Abstract
TCR engagement by peptide-MHC class I (pMHC) ligands induces a conformational change (Δc) in CD3 (CD3Δc) that contributes to T cell signaling. We found that when this interaction took place between primary T lineage cells and APCs, the CD8 coreceptor was required to generate CD3Δc. Interestingly, neither enhancement of Ag binding strength nor Src kinase signaling explained this coreceptor activity. Furthermore, Ag-induced CD3Δc was developmentally attenuated by the increase in sialylation that accompanies T cell maturation and limits CD8 activity. Thus, both weak and strong ligands induced CD3Δc in preselection thymocytes, but only strong ligands were effective in mature T cells. We propose that CD8 participation in the TCR/pMHC interaction can physically regulate CD3Δc induction by “translating” productive Ag encounter from the TCR to the CD3 complex. This suggests one mechanism by which the developmentally regulated variation in CD8 sialylation may contribute to the developmental tuning of T cell sensitivity.
Throughout ontogeny, homeostasis, and immune function, the αβ TCR recognizes a variety of peptide-MHC class I (pMHC)3 ligands to which distinct cellular responses are generated. Weak ligands induce positive selection in thymocytes, while promoting T cell survival in the periphery (1–3). Strong ligands induce negative selection when encountered by thymocytes, but clonal expansion, immune function, and memory when encountered by mature T cells (4, 5). To achieve these responses, the αβ TCR must communicate the strength of bound ligands to the noncovalently associated γδεζ CD3 subunits, which in turn initiate the biochemical signaling cascade (6). How does the TCR communicate ligand potency to the CD3 complex? What mechanisms allow the cell maturation state to determine the nature of the response?
Recently, TCR stimulation with either Abs or antigenic pMHC was shown to induce a conformational change (Δc) in CD3 (CD3Δc) that is required for full TCR activation, synapse formation, and functional responses (7–10). CD3Δc has the following characteristics: 1) it involves the exposure of a highly conserved, cryptic binding site on CD3ε; 2) it precedes and is independent of enzymatic signaling activity, including that of Src kinases; and 3) its induction correlates with efficient TCR/CD3 signaling, synapse formation, and functional responses. It was proposed that CD3Δc could be involved in the discrimination between weak and strong pMHC ligands (11, 12). However, this question has produced seemingly contradictory results. In thymocytes, one study found that both weak (positive selection) and strong (negative selection) ligands induced CD3Δc, thus eliminating the possibility that CD3Δc is involved in sensing ligand potency during thymic selection (8). However, a second study concluded that primarily negative selection ligands induce CD3Δc in thymocytes (13). In mature T cells, a third study showed that a weak pMHC ligand did not induce CD3Δc, but a strong antigenic ligand did (9). In the current work, we investigate the possibility that all three studies are correct, but that the disparate results are due to the fact that Ag recognition itself, reflected by CD3Δc induction, is subject to “developmental tuning.”
Developmental tuning is a widely acknowledged but incompletely understood process describing the decrease in responsiveness to weak pMHC ligands that accompanies the progression from thymocyte to mature T cell (14–17). Prolonged TCR engagement in thymocytes (18) may program alterations in the expression of key signaling molecules such as Fyn (19), Syk (20), CD5 (21), Src homology protein-1 and others (22), raising the signaling threshold necessary to respond to weak ligands, and preventing mature T cells from autoimmune activity (22, 23). Part of this effect was recently shown to be caused by the micro RNA miR-181, whose decreased levels after positive selection favors the expression of multiple phosphatases that down-modulate T cell signaling, including Src homology protein-2 (24). Another known consequence of positive selection is the modified glycosylation of the CD8 coreceptor, increasing the terminal sialylation of its O-linked glycans, which diminishes CD8 TCR-independent (noncognate) MHC binding (25–27). Pleiotropic desialylation of surface T cell glycoproteins and lipids results in enhanced T cell signaling (28, 29). However, it remains unclear whether sialylation directly regulates Ag recognition by altering cognate CD8/TCR/pMHC binding.
In this study, we considered the impact of the CD8 coreceptor on cognate Ag recognition as measured by CD3Δc induction in primary T lineage cells of different maturation states. The results from this study shed light on how CD3Δc is induced, how CD3Δc reflects ligand discrimination, and how developmental tuning directly affects Ag recognition.
Materials and Methods
DNA constructs and mice
The construct pGEX-4T1-GST-SH3.1α (SH3.1 derived from human Nck-α) was provided by Dr. R. Geha (Children’s Hospital, Harvard Medical School, Boston, MA). OT-I and OT-I β2-microglobulin-deficient (β2m−/−) RAG2−/− mice were bred and maintained on C57BL/6 back-ground and used between 6 and 12 wk of age. Animal procedures were performed in accordance with the guidelines and regulations of the University Hospital-Basel and the Canton of Basel-Stadt (Basel, Switzerland).
Abs, peptides, and other reagents
Rabbit anti-CD3ζ antiserum s448 was used to detect mature TCR/CD3 complexes by Western blotting as previously described (30). Monoclonal anti-CD3ζ (H146) was used to immunoprecipitate the TCR/CD3 complex (31). Abs from BD Biosciences included anti-H-2Kb/H-2Db (28.8-6), anti-Thy1.2 (53-2.1), anti-CD4 (RM4-5), anti-CD8α (53.6.7), anti-CD8β (53.5.8), anti-CD69 (H12F3), rat IgG isotype control (R35-38), anti-TCR Vα2 (B20.1), and anti-CD3-ε (2C11). Abs from Upstate Biotechnology included mouse anti-phosphotyrosine (4G10) and rabbit anti-linker for activation of T cell (anti-LAT). The peptides pFARL (SSIEFARL), pVSV (RGYVYQGL), pE1 (EIINFEKL), pG4 (SIIGFEKL), pQ7 (SIINFEQL), pQ4H7 (SIIQFEHL), pT4 (SIITFEKL), pQ4 (SIIQFEKL), pKB (SIINFE-diaminobutyrate-L), and pOVA (SIINFEKL) were synthesized as previously described (32). Other reagents included the Src kinase inhibitor PP2 (Calbiochem), protein G-Sepharose (GE Biosciences), type II neuraminidase from Vibrio cholerae and the detergent Brij 58 (Sigma-Aldrich), streptavidin (Molecular Probes), and the peanut lectin agglutinin (PNA)-FITC (Vector Laboratories).
Thymocyte and mature T cell stimulation
Without performing further purification procedures, thymi and lymph nodes from OT-I mice described were used as source of thymocytes (≥95% pure) and mature T cells (50–65% pure), respectively. T2-Kb wild-type (WT) cells or T2-Kb MUT (H2-Kb molecule mutant at 227 residue) cells (33), provided by T. Potter (National Jewish Medical and Research Center, Denver, CO), which express the WT or the point mutation D227K within H-2Kb, were used as APCs in all experiments. APCs were cultured with 2 μM exogenous peptide (3 h, 37°C), washed, verified for equal peptide loading by staining with anti-H-2Kb mAb 28.8-6, and cocultured with thymocytes or mature T cells (30 min, 37°C, 1:1 ratio). In some experiments, thymocytes were stimulated with 10 μg/ml soluble Ab in RPMI 1640 medium supplemented with 10% FBS (15 min, 37°C). Where indicated, PP2 treatment (20 μM) was performed as previously described (8).
Surface staining with PE-MHC tetramers
Kb WT and Kb MUT D227K PE-labeled tetramers were produced following standard refolding, biotinylation, and tetramerization protocols. Kb plasmids were gifts of P. Holman and S. Jameson (University of Minnesota, Minneapolis, MN) (34). Tetramers were purified via gel filtration and FPLC just before use. For surface staining of OT-I cells with the tetramers, 0.5 × 106 thymocytes, or mature T cells were incubated with the corresponding peptide tetramer (50 nM) together with mAbs that do not inhibit tetramer binding, anti-CD8α-allophycocyanin, anti-CD4-PerCP (data not shown and 35). Mean fluorescence intensity (MFI) and SEM are indicated.
CD3 pull-down (CD3-PD) assay and Western blots
The CD3-PD assay was previously described and proven as a means to detect CD3Δc (7). Briefly, cells were lysed in 0.3% Brij 58 isotonic buffer, and postnuclear fractions were obtained. Samples were precleared by incubation with GST-beads (1 h, 4°C) before specific pull-down with GST-SH3.1 beads (4–12 h, 4°C), followed by reducing SDS-PAGE (13%), polyvinylidene difluoride transfer, and Western blotting for CD3-ζ (s448). Where indicated, immunoprecipitations were performed with anti-CD3ζ (H146)/protein G-Sepharose beads, subjected to reducing SDS-PAGE (13%), transferred to nitrocelullose membranes, and blotted as indicated.
