubMed] [Google Scholar]
- 28.Bruegge K, Jelkmann W, Metzen E. Hydroxylation of hypoxia-inducible transcription factors and chemical compounds targeting the HIF-alpha hydroxylases. Curr Med Chem. 2007;14(17):1853–1862. doi: 10.2174/092986707781058850. [DOI] [PubMed] [Google Scholar]
- 29.Hewitson KS, Lienard BM, McDonough MA, et al. Structural and mechanistic studies on the inhibition of the hypoxia-inducible transcription factor hydroxylases by tricarboxylic acid cycle intermediates. J Biol Chem. 2007;282(5):3293–3301. doi: 10.1074/jbc.M608337200. [DOI] [PubMed] [Google Scholar]
- 30.Koivunen P, Hirsila M, Remes AM, Hassinen IE, Kivirikko KI, Myllyharju J. Inhibition of hypoxia-inducible factor (HIF) hydroxylases by citric acid cycle intermediates: possible links between cell metabolism and stabilization of HIF. J Biol Chem. 2007;282(7):4524–4532. doi: 10.1074/jbc.M610415200. [DOI] [PubMed] [Google Scholar]
- 31.Lu H, Dalgard CL, Mohyeldin A, McFate T, Tait AS, Verma A. Reversible inactivation of HIF-1 prolyl hydroxylases allows cell metabolism to control basal HIF-1. J Biol Chem. 2005;280(51):41928–41939. doi: 10.1074/jbc.M508718200. [DOI] [PubMed] [Google Scholar]
- 32.Payne SR, Kemp CJ. Tumor suppressor genetics. Carcinogenesis. 2005;26(12):2031–2045. doi: 10.1093/carcin/bgi223. [DOI] [PubMed] [Google Scholar]
- 33.Handel-Fernandez ME, Nassiri M, Arana M, et al. Mapping of genetic deletions on the long arm of chromosome 22 in human pancreatic adenocarcinomas. Anticancer Res. 2000;20(6B):4451–4456. [PubMed] [Google Scholar]
- 34.McDonald-McGinn DM, Reilly A, Wallgren-Pettersson C, et al. Malignancy in chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome) Am J Med Genet. 2006;140(8):906–909. doi: 10.1002/ajmg.a.31199. [DOI] [PubMed] [Google Scholar]
- 35.Schmucker B, Tang Y, Kressel M. Novel alternatively spliced isoforms of the neurofibromatosis type 2 tumor suppressor are targeted to the nucleus and cytoplasmic granules. Hum Mol Genet. 1999;8(8):1561–1570. doi: 10.1093/hmg/8.8.1561. [DOI] [PubMed] [Google Scholar]
- 36.Versteege I, Sevenet N, Lange J, et al. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature. 1998;394(6689):203–206. doi: 10.1038/28212. [DOI] [PubMed] [Google Scholar]
- 37.Alonso ME, Bello MJ, de Campos JM, et al. No evidence of INI1hSNF5 (SMARCB1) and PARVG point mutations in oligodendroglial neoplasms. Cancer Genet Cytogenet. 2005;160(2):169–173. doi: 10.1016/j.cancergencyto.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 38.Kruzelock RP, Cuevas BD, Wiener JR, et al. Functional evidence for an ovarian cancer tumor suppressor gene on chromosome 22 by microcell-mediated chromosome transfer. Oncogene. 2000;19(54):6277–6285. doi: 10.1038/sj.onc.1204013. [DOI] [PubMed] [Google Scholar]
- 39.Manda R, Kohno T, Hamada K, Takenoshita S, Kuwano H, Yokota J. Absence of hSNF5/INI1 mutation in human lung cancer. Cancer Lett. 2000;153(1–2):57–61. doi: 10.1016/s0304-3835(00)00342-6. [DOI] [PubMed] [Google Scholar]
- 40.Huang B, Starostik P, Kuhl J, Tonn JC, Roggendorf W. Loss of heterozygosity on chromosome 22 in human ependymomas. Acta Neuropathol. 2002;103(4):415–420. doi: 10.1007/s00401-001-0479-3. [DOI] [PubMed] [Google Scholar]
- 41.Sevenet N, Lellouch-Tubiana A, Schofield D, et al. Spectrum of hSNF5/INI1 somatic mutations in human cancer and genotype-phenotype correlations. Hum Mol Genet. 1999;9(13):2359–2368. doi: 10.1093/hmg/8.13.2359. [DOI] [PubMed] [Google Scholar]
- 42.Tretter L, Adam-Vizi V. Alpha-ketoglutarate dehydrogenase: a target and generator of oxidative stress. Philosoph Trans Royal Soc London. 2005;360(1464):2335–2345. doi: 10.1098/rstb.2005.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hagedorn CH, Phang JM. Catalytic transfer of hydride ions from NADPH to oxygen by the interconversions of proline and delta 1-pyrroline-5-carboxylate. Arch Biochem Biophy. 1986;248(1):166–174. doi: 10.1016/0003-9861(86)90413-3. [DOI] [PubMed] [Google Scholar]
- 44.Cooper SK, Pandhare J, Donald SP, Phang JM. A novel function for hydroxyproline oxidase in apoptosis through generation of reactive oxygen species. J Biol Chem. 2008;283(16):10485–10492. doi: 10.1074/jbc.M702181200. [DOI] [PMC free article] [PubMed] [Google Scholar]