Abstract
Objectives
The aim of this study was to analyse the influence of the microtopography and hydrophilicity of titanium (Ti) substrates on initial oral biofilm formation.
Materials and methods
Nine bacterial species belonging to the normal oral microbiota, including: Aggregatibacter actinomycetemcomitans, Actinomyces israelii, Campylobacter rectus, Eikenella corrodens, Fusobacterium nucleatum, Parvimonas micra, Porphyromonas gingivalis, Prevotella intermedia, and Streptococcus sanguinis were tested on Ti surfaces: pretreatment (PT [Ra<0.2 μm]), acid-etched (A [Ra<0.8 μm]), A modified to be hydrophilic (modA), sand-blasted/acid-etched (SLA [Ra = 4 μm]), and hydrophilic SLA (modSLA). Disks were incubated for 24 h in anaerobic conditions using a normal culture medium (CM) or human saliva (HS). The total counts of bacteria and the proportion of each bacterial species were analysed by checkerboard DNA–DNA hybridization. Results: Higher counts of bacteria were observed on all surfaces incubated with CM compared with the samples incubated with HS. PT, SLA, and modSLA exhibited higher numbers of attached bacteria in CM, whereas SLA and modSLA had a significant increase in bacterial adhesion in HS. The proportion of the species in the initial biofilms was also influenced by the surface properties and the media used: SLA and modSLA increased the proportion of species like A. actinomycetemcomitans and S. sanguinis in both media, while the adhesion of A. israelii and P. gingivalis on the same surfaces was affected in the presence of saliva.
Conclusions
The initial biofilm formation and composition were affected by the microtopography and hydrophilicity of the surface and by the media used.
Keywords: biofilm, hydrophilicity, microstructure, titanium
Biofilm formation on dental implants is a persistent problem that can cause implant failure. Once a biofilm is formed, bacterial cells become highly resistant to antibiotics and host defenses (Costerton et al. 1999), and clinical experience has shown that biofilms must be removed physically before the infection can be resolved (Costerton 2005). There is an apparent clinical and microbiological similarity between peri-implantitis and periodontitis (Papaioannou et al. 1996; Listgarten & Lai 1999). However, it remains unclear whether the first steps in biofilm formation on titanium (Ti) implants are similar to biofilm formation on teeth.
The biofilm formation process is extremely complicated and this is particularly true when multiple species are present in the biofilm as in dental plaque. This process is affected by many factors including environment, bacterial properties, and material surface characteristics, such as chemical composition, surface energy, hydrophilicity, and topography (Merritt & Chang 1991; An & Friedman 1998; Katsikogianni & Missirlis 2004). in vitro studies of biofilm formation on Ti surfaces have focused on the effects of surface morphology and surface chemistry (Yoshinari et al. 2000; Grossner-Schreiber et al. 2001; Barbour et al. 2007), but most biofilm models in these studies have only included one or two bacterial strains. In the oral cavity, however, the microbial ecology is complex and can consist of hundreds of species, each with a preference for specific microenvironmental properties.
Studies investigating peri-implant microbiota in vivo have examined the influence of oral health status on the presence of specific bacterial species. Some of these studies report similar supra- and sub-gingival microbiota on teeth and Ti implants (Groessner-Schreiber et al. 2004; Furst et al. 2007; Shibli et al. 2008). In contrast, some studies found an absence of periodontal pathogens like Aggregatibacter (formerly Actinobacillus) actinomycetemcomitans and Porphyromonas gingivalis (Heuer et al. 2007).
On natural dental surfaces like enamel, the composition of the primary acquired pellicle plays an important role in determining the type and amount of bacteria that will attach (Gibbons 1996; Steinberg et al. 1998; Sela et al. 2007). Salivary and serum constituents can also adsorb onto Ti surfaces (Kohavi et al. 1995, 1997), and this is influenced by the structural and chemical properties of the surface (Katsikogianni & Missirlis 2004; Mabboux et al. 2004; Jeyachandran et al. 2006). Recent iterations of dental implant design are based on in vitro and in vivo studies showing that micron-scale and submicron-scale structural features increase osteoblast differentiation and peri-implant bone formation, which can be further enhanced by increased hydrophilicity (Cochran 1999; Buser et al. 2004; Lossdorfer et al. 2004; Schwarz et al. 2005, 2007a, 2007b; Zhao et al. 2005; Jimbo et al. 2008). Hydrophilic surfaces have a wicking effect on tissue fluids including blood, leading to adsorption of cell attachment proteins like fibronectin. While many cells involved in tissue regeneration use these proteins to adhere to the implant, the same proteins could also possess binding sites for bacteria (Quirynen et al. 2001; Plummer & Douglas 2006). However, the effect of these surface modifications on the attachment and growth of oral microorganisms, particularly under conditions that simulate the oral environment, is not known. The purpose of this study was to evaluate how biofilm formation and composition are affected by implant surface properties like microtopography and hydrophilicity using a regular culture medium (CM) or human saliva (HS).
