Abstract
The Wnt signaling pathway inhibitor Dickkopf-2 (Dkk2) regulates osteoblast differentiation on microstructured titanium (Ti) surfaces, suggesting involvement of Wnt signaling in this process. To test this, human osteoblast-like MG63 cells were cultured on tissue culture polystyrene or Ti (smooth PT (Ra = 0.2 μm), sand-blasted and acid-etched SLA (Ra = 3.22 μm), modSLA (hydrophilic SLA)). Expression of Wnt pathway receptors, activators and inhibitors was measured by qPCR. Non-canonical pathway ligands, receptors and intracellular signaling molecules, as well as bone morphogenetic proteins BMP2 and BMP4, were upregulated on SLA and modSLA, whereas canonical pathway members were downregulated. To confirm that non-canonical signaling was involved, cells were cultured daily with exogenous Wnt3a (canonical pathway) or Wnt5a (non-canonical pathway). Alternatively, cells were cultured with antibodies to Wnt3a or Wnt5a to validate that Wnt proteins secreted by the cells were mediating cell responses to the surface. Wnt5a, but not Wnt3a, increased MG63 cell differentiation and BMP2 and BMP4 proteins, suggesting Wnt5a promotes osteogenic differentiation through production of BMPs. Effects of exogenous and endogenous Wnt5a were synergistic with surface microstructure, suggesting the response also depends on cell maturation state. These results indicate a major role for the non-canonical, calcium-dependent Wnt pathway in differentiation of osteoblasts on microstructured titanium surfaces during implant osseointegration.
Keywords: Cell signaling, Titanium surface roughness, Osteoblast differentiation, Gene expression, Regulatory factors
1. Introduction
In vitro studies have shown that osteoblasts are sensitive to surface microtopography and chemistry of their substrate [1–3]. When grown on titanium substrates presenting complex micron and sub-micron topographic features, termed microstructured Ti, they exhibit increased osteoblast maturation and terminal differentiation than when grown on tissue culture polystyrene (TCPS) or smooth Ti surfaces [4,5]. This process depends on production of the Wnt pathway inhibitor Dickkopf-2 (Dkk2). Studies using Dkk2 silenced cells show that they fail to differentiate when grown on microstructured Ti; osteoblastic differentiation can be restored when Dkk2 but not Dkk1 is added to the culture medium [6]. Moreover, adding anti-Dkk2 antibodies to the medium blocks the effect of microtexture on osteoblast differentiation, indicating that endogenous Dkk2 acts in an autocrine/paracrine manner. Involvement of Dkk2 in the response of osteoblasts to microtextured Ti suggests that differentiation on these surfaces is mediated by the Wnt signaling pathways.
Wnt family genes encode a large number of proteins that regulate patterning, development, proliferation and differentiation in a large variety of organs and tissues [7]. Wnt proteins function in both an autocrine and paracrine manner, binding to specific G-protein coupled receptors of the Frizzled family (Fzd) and triggering signaling pathways according to the ligand–receptor combination. The functional effect of Wnt proteins is determined not only by the ligand but also by the specific receptor-coreceptor interaction. Fzds function as receptors and low-density lipoprotein receptor-related proteins (Lrp5/6), receptor tyrosine kinase-like orphan receptor 2 (Ror2) and receptor-like tyrosine kinase (Ryk) have been shown to function as co-receptors [8].
In the present study, we focused on two Wnt signaling pathways involved in bone formation and osteoblast differentiation, the Wnt/β-catenin pathway, also known as the canonical pathway, and the Wnt calcium-dependent pathway, one of two non-canonical Wnt pathways. In the Wnt canonical pathway, a Wnt protein binds to a Fzd receptor and a co-receptor (Lrp5/6), resulting in Dishevelled (Dsh) activation and inhibition of a complex consisting of Axin, glycogen synthase kinase 3β (GSK3β) and adenomatous polyposis coli (APC). When this occurs, GSK3β is unable to phosphorylate β-catenin and, instead, non-phosphorylated β-catenin accumulates in the cytoplasm, translocates into the nucleus and modulates gene transcription. Several studies have shown the importance of the activation of the Wnt/β-catenin pathway in mesenchymal stem cell differentiation towards an osteogenic phenotype, osteoblast maturation, and bone formation and development [9–12].
The Wnt calcium-dependent pathway relies on Wnts to stimulate the intracellular release of calcium to activate calmodulin-dependent kinase II (CamKII), protein kinase C (PKC) and calcineurin. These activated proteins trigger a signaling cascade that modulates gene expression. We showed previously that PKC activity is increased in osteoblasts cultured on microtextured Ti compared to smooth Ti and TCPS and inhibition of PKC blocks the stimulatory effects of the surface on osteoblast differentiation [13,14]. In vivo studies also support a role for the non-canonical pathway in bone formation. Wnt5a+/− mice present a reduced bone mass phenotype, decreased osteoblast number and increased adipocytes [15], suggesting that Wnt5a blocks PPAR-gamma-induced adipogenesis in bone marrow cells and plays an important role in increasing the commitment of mesenchymal stem cells to the osteoblast phenotype [15–17].
In this study, we first determined which components of the canonical and non-canonical Wnt signaling pathways are expressed in osteoblasts cultured on Ti in comparison to TCPS and if expression on Ti was sensitive to surface microtexture and/or surface energy. In order to address the role of canonical vs. non-canonical Wnt signaling, we examined the potential contributions of Wnt3a and Wnt5a in the maturation of osteoblast-like cells on microstructured titanium surfaces using exogenous proteins. Furthermore, to validate that Wnt proteins are secreted into the media and act in an autocrine/paracrine manner, we blocked the endogenous proteins with specific antibodies. The results of this study show for the first time that expression of Wnt pathways is surface-dependent and that Wnt5a plays an important role in mediating the effects of Ti surface microstructure on osteoblast differentiation.
2. Materials and methods
2.1. Ti disk preparation
Institut Straumann AG (Basel, Switzerland) supplied the Ti disks used in this study. Sheets of grade 2 unalloyed Ti, 1 mm thick, were punched to create disks 15 mm in diameter, which fitted into wells of 24-well tissue culture plates. The production methods and surface characterization have been described previously [4,18]. The hydrophilicity of disks with pretreatment (PT, Ra = 0.2 μm), sandblasted and acid-etched (SLA, Ra = 3.22 μm) and hydrophilic SLA (modSLA, Ra = 3.22 μm) surfaces was determined using advancing contact angles: PT, 95.8°; SLA, 139.8°; and modSLA ~0° [19].
2.2. Cell culture methods
Human osteoblast-like MG63 cells (American Type Culture Collection, Manassas, VA) were plated at 10,000 cells cm−2 on TCPS or Ti surfaces. Cells were cultured in Dulbecco’s modified Eagle’s medium (Mediatech, Manassas, VA) supplemented with 10% fetal bovine serum (Hyclone, Waltham, MA) and 1% penicillin–streptomycin (Invitrogen, Carlsbad, CA). All cells were cultured at 37 °C with 5% CO2 and 100% humidity. To validate the observations using MG63 cells, primary human osteoblasts were isolated from a 15-year-old male patient at Children’s Healthcare of Atlanta (CHOA) under Institutional Review Board approval at Georgia Tech and CHOA, and were cultured in a comparable manner.