Early T cell response monitored by flow cytometry
Early responses of thymocytes and mature T cells were monitored by flow cytometry analysis of TCR down-regulation and CD69 up-regulation. For TCR down-regulation, cells were stimulated for 30 min as indicated and stained with anti-CD8-allophycocyanin, anti-CD4-PerCP, anti-Thy1.2-FITC, and anti-Vα2-PE. For CD69 expression, cells were stimulated for 20 h as indicated and stained with anti-CD8-allophycocyanin, anti-CD4-PerCP, anti-Vα2-FITC, and anti-CD69-PE. Staining took place for 30–45 min on ice, and washed cells were analyzed on a BD FACSCalibur flow cytometer calibrated with RCP-30-5A beads (Spherotech). MFI and SEM are indicated.
Neuraminidase treatment
Thymocytes or mature T cells were incubated with or without neuraminidase (0.007 U/106 cells) in RPMI 1640 medium at a density of 30 × 106 cells/ml (45 min, 37°C), followed by addition of 10% FBS to quench enzymatic activity. Washed cells were either analyzed by flow cytometry or stimulated by APC as described.
Results
T cell sensitivity to undergo CD3Δc decreases upon thymic selection
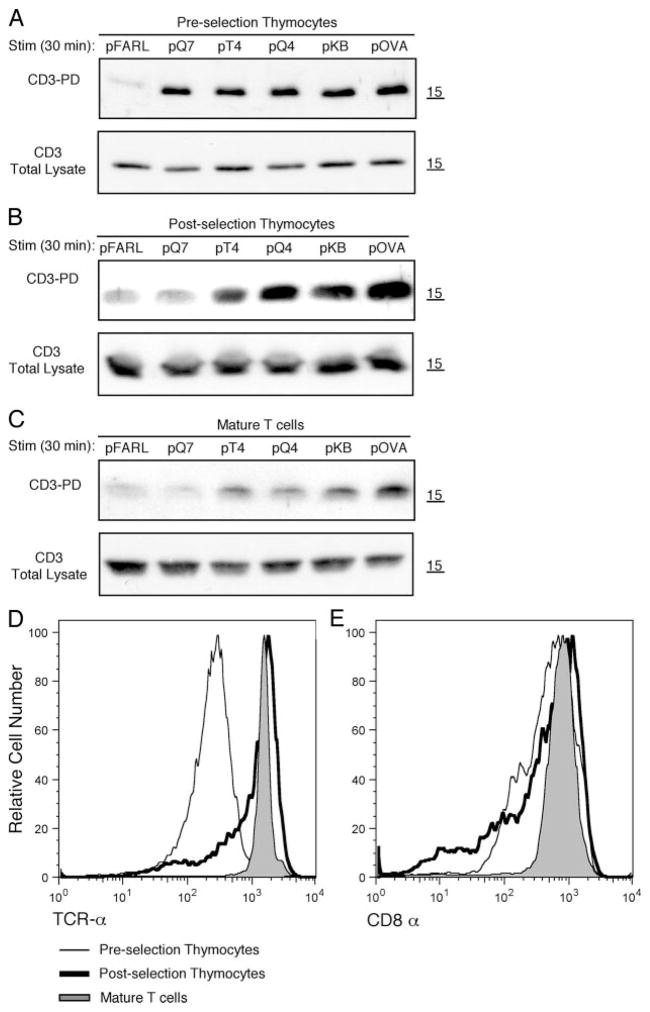
To assess whether the sensitivity of Ag recognition may be developmentally regulated, we examined the ability of T lineage cells to undergo CD3Δc when presented with pMHC ligands. The following three cell maturation states were tested: preselection, CD4+CD8+ double positive (DP) thymocytes from OT-I β2m−/− RAG2−/− mice, which lack pMHC presentation (36); postselection thymocytes, DP and CD4−CD8+ single positive thymocytes from OT-I β2m+/+ mice, which express pMHC ligands that mediate positive selection of the OT-I TCR (37); and mature, CD8+ T cells from OT-I β2m+/+ mice. Cells were cocultured with T2-Kb WT APCs preloaded with the following OVA variant peptides of increasing affinity for the OT-I TCR: pFARL (null), pQ7 (positive selector/antagonist), pT4 (mixed agonist/antagonist), pQ4, pKB, or pOVA (negative selectors/agonists) (8, 38–40). The induction of CD3Δc was assessed by the CD3-PD assay, which detects the exposure of a cryptic polyproline site in CD3ε upon productive TCR engagement by pMHC ligands, allowing the SH3.1 domain of Nck to bind and capture TCR/CD3 complexes, as described in Materials and Methods (7, 8). Like TCR down-regulation, the measurement of CD3Δc relies on the accumulation of engaged TCRs, which increases with the time of T cell to APC contact. Therefore, although induction of CD3Δc has been shown to occur immediately upon TCR engagement (7, 8), thymocytes were stimulated during 30 min to ensure maximal CD3Δc detection in the CD3-PD assay, as reported previously (8). We found that preselection DP thymocytes underwent CD3Δc when presented with both weak and strong pMHC ligands, but postselection thymocytes and mature T cells were productively engaged only by negative selectors/agonists (Fig. 1, A–C). In this and other experiments, including more variant peptides (data not shown), postselection thymocytes and mature T cells never induced CD3Δc when engaged by positive selector/antagonist ligands. These data demonstrate that the ability of weak (i.e., positive selecting) ligands to induce CD3Δc is substantially reduced after T lineage cells have undergone positive selection. This reduction in sensitivity cannot be explained by the expression level of either TCR or CD8 present at each maturation stage because preselection DP thymocytes show the highest sensitivity despite the lowest levels of both molecules at the surface (Fig. 1, D and E).
FIGURE 1.
T cell sensitivity to undergo CD3Δc decreases upon thymic selection. Murine T lineage OT-I cells were cocultured with T2-Kb WT APCs loaded with the peptides pFARL, pQ7, pT4, pQ4, pKB, or pOVA using three cell maturation states: preselection OT-I DP thymocytes from OT-I β2m−/− RAG2−/− (A); post-election OT-I thymocytes from OT-I β2m+/+ (B); and mature CD8+ T cells from OT-I β2m+/+ lymph nodes (C). The CD3-PD assay was performed on postnuclear lysates to detect the induction of CD3Δc (top panels), while a fraction of these postnuclear lysates was used to determine the total CD3 content by Western blot of CD3ζ (bottom panels) (A–C). As expected, CD3ζ migrates just larger than the 15 kDa molecular mass marker. Surface expression levels of Vα2(TCRα) (D) or CD8α (E) are represented by fluorescence intensity on the x-axis gated on Thy1.2+ cells from preselection OT-I DP thymocytes, postselection OT-I thymocytes, or mature OT-I T cells.
Disruption of CD8/pMHC interaction inhibits the induction of CD3Δc in preselection DP thymocytes
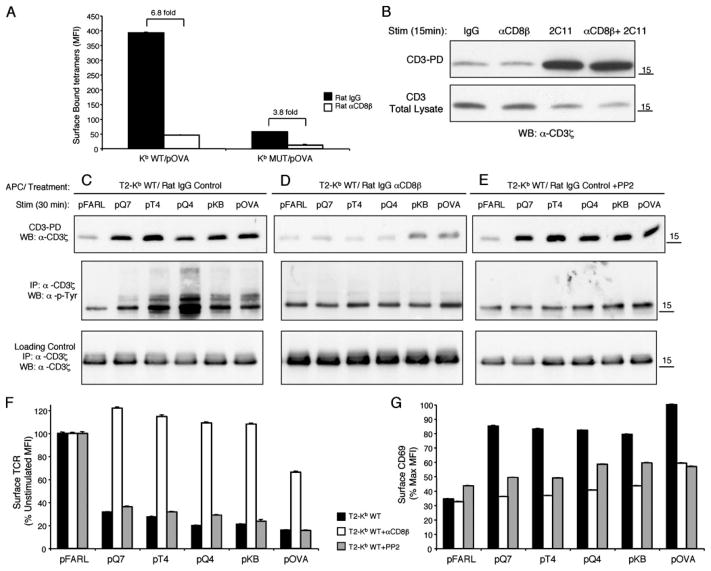
Because the TCR-independent noncognate interaction of CD8 with MHC is known to decrease after positive selection (26, 27, 41, 42), we studied whether CD8 plays a role in CD3Δc induction. Preselection DP thymocytes from OT-I β2m−/− RAG2−/− mice were incubated with the anti-CD8β mAb 53.5.8 (27, 34) and stained with either pOVA-loaded H-2Kb WT tetramers (Kb WT/pOVA) or H-2Kb D227K mutant tetramers, defective in CD8 binding (Kb MUT/pOVA) (33). Both the blocking mAb and the tetramer mutation inhibited pMHC binding to the OT-I TCR (Fig. 2A). We confirmed that blocking with anti-CD8β mAb neither autonomously induces nor inhibits the CD3-PD assay; i.e., treatment with anti-CD8β mAb alone or together with 2C11 (anti-CD3ε mAb) had no effect on CD3Δc detection in thymocytes (Fig. 2B).