Material and methods
Ti-disk surfaces
Ti disks with diameters of 15 mm were prepared from 1-mm-thick sheets of grade 2 unalloyed Ti (ASTM F67). The methods used to produce the pretreatment (PT), sand-blasted and acid-etched (SLA) and hydrophilic SLA (modSLA) surfaces have been reported previously (Schwarz et al. 2007a, 2007b). The PT surface is relatively smooth with a mean peak to valley roughness (Ra) <0.2 μm. Submicron rough A surfaces were produced by treating PT with heated concentrated acid, resulting in an Ra of 0.8 μm, based on surface profilometry. PT surfaces were also sand-blasted and acid-etched to produce SLA surfaces with a Ra of 3.2 μm. Before use, PT, A, and SLA surfaces were washed in ultrasonic cleaner and sterilized in an oxygen plasma cleaner (PDC-32G, Harrick Plasma, Ithaca, NY, USA). The modA and modSLA surfaces were produced with same mechanical or chemical treatments as A and SLA surfaces, respectively. However, the modA and modSLA surfaces were rinsed under nitrogen protection to prevent exposure to air during the procedure and then stored in a sealed glass tube containing isotonic NaCl solution, reducing adsorption of atmospheric hydrocarbons, and therefore, retaining a higher surface energy (Rupp et al. 2006). These sealed disks were sterilized by g-irradiation at 25 kGy over night. All disks were fabricated by Institut Straumann AG (Basel, Switzerland) and were shipped to us ready for use. Each surface was used in triplicate for the experiments described below.
Bacterial strains
Unless otherwise noted, chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA). Nine bacterial species were tested on each surface. The reference strain for each species is shown in Table 1. The nine selected species are representative of the normal subgingival dental plaque. We used Actinomyces israelii and Streptococcus sanguinis as early colonizers. To represent the second group of bacteria that functions as a bridge between the early and late colonizers, we used Fusobacterium nucleatum, Eikenella corrodens, and Prevotella intermedia. Finally, P. gingivalis was used as a representative of the third group of species that appears at late stages of biofilm development. Lyophilized bacterial stocks (American Type Culture Collection, Rockville, MD, USA) were rehydrated in Mycoplasma broth base (BBL, Becton-Dickinson and Co., Sparks, MD, USA). All strains were grown on Mycoplasma agar base (BBL, Becton-Dickinson and Co.) supplemented with 5% defibrinated sheep blood, 5 μg/ml hemin, and 0.3 μg/ml menadione under anaerobic conditions (80% N2, 10% CO2, and 10% H2).
Table 1.
Reference strains used for the biofilm model and for the preparation of DNA probes
| Species | Strain* |
|---|---|
| Actinomyces israelii | 12102 |
| Aggregatibacter | 43718 |
|
actinomycetemcomitans
serotype b |
|
| Campylobacter rectus | 33238 |
| Eikenella corrodens | 23834 |
|
Fusobacterium nucleatum ss
nucleatum |
25586 |
| Parvimonas micra | 33270 |
| Porphyromonas gingivalis | 33277 |
| Prevotella intermedia | 25611 |
| Streptococcus sanguinis | 10556 |
American Type Culture Collection (Rockville, MD, USA).
Initial biofilm formation assay
Bacterial growth from 5- to 7-day cultures of each strain was harvested and the optical density (OD) in each tube was adjusted to 1 at 600 nm in a spectrophotometer. Sterile disks were placed individually in 24-well plates and a total of 106 cells/ml suspension of each reference strain was added, in order to obtain a mixed culture, in a total volume of 1 ml. Plates were incubated for 24 h at 35°C under anaerobic conditions using enriched Mycoplasma broth-media (5 μg/ml hemin and 0.3 μg/ml menadione) (CM) or HS. HS was processed as reported previously (Guggenheim et al. 2001), except that after centrifugation, supernatants were sterilized by filtration. All experiments were performed in triplicate. After anaerobic incubation, each sample was washed twice with Mycoplasma broth. After washing, 1 ml of enriched Mycoplasma broth was added and the samples were sonicated for five periods of 10 s each in order to detach the adherent bacteria from each surface.
Biofilm composition
One hundred microliters of the bacterial suspension obtained after sonication of each sample were placed in individual Eppendorf tubes with 900 μl of TE buffer pH 7.6 (10 mM Tris-HCl, 1 mM EDTA). The suspensions were mixed by vortexing and 100 μl were placed in new Eppendorf tubes with 100 μl of 0.5 M NaOH. Bacterial species were identified and quantified using the checkerboard DNA–DNA hybridization technique as described previously (Socransky et al. 1994). In brief, DNA probes were prepared using the growth from 3- to 7-day cultures of the nine reference strains used in the biofilm model (Table 1). Bacterial growth was harvested and placed in tubes containing 1 ml of TE buffer. Cells were washed twice and lysed at 37°C for 1 h with either 10% sodium dodecyl sulfate (SDS) plus proteinase K (20 mg/ml) for Gram-negative strains or lysozyme (15 mg/ml) plus achromopeptidase (5 mg/ml) for Gram-positive strains. DNA was isolated and purified using the method described by Smith