2.3. Expression of Wnt pathway genes
When cells grown on TCPS reached confluence, fresh media were added to all cultures. RNA was isolated 12 h later using Trizol and quantified using a Nanodrop spectrophotometer (Thermo Scientific, Waltham, MA). mRNA levels for the following proteins were assessed: Wnt ligands WNT1, WNT3A, WNT5A, WNT7B, WNT10B and WNT11; Wnt receptors LRP5, LRP6, KREM1 and ROR2; Wnt pathway members β-catenin (CTNNB) and AXIN2; and Wnt pathway inhibitors WIF1, SFRP1, SFRP2, CER1, DKK1 and DKK2. We did not measure secreted protein levels for the Wnts for several reasons. Validated immunoassay kits are not available for these proteins. Moreover, Wnt proteins are secreted glycoproteins that bind strongly to heparin sulfate molecules in the extracellular matrix. As a result, measurement of Wnts in conditioned media may be inaccurate due to the possibility of proteins remaining tethered to the extracellular matrix. Importantly, previous studies showed that secreted Dkk1 and Dkk2 protein levels were regulated in a surface-dependent manner and to the same extent as mRNA levels for these proteins [6]. In addition, we verified that expression of the downstream protein osteocalcin (OCN) was modulated by surface properties, as noted in our previously published work [20,21].
To create a cDNA template, 1 μg of RNA was reverse transcribed using random primers (Promega, Madison, WI) and Omniscript reverse transcriptase (Qiagen, Valencia, CA). To quantify expression, cDNA was used for real-time qPCR with gene-specific primers using an iQ5 (Bio-Rad, Hercules, CA). Fluorescence values were quantified as starting quantities using known dilutions of MG63 cells grown on TCPS. mRNAs for these genes were normalized to expression of glyceraldehyde-3-phophate dehydrogenase (GAPDH) mRNAs. Primers (Table 1) were designed using the Beacon designer software and synthesized by Eurofins MWG Operon (Huntsville, AL).
Table 1.
Primer sequences used for real-time PCR analysis of gene expression.
| AXIN-2 | F | GGA GAA ATG CGT GGA TAC C |
| R | GCT GCT TGG AGA CAA TGC | |
| CER-1 | F | TAC CTC CTG CTC TCA CTG TT G |
| R | ATG CTC CGT CTT CAC CTT GC | |
| CTNNB | F | GGC AGC AAC AGT CTT ACC |
| R | TCC ACA TCC TCT TCC TCA | |
| DKK1 | F | CCA GAC CAT TGA CAA CTA CC |
| R | CAG GCG AGA CAG ATT TGC | |
| DKK2 | F | TGA CTT GGG ATG GCA GAA TC |
| R | CAG AAA TGA CGA GCA CAG C | |
| FZD-1 | F | TGC CAA TCC TGA CAT CTC G |
| R | GCA AGA GGT CTG TCC ATC C | |
| FZD-2 | F | TCT GGG CGA GCG TGA TTG |
| R | GAC AGT GAA GAA GGT GGA AGC | |
| FZD-3 | F | GCT CTC ATA GTT GGC ATT CC |
| R | TAC CTG TCG GCT CTC ATT C | |
| FZD-4 | F | CAT CTC TCA TCC CTT TCC C |
| R | GCC TAC TCT CAT AGT CTT CC | |
| FZD-5 | F | TTG GTA TTC ATT AGG CTG TTG C |
| R | GTC ACG GAT GCT GTT ATT AAG G | |
| FZD-6 | F | GCG GAG TGA AGG AAG GAT TAG |
| R | ACA AGC AGA GAT GTG GAA CC | |
| FZD-7 | F | AAG TGA CCT GGA AGT GAG AAG |
| R | CAC ATC GCC GTT ATC ATC ATC | |
| FZD-8 | F | CTT GAT GGG CTG AGG TTC C |
| R | GTC TGG GAG GCT TCA ATG C | |
| FZD-9 | F | CAT CGG CTA CAA CCT GAC C |
| R | GCG AGC ACA GGA AGA AGC | |
| GAPDH | F | GCT CTC CAG AAC ATC ATC C |
| R | TGC TTC ACC ACC TTC TTG | |
| KREMEN-1 | F | CAA CAA GAT GAC CGC AAT CC |
| R | GAG AAG AAC CAC AGG AGA GG | |
| LRP-5 | F | TGG ATT TGA ACT CGG ACT C |
| R | GGG AAG AGA TGG AAG TAG C | |
| LRP-6 | F | GCA GAG GAG AAC TAT GAA AGC |
| R | GTT GGA GGC AGT CAG AGG | |
| OCN | F | GTG ACG AGT TGG CTG ACC |
| R | TGG AGA GGA GCA GAA CTG G | |
| ROR2 | F | GTG CCT TCA AAG AAA CTG |
| R | TAC AAT ACT GCT TCC TCT G | |
| SFRP1 | F | ATC AGC CAG TCT CAG ATG CC |
| R | AAA TCG CCG TCT CTC TCA GG | |
| SFRP2 | F | AAG GAA AAG CCC ACC CGA ATC |
| R | ACA ACA ACC AAC CAG ACC CAA G | |
| WIF1 | F | GCG GCA CGA GGA GTT TTC |
| R | AGC AGG CAC AGG AGG ATG | |
| WNT1 | F | CAG GAG GTG AGA GAA GGA TG |
| R | TGG AGC AGG CAG AAT GAC | |
| WNT3A | F | GTC CCG TCC CTC CCT TTC |
| R | ACC TCT CTT CCT ACC TTT CCC | |
| WNT5A | F | TCT CAG CCC AAG CAA CAA GG |
| R | GCC AGC ATC ACA TCA CAA CAC | |
| WNT7B | F | TCG GCA CTA ACA CAT AGC |
| R | CGG GAA AGG AAA CAG AGG | |
| WNT10B | F | TTC TCT CGG GAT TTC TTG GAT TC |
| R | GTT GTT GTG GAT TCG CAT TCG | |
| WNT 11 | F | ACT GAT GCG TCT ACA CAA C |
| R | AGGTATCGGGTCTTGAGG |
2.4. Autocrine and paracrine effects of Wnt3a and Wnt5a
To determine if the cells were sensitive to Wnts in our model system, we treated cultures with either 50 ng ml−1 recombinant human Wnt3a (R&D Systems, Minneapolis, MN) [22] or 125 ng ml−1 recombinant mouse Wnt5a (R&D Systems) [23]. To test the effects of endogenous Wnts, we treated cultures with 1:200 dilutions of either a rabbit polyclonal antibody to Wnt3a (AbWnt3a; Abcam ab28472, Cambridge, MA) or a rabbit polyclonal antibody to Wnt5a (AbWnt5a; Abcam ab72583).
Media were changed every 48 h and cells treated with either proteins or antibodies to Wnts until they reached confluence on TCPS (about 7 days). Both cell types were treated with 1:200 dilutions of mouse IgG (Santa Cruz, Santa Cruz, CA) as controls. These cells behaved like the untreated cultures for all parameters tested (data not shown). At confluence, cells were incubated in full media without treatment for 24 h. Cells were harvested from the surfaces by two sequential trypsinizations and counted using a cell counter (Z2 Particle counter, Beckman Coulter, Fullerton, CA) [4].