FIGURE 2.
Disruption of CD8/pMHC interaction inhibits the induction of CD3Δc in preselection DP thymocytes. A, OT-I DP thymocytes from OT-I β2m−/− RAG2−/− mice were incubated with either rat IgG control (■) or the anti-CD8β blocking mAb, 53.5.8 (□) (30 min, 37°C), and stained with Kb WT/pOVA or Kb MUT/pOVA tetramers (45 min on ice) before analysis by flow cytometry. Tetramer identity is indicated on the x-axis, and the quantity of surface bound tetramers gated on CD4+CD8+ DP thymocytes is represented as MFI on the y-axis. The fold difference in tetramer binding is indicated. B, The CD3-PD assay was performed on C57BL/6 thymocytes stimulated with the following Abs: IgG control, anti-CD8β (53.5.8), 2C11 (anti-CD3ε), or anti-CD8β+ 2C11 (top). A fraction of the postnuclear lysate of each sample was used to determine the total CD3 content by Western blot of CD3ζ (bottom). C–E, OT-I DP thymocytes from OT-I β2m−/− RAG2−/− mice were either pretreated with control rat IgG (C), anti-CD8β blocking mAb 53.5.8 (D), or control rat IgG plus PP2 Src kinase inhibitor (30 min, 37°C) (E). Then thymocytes were cocultured with T2-Kb WT APCs loaded with pFARL, pQ7, pT4, pQ4, pKB, or pOVA (2 μM, 30 min, 37°C). Cells were lysed and subjected to either the CD3-PD assay (upper panels), or anti-CD3ζ immunoprecipitation and Western blotting with anti-CD3ζ (upper and lower panels) or anti-phosphotyrosine (α-p-Tyr) (middle panels). TCR down-regulation (F) and CD69 up-regulation (G) were analyzed by flow cytometry. Peptides are represented on the x-axis and surface TCR expression (F) on the y-axis as a percentage of the expression level gated on Thy1.2+ CD4+CD8+ DP thymocytes cocultured with the null peptide pFARL. G, CD69 expression is represented on the y-axis as a percentage of the maximum induction on Thy1.2+ CD4+CD8+ DP thymocytes cocultured with peptide pOVA.
To study CD3Δc induction when CD8/pMHC interaction is blocked, T2-Kb WT APCs were preloaded with OVA variant peptides and cocultured with preselection OT-I DP thymocytes that had been preincubated either with control Ig, anti-CD8β, or the Src kinase inhibitor, PP2. As expected, all cognate peptides induced CD3Δc in control thymocytes (Fig. 2C, upper) even when Src kinase activity was inhibited (Fig. 2E, upper). However, CD8 blockade dramatically inhibited CD3Δc induction by all peptides (Fig. 2D, upper). Similar to Src kinase inhibition, CD8 blockade inhibited CD3ζ phosphorylation (Fig. 2, C–E, middle panel), TCR down-regulation (Fig. 2F), and CD69 up-regulation (Fig. 2G), consistent with the idea that CD8 normally contributes to Src kinase delivery and activation during cognate pMHC recognition (43, 44). We conclude that CD8/MHC class I interaction during cognate pMHC stimulation of preselection OT-I thymocytes is required for CD3Δc induction, whereas Src kinase activity is not.
Involvement of CD8 in CD3Δc induction by cognate ligand has a role beyond affinity
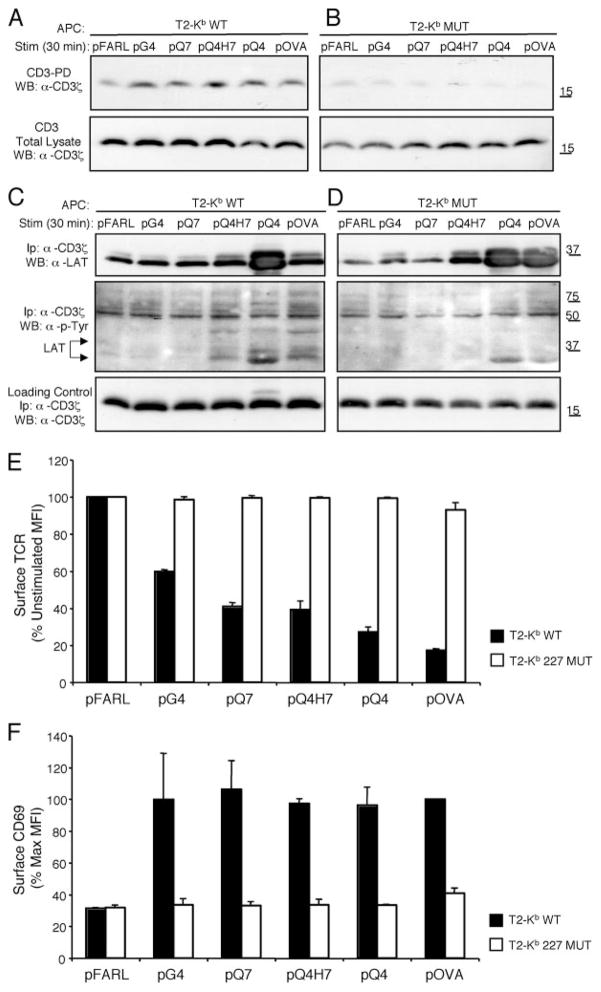
We wished to determine whether CD8 helps induce CD3Δc by enhancing the binding strength/affinity of TCR/pMHC interaction. The relative affinity of various Kb WT or Kb MUT/peptide complexes for the OT-I TCR is: Kb WT/pFARL < Kb WT/pG4 < Kb WT/pQ7 < Kb WT/pQ4H7 < Kb MUT/pOVA < Kb WT/pOVA (Fig. 3) (40, 45–47). When preselection OT-I DP thymocytes were stimulated by APCs expressing these pMHC ligands, CD3Δc was poorly induced by Kb MUT/pOVA despite efficient CD3Δc induction by three ligands (Kb WT/pG4, Kb WT/pQ7, and Kb WT/pQ4H7) with lower affinity for the OT-I TCR (Fig. 3). When other cognate peptides were presented by either T2-Kb WT APCs or T2-Kb MUT APCs, CD3Δc induction was always poorly induced by the mutant Kb ligands (Fig. 4, A and B). The mutant APCs did not fail to contact the OT-I TCR because stimulation by the stronger pMHC ligands induced some LAT phosphorylation and recruitment to CD3 (Fig. 4, C and D). This reveals that some signal transduction occurred when CD8/pMHC interaction was impaired and CD3Δc was inhibited; however, because the T2-Kb 227 MUT APCs also failed to induce TCR down-regulation (Fig. 4E) and CD69 up-regulation (Fig. 4F), this signaling was ineffective. We conclude that CD8 is required in order for cognate TCR/pMHC binding to induce CD3Δc, and that this coreceptor activity cannot be explained solely by its contribution to the affinity of cognate interactions (Figs. 3 and 4).
FIGURE 3.
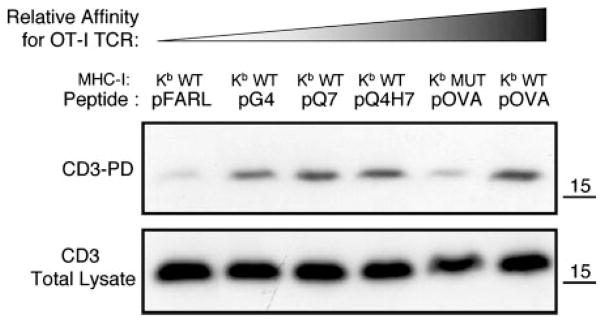
Involvement of CD8 in CD3Δc induction by cognate ligand in a role beyond affinity. The relative affinity for the OT-I TCR of variant peptides when loaded either in Kb WT or Kb MUT molecules is: Kb WT/pFARL < Kb WT/pG4 < Kb WT/pQ7 < Kb WT/pQ4H7 < Kb MUT/pOVA < Kb WT/pOVA. Preselection OT-I DP thymocytes were cocultured with these peptide-loaded APCs and the CD3-PD assay was performed (top). A fraction of the postnuclear lysate of each sample was used to determine the total CD3 content by Western blot of CD3ζ (bottom).