et al. (1989). Whole-genomic DNA probes were prepared for each species by labeling 1 mg of DNA with digoxigenin (Roche Diagnostics, Mannheim, Germany) using a random primer technique (Feinberg & Vogelstein 1983). The specificity and sensitivity of the nine DNA probes were assessed by hybridizing each DNA probe against individual pure cultures of all of the species adjusted to 104, 105, 106, and 107 cells. The sensitivity of the assay was set to allow the detection of approximately 104 cells of a given species by adjusting the concentration of each individual DNA probe. For the DNA–DNA hybridization, each sample was thawed at room temperature, boiled for 10 min, and neutralized with 800 μl of 5 M ammonium acetate. The released DNA from each sample was then placed into individual lanes (Minislot-30, Immunetics Inc., Cambridge, MA, USA), concentrated onto a 15 cm × 15 cm positively charged nylon membrane (Roche Diagnostics), and fixed to the membrane by cross-linking under ultraviolet light. Two lanes on each membrane contained standards consisting of a mixture at 105 and 106 cells of each bacterial species tested. The membranes were pre-hybridized at 42°C for 2 h in 50% formamide, 5 × standard saline citrate (SSC) (1 × SSC = 150 mM NaCl and 15 mM Na citrate), 1% casein (Sigma-Aldrich), 5 × Denhardt’s solution, 25 mM sodium phosphate (pH 6.5), and 0.5 mg/ml yeast RNA (Roche Diagnostics). Each membrane was placed in a second device (Miniblotter-45, Immunetics) with the sample lanes rotated 90° to the channels of the apparatus. The probes were diluted to ~20 ng/ml in hybridization solution (45% formamide, 5 × SSC, 1 × Denhardt’s solution, 20 mM Na phosphate (pH 6.5), 0.2 mg/ml yeast RNA, 10% dextran sulfate, and 1% casein), placed in individual channels of the device, and hybridized overnight at 421C. Probes were hybridized in two sets of nine consecutive channels, leaving empty channels (hybridization solution only) to allow noise and background correction of signals. The membranes were washed twice at high stringency for 20 m each time at 68°C in phosphate buffer (0.1 × SSC and 0.1% SDS). Membranes were blocked by 1 h incubation in maleate buffer (100 mM maleic acid and 150 mM NaCl, pH 7.5) containing 1% casein. Hybrids were detected by exposing the membranes to a 1 : 50,000 dilution of anti-digoxigenin antibody conjugated to alkaline phosphatase (Roche Diagnostics) for 30 m, using a previously described modification (Engler-Blum et al. 1993). Signals were detected by chemiluminescence using a chemiluminescent agent (CDP-Star, Roche Diagnostics) for 30 m on the membranes at room temperature and exposed to films in autoradiographic cassettes for 30 m. Films were developed and then photographed using a digital photodocumentation system (DigiDoc, BioRad Laboratories, Hercules, CA, USA). Signals were detected with specialized software (Quantity One, BioRad Laboratories), adjusted by subtracting the average plus two standard deviations of the noise and background detected in the empty lanes, and converted to absolute counts by comparison with the standards on the membrane. Failure to detect a signal was recorded as zero.
Biofilm morphology
In order to observe biofilm morphology in each of the test substrates, samples were prepared for scanning electron microscopy (SEM). Specimens were fixed in 2% glutaraldehyde 24 h at room temperature, then washed three times with phosphate buffer solution (pH 7.4) and dehydrated through a series of graded ethanol solutions (20%, 40%, 60%, 80%, and 100%). Samples were subsequently vacuum dried, sputter-coated with Au, and observed using a scanning electron microscope (Stereoscan 440, Cambridge-Leica, Wetzlar, Germany) at 20 kV.
Data analysis
Total counts of bacteria are presented as mean standard error of the mean (SEM) × 106 per ml. The proportion of each strain in the biofilms is presented as a percentage based on the total numbers of bacteria that were obtained. Data were analysed using analysis of variance (ANOVA) followed by Bonferroni’s correction for multiple comparisons and significant differences were determined. Significance was set at P<0.05.
Results
The total number of bacterial counts varied with the titanium test substrate and medium used (Fig. 1). There were consistently more bacteria on disks cultivated in the CM than in HS. In addition, when CM was used, significantly lower numbers of bacteria were detected on A and modSLA substrates when compared with PT (P<0.05). SLA and modSLA substrates had greater numbers of bacteria than the A or modA surfaces in both CM and HS (P%lt;0.05). When bacteria were cultured with HS, the greatest adhesion of bacteria was observed on the modSLA surfaces (P<0.05).
Fig. 1.
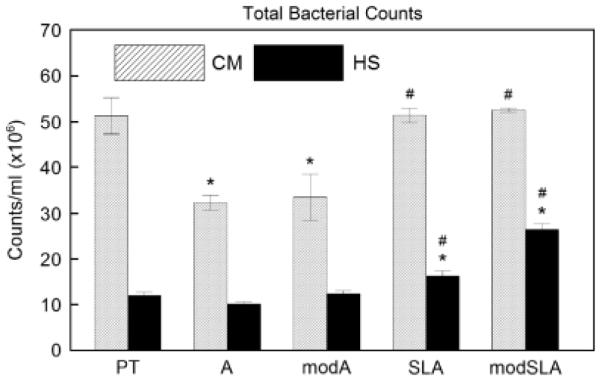
Total bacterial counts (bacterial counts/ml × 106) on PT, A, modA, SLA, and modSLA surfaces after 24 h of anaerobic incubation in culture medium (CM) or human saliva (HS). *P<0.05, vs. PT for CM or HS; # P<0.05, vs. A or modA for CM or HS. PT, pretreatment; A, acid-etched; modA, A modified to be hydrophilic; SLA, sand-blasted/acid-etched; modSLA, hydrophilic SLA.