We did not assess the effects of exogenous or endogenous Wnts on mineralization of the cultures. MG63 cells do not calcify their extracellular matrix under the culture conditions that we used [24,25]. Osteoblasts and precursor cells can be induced to mineralize by treating them with osteogenic media containing combinations of beta-glycerophosphate, ascorbic acid and dexamethasone [26–28], but addition of these factors was an additional variable that had potential to obscure the specific roles of each Wnt protein in osteoblast differentiation. The same reasoning applied to the normal human osteoblast cultures. To assess the effects of the Wnts on osteoblast differentiation, we examined alkaline phosphatase activity, an early marker of osteoblast maturation that reaches a peak before matrix mineralization [29], and osteocalcin, a late marker of osteoblast differentiation and an important modulator of hydroxyapatite crystal formation [30]. Cells were lysed and alkaline phosphatase specific activity and protein levels measured in the lysate, as described previously [4]. The conditioned media were collected and osteocalcin levels measured by radioimmunoassay (Biomedical Technologies Inc., Stoughton, MA). In addition, levels of osteoprotegerin (OPG; R&D Systems), vascular endothelial growth factor (VEGF; R&D Systems), transforming growth factor beta-1 (TGF-β1; R&D Systems), bone morphogenetic protein-2 (BMP2; PeproTech, Rocky Hill, NJ) and BMP4 (R&D Systems) in the conditioned media were measured by ELISA per manufacturer’s instructions, as described previously [31,32].
2.5. Statistical analysis
The data presented are from one of two sets of experiments, with comparable results. Data are mean ± SEM of six independent cultures per variable. Data were first analyzed by analysis of variance. Significant differences between groups were determined using Bonferroni’s modification of Student’s t-test. p < 0.05 was considered to be significant.
3. Results
3.1. Surface-dependent regulation of Wnt pathway gene expression
Expression of Wnt ligands WNT1 (Fig. 1A), WNT3A (Fig. 1B) and WNT7B (Fig. 1D) mRNAs in MG63 cells on PT was similar to expression on TCPS; however, cells grown on rough SLA and modSLA surfaces had lower expression. WNT5A mRNA was increased 100% on rough SLA surfaces in comparison to TCPS or PT surfaces and was further increased by the high surface energy of modSLA surfaces (Fig. 1C). Expression of WNT10B mRNA decreased on SLA in comparison to TCPS, with a further decrease on modSLA substrates (Fig. 1E). Both WNT11 (Fig. 1F) and AXIN2 (Fig. 1H) mRNAs had 100% higher expression on SLA and modSLA surfaces than cells on TCPS or PT. There was no difference in CTNNB mRNA expression between the groups (Fig. 1G). OCN mRNA was measured as an indicator of cell maturation and was higher on SLA and modSLA surfaces than on the smooth TCPS or PT (Fig. 1I).
Fig. 1.
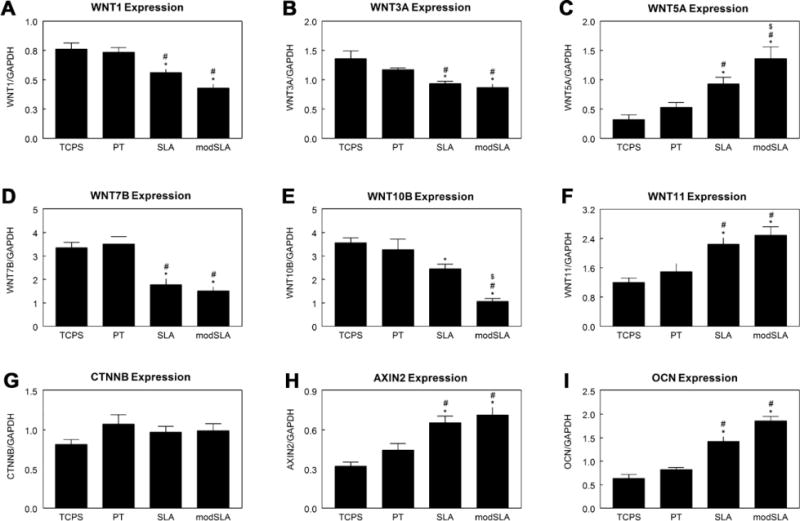
Regulation of Wnt pathway activators and canonical molecules in MG63 cells grown on microstructured Ti surfaces. Expression of Wnt pathway activators WNT1 (A), WNT3A (B), WNT5A (C), WNT7B (D), WNT10B (E), and WNT11 (F) were measured by real-time PCR. Expression of activation (CTNNB (G)) and inhibition (AXIN2 (H)) of canonical Wnt signaling were also measured. Osteoblast maturation was confirmed by OCN expression (I). *p < 0.05, vs. TCPS; #p < 0.05, vs. PT; $p < 0.05, vs. SLA.
Similar results were seen in cultures of primary human osteoblasts. CTNNB/GAPDH was lower on rough SLA (0.85 ± 0.04) and modSLA (0.82 ± 0.04) in comparison to PT (1.35 ± 0.06). WNT3A/GAPDH was lower on SLA (0.93 ± 0.04) and modSLA (0.90 ± 0.04) than on PT (1.24 ± 0.05). However, there was a 2-fold increase in WNT5A/GAPDH on modSLA compared to osteoblasts cultured on PT (3.21 ± 0.14 vs. 1.61 ± 0.08).
Expression of mRNAs for Wnt receptors was also sensitive to surface properties. Both FZD1 (Fig. 2A) and FZD3 (Fig. 2C) had higher expression on Ti substrates than on TCPS. Expression of FZD2 (Fig. 2B) and FZD6 (Fig. 2F) was higher on rough SLA and modSLA surfaces. In contrast, expression of FZD4 (Fig. 2D) was lower on SLA and modSLA than on TCPS or PT. FZD5 expression was higher on SLA surfaces than TCPS and was upregulated on modSLA surfaces in comparison to both TCPS and PT (Fig. 2E). SLA surface had higher FZD expression than TCPS, but expression on modSLA was increased 100% in comparison to the other substrates examined (Fig. 2G). FZD8 expression was higher on PT substrates than on TCPS, but roughness had no effect on expression (Fig. 2H). While FZD9 expression was increased on PT and SLA in comparison to TCPS, expression on modSLA was upregulated in comparison to all other surfaces (Fig. 2I).
Fig. 2.

Regulation of Frizzleds in MG63 cells grown on microstructured Ti surfaces. Expression of FZD receptors FZD1-9 (A–I) were measured using real-time PCR. *p < 0.05, vs. TCPS; #p < 0.05, vs. PT; $p < 0.05, vs. SLA.
Levels of mRNAs for the co-receptors were substrate-dependent as well. Ti surfaces had no effect on LRP5 expression (Fig. 3A), but LRP6 was increased 50% in cells on SLA substrates in comparison to TCPS and was significantly higher on modSLA in comparison to PT (Fig. 3B). Expression of KREM1 increased on Ti surfaces, and was higher on modSLA than on PT (Fig. 3C). ROR2 expression was increased on SLA and modSLA in comparison to TCPS and PT, but was highest on modSLA (Fig. 3D).
Fig. 3.

Regulation of Wnt pathway co-receptors in MG63 cells grown on microstructured Ti surfaces. Expression of Wnt pathway co-receptors LRP5 (A), LRP6 (B), KREM1 (C) and ROR2 (D) were measured by real-time PCR. *p < 0.05, vs. TCPS; #p < 0.05, vs. PT; $p < 0.05, vs. SLA.