FIGURE 4.
Peptide presentation by T2-Kb D227K MUT APCs inhibits CD3Δc induction in preselection DP thymocytes. Preselection OT-I DP thymocytes were cocultured with T2-Kb WT (A) or T2-Kb MUT (B) APC preloaded with pFARL, pG4, pQ7, pQ4H7, pQ4, or pOVA, and the CD3-PD assay was performed (top). Total CD3ζ content in the postnuclear lysates is also shown (bottom). C and D, Samples from A and B were immuno-precipitated with anti-CD3ζ and associated proteins were detected by Western blot. Linker for activation of T cell (LAT) association with CD3ζ was inducible by either T2-Kb WT (C) or T2-Kb MUT (D) APC (upper panels), and some of this LAT was phosphorylated when blotted with anti-phosphotyrosine (α-p-Tyr) (middle panels). Total CD3ζ content in the postnuclear lysates is also shown (lower panels). TCR down-regulation (E) and CD69 up-regulation (F) were analyzed by flow cytometry for the experiments shown in A and B. Peptides are listed on the x-axis, whereas surface TCR expression (E) is represented on the y-axis as a percentage of the expression level on DP thymocytes cocultured with the null peptide pFARL. F, CD69 expression is represented on the y-axis as a percentage of the maximum induction on DP thymocytes cocultured with peptide pOVA.
Induction of CD3Δc by agonist ligands in mature T cells requires CD8
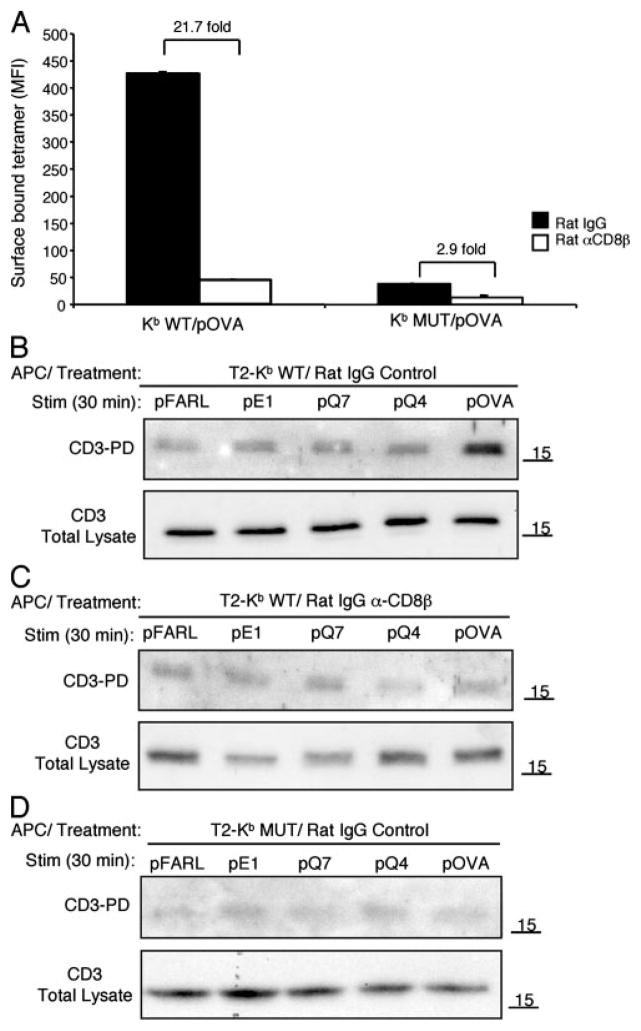
Lymph node CD8+ cells from OT-I β2m+/+ mice were incubated with anti-CD8β mAb, and stained with either Kb WT or Kb MUT/pOVA tetramers. As observed with preselection thymocytes, both the blocking mAb and the H-2Kb D227K mutation inhibited binding to the OT-I TCR on mature T cells (Fig. 5A). To study CD3Δc induction when CD8/pMHC-I interaction is blocked, mature CD8+ T cells that had been preincubated with either control Ig or anti-CD8β mAb were cocultured with T2-Kb WT or T2-Kb MUT APCs preloaded with null (pFARL), antagonist (pE1, pQ7), or agonist (pQ4, pOVA) peptides. Compared with preselection DP thymocytes (Fig. 1A), mature T cells displayed a lower sensitivity for cognate stimulation, inducing CD3Δc only to agonist peptides, with pOVA inducing the strongest response (Figs. 5B, top, and 1C, bottom). When mature T cells were pretreated with anti-CD8β, or the peptides were presented by T2-Kb MUT APCs, CD3Δc induction was inhibited for all peptides tested (Fig. 5, C and D). These data demonstrate that although peripheral T cells are less sensitive than preselection thymocytes to cognate pMHC ligands, both cell types require CD8 to induce CD3Δc.
FIGURE 5.
Induction of CD3Δc by agonist ligands in mature T cells requires CD8. A, Mature CD8+ T cells from OT-I β2m+/+ lymph nodes were incubated with either rat IgG control (■) or the anti-CD8β blocking mAb, 53.5.8 (□) (30 min, 37°C), and stained with Kb WT/pOVA or Kb MUT/pOVA tetramers (45 min on ice) before analysis by flow cytometry. Tetramer identity is indicated on the x-axis, and the quantity of surface bound tetramers gated on CD8+ cells is represented as MFI on the y-axis. The fold difference in tetramer binding is indicated. B–D, Mature CD8+ T cells from OT-I β2m+/+ lymph nodes were either pretreated with control rat IgG (B and D) or anti-CD8β blocking mAb 53.5.8 (30 min, 37°C) (C). T cells were then cocultured with T2-Kb WT (B and C) APCs or T2-Kb MUT (D) APCs loaded with pFARL, pQ7, pT4, pQ4, pKB, or pOVA (2 μM, 30 min, 37°C). Cells were lysed and subjected to the CD3-PD assay (top panels). Total CD3ζ content in the postnuclear lysates is also shown (bottom panels).