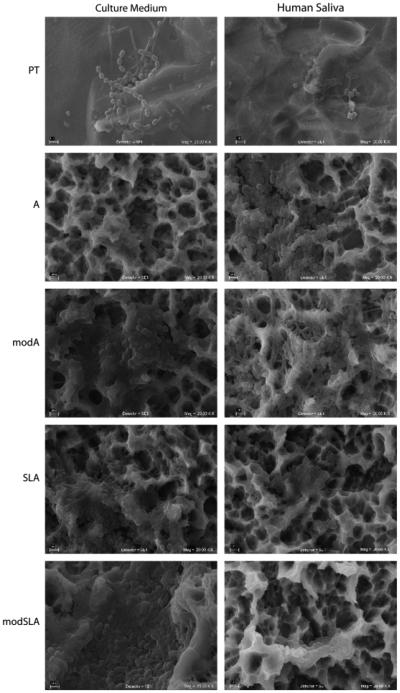
The differences in bacterial distribution on the test substrates were confirmed in scanning electron micrographs of the biofilms (Fig. 2). Greater amounts of biofilm were found on the PT disks cultured in CM compared with HS. Bacteria growing in CM appeared to conform to sheet-like regions rather than to the occasional projections. Chains of bacilli-shaped bacteria predominated. In contrast, in HS bacteria coated the irregular surface and cocci filled the pits. When bacteria were grown in CM on the modA surface, the structural elements appeared to be coated with filaments with regions of cocci interspersed. This was more pronounced in cultures grown in HS. It was difficult to distinguish individual colonies or bacteria on SLA substrates. In CM, filaments, and cocci were interspersed with extracellular matrix and this was more pronounced on the modSLA surface, where the matrix formed rope-like structures along the substrate ridges. This matrix covered the bacteria in cultures grown in HS on SLA and modSLA.
Fig. 2.
Scanning electron micrographs of biofilms formed on PT, A, modA, SLA, and modSLA surfaces. Biofilms were grown in culture medium (CM) or human saliva (HS). Scale bar represents 2 μm. PT, pretreatment; A, acid-etched; modA, A modified to be hydrophilic; SLA, sand-blasted/acid-etched; modSLA, hydrophilic SLA.
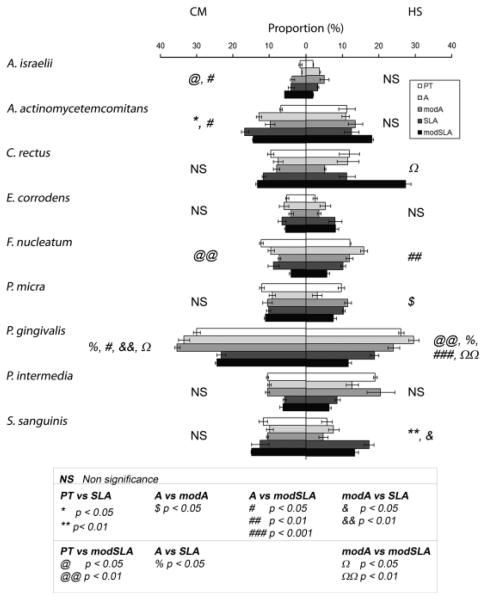
The composition of the biofilm was also sensitive to substrate properties in a media-dependent manner. Figure 3 shows the proportion of each species in the biofilms formed on the test substrates. When bacteria were grown in CM, P. gingivalis represented the highest proportion on the biofilms in all surfaces tested, while A. israelii represented the lowest proportion of the total biofilm. P. gingivalis was found at significant higher levels on A and modA surfaces than on SLA (P<0.05, P<0.01, respectively) or on modSLA (P<0.05, P<0.05, respectively). A. israelii, which represented the lowest proportion of the initial biofilms formed on the test substrates, showed clear substrate specific differences, with increasing levels as the surfaces became rougher and more hydrophilic (PT vs. modSLA, P<0.05; A vs. modSLA, P<0.05). A. actinomycetemcomitans and F. nucleatum exhibited roughness-dependent increases, with reduced levels on the hydrophilic substrates. Differences in the proportion of A. actinomycetemcomitans were found on the PT surfaces compared with SLA or modSLA (P<0.05). Differences in the amount of F. nucleatum were found when PT was compared with modSLA. In contrast, surface topography and energy had no effect on the proportion of C. rectus, E. corrodens, Parvimonas micra, P. intermedia, and S. sanguinis. Different patterns of adhesion were observed when the bacteria were cultured in HS (Fig. 3). Moreover, the differential effects of substrate topography and hydrophilicity were amplified. P. gingivalis proportions were elevated on PT in comparison with modSLA (P<0.01), on A in comparison to SLA (P<0.05) and modSLA (P<0.001), and on modA in comparison with modSLA (P<0.01). F. nucleatum was higher on A when compared with modSLA (P<0.01). C. rectus was markedly elevated on modSLA in comparison to modA (P<0.05). S. sanguinis exhibited increased numbers on SLA in comparison to PT (P<0.01) and on modA vs. modSLA substrates. In HS, surface topography and energy had no effect on the proportion of A. israelii, A. actinomycetemcomitans, E. corrodens, and P. intermedia.
Fig. 3.
Proportion of the bacterial strains in the initial biofilms formed on pretreatment (PT ), A, modA, SLA, and modSLA surfaces using culture medium (CM) or human saliva (HS). PT, pretreatment; A, acid-etched; modA, A modified to be hydrophilic; SLA, sand-blasted/acid-etched; modSLA, hydrophilic SLA.
Discussion
In this study, we examined initial biofilm formation on Ti implant surfaces with different microtopography and hydrophilicity. We also tested the influence of different incubation media by growing these biofilms with normal CM or HS. Our biofilm model included nine bacterial species representative of all the complexes of the subgingival plaque described by Socransky et al. (1998) and Socransky & Haffajee (2005).