Levels of mRNAs for Wnt pathway inhibitors also depended on the surface. WIF1 (Fig. 4A) increased on Ti and in a roughness and surface energy-dependent manner (TCPS < PT < SLA < modSLA). Both SFRP1 (Fig. 4B) and SFRP2 (Fig. 4C) were higher on rough surfaces (SLA and modSLA) in comparison to smooth TCPS and PT surfaces. Culture on Ti substrates increased expression of CER1 (Fig. 4D). DKK1 expression was upregulated on PT, but was further increased on SLA and modSLA surfaces (Fig. 4E). Expression of DKK2 was higher on Ti substrates than on TCPS, but the combined high surface energy and roughness of modSLA increased expression over smooth PT (Fig. 4F).
Fig. 4.
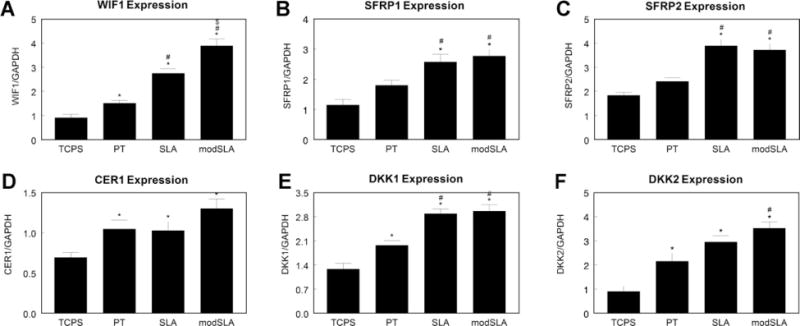
Regulation of Wnt pathway inhibitors in MG63 cells grown on microstructured Ti surfaces. Expression of Wnt pathway inhibitors WIF1 (A), SFRP1 (B), SFRP2 (C), CER1 (D), DKK1 (E) and DKK2 (F) were measured by real-time PCR. *p < 0.05, vs. TCPS; #p < 0.05, vs. PT; $p < 0.05, vs. SLA.
3.2. Effect of Wnt3a on MG63 cells
In untreated MG63 cells, cell number on TCPS and PT was comparable, but was decreased on SLA and further decreased on modSLA surfaces (Fig. 5A). Treatment with Wnt3a had no effect on cell number on TCPS, PT or SLA, but slightly decreased cell number on modSLA. AbWnt3a-treated cells had similar cell numbers to untreated cells. Alkaline phosphatase specific activity and osteocalcin both increased on titanium surfaces, with highest levels on the rough SLA and modSLA surfaces (Fig. 5B and C). Treatment with Wnt3a or AbWnt3a did not change alkaline phosphatase or osteocalcin levels as compared to untreated cells. The same effect was seen in levels of OPG (Fig. 5D).
Fig. 5.

Effect of Wnt3a on MG63 cell response to surface microstructure and surface energy. MG63 cells plated on TCPS, PT, SLA or modSLA surfaces were cultured with 50 ng ml−1 Wnt3a or 1:200 AbWnt3a until confluence. At confluence, cell number (A), alkaline phosphatase specific activity (B), osteocalcin levels (C) and OPG levels (D) were determined. *p < 0.05, Ti vs. TCPS; #p < 0.05, treatment with either Wnt3a or AbWnt3a vs. untreated MG63 cells on each surface.
BMP2 increased about 50% in the conditioned media of cells grown on SLA and modSLA from levels on TCPS and PT surfaces (Fig. 6A). Cells treated with Wnt3a increased levels on PT and decreased BMP2 on modSLA in comparison to untreated MG63 cells. AbWnt3a increased BMP2 levels over untreated cells only on SLA and modSLA substrates. BMP4 levels increased in MG63 cells grown on SLA and modSLA surfaces by 50–100% as compared to TCPS and PT surfaces (Fig. 6B). Addition of Wnt3a did not affect BMP4 levels on any surface. However, blocking endogenous Wnt3a with AbWnt3a increased BMP4 levels on all surfaces by 25–30% over control levels. Levels of VEGF and latent TGF-β1 increased on titanium surfaces, with the highest levels on the rough SLA and modSLA surfaces (Fig. 6C and D). There was no change in levels of either factor from control after treatment with Wnt3a or AbWnt3a. A similar effect was seen on active TGF-β1 (Supplemental Fig. 1A).
Fig. 6.
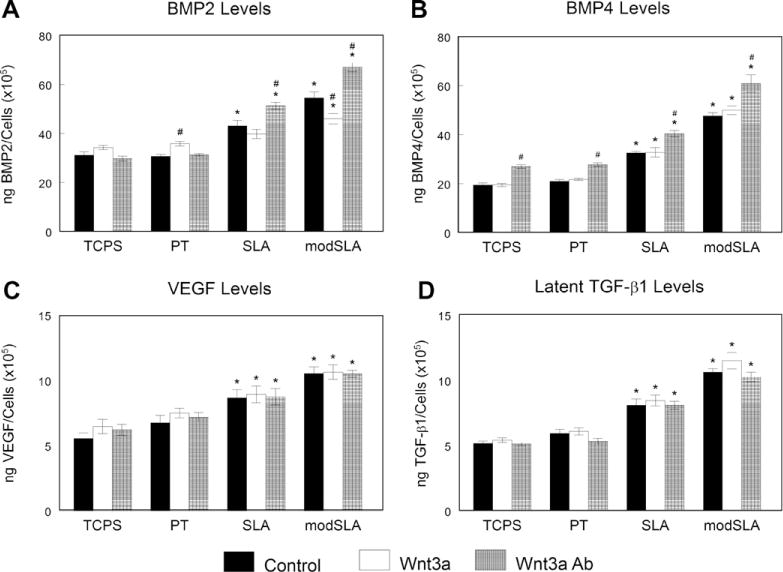
Effect of Wnt3a on MG63 cell response to surface microstructure and surface energy. MG63 cells plated on TCPS, PT, SLA or modSLA surfaces were cultured with 50 ng ml−1 Wnt3a or 1:200 AbWnt3a until confluence. At confluence, levels of BMP2 (A), BMP4 (B), VEGF (C) and latent TGF-β1 (D) were determined. *p < 0.05, Ti vs. TCPS; #p < 0.05, treatment with either Wnt3a or AbWnt3a vs. untreated MG63 cells on each surface.
3.3. Effect of Wnt5a on MG63 cells
MG63 cell number decreased on SLA surfaces in comparison to TCPS and PT surfaces, with a 50% reduction in number on modSLA from TCPS (Fig. 7A). While treatment with AbWnt5a did not affect cell number in comparison to control, Wnt5a protein reduced cell number by 10% on PT and 50% on SLA and modSLA surfaces. Alkaline phosphatase specific activity increased in MG63 cells grown on titanium surfaces, with highest activity seen on the rough SLA and modSLA surfaces (Fig. 7B). Addition of exogenous Wnt5a increased alkaline phosphatase specific activity on all surfaces in comparison to control. Treatment with AbWnt5a slightly decreased levels in comparison to untreated cells, but this difference was not significant. Osteocalcin levels were increased by 100% in MG63 cells grown on SLA and modSLA surfaces over TCPS and PT surfaces (Fig. 7C). Wnt5a treatment increased osteocalcin on SLA and increased levels by almost 100% on modSLA. AbWnt5a did not affect osteocalcin production on TCPS and PT substrates, but decreased levels on SLA and modSLA when compared to the untreated cells. Levels of OPG were increased by 50% in MG63 cells grown on SLA and 100% in cells on modSLA over TCPS and PT (Fig. 7D). Treatment with Wnt5a increased OPG on all surfaces, but its effect was greatest on the rough SLA and modSLA substrates. AbWnt5a did not affect OPG production on TCPS and PT substrates, but slightly decreased levels on SLA and modSLA when compared to the untreated cells.