Surface desialylation of mature T cells re-establishes preselection sensitivity for cognate ligand recognition and CD3Δc induction
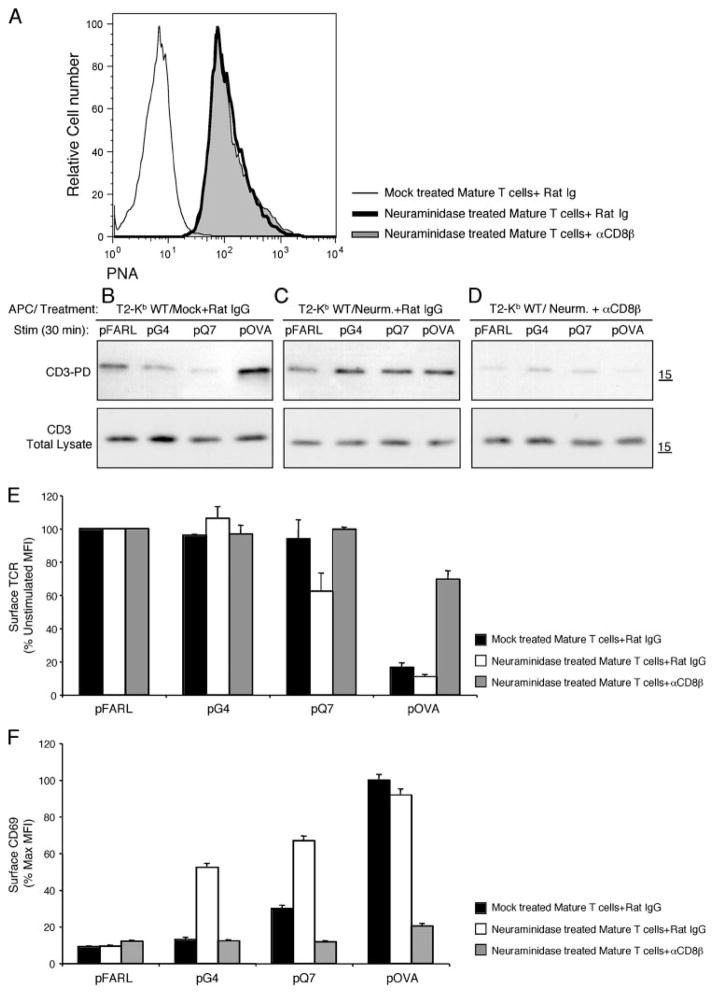
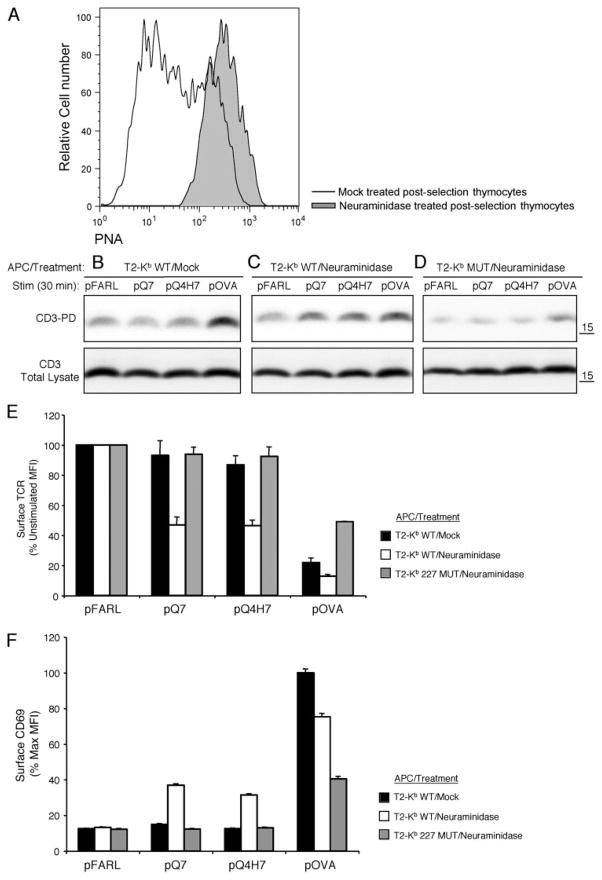
Increased sialylation in mature T cells has been shown to minimize TCR-independent noncognate CD8/MHC interactions. Furthermore, cleavage of surface sialic acid on mature T cells by neur-aminidase restores noncognate CD8 binding to levels observed with preselection DP thymocytes (26, 27). To study the impact of surface sialylation on the sensitivity of mature T cells, lymph node T cells from OT-I β2m+/+ mice were either untreated or treated with the enzyme neuraminidase. As expected, neuraminidase treatment resulted in increased PNA staining, which binds unsialylated O-linked core-1 glycans (25) (Fig. 6A), and increased the binding of Kb WT/pQ7 and Kb WT/OVA tetramers in a CD8-dependent fashion (data not shown). Mature T cells were cocultured with T2-Kb WT APCs preloaded with null (pFARL), antagonist (pG4, pQ7), or agonist (pOVA) peptides in the presence or absence of anti-CD8β. Control T cells displayed the expected low sensitivity for CD3Δc induction, responding only to pOVA (Fig. 6B). However, neuraminidase treatment of T cells restored CD3Δc induction by the weak ligands (Fig. 6C) by a mechanism dependent on CD8 (Fig. 6D). Neuraminidase treatment also enhanced functional responsiveness, as measured by TCR down-regulation (Fig. 6E) and CD69 up-regulation (Fig. 6F). Similar experiments were performed with postselection thymocytes from OT-I β2m+/+ mice. Likewise, the induction of CD3Δc was restored and functional responsiveness to the positive selectors pQ7 and pQ4H7 was enhanced in a CD8-dependent manner after neuraminidase treatment in postselection thymocytes (Fig. 7). Neuraminidase treatment did not enhance CD3Δc induction in preselection DP thymocytes (data not shown), as these cells express low basal levels of sialic acid (48) and maximal sensitivity for CD3Δc induction (Fig. 1). We conclude that the level of membrane sialylation on postselection thymocytes and mature T cells limits the sensitivity of cognate Ag recognition to agonist ligands by a mechanism dependent on CD8.
FIGURE 6.
Surface desialylation of mature T cells re-establishes preselection sensitivity for cognate ligand recognition and CD3Δc induction. A, Surface PNA staining (MFI) of CD8+ Kb WT/pOVA tetramer plus mature T cells from OT-I β2m+/+ lymph nodes that were mock and rat IgG-treated (B), neuraminidase and rat IgG-treated (C), or neuraminidase and anti-CD8β-treated (D). These T cells were cocultured with T2-Kb WT APC preloaded with pFARL, pQ7, pQ4H7, or pOVA (2 μM, 30 min, 37°C). The induction of CD3Δc was detected using the CD3-PD assay (top). Total CD3ζ content in the post-nuclear lysates is also shown (bottom). TCR down-regulation (E) and CD69 up-regulation (F) were analyzed by flow cytometry for the experiments shown in B–D. Peptides are listed on the x-axis, whereas surface TCR expression (E) is represented on the y-axis as a percentage of the expression level gated on Thy1+ CD4+CD8+ DP thymocytes cocultured with the null peptide pFARL. F, CD69 expression is represented on the y-axis as a percentage of the maximum induction on Thy1+ CD4+CD8+ DP thymocytes cocultured with peptide pOVA.
FIGURE 7.
Neuraminidase treatment of postselection thymocytes reestablishes preselection sensitivity for cognate ligand recognition and CD3Δc induction. A, Surface PNA staining (MFI) of CD8+ Kb WT/pOVA tetramer plus thymocytes from OT-I β2m+/+ mice that were either mock-treated (B) or treated with neuraminidase (C and D). These thymocytes were cocultured with T2-Kb WT (B and C) APC or T2-Kb MUT (D) APC preloaded with pFARL, pQ7, pQ4H7, or pOVA (2 μM, 30 min, 37°C). The induction of CD3Δc was detected using the CD3-PD assay (top panels). Total CD3ζ content in the postnuclear lysates is also shown (bottom panels). TCR down-regulation (E) and CD69 up-regulation (F) were analyzed by flow cytometry for the experiments shown in B–D. Peptides are listed on the x-axis, whereas surface TCR expression (E) is represented on the y-axis as a percentage of the expression level gated on Thy1+ CD4+CD8+ DP thymocytes cocultured with the null peptide pFARL. F, CD69 expression is represented on the y-axis as a percentage of the maximum induction on Thy1+ CD4+CD8+ thymocytes cocultured with peptide pOVA.
Discussion
We found that the induction of CD3Δc by cognate pMHC ligands for the OT-I TCR required CD8/TCR/pMHC interaction (Figs. 2–5). CD8 was required, whereas Src kinase activity was not (Fig. 2). Moreover, a high affinity for the OT-I TCR did not overcome the CD8 requirement for CD3Δc induction (Fig. 3). Previous studies have not resolved whether the CD3Δc is induced by an upstream conformational change in the αβ TCR heterodimer (TCRΔc). The majority of bound TCR/pMHC crystal structures reveal “induced-fit” TCRΔc that do not extend outside of the ligand binding interface and do not involve observable rearrangements of the TCR constant regions (49). We propose a model where CD8 physically induces and/or stabilizes CD3Δc during cognate TCR/pMHC interaction, “translating” productive Ag encounter from the TCR to the CD3 complex (Fig. 8). Importantly, this model does not require the αβ TCR to directly transmit a stable conformational change to the CD3 complex, consistent with the vast majority of TCR/pMHC crystal structures.
FIGURE 8.
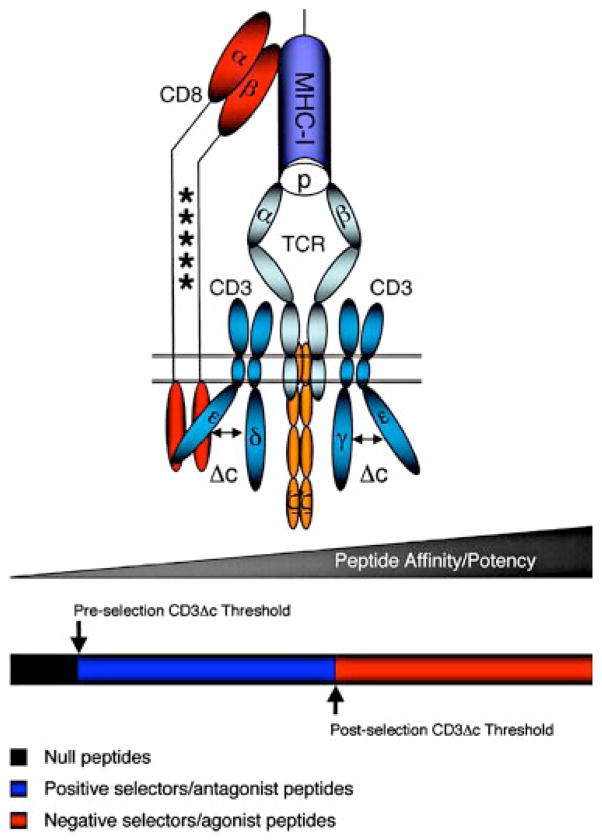
Model of developmental tuning of T cell Ag recognition mediated by changes in the sialylation state of CD8. The illustration represents the tripartite interaction between the TCR, pMHC, and the CD8 coreceptor required to induce CD3Δc in the OT-I TCR. The asterisk marks within the stalk of CD8 schematically represent glycan adducts that become differentially sialylated upon positive selection, compromising the ability of CD8 to bind MHC class I. The multicolor bar below the TCR/CD3 complex symbolizes ligands of various affinity: black, null ligands; blue, positive selector/antagonist ligands; and red, negative selector/agonist ligands. The arrow indicates the relative affinity threshold required by cognate peptides to induce CD3Δc before and after positive selection.