The numbers of bacteria on all surfaces were dependent on the incubation media, revealing the strong influence of the “bulk fluid” in the initial formation and consolidation of a biofilm. The major bulk fluids in the oral cavity are saliva and the gingival crevicular fluid, and many studies indicate that these fluids are critical for the colonization of certain taxa (De Jong & Van der Hoeven 1987; Gibbons 1996). Our results showed that a higher number of bacteria attached to all surfaces when CM was used. A possible explanation could be that saliva contains some antimicrobial substances, such lysozyme, lactoferrin, lactoperoxidase, and secretory IgA (Tenovuo 1998). Scanning electron micrographs of the biofilms suggest that biofilm morphology is also affected by the CM. Coccal forms were more abundant in CM cultures and they tended to congregate in focal regions. Less special variation was evident in the HS cultures, in part because of the thick layer or extracellular matrix, which followed the contours of the micron-scale topographic features of the underlying Ti disk.
Surface microtopography is an important influence in oral bacterial colonization. A positive correlation between surface roughness and bacterial attachment in vitro has been shown (Quirynen et al. 1996), and a recent pilot study revealed that initial supra-gingival plaque biolmformation in vivo was influenced by microtopography (Schwarz et al. 2007a, 2007b). This pilot study also suggested that hydrophilicity was less important than roughness in the formation of supra-gingival plaque. Other studies suggest that roughness appears to be a minor factor in initial biofilm formation (Bos et al. 1999; BoulangePetermann et al. 1997). In our study, based on analysis of the total number of bacteria attached to surfaces with different microtopographies (PT, A, and SLA), no differences were found when samples were incubated with saliva, supporting the hypothesis that microtexture is not a major determinant in biofilm formation. However, samples incubated with CM had a greater number of bacterial counts on PT and SLA than on the A surfaces, discounting this notion.
Regarding the surface-free energy effect on the bacterial adhesion, we observed more bacteria on modSLA than on SLA in cultures grown with HS. This correlation between surface energy and bacterial adhesion has been studied previously (van Dijk et al. 1987; Quirynen & Bollen 1995). However, we did not assess the hydrophilicity of the bacterial cell surface, which also has been reported to have an influence in bacterial adhesion (Strevett & Chen 2003), because low surface free energy materials attracted microorganisms with relatively low surface free energy (Minagi et al. 1985; Mabboux et al. 2004).
The composition of the biofilms that formed on each surface was both substrate dependent and media dependent demonstrating the interrelationship between physical and chemical properties of the surface and biological properties of the environment in initial biofilm formation. For example, P. gingivalis, a very important periodontal pathogen (Moore 1987; Holt & Ebersole 2005), was found in high proportions in the experimental biofilms on all surfaces tested, particularly when CM was used. This finding is consistent with those reported previously showing that P. gingivalis is capable of colonizing Ti surfaces at a very high rate (Yoshinari et al. 2000; Pier-Francesco et al. 2006). S. sanguinis is an “early colonizer” (Gibbons 1996); thus, it was not surprising to find this microorganism in high proportions. Some studies also report significant adhesion of this microorganism on Ti surfaces (Hauser-Gerspach et al. 2007). We also found significant adhesion of A. actinomycetemcomitans on all surfaces, particularly on the SLA and modSLA surfaces in both CM and HS. A. actinomycetemcomitans is a well-known periodontal pathogen (Crossner et al. 1990; Fives-Taylor et al. 2000), and the adhesion of this microorganism to implant surfaces has also been studied (Yoshinari et al. 2000; Callan et al. 2005). Although it is intriguing that A. actinomycetemcomitans was found in higher numbers on SLA and modSLA surfaces, the reason for this is not known, but opens an interesting field of study.
The effects of surface microstructure and surface energy vary among the test species. This is consistent with numerous studies showing the critical role of microenvironment on attachment and growth of individual constituents of the oral microbiota (Grossner-Schreiber et al. 2001; Scarano et al. 2003; Pier-Francesco et al. 2006). The effect of the incubation media was also an important factor; differences in the bacterial adhesion and biofilm composition were observed. Using saliva as a CM could be adequate to simulate an in vivo situation. However, the error associated with the use of saliva could be also larger, because saliva components can vary from donor to donor and even from the same donor (Crosley et al. 2009). Therefore, for the in vitro study of bacterial adhesion and biofilm formation on implant surfaces, it is more reliable and convenient to use a well-controlled CM.
Taken together, the data show that initial biofilm formation and composition are affected by surface microtopography and hydrophilicity, as well as the CM used. The method used here provides a powerful tool to study bacterial interaction with biomaterials in vitro using a complex biofilm model. By tailoring surface properties, we may be able to control the initial biofilm formation, directing the formation of a less pathogenic biofilm on the implant surface and potentially avoiding peri-implant infections.
Acknowledgements
This study was supported by US PHS Grant AR052102. The authors thank Omar Novelo for SEM images, Silvia Antuna for technical support and Institut Straumann for supplying the surfaces. A. Almaguer-Flores and R. Olivares-Navarrete thank DGAPA and PROFIP program of the Universidad Nacional Autónoma de Mé xico.