Fig. 7.

Effect of Wnt5a on MG63 cell response to surface microstructure and surface energy. MG63 cells plated on TCPS, PT, SLA or modSLA surfaces were cultured with 250 ng ml−1 Wnt5a or 1:200 AbWnt5a until confluence. At confluence, cell number (A), alkaline phosphatase specific activity (B), osteocalcin levels (C) and OPG levels (D) were determined. *p < 0.05, Ti vs. TCPS; #p < 0.05, treatment with either Wnt5a or AbWnt5a vs. untreated MG63 cells on each surface.
BMP2 levels increased in the media of cultures on SLA and modSLA surfaces in comparison to TCPS and PT (Fig. 8A). Addition of Wnt5a increased levels by 80% on SLA and modSLA surfaces over untreated levels, but there was no effect on TCPS or PT surfaces. AbWnt5a did not affect BMP2 levels on any of the surfaces examined. Levels of BMP4 increased in MG63 cells on SLA and modSLA surfaces in comparison to TCPS and PT (Fig. 8B). Addition of Wnt5a increased BMP4 levels by 50% over untreated cells and this effect was not substrate dependent. There was no difference in BMP4 levels between untreated and AbWnt5a-treated MG63 cells on any of the surfaces examined. VEGF production increased in MG63 cells grown on the rough SLA and modSLA surfaces (Fig. 8C). Wnt5a-treated cells increased VEGF on all surfaces, but this effect was additive to the surface effect seen in untreated cells. AbWnt5a treatment had no effect on the smooth surfaces or on SLA, but decreased VEGF on the modSLA surfaces in comparison to control. Latent TGF-β1 increased in the conditioned media of MG63 cells on rough surfaces (Fig. 8D). This effect increased with addition of Wnt5a, increasing levels by 50% on SLA surfaces and by 100% on modSLA surfaces. Addition of AbWnt5a decreased latent TGF-β1 on modSLA substrates, but had no effect on the other surfaces. Active TGF-β1 increased in response to Wnt5a on all Ti substrates; however, there was no effect after treatment with AbWnt5a (Supplemental Fig. 1B).
Fig. 8.

Effect of Wnt5a on MG63 cell response to surface microstructure and surface energy. MG63 cells plated on TCPS, PT, SLA or modSLA surfaces were cultured with 250 ng ml−1 Wnt5a or 1:200 AbWnt5a until confluence. At confluence, levels of BMP2 (A), BMP4 (B), VEGF (C) and latent TGF-β1 (D) were determined. *p < 0.05, Ti vs. TCPS; #p < 0.05, treatment with either Wnt5a or AbWnt5a vs. untreated MG63 cells on each surface.
4. Discussion
The canonical Wnt pathway (β-catenin dependent) and calcium-dependent Wnt pathway have been shown to participate in osteoblast differentiation, maturation, and bone formation, based on in vitro studies as well as through analysis of knockout mouse models [33–36]. Most of the in vitro studies have been conducted using traditional cell culture methods. While these studies have provided information concerning potential mechanisms involved in osteoblastic differentiation, they do not address the important questions related to how materials used clinically might affect osteoblast behavior. Recent studies in our laboratory examining the relative roles of Dkk1 and Dkk2 in regulating enhanced osteoblastic differentiation on microtextured titanium surfaces suggested that it is mediated by Wnt signaling [6]. The results of the present study confirm that Wnt signaling is sensitive to substrate properties and demonstrate that osteoblast response to Ti microtexture is through non-canonical Wnt mechanisms.
We found that mRNA expression for several Wnt ligands was modulated by surface roughness and energy in MG63 cells as well as normal human osteoblasts. Wnt1, Wnt2, Wnt3a, Wnt8, Wnt8b are members of the Wnt1 class, which induce a secondary body axis in Xenopus embryos and activate the Wnt/β-catenin signaling cascade [37]. However, Wnt7b and Wnt10b also activate the same signaling pathway [38–40].
Wnts are lipid-modified, secreted glycoproteins that interact with cell surface and extracellular matrix sulfated glycosaminoglycans, modulating their biodistribution and activity [41,42]. Due to their hydrophobic nature and affinity for heparan and chondroitin sulfates, analysis of Wnts by conventional quantitative ELISA techniques has not been successful and is still in development [43]. For the same reason, analysis of Wnts by Western blot in these kinds of experiments could be inaccurate due to the accumulation of Wnts in the extracellular matrix over time. Thus, our results cannot make definitive statements concerning the effects of the Ti surfaces on the levels of each of these proteins or their specific impact on Wnt signaling.
The canonical Wnt/β-catenin pathway has been extensively studied as a promoter for bone health and as a potential therapeutic target. Several studies have demonstrated that Wnt1 and Wnt3a induce alkaline phosphatase activity during osteoblast differentiation in osteoprogenitor cells [44,45]. Rawadi and colleagues also showed that Wnt3a increased expression of important osteogenic markers as Runx2, osteocalcin, and collagen type I [44]. Wnt7b is expressed after osteogenic induction [46], and a skeletal phenotype is not present in Wnt7b−/− embryo [46]. Wnt10b−/− mutants present a skeletal phenotype, with a decrease in trabecular bone and osteocalcin levels [47]. However, in our study we found that expression of mRNAs for canonical Wnts decreased with increasing osteoblast maturation on SLA and modSLA, suggesting that canonical Wnt signaling is repressed during osteoblast maturation on microstructured Ti surfaces (Fig. 9).
Fig. 9.

Schematic of regulation of Wnt canonical and calcium-dependent pathway molecules in microstructured Ti surfaces. Comparison of regulation of canonical (left) and calcium-dependent (right) Wnt signaling pathways in cells grown on PT and modSLA surfaces. Green arrow indicates upregulation and red arrow indicates downregulation of expression on modSLA surfaces in comparison to PT surfaces.
Wnt/β-catenin signaling is regulated by many receptors, co-receptors and inhibitors. Fzd proteins act as Wnt receptors and structurally have seven transmembrane domains. While they are suggested to be G-protein coupled receptors, only some Fzds activate heterotrimeric G-proteins to increase intracellular calcium [48]. Wnts interact with Fzds through a cysteine-rich domain, but the Wnt ligand specificity is unclear [49]. Lrp5 and Lrp6 participate as co-receptors for the Wnt/β-catenin signaling. Mutations in the LRP5 gene have shown to cause osteoporosis and osteoporosis pseudoglioma syndrome characterized by blindness [50]. Moreover, recently Yadav and Ducy generated an LRP5−/− mouse [51]. In that experimental model, they observed a decrease in the amount of bone volume, formation rate and osteoblast number. In contrast, a gain of function mutation in LRP5 causes an increase in bone mass characterized by elevated bone formation coupled with normal resorption [52]. LRP6 mutations also result in a delayed ossification at birth and a low-bone-mass phenotype [53]. While these studies indicate that Wnt/β-catenin activation is critical for osteoblast differentiation, maturation and bone formation, recent studies suggest a possible role of Wnt/β-catenin pathway as a promoter of mesenchymal stem cell renewal and inhibitor of osteoblast differentiation [16,17,54]. Our data demonstrate that expression of mRNAs for Wnt/β-catenin signaling pathway molecules was sensitive to microstructured titanium surfaces, with a downregulation of the activators and an upregulation of the inhibitors, suggesting that Wnt/β-catenin signaling pathway inhibition is needed in osteoblast maturation.