We used the OT-I TCR to describe how αβ TCR and CD3 can communicate through CD8. However, because CD3Δc induction is required for optimal signaling (7, 10), we suspect that coreceptor-independent T cells (50–52) may induce CD3Δc by an alternative mechanism. For example, the LC13 αβ TCR is specific for a viral peptide presented by HLA-B8 (53) and is CD8-independent (54). The crystal structure of this ligand-receptor pair provides the only case to date of an allosteric conformational change in the extracellular constant region of the TCR α-chain where CD3ε likely associates (55). Other recent experiments showed that the class I-restricted “T1” TCR, previously described as functionally independent of CD8 (56), can induce CD3Δc in response to a strong pMHC ligand in hybridomas lacking CD8 expression (10). However, because most pMHC-restricted T cell responses are CD8-dependent (52), we suspect that CD8 usually plays a major role inducing CD3Δc.
CD3Δc seems to clearly reflect productive TCR engagement, but whether CD3Δc also plays a role in discerning ligand potency has been less clear. We studied the sensitivity of T lineage cells to undergo CD3Δc and found that, although weak/positive selecting ligands induced CD3Δc in preselection DP thymocytes, these weak ligands failed to induce CD3Δc once thymocytes had begun positive selection (Figs. 1 and 7B). Though first observable in post-selection thymocytes, this reduced sensitivity to CD3Δc induction was further maintained in mature peripheral T cells (Figs. 1, 5B, and 6B), and correlated with a decreased cellular response to weak ligands (Figs. 6, E and F, and 7, E and F).
The fact that both positive and negative selecting ligands induce CD3Δc in preselection thymocytes rules out CD3Δc induction as the universal read-out of ligand affinity. However, another possibility is that the duration of CD3Δc could play a decisive role in this regard. We speculate that the length of the CD8/TCR/pMHC interaction (57–59) might determine the duration of CD3Δc and the extent to which the cell signaling machinery is activated. This idea is consistent with the finding that CD4 (60) and CD8 (61) activity can determine the strength of signal delivered by pMHC ligands. The latter study showed that the kinetics of CD8 association with the OT-I TCR predicted the strength of ligand better than the affinity of the TCR/pMHC interaction itself (61). Future experiments will need to address whether coreceptors impact ligand discrimination by controlling the duration of CD3Δc.
The attenuation of CD3Δc induction by weak ligands following positive selection establishes an interesting relationship between productive TCR engagement and the developmental tuning of T cell sensitivity. Following positive selection, CD3Δc induction and productive Ag recognition are confined to agonist ligands. This limitation is reversed by desialylation with neuraminidase (Figs. 6 and 7). Although desialylation may affect other surface molecules relevant to TCR/CD3 signaling (like CD45 (48)), the direct effect we observe on cognate Ag recognition provides at least a partial explanation for how sialylation contributes to the regulation of T cell sensitivity via CD8 (Figs. 6 and 7). This observation is supported by previous work, which showed that the TCR-independent noncognate CD8/MHC binding characteristic of preselection thymocytes was down-regulated by sialylation after positive selection (26, 27). The regulation of ligand sensitivity (developmental tuning) is controlled on several levels, including the regulation of CD8 expression (62) and the regulation of phosphatase activity (19–24). The change in sialylation and subsequent regulation of CD3Δc is one proximal aspect of this process. How CD8 sialylation contributes to an alteration in ligand binding and an alteration in signal initiation is currently under investigation.
Our results reconcile previous discrepancies regarding the efficiency with which positive selection pMHC ligands induce CD3Δc in thymocytes. Previous work from our laboratory demonstrated that positive and negative selecting ligands similarly induce CD3Δc in preselection (β2m−/−) thymocytes (8). However, Risueno et al. (13) demonstrated an increased number of thymocytes displaying CD3Δc in negative-selecting male H-Y mice when compared with positive-selecting female H-Y mice, arguing that the efficiency of detecting CD3Δc is increased under negative selection. The fact that postselection thymocytes from β2m+/+ animals were used in their studies may partially explain the lower detection of CD3Δc in positive selecting H-Y mice. The increased levels of surface sialylation on the thymocytes in theseβ2m+/+ mice would minimize their reactivity to positive selecting ligands, as we observed with OT-I postselection thymocytes (Figs. 1 and 7). Furthermore, it is not clear whether the H-Y negative selecting peptide is expressed on more thymic APCs compared with the positive-selecting peptide. For these reasons, positively selected thymocytes with a detectable CD3Δc may be difficult to visualize in β2m+/+ mice. However, when bona fide preselection thymocytes fromβ2m−/− mice are stimulated with positive selection ligands in vitro or in vivo, CD3Δc is efficiently induced (8) (Figs. 1–4).
In conclusion, we tested the idea that changes in the glycosylation pattern of developing T cells influences Ag recognition via CD3Δc induction. When enzymatically desialylated with neuraminidase, mature T cells and postselection thymocytes recovered CD3Δc induction by weak cognate stimuli (Figs. 6B and 7B). As expected, this treatment also enhanced responses to weak stimulation in mature T cells and postselection thymocytes (Figs. 6, E and F, and 7, E and F). Moreover, the positive effect of the neuraminidase treatment on CD3Δc induction and T cell responses was always dependent on the integrity of CD8/MHC interaction (Figs. 6D and 7D). These observations support the idea that CD8 sialylation regulates the developmental tuning of cognate Ag recognition and CD3Δc induction (Fig. 8). Throughout T cell maturation, the induction of CD3Δc is a feature of productively triggered TCRs, likely involved in a basic mechanism to enable signaling activity. Future experiments will focus on quantifying the interplay between coreceptors, TCR, pMHC, and the induction of CD3Δc.
Acknowledgments
We thank F. Carbone, M. Bevan, and D. Kioussis for OT-I transgenic mice; T. Potter for T2-Kb cells; R. Geha for the GST-SH3.1 construct; M. Mallaun, D. Naeher, E. M. Naves, J. R. Regueiro, and E. Teixeiro for helpful discussions; and B. Hausmann and D. Roubaty for excellent technical assistance.
Footnotes
This work was supported by Dirección General de Universidades e Investigación and Universidad Complutense de Madrid (UCM) program (reference no. 920631), by Ramón y Cajal program (UCM), and by Grant PI060057 from the Instituto de Salud Carlos III. This work is also supported with start-up funds from the Mayo Foundation (to D.G.), the Ruth L. Kirschstein National Research Service Award from the National Cancer Institute, National Institutes of Health, and start-up funds from the Mayo Foundation (to A.G.S.), and by the Cancer Research Foundation (to M.A.D.), EURAPS Novartis, the Swiss National Science Foundation, and Hoffman-LaRoche, Ltd. (to E.P.).