Contributor Information
R. Olivares-Navarrete, Department of Biomedical Engineering, Georgia Institute of Technology, Atlanta, GA, USA
M. Wieland, MyoPowers Medical Technologies SA, Lausanne, Switzerland
L. A. Ximénez-Fyvie, Instituto de Investigaciones en Materiales, Universidad Nacional, Autónoma de México, Ciudad Universitaria, México D. F., México
Z. Schwartz, Department of Biomedical Engineering, Georgia Institute of Technology, Atlanta, GA, USA
B. D. Boyan, Department of Biomedical Engineering, Georgia Institute of Technology, Atlanta, GA, USA
References
- An YH, Friedman RJ. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. Journal of Biomedical Materials Research. 1998;43:338–348. doi: 10.1002/(sici)1097-4636(199823)43:3<338::aid-jbm16>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Barbour ME, O’Sullivan DJ, Jenkinson HF, Jagger DC. The effects of polishing methods on surface morphology, roughness and bacterial colo- nisation of titanium abutments. Journal of Materials Science: Materials in Medicine. 2007;18:1439–1447. doi: 10.1007/s10856-007-0141-2. [DOI] [PubMed] [Google Scholar]
- Bos R, van der Mei HC, Busscher HJ. Physico-chemistry of initial microbial adhesive interactions – its mechanisms and methods for study. FEMS Microbiology Reviews. 1999;23:179–230. doi: 10.1111/j.1574-6976.1999.tb00396.x. [DOI] [PubMed] [Google Scholar]
- BoulangePetermann L, Rault J, BellonFontaine MN. Adhesion of Streptococcus thermophilus to stainless steel with different surface topography and roughness. Biofouling. 1997;11:201–216. [Google Scholar]
- Buser D, Broggini N, Wieland M, Schenk RK, Denzer AJ, Cochran DL, Hoffmann B, Lussi A, Steinemann SG. Enhanced bone apposition to a chemically modified SLA titanium surface. Journal of Dental Research. 2004;83:529–533. doi: 10.1177/154405910408300704. [DOI] [PubMed] [Google Scholar]
- Callan DP, Cobb CM, Williams KB. DNA probe identification of bacteria colonizing internal surfaces of the implant–abutment interface: a preliminary study. Journal of Periodontology. 2005;76:115–120. doi: 10.1902/jop.2005.76.1.115. [DOI] [PubMed] [Google Scholar]
- Cochran DL. A comparison of endosseous dental implant surfaces. Journal of Periodontology. 1999;70:1523–1539. doi: 10.1902/jop.1999.70.12.1523. [DOI] [PubMed] [Google Scholar]
- Costerton JW. Biofilm theory can guide the treatment of device-related orthopaedic infections. Clinical Orthopaedics and Related Research. 2005;437:7–11. doi: 10.1097/00003086-200508000-00003. [DOI] [PubMed] [Google Scholar]
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- Crosley LK, Duthie SJ, Polley AC, Bouwman FG, Heim C, Mulholland F, Horgan G, Johnson IT, Mariman EC, Elliott RM, Daniel H, de Roos B. Variation in protein levels obtained from human blood cells and biofluids for platelet, peripheral blood mononuclear cell, plasma, urine and saliva proteomics. Genes and Nutrition. 2009;4:95–102. doi: 10.1007/s12263-009-0121-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossner CG, Carlsson J, Sjodin B, Tarnvik A, Unell L, Venge P, Wranne L. Periodontitis in the primary dentition associated with Actinobacillus actinomycetemcomitans infection and leukocyte dysfunction. A 3½ year follow-up. Journal of Clinical Periodontology. 1990;17:264–267. doi: 10.1111/j.1600-051x.1990.tb00023.x. [DOI] [PubMed] [Google Scholar]
- De Jong MH, Van der Hoeven JS. The growth of oral bacteria on saliva. Journal of Dental Research. 1987;66:498–505. doi: 10.1177/00220345870660021901. [DOI] [PubMed] [Google Scholar]
- Engler-Blum G, Meier M, Frank J, Muller GA. Reduction of background problems in nonradioactive northern and southern blot analyses enables higher sensitivity than 32P-based hybridizations. Analytical Biochemistry. 1993;210:235–244. doi: 10.1006/abio.1993.1189. [DOI] [PubMed] [Google Scholar]
- Feinberg AP, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Analytical Biochemistry. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fives-Taylor PM, Meyer DH, Mintz KP, Brissette C. Virulence factors of Actinobacillus actinomycetemcomitans. Periodontology. 2000;20:136–167. doi: 10.1111/j.1600-0757.1999.tb00161.x. [DOI] [PubMed] [Google Scholar]
- Furst MM, Salvi GE, Lang NP, Persson GR. Bacterial colonization immediately after installation on oral titanium implants. Clinical Oral Implants Research. 2007;18:501–508. doi: 10.1111/j.1600-0501.2007.01381.x. [DOI] [PubMed] [Google Scholar]
- Gibbons RJ. Role of adhesion in microbial colonization of host tissues: a contribution of oral microbiology. Journal of Dental Research. 1996;75:866–870. doi: 10.1177/00220345960750030201. [DOI] [PubMed] [Google Scholar]
- Groessner-Schreiber B, Hannig M, Duck A, Griepentrog M, Wenderoth DF. Do different implant surfaces exposed in the oral cavity of humans show different biofilm compositions and activities? European Journal of Oral Sciences. 2004;112:516–522. doi: 10.1111/j.1600-0722.2004.00171.x. [DOI] [PubMed] [Google Scholar]