β-Catenin translocation to the nucleus is frequently associated with canonical Wnt pathway activation; however, β-catenin translocation can also be regulated by cadherin signaling [55]. Here we found no change in β-catenin expression, providing additional evidence that this pathway does not control osteoblast differentiation on these surfaces. We have also previously demonstrated that Dkk1 and Dkk2, Wnt/β-catenin pathway inhibitors, modulated osteoblast maturation in cells grown on microstructured titanium surfaces [6], a result confirmed in the current study. It has also been shown that osterix, a master regulator of osteoblast differentiation, inhibits Wnt/β-catenin signaling [56]. Taken together, these data suggest that Wnt/β-catenin signaling is repressed in osteoblasts on microstructured Ti surfaces.
The Wnt calcium-dependent pathway, which does not involve β-catenin, is transduced through Fzd proteins and co-receptors Ror2 and Ryk [57,58]. This less studied pathway is not completely understood, but Wnt5a has been shown to activate this signaling pathway. Members of the Wnt5a class (Wnt4, Wn5a, Wnt5b, Wnt6, Wnt7a and Wnt11) cannot induce secondary axis formation, activate heterotrimeric G-proteins or increase intracellular calcium levels [37]. Wnt5a has also been shown to inhibit the Wnt/β-catenin pathway, but the mechanism is not clear [59]. We found upregulation of mRNAs for the non-canonical Wnts Wnt5a and Wnt11 in cells on SLA and modSLA surfaces. The role of Wnt5a in skeletal development was underscored in the Wnt5a−/− mouse, which showed deformities in the developing face, including cleft palate, truncated snout, mandible and tongue, significant limb shortening, and absence of distal digits [35]. We also found upregulation of ROR2 in the more mature cells on SLA and modSLA surfaces. The Ror2−/− mouse model exhibited a similar phenotype to the Wnt5a−/− mouse, including shortened limbs and facial abnormalities, suggesting that Wnt5a and Ror2 are required for osteoblast differentiation and maturation [58]. Wnt5a has been also implicated in the activation of the planar cell polarity pathway through the phosphorylation of Dsh and activation of RhoA, Rac and JNK [60,61]. However, the same group demonstrated that activation of JNK by Wnt5a is regulated by PKC, and that inhibition of PKC blocked JNK activation [62].
The results of the gene expression studies showed that both canonical and calcium-dependent Wnt pathways were tightly regulated in MG63 cells and normal human osteoblasts in response to surface roughness and energy, but subsequent osteoblast maturation was controlled by calcium-dependent Wnt signaling (Fig. 9). This hypothesis was supported by our studies assessing the specific roles of Wnt3a and Wnt5a in regulating osteoblast differentiation. The MG63 cells responded to the surface microstructure with a decrease in cell number in a roughness-dependent manner. However, this effect was not modified by the addition of exogenous Wnt3a or blocking the endogenous Wnt3a. Neither treatment had an effect on alkaline phosphatase activity or levels of osteocalcin, important factors in matrix mineralization [29,30]. In addition, Wnt3a had no effect on production of OPG, TGF-β1 or VEGF, which are associated with reduced osteoclastic activity and enhanced vascularization required for peri-implant bone formation [31,63].
The lack of effect of either exogenous or endogenous Wnt3a may be attributed to the high levels of Wnt antagonists Dkk1 and Dkk2 produced by MG63 cells and normal human osteoblasts on microstructured titanium surfaces, as we demonstrated previously [6]. A second hypothesis is that the Wnt/β-catenin pathway is inactive in MG63 cells [64]. Due to the increase in BMP2 and BMP4 levels after blocking endogenous Wnt3a, our data support the hypothesis that Wnt3a inhibits osteoblastogenesis and osteoblast maturation and confirms similar results from a study in which Dkk1 blocked the induction of BMP promoters [65].
Our results support the hypothesis that non-canonical Wnt signaling mediates osteoblast differentiation on Ti in general and on microstructured Ti specifically. Wnt5a treatment significantly reduced MG63 cell number on all titanium surfaces, with a very robust cell number reduction on the rough SLA and modSLA substrates. Conversely, osteoblast maturation was enhanced as demonstrated by increased alkaline phosphatase activity and production of osteocalcin. Levels of BMP2, BMP4, VEGF, latent and active TGF-β1, and OPG were upregulated, suggesting that Wnt5a induced autocrine/paracrine regulation of osteoblast differentiation together with an osteogenic environment conducive to net bone formation in vivo. Interestingly, OPG, VEGF, active TGF-β1 and BMP4 levels increased with the treatment of exogenous Wnt5a on all surfaces tested, with a synergistic effect on the roughest surfaces whereas alkaline phosphatase activity, osteocalcin, BMP2 and latent TGF-β1 levels increased only on the microstructured surfaces. These results indicate that Wnt5a affects maturation of MG63 cells grown on microstructured titanium surfaces. Additionally, our data suggest that this effect may be mediated through local factors, particularly through BMPs. In addition, the effect on OPG levels suggests that Wnt5a is important in regulation of bone turnover, particularly on rough surfaces. Our data also support our previous observations showing that PKC activity is increased in MG63 cells grown on microtextured Ti and is required for their differentiation [13,14].
Differences noted in rate of osseointegration of smooth and microtextured Ti implants in vivo [66,67] may reflect these differences in gene expression. However, there are not enough data demonstrating the role of Wnt5a in osteoblasts, and only a few studies have examined Wnt5a in bone marrow cells or osteoblast precursor cells. The Wnt5a−/− [35] and Ror−/− mouse models [68,69] exhibit similar phenotypes, including dwarfism, cartilage and bone defects, limb shortening, and facial abnormalities. Wnt5a has also been shown to be important in fracture healing and bone regeneration, indicating its importance of this molecule in bone formation and homeostasis [56]. One study suggested that Wnt5a is involved in osteogenic differentiation on titanium surfaces; however, this study focused only on gene expression, and the increase in Wnt5a expression was observed at just one of the four time points measured [70].
Our data indicate that Wnt5a acts in an autocrine/paracrine manner, raising the question of how its production is regulated. Antibodies to Wnt5a blocked its effects in cultures grown on SLA and modSLA. Thus, endogenously generated Wnt5a was unable to bind its receptor and activate the non-canonical signaling pathway. We previously showed that production of Dkk2 depends on signaling via integrin heterodimer α2β1 [6], but whether Wnt5a is mediated by this mechanism is not known. α2β1 is also required for production of TGF-β1 and TGF-β1 modulates OPG production on SLA and modSLA [71], suggesting the possibility that factors like the BMPs and TGF-β1 induced by Wnt5a signaling may participate in the overall responses observed in the present study. Failure of the AbWnt5a cells to increase production of TGF-β1 in the present study may account for the reduction in OPG, but the results do not tell us if α2β1 is downstream of Wnt5a or if the effects are regulated by the α2β1 and Wnt5a pathways independently.
Our study highlights the importance of physiologically relevant culture substrates in elucidating signaling pathways. For most of the factors examined, we saw no difference between treated and untreated cells on TCPS. It was only when the cells were cultured on substrates similar to topographies seen in the body that the contributions of the signaling pathway were apparent.
5. Conclusions
Collectively, our results suggest that osseointegration requires Wnt signaling from both the canonical and calcium-dependent Wnt pathways, and that these pathways are regulated in an autocrine and paracrine manner on microstructured titanium surfaces. Wnt activators, inhibitors and receptors may play an important role around implants, and Wnt signaling pathways must be tightly regulated for successful osteoblast differentiation, maturation and eventual bone formation.