Abbreviations used in this paper: pMHC, peptide-MHC class I; CD3-PD, CD3 pull-down; CD3Δc, conformational change in CD3; DP, double positive; MFI, mean fluorescence intensity; β2m, β2-microglobulin; WT, wild type.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 2.Wong P, Barton GM, Forbush KA, Rudensky AY. Dynamic tuning of T cell reactivity by self-peptide-major histocompatibility complex ligands. J Exp Med. 2001;193:1179–1187. doi: 10.1084/jem.193.10.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Germain RN, Stefanová I. The dynamics of T cell receptor signaling: complex orchestration and the key roles of tempo and cooperation. Annu Rev Immunol. 1999;17:467–522. doi: 10.1146/annurev.immunol.17.1.467. [DOI] [PubMed] [Google Scholar]
- 4.Palmer E. Negative selection–clearing out the bad apples from the T-cell repertoire. Nat Rev Immunol. 2003;3:383–391. doi: 10.1038/nri1085. [DOI] [PubMed] [Google Scholar]
- 5.Pircher H, Rohrer UH, Moskophidis D, Zinkernagel RM, Hengartner H. Lower receptor avidity required for thymic clonal deletion than for effector T-cell function. Nature. 1991;351:482–485. doi: 10.1038/351482a0. [DOI] [PubMed] [Google Scholar]
- 6.Terhorst C, Exley M, Franco R, Hall C, Kang J, Mueller B, Sancho J, She J, Wileman T. Coupling of T-cell activation with T-cell receptor assembly. Year Immunol. 1993;7:1–24. [PubMed] [Google Scholar]
- 7.Gil D, Schamel WW, Montoya M, Sánchez-Madrid F, Alarcón B. Recruitment of Nck by CD3ε reveals a ligand-induced conformational change essential for T cell receptor signaling and synapse formation. Cell. 2002;109:901–912. doi: 10.1016/s0092-8674(02)00799-7. [DOI] [PubMed] [Google Scholar]
- 8.Gil D, Schrum AG, Alarcón B, Palmer E. T cell receptor engagement by peptide-MHC ligands induces a conformational change in the CD3 complex of thymocytes. J Exp Med. 2005;201:517–522. doi: 10.1084/jem.20042036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Risueño RM, Gil D, Fernández E, Sánchez-Madrid F, Alarcón B. Ligand-induced conformational change in the T-cell receptor associated with productive immune synapses. Blood. 2005;106:601–608. doi: 10.1182/blood-2004-12-4763. [DOI] [PubMed] [Google Scholar]
- 10.Minguet S, Swamy M, Alarcón B, Luescher IF, Schamel WW. Full activation of the T cell receptor requires both clustering and conformational changes at CD3. Immunity. 2007;26:43–54. doi: 10.1016/j.immuni.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Schamel WW, Risueño RM, Minguet S, Ortíz AR, Alarcón B. A conformation- and avidity-based proofreading mechanism for the TCR-CD3 complex. Trends Immunol. 2006;27:176–182. doi: 10.1016/j.it.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–782. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- 13.Risueño RM, van Santen HM, Alarcón B. A conformational change senses the strength of T cell receptor-ligand interaction during thymic selection. Proc Natl Acad Sci USA. 2006;103:9625–9630. doi: 10.1073/pnas.0601785103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marquez ME, Ellmeier W, Sanchez-Guajardo V, Freitas AA, Acuto O, Di Bartolo V. CD8 T cell sensory adaptation dependent on TCR avidity for self-antigens. J Immunol. 2005;175:7388–7397. doi: 10.4049/jimmunol.175.11.7388. [DOI] [PubMed] [Google Scholar]
- 15.Bevan MJ, Hogquist KA, Jameson SC. Selecting the T cell receptor repertoire. Science. 1994;264:796–797. doi: 10.1126/science.8171333. [DOI] [PubMed] [Google Scholar]
- 16.Davey GM, Schober SL, Endrizzi BT, Dutcher AK, Jameson SC, Hogquist KA. Preselection thymocytes are more sensitive to T cell receptor stimulation than mature T cells. J Exp Med. 1998;188:1867–1874. doi: 10.1084/jem.188.10.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grossman Z, Singer A. Tuning of activation thresholds explains flexibility in the selection and development of T cells in the thymus. Proc Natl Acad Sci USA. 1996;93:14747–14752. doi: 10.1073/pnas.93.25.14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eck SC, Zhu P, Pepper M, Bensinger SJ, Freedman BD, Laufer TM. Developmental alterations in thymocyte sensitivity are actively regulated by MHC class II expression in the thymic medulla. J Immunol. 2006;176:2229–2237. doi: 10.4049/jimmunol.176.4.2229. [DOI] [PubMed] [Google Scholar]
- 19.Cooke MP, Abraham KM, Forbush KA, Perlmutter RM. Regulation of T cell receptor signaling by a src family protein-tyrosine kinase (p59fyn) Cell. 1991;65:281–291. doi: 10.1016/0092-8674(91)90162-r. [DOI] [PubMed] [Google Scholar]
- 20.Chan AC, van Oers NS, Tran A, Turka L, Law CL, Ryan JC, Clark EA, Weiss A. Differential expression of ZAP-70 and Syk protein tyrosine kinases, and the role of this family of protein tyrosine kinases in TCR signaling. J Immunol. 1994;152:4758–4766. [PubMed] [Google Scholar]
- 21.Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, Love PE. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J Exp Med. 1998;188:2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lucas B, Stefanová I, Yasutomo K, Dautigny N, Germain RN. Divergent changes in the sensitivity of maturing T cells to structurally related ligands underlies formation of a useful T cell repertoire. Immunity. 1999;10:367–376. doi: 10.1016/s1074-7613(00)80036-9. [DOI] [PubMed] [Google Scholar]
- 23.Dautigny N, Lucas B. Developmental regulation of TCR efficiency. Eur J Immunol. 2000;30:2472–2478. doi: 10.1002/1521-4141(200009)30:9<2472::AID-IMMU2472>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 24.Li QJ, Chau J, Ebert PJ, Sylvester G, Min H, Liu G, Braich R, Manoharan M, Soutschek J, Skare P, et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129:147–161. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Wu W, Harley PH, Punt JA, Sharrow SO, Kearse KP. Identification of CD8 as a peanut agglutinin (PNA) receptor molecule on immature thymocytes. J Exp Med. 1996;184:759–764. doi: 10.1084/jem.184.2.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moody AM, Chui D, Reche PA, Priatel JJ, Marth JD, Reinherz EL. Developmentally regulated glycosylation of the CD8αβ coreceptor stalk modulates ligand binding. Cell. 2001;107:501–512. doi: 10.1016/s0092-8674(01)00577-3. [DOI] [PubMed] [Google Scholar]
- 27.Daniels MA, Devine L, Miller JD, Moser JM, Lukacher AE, Altman JD, Kavathas P, Hogquist KA, Jameson SC. CD8 binding to MHC class I molecules is influenced by T cell maturation and glycosylation. Immunity. 2001;15:1051–1061. doi: 10.1016/s1074-7613(01)00252-7. [DOI] [PubMed] [Google Scholar]
- 28.Starr TK, Daniels MA, Lucido MM, Jameson SC, Hogquist KA. Thymocyte sensitivity and supramolecular activation cluster formation are developmentally regulated: a partial role for sialylation. J Immunol. 2003;171:4512–4520. doi: 10.4049/jimmunol.171.9.4512. [DOI] [PubMed] [Google Scholar]
- 29.Pappu BP, Shrikant PA. Alteration of cell surface sialylation regulates antigen-induced naive CD8+ T cell responses. J Immunol. 2004;173:275–284. doi: 10.4049/jimmunol.173.1.275. [DOI] [PubMed] [Google Scholar]
- 30.San José E, Sahuquillo AG, Bragado R, Alarcón B. Assembly of the TCR/CD3 complex: CD3 epsilon/delta and CD3 ε/γ dimers associate indistinctly with both TCR α and TCR β chains: evidence for a double TCR heterodimer model. Eur J Immunol. 1998;28:12–21. doi: 10.1002/(SICI)1521-4141(199801)28:01<12::AID-IMMU12>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 31.Rozdzial MM, Kubo RT, Turner SL, Finkel TH. Developmental regulation of the TCR ζ-chain: differential expression and tyrosine phosphorylation of the TCR ζ-chain in resting immature and mature T lymphocytes. J Immunol. 1994;153:1563–1580. [PubMed] [Google Scholar]
- 32.Stotz SH, Bolliger L, Carbone FR, Palmer E. T cell receptor (TCR) antagonism without a negative signal: evidence from T cell hybridomas expressing two independent TCRs. J Exp Med. 1999;189:253–264. doi: 10.1084/jem.189.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Potter TA, Rajan TV, Dick RF, II, Bluestone JA. Substitution at residue 227 of H-2 class I molecules abrogates recognition by CD8-dependent, but not CD8-independent, cytotoxic T lymphocytes. Nature. 1989;337:73–75. doi: 10.1038/337073a0. [DOI] [PubMed] [Google Scholar]