- Grossner-Schreiber B, Griepentrog M, Haustein I, Muller WD, Lange KP, Briedigkeit H, Gobel UB. Plaque formation on surface modified dental implants. An in vitro study. Clinical Oral Implants Research. 2001;12:543–551. doi: 10.1034/j.1600-0501.2001.120601.x. [DOI] [PubMed] [Google Scholar]
- Guggenheim B, Giertsen E, Schupbach P, Shapiro S. Validation of an in vitro biofilm model of supragingival plaque. Journal of Dental Research. 2001;80:363–370. doi: 10.1177/00220345010800011201. [DOI] [PubMed] [Google Scholar]
- Hauser-Gerspach I, Kulik EM, Weiger R, Decker EM, Von Ohle C, Meyer J. Adhesion of Streptococcus sanguinis to dental implant and restorative materials in vitro. Dental Materials Journal. 2007;26:361–366. doi: 10.4012/dmj.26.361. [DOI] [PubMed] [Google Scholar]
- Heuer W, Elter C, Demling A, Neumann A, Suerbaum S, Hannig M, Heidenblut T, Bach FW, Stiesch-Scholz M. Analysis of early biofilm formation on oral implants in man. Journal of Oral Rehabilitation. 2007;34:377–382. doi: 10.1111/j.1365-2842.2007.01725.x. [DOI] [PubMed] [Google Scholar]
- Holt SC, Ebersole JL. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontology 2000. 2005;38:72–122. doi: 10.1111/j.1600-0757.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- Jeyachandran YL, Narayandass SK, Mangalaraj D, Bao CY, Martin PJ. The effect of surface composition of titanium films on bacterial adhesion. Biomedical Materials. 2006;1:L1–L5. doi: 10.1088/1748-6041/1/1/L01. [DOI] [PubMed] [Google Scholar]
- Jimbo R, Sawase T, Baba K, Kurogi T, Shibata Y, Atsuta M. Enhanced initial cell responses to chemically modified anodized titanium. Clinical Implant Dentistry and Related Research. 2008;10:55–61. doi: 10.1111/j.1708-8208.2007.00061.x. [DOI] [PubMed] [Google Scholar]
- Katsikogianni M, Missirlis YF. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria–material interactions. European Cells and Materials. 2004;8:37–57. doi: 10.22203/ecm.v008a05. [DOI] [PubMed] [Google Scholar]
- Kohavi D, Klinger A, Steinberg D, Mann E, Sela NM. Alpha-amylase and salivary albumin adsorption onto titanium, enamel and dentin: an in vivo study. Biomaterials. 1997;18:903–906. doi: 10.1016/s0142-9612(97)00026-4. [DOI] [PubMed] [Google Scholar]
- Kohavi D, Klinger A, Steinberg D, Sela MN. Adsorption of salivary proteins onto prosthetic titanium components. Journal of Prosthetic Dentistry. 1995;74:531–534. doi: 10.1016/s0022-3913(05)80357-9. [DOI] [PubMed] [Google Scholar]
- Listgarten MA, Lai CH. Comparative microbiological characteristics of failing implants and periodontally diseased teeth. Journal of Periodontology. 1999;70:431–437. doi: 10.1902/jop.1999.70.4.431. [DOI] [PubMed] [Google Scholar]
- Lossdorfer S, Schwartz Z, Wang L, Lohmann CH, Turner JD, Wieland M, Cochran DL, Boyan BD. Microrough implant surface topographies increase osteogenesis by reducing osteoclast formation and activity. Journal of Biomedical Materials Research Part A. 2004;70:361–369. doi: 10.1002/jbm.a.30025. [DOI] [PubMed] [Google Scholar]
- Mabboux F, Ponsonnet L, Morrier JJ, Jaffrezic N, Barsotti O. Surface free energy and bacterial retention to saliva-coated dental implant materials–an in vitro study. Colloids and Surfaces B: Biointerfaces. 2004;39:199–205. doi: 10.1016/j.colsurfb.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Merritt K, Chang CC. Factors influencing bacterial adherence to biomaterials. Journal of Bio-materials Applications. 1991;5:185–203. doi: 10.1177/088532829100500304. [DOI] [PubMed] [Google Scholar]
- Minagi S, Miyake Y, Inagaki K, Tsuru H, Suginaka H. Hydrophobic interaction in Candida albicans and Candida tropicalis adherence to various denture base resin materials. Infection and Immunity. 1985;47:11–14. doi: 10.1128/iai.47.1.11-14.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore WE. Microbiology of periodontal disease. Journal of Periodontal Research. 1987;22:335–341. doi: 10.1111/j.1600-0765.1987.tb01595.x. [DOI] [PubMed] [Google Scholar]
- Papaioannou W, Quirynen M, Van Steenberghe D. The influence of periodontitis on the subgingival flora around implants in partially edentulous patients. Clinical Oral Implants Research. 1996;7:405–409. doi: 10.1034/j.1600-0501.1996.070415.x. [DOI] [PubMed] [Google Scholar]
- Pier-Francesco A, Adams RJ, Waters MG, Williams DW. Titanium surface modification and its effect on the adherence of Porphyromonas gingivalis: an in vitro study. Clinical Oral Implants Research. 2006;17:633–637. doi: 10.1111/j.1600-0501.2006.01274.x. [DOI] [PubMed] [Google Scholar]
- Plummer C, Douglas CW. Relationship between the ability of oral streptococci to interact with platelet glycoprotein ibalpha and with the salivary low-molecular-weight mucin, MG2. FEMS Immunology and Medical Microbiology. 2006;48:390–399. doi: 10.1111/j.1574-695X.2006.00161.x. [DOI] [PubMed] [Google Scholar]
- Quirynen M, Bollen CM. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. A review of the literature. Journal of Clinical Periodontology. 1995;22:1–14. doi: 10.1111/j.1600-051x.1995.tb01765.x. [DOI] [PubMed] [Google Scholar]
- Quirynen M, Bollen CM, Papaioannou W, Van Eldere J, van Steenberghe D. The influence of titanium abutment surface roughness on plaque accumulation and gingivitis: short-term observations. The International Journal of Oral & Maxillofacial Implants. 1996;11:169–178. [PubMed] [Google Scholar]
- Quirynen M, De Soete M, Dierickx K, van Steenberghe D. The intra-oral translocation of periodontopathogens jeopardises the outcome of periodontal therapy. A review of the literature. Journal of Clinical Periodontology. 2001;28:499–507. doi: 10.1034/j.1600-051x.2001.028006499.x. [DOI] [PubMed] [Google Scholar]
- Rupp F, Scheideler L, Olshanska N, de Wild M, Wieland M, Geis-Gerstorfer J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. Journal of Biomedical Materials Research Part A. 2006;76:323–334. doi: 10.1002/jbm.a.30518. [DOI] [PubMed] [Google Scholar]
- Scarano A, Piattelli M, Vrespa G, Caputi S, Piattelli A. Bacterial adhesion on titanium nitride-coated and uncoated implants: an in vivo human study. Journal of Oral Implantology. 2003;29:80–85. doi: 10.1563/1548-1336(2003)029<0080:BAOTNA>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- Schwartz Z, Nasazky E, Boyan BD. Surface microtopography regulates osteointegration: the role of implant surface microtopography in osteointegration. Alpha Omegan. 2005;98:9–19. [PubMed] [Google Scholar]
- Schwarz F, Ferrari D, Herten M, Mihatovic I, Wieland M, Sager M, Becker J. Effects of surface hydrophilicity and microtopography on early stages of soft and hard tissue integration at non-submerged titanium implants: an immunohisto-chemical study in dogs. Journal of Periodontology. 2007a;78:2171–2184. doi: 10.1902/jop.2007.070157. [DOI] [PubMed] [Google Scholar]
- Schwarz F, Sculean A, Wieland M, Horn N, Nuesry E, Bube C, Becker J. Effects of hydrophilicity and microtopography of titanium implant surfaces on initial supragingival plaque biofilm formation. A pilot study. Mund Kiefer Gesichtschirurgie. 2007b;11:333–338. doi: 10.1007/s10006-007-0079-z. [DOI] [PubMed] [Google Scholar]
- Sela MN, Badihi L, Rosen G, Steinberg D, Kohavi D. Adsorption of human plasma proteins to modified titanium surfaces. Clinical Oral Implants Research. 2007;18:630–638. doi: 10.1111/j.1600-0501.2007.01373.x. [DOI] [PubMed] [Google Scholar]
- Shibli JA, Melo L, Ferrari DS, Figueiredo LC, Faveri M, Feres M. Composition of supra- and subgingival biofilm of subjects with healthy and diseased implants. Clinical Oral Implants Research. 2008;19:975–982. doi: 10.1111/j.1600-0501.2008.01566.x. [DOI] [PubMed] [Google Scholar]
- Smith GL, Socransky SS, Smith CM. Rapid method for the purification of DNA from subgingival microorganisms. Oral Microbiology and Immunology. 1989;4:47–51. doi: 10.1111/j.1399-302x.1989.tb00406.x. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontology 2000. 2005;38:135–187. doi: 10.1111/j.1600-0757.2005.00107.x. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. Journal of Clinical Periodontology. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Smith C, Martin L, Paster BJ, Dewhirst FE, Levin AE. “Checkerboard” DNA–DNA hybridization. Biotechniques. 1994;17:788–792. [PubMed] [Google Scholar]
- Steinberg D, Sela MN, Klinger A, Kohavi D. Adhesion of periodontal bacteria to titanium, and titanium alloy powders. Clinical Oral Implants Research. 1998;9:67–72. doi: 10.1034/j.1600-0501.1998.090201.x. [DOI] [PubMed] [Google Scholar]
- Strevett KA, Chen G. Microbial surface thermodynamics and applications. Research in Microbiology. 2003;154:329–335. doi: 10.1016/S0923-2508(03)00038-X. [DOI] [PubMed] [Google Scholar]
- Tenovuo J. Antimicrobial function of human saliva – how important is it for oral health? Acta Odontologica Scandinavica. 1998;56:250–256. doi: 10.1080/000163598428400. [DOI] [PubMed] [Google Scholar]
- van Dijk J, Herkstroter F, Busscher H, Weerkamp A, Jansen H, Arends J. Surface-free energy and bacterial adhesion. An in vivo study in beagle dogs. Journal of Clinical Periodontology. 1987;14:300–304. doi: 10.1111/j.1600-051x.1987.tb01537.x. [DOI] [PubMed] [Google Scholar]
- Yoshinari M, Oda Y, Kato T, Okuda K, Hirayama A. Influence of surface modifications to titanium on oral bacterial adhesion in vitro. Journal of Biomedical Materials Research. 2000;52:388–394. doi: 10.1002/1097-4636(200011)52:2<388::aid-jbm20>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Zhao G, Schwartz Z, Wieland M, Rupp F, Geis-Gerstorfer J, Cochran DL, Boyan BD. High surface energy enhances cell response to titanium substrate microstructure. Journal of Biomedical Materials Research Part A. 2005;74:49–58. doi: 10.1002/jbm.a.30320. [DOI] [PubMed] [Google Scholar]