Supplementary Material
Acknowledgments
This study was supported by a NIH USPHS Grant AR052102. Ti disks were provided by Institut Straumann AG (Basel, Switzerland) as a gift.
This project was supported by a grant from the National Institute of Arthritis, Musculoskeletal, and Skin Diseases at the National Institutes of Health (USPHS Grant AR052102). Titanium disks were provided by Institut Straumann AG (Basel, Switzerland) as a gift. The funding source had no role in the study design, data collection and analysis, data interpretation or manuscript preparation.
Appendix A. Figures with essential colour discrimination
Certain figures in this article, particularly Fig. 9 is difficult to interpret in black and white. The full colour images can be found in the on-line version, at doi: 10.1016/j.actbio.2011.02.030.
Appendix B. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.actbio.2011.02.030.
References
- 1.Schwartz Z, Kieswetter K, Dean DD, Boyan BD. Underlying mechanisms at the bone-surface interface during regeneration. J Periodontal Res. 1997;32:166–71. doi: 10.1111/j.1600-0765.1997.tb01399.x. [DOI] [PubMed] [Google Scholar]
- 2.Bachle M, Kohal RJ. A systematic review of the influence of different titanium surfaces on proliferation, differentiation and protein synthesis of osteoblastlike MG63 cells. Clin Oral Implants Res. 2004;15:683–92. doi: 10.1111/j.1600-0501.2004.01054.x. [DOI] [PubMed] [Google Scholar]
- 3.Meyer U, Buchter A, Wiesmann HP, Joos U, Jones DB. Basic reactions of osteoblasts on structured material surfaces. Eur Cell Mater. 2005;9:39–49. doi: 10.22203/ecm.v009a06. [DOI] [PubMed] [Google Scholar]
- 4.Zhao G, Raines AL, Wieland M, Schwartz Z, Boyan BD. Requirement for both micron- and submicron scale structure for synergistic responses of osteoblasts to substrate surface energy and topography. Biomaterials. 2007;28:2821–9. doi: 10.1016/j.biomaterials.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao G, Zinger O, Schwartz Z, Wieland M, Landolt D, Boyan BD. Osteoblast-like cells are sensitive to submicron-scale surface structure. Clin Oral Implants Res. 2006;17:258–64. doi: 10.1111/j.1600-0501.2005.01195.x. [DOI] [PubMed] [Google Scholar]
- 6.Olivares-Navarrete R, Hyzy S, Wieland M, Boyan BD, Schwartz Z. The roles of Wnt signaling modulators Dickkopf-1 (Dkk1) and Dickkopf-2 (Dkk2) and cell maturation state in osteogenesis on microstructured titanium surfaces. Biomaterials. 2010;31:2015–24. doi: 10.1016/j.biomaterials.2009.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Easwaran V, Lee SH, Inge L, Guo L, Goldbeck C, Garrett E, et al. Beta-Catenin regulates vascular endothelial growth factor expression in colon cancer. Cancer Res. 2003;63:3145–53. [PubMed] [Google Scholar]
- 8.Billiard J, Way DS, Seestaller-Wehr LM, Moran RA, Mangine A, Bodine PV. The orphan receptor tyrosine kinase Ror2 modulates canonical Wnt signaling in osteoblastic cells. Mol Endocrinol. 2005;19:90–101. doi: 10.1210/me.2004-0153. [DOI] [PubMed] [Google Scholar]
- 9.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–50. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Hu B, Nikolakopoulou AM, Cohen-Cory S. BDNF stabilizes synapses and maintains the structural complexity of optic axons in vivo. Development. 2005;132:4285–98. doi: 10.1242/dev.02017. [DOI] [PubMed] [Google Scholar]
- 11.Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8:727–38. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133:3231–44. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz Z, Lohmann CH, Sisk M, Cochran DL, Sylvia VL, Simpson J, et al. Local factor production by MG63 osteoblast-like cells in response to surface roughness and 1,25-(OH)2D3 is mediated via protein kinase C- and protein kinase A-dependent pathways. Biomaterials. 2001;22:731–41. doi: 10.1016/s0142-9612(00)00241-6. [DOI] [PubMed] [Google Scholar]
- 14.Fang M, Olivares-Navarrete R, Wieland M, Cochran DL, Boyan BD, Schwartz Z. The role of phospholipase D in osteoblast response to titanium surface microstructure. J Biomed Mater Res A. 2010;93:897–909. doi: 10.1002/jbm.a.32596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takada I, Mihara M, Suzawa M, Ohtake F, Kobayashi S, Igarashi M, et al. A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-gamma transactivation. Nat Cell Biol. 2007;9:1273–85. doi: 10.1038/ncb1647. [DOI] [PubMed] [Google Scholar]
- 16.Baksh D, Boland GM, Tuan RS. Cross-talk between Wnt signaling pathways in human mesenchymal stem cells leads to functional antagonism during osteogenic differentiation. J Cell Biochem. 2007;101:1109–24. doi: 10.1002/jcb.21097. [DOI] [PubMed] [Google Scholar]
- 17.Baksh D, Tuan RS. Canonical and non-canonical Wnts differentially affect the development potential of primary isolate of human bone marrow mesenchymal stem cells. J Cell Physiol. 2007;212:817–26. doi: 10.1002/jcp.21080. [DOI] [PubMed] [Google Scholar]
- 18.Rupp F, Scheideler L, Olshanska N, de Wild M, Wieland M, Geis-Gerstorfer J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J Biomed Mater Res A. 2006;76:323–34. doi: 10.1002/jbm.a.30518. [DOI] [PubMed] [Google Scholar]
- 19.Zhao G, Schwartz Z, Wieland M, Rupp F, Geis-Gerstorfer J, Cochran DL, et al. High surface energy enhances cell response to titanium substrate microstructure. J Biomed Mater Res A. 2005;74:49–58. doi: 10.1002/jbm.a.30320. [DOI] [PubMed] [Google Scholar]
- 20.Raz P, Lohmann CH, Turner J, Wang L, Poythress N, Blanchard C, et al. 1alpha,25(OH)2D3 regulation of integrin expression is substrate dependent. J Biomed Mater Res A. 2004;71:217–25. doi: 10.1002/jbm.a.30134. [DOI] [PubMed] [Google Scholar]
- 21.Olivares-Navarrete R, Raz P, Zhao G, Chen J, Wieland M, Cochran DL, et al. Integrin alpha2beta1 plays a critical role in osteoblast response to micron-scale surface structure and surface energy of titanium substrates. Proc Natl Acad Sci U S A. 2008;105:15767–72. doi: 10.1073/pnas.0805420105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer L, Boland G, Tuan RS. Wnt-3A enhances bone morphogenetic protein-2-mediated chondrogenesis of murine C3H10T1/2 mesenchymal cells. J Biol Chem. 2002;277:30870–8. doi: 10.1074/jbc.M109330200. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Rubin B, Bodine PV, Billiard J. Wnt5a induces homodimerization and activation of Ror2 receptor tyrosine kinase. J Cell Biochem. 2008;105:497–502. doi: 10.1002/jcb.21848. [DOI] [PubMed] [Google Scholar]
- 24.Pierschbacher MD, Dedhar S, Ruoslahti E, Argraves S, Suzuki S. An adhesion variant of the MG-63 osteosarcoma cell line displays an osteoblast-like phenotype. Ciba Found Symp. 1988;136:131–41. doi: 10.1002/9780470513637.ch9. [DOI] [PubMed] [Google Scholar]