- 34.Daniels MA, Jameson SC. Critical role for CD8 in T cell receptor binding and activation by peptide/major histocompatibility complex multimers. J Exp Med. 2000;191:335–346. doi: 10.1084/jem.191.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Devine L, Hodsdon ME, Daniels MA, Jameson SC, Kavathas PB. Location of the epitope for an anti-CD8α antibody 53.6.7 which enhances CD8α-MHC class I interaction indicates antibody stabilization of a higher affinity CD8 conformation. Immunol Lett. 2004;93:123–130. doi: 10.1016/j.imlet.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Zijlstra M, Bix M, Simister NE, Loring JM, Raulet DH, Jaenisch R. β2-microglobulin deficient mice lack CD4−8+ cytolytic T cells. Nature. 1990;344:742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]
- 37.Clarke SR, Barnden M, Kurts C, Carbone FR, Miller JF, Heath WR. Characterization of the ovalbumin-specific TCR transgenic line OT-I: MHC elements for positive and negative selection. Immunol Cell Biol. 2000;78:110–117. doi: 10.1046/j.1440-1711.2000.00889.x. [DOI] [PubMed] [Google Scholar]
- 38.Puls KL, Hogquist KA, Reilly N, Wright MD. CD53, a thymocyte selection marker whose induction requires a lower affinity TCR-MHC interaction than CD69, but is up-regulated with slower kinetics. Int Immunol. 2002;14:249–258. doi: 10.1093/intimm/14.3.249. [DOI] [PubMed] [Google Scholar]
- 39.Stefanski HE, Mayerova D, Jameson SC, Hogquist KA. A low affinity TCR ligand restores positive selection of CD8+ T cells in vivo. J Immunol. 2001;166:6602–6607. doi: 10.4049/jimmunol.166.11.6602. [DOI] [PubMed] [Google Scholar]
- 40.Daniels MA, Teixeiro E, Gill J, Hausmann B, Roubaty D, Holmberg K, Werlen G, Hollander GA, Gascoigne NR, Palmer E. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 41.Kao C, Sandau MM, Daniels MA, Jameson SC. The sialyltransferase ST3Gal-I is not required for regulation of CD8-class I MHC binding during T cell development. J Immunol. 2006;176:7421–7430. doi: 10.4049/jimmunol.176.12.7421. [DOI] [PubMed] [Google Scholar]
- 42.Moody AM, North SJ, Reinhold B, Van Dyken SJ, Rogers ME, Panico M, Dell A, Morris HR, Marth JD, Reinherz EL. Sialic acid capping of CD8β core 1-O-glycans controls thymocyte-major histocompatibility complex class I interaction. J Biol Chem. 2003;278:7240–7246. doi: 10.1074/jbc.M210468200. [DOI] [PubMed] [Google Scholar]
- 43.Thome M, Germain V, DiSanto JP, Acuto O. The p56lck SH2 domain mediates recruitment of CD8/p56lck to the activated T cell receptor/CD3/ζ complex. Eur J Immunol. 1996;26:2093–2100. doi: 10.1002/eji.1830260920. [DOI] [PubMed] [Google Scholar]
- 44.Miceli MC, Parnes JR. Role of CD4 and CD8 in T cell activation and differentiation. Adv Immunol. 1993;53:59–122. doi: 10.1016/s0065-2776(08)60498-8. [DOI] [PubMed] [Google Scholar]
- 45.Alam SM, Davies GM, Lin CM, Zal T, Nasholds W, Jameson SC, Hogquist KA, Gascoigne NR, Travers PJ. Qualitative and quantitative differences in T cell receptor binding of agonist and antagonist ligands. Immunity. 1999;10:227–237. doi: 10.1016/s1074-7613(00)80023-0. [DOI] [PubMed] [Google Scholar]
- 46.Rosette C, Werlen G, Daniels MA, Holman PO, Alam SM, Travers PJ, Gascoigne NR, Palmer E, Jameson SC. The impact of duration versus extent of TCR occupancy on T cell activation: a revision of the kinetic proofreading model. Immunity. 2001;15:59–70. doi: 10.1016/s1074-7613(01)00173-x. [DOI] [PubMed] [Google Scholar]
- 47.Gascoigne NR, Zal T, Alam SM. T-cell receptor binding kinetics in T-cell development and activation. Expert Rev Mol Med. 2001;2001:1–17. doi: 10.1017/S1462399401002502. [DOI] [PubMed] [Google Scholar]
- 48.Daniels MA, Hogquist KA, Jameson SC. Sweet ‘n’ sour: the impact of differential glycosylation on T cell responses. Nat Immunol. 2002;3:903–910. doi: 10.1038/ni1002-903. [DOI] [PubMed] [Google Scholar]
- 49.Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol. 2006;24:419–466. doi: 10.1146/annurev.immunol.23.021704.115658. [DOI] [PubMed] [Google Scholar]
- 50.Knall C, Smith PA, Potter TA. CD8-dependent CTL require co-engagement of CD8 and the TCR for phosphatidylinositol hydrolysis, but CD8-independent CTL do not and can kill in the absence of phosphatidylinositol hydrolysis. Int Immunol. 1995;7:995–1004. doi: 10.1093/intimm/7.6.995. [DOI] [PubMed] [Google Scholar]
- 51.Gray PM, Parks GD, Alexander-Miller MA. A novel CD8-independent high-avidity cytotoxic T-lymphocyte response directed against an epitope in the phosphoprotein of the paramyxovirus simian virus 5. J Virol. 2001;75:10065–10072. doi: 10.1128/JVI.75.21.10065-10072.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holler PD, Kranz DM. Quantitative analysis of the contribution of TCR/pepMHC affinity and CD8 to T cell activation. Immunity. 2003;18:255–264. doi: 10.1016/s1074-7613(03)00019-0. [DOI] [PubMed] [Google Scholar]
- 53.Kjer-Nielsen L, Clements CS, Brooks AG, Purcell AW, McCluskey J, Rossjohn J. The 1.5 A crystal structure of a highly selected antiviral T cell receptor provides evidence for a structural basis of immunodominance. Structure. 2002;10:1521–1532. doi: 10.1016/s0969-2126(02)00878-x. [DOI] [PubMed] [Google Scholar]
- 54.Buslepp J, Wang H, Biddison WE, Appella E, Collins EJ. A correlation between TCR Vα docking on MHC and CD8 dependence: implications for T cell selection. Immunity. 2003;19:595–606. doi: 10.1016/s1074-7613(03)00269-3. [DOI] [PubMed] [Google Scholar]
- 55.Kjer-Nielsen L, Clements CS, Purcell AW, Brooks AG, Whisstock JC, Burrows SR, McCluskey J, Rossjohn J. A structural basis for the selection of dominant αβ T cell receptors in antiviral immunity. Immunity. 2003;18:53–64. doi: 10.1016/s1074-7613(02)00513-7. [DOI] [PubMed] [Google Scholar]
- 56.Kessler BM, Bassanini P, Cerottini JC, Luescher IF. Effects of epitope modification on T cell receptor-ligand binding and antigen recognition by seven H-2Kd-restricted cytotoxic T lymphocyte clones specific for a photoreactive peptide derivative. J Exp Med. 1997;185:629–640. doi: 10.1084/jem.185.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rabinowitz JD, Beeson C, Lyons DS, Davis MM, McConnell HM. Kinetic discrimination in T-cell activation. Proc Natl Acad Sci USA. 1996;93:1401–1405. doi: 10.1073/pnas.93.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lyons DS, Lieberman SA, Hampl J, Boniface JJ, Chien Y, Berg LJ, Davis MM. A TCR binds to antagonist ligands with lower affinities and faster dissociation rates than to agonists. Immunity. 1996;5:53–61. doi: 10.1016/s1074-7613(00)80309-x. [DOI] [PubMed] [Google Scholar]
- 59.McKeithan TW. Kinetic proofreading in T-cell receptor signal transduction. Proc Natl Acad Sci USA. 1995;92:5042–5046. doi: 10.1073/pnas.92.11.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Madrenas J, Chau LA, Smith J, Bluestone JA, Germain RN. The efficiency of CD4 recruitment to ligand-engaged TCR controls the agonist/partial agonist properties of peptide-MHC molecule ligands. J Exp Med. 1997;185:219–229. doi: 10.1084/jem.185.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yachi PP, Ampudia J, Zal T, Gascoigne NR. Altered peptide ligands induce delayed CD8-T cell receptor interaction–a role for CD8 in distinguishing antigen quality. Immunity. 2006;25:203–211. doi: 10.1016/j.immuni.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 62.Park JH, Adoro S, Lucas PJ, Sarafova SD, Alag AS, Doan LL, Erman B, Liu X, Ellmeier W, Bosselut R, Feigenbaum L, Singer A. ‘Coreceptor tuning’: cytokine signals transcriptionally tailor CD8 coreceptor expression to the self-specificity of the TCR. Nat Immunol. 2007;8:1049–1059. doi: 10.1038/ni1512. [DOI] [PubMed] [Google Scholar]