- 25.Hattar S, Berdal A, Asselin A, Loty S, Greenspan DC, Sautier JM. Behaviour of moderately differentiated osteoblast-like cells cultured in contact with bioactive glasses. Eur Cell Mater. 2002;4:61–9. doi: 10.22203/ecm.v004a05. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida T, Kikuchi M, Koyama Y, Takakuda K. Osteogenic activity of MG63 cells on bone-like hydroxyapatite/collagen nanocomposite sponges. J Mater Sci Mater Med. 2010;21:1263–72. doi: 10.1007/s10856-009-3938-3. [DOI] [PubMed] [Google Scholar]
- 27.Maniatopoulos C, Sodek J, Melcher AH. Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell Tissue Res. 1988;254:317–30. doi: 10.1007/BF00225804. [DOI] [PubMed] [Google Scholar]
- 28.Peter SJ, Liang CR, Kim DJ, Widmer MS, Mikos AG. Osteoblastic phenotype of rat marrow stromal cells cultured in the presence of dexamethasone, beta-glycerolphosphate, and L-ascorbic acid. J Cell Biochem. 1998;71:55–62. doi: 10.1002/(sici)1097-4644(19981001)71:1<55::aid-jcb6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 29.Stein GS, Lian JB, Owen TA. Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation. FASEB J. 1990;4:3111–23. doi: 10.1096/fasebj.4.13.2210157. [DOI] [PubMed] [Google Scholar]
- 30.Boskey AL, Gadaleta S, Gundberg C, Doty SB, Ducy P, Karsenty G. Fourier transform infrared microspectroscopic analysis of bones of osteocalcin-deficient mice provides insight into the function of osteocalcin. Bone. 1998;23:187–96. doi: 10.1016/s8756-3282(98)00092-1. [DOI] [PubMed] [Google Scholar]
- 31.Lossdorfer S, Schwartz Z, Wang L, Lohmann CH, Turner JD, Wieland M, et al. Microrough implant surface topographies increase osteogenesis by reducing osteoclast formation and activity. J Biomed Mater Res A. 2004;70:361–9. doi: 10.1002/jbm.a.30025. [DOI] [PubMed] [Google Scholar]
- 32.Kieswetter K, Schwartz Z, Hummert TW, Cochran DL, Simpson J, Dean DD, et al. Surface roughness modulates the local production of growth factors and cytokines by osteoblast-like MG-63 cells. J Biomed Mater Res. 1996;32:55–63. doi: 10.1002/(SICI)1097-4636(199609)32:1<55::AID-JBM7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 33.Grigoryan T, Wend P, Klaus A, Birchmeier W. Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev. 2008;22:2308–41. doi: 10.1101/gad.1686208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamura M, Nemoto E, Sato MM, Nakashima A, Shimauchi H. Role of the Wnt signaling pathway in bone and tooth. Front Biosci (Elite Ed) 2010;2:1405–13. doi: 10.2741/e201. [DOI] [PubMed] [Google Scholar]
- 35.Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–23. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- 36.Yoshikawa Y, Fujimori T, McMahon AP, Takada S. Evidence that absence of Wnt-3a signaling promotes neuralization instead of paraxial mesoderm development in the mouse. Dev Biol. 1997;183:234–42. doi: 10.1006/dbio.1997.8502. [DOI] [PubMed] [Google Scholar]
- 37.Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 38.Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A. 2009;106:641–6. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng SL, Shao JS, Cai J, Sierra OL, Towler DA. Msx2 exerts bone anabolism via canonical Wnt signaling. J Biol Chem. 2008;283:20505–22. doi: 10.1074/jbc.M800851200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stevens JR, Miranda-Carboni GA, Singer MA, Brugger SM, Lyons KM, Lane TF. Wnt10b deficiency results in age dependent loss of bone mass and progressive reduction of mesenchymal progenitor cells. J Bone Miner Res. 2010 doi: 10.1002/jbmr.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reichsman F, Smith L, Cumberledge S. Glycosaminoglycans can modulate extracellular localization of the wingless protein and promote signal transduction. J Cell Biol. 1996;135:819–27. doi: 10.1083/jcb.135.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ford CE, Ekstrom EJ, Andersson T. Wnt-5a signaling restores tamoxifen sensitivity in estrogen receptor-negative breast cancer cells. Proc Natl Acad Sci U S A. 2009;106:3919–24. doi: 10.1073/pnas.0809516106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Kummitha CM, Mayle KM, Christman MA, 2nd, Deosarkar SP, Schwartz AL, McCall KD, et al. A sandwich ELISA for the detection of Wnt5a. J Immunol Methods. 2010;352:38–44. doi: 10.1016/j.jim.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rawadi G, Vayssiere B, Dunn F, Baron R, Roman-Roman S. BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J Bone Miner Res. 2003;18:1842–53. doi: 10.1359/jbmr.2003.18.10.1842. [DOI] [PubMed] [Google Scholar]
- 45.Caverzasio J, Manen D. Essential role of Wnt3a-mediated activation of mitogen-activated protein kinase p38 for the stimulation of alkaline phosphatase activity and matrix mineralization in C3H10T1/2 mesenchymal cells. Endocrinology. 2007;148:5323–30. doi: 10.1210/en.2007-0520. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Wang Y, Li X, Zhang J, Mao J, Li Z, et al. The LRP5 high-bone-mass G171V mutation disrupts LRP5 interaction with Mesd. Mol Cell Biol. 2004;24:4677–84. doi: 10.1128/MCB.24.11.4677-4684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD, et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci U S A. 2005;102:3324–9. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang H, Lee Y, Malbon CC. PDE6 is an effector for the Wnt/Ca2+/cGMP-signalling pathway in development. Biochem Soc Trans. 2004;32:792–6. doi: 10.1042/BST0320792. [DOI] [PubMed] [Google Scholar]
- 49.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–77. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 50.Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–23. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 51.Yadav VK, Ducy P. Lrp5 and bone formation: a serotonin-dependent pathway. Ann N Y Acad Sci. 2010;1192:103–9. doi: 10.1111/j.1749-6632.2009.05312.x. [DOI] [PubMed] [Google Scholar]
- 52.Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346:1513–21. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 53.Holmen SL, Giambernardi TA, Zylstra CR, Buckner-Berghuis BD, Resau JH, Hess JF, et al. Decreased BMD and limb deformities in mice carrying mutations in both Lrp5 and Lrp6. J Bone Miner Res. 2004;19:2033–40. doi: 10.1359/JBMR.040907. [DOI] [PubMed] [Google Scholar]
- 54.Dong YF, Soung do Y, Schwarz EM, O’Keefe RJ, Drissi H. Wnt induction of chondrocyte hypertrophy through the Runx2 transcription factor. J Cell Physiol. 2006;208:77–86. doi: 10.1002/jcp.20656. [DOI] [PubMed] [Google Scholar]
- 55.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–7. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim JB, Leucht P, Lam K, Luppen C, Ten Berge D, Nusse R, et al. Bone regeneration is regulated by wnt signaling. J Bone Miner Res. 2007;22:1913–23. doi: 10.1359/jbmr.070802. [DOI] [PubMed] [Google Scholar]
- 57.Keeble TR, Halford MM, Seaman C, Kee N, Macheda M, Anderson RB, et al. The Wnt receptor Ryk is required for Wnt5a-mediated axon guidance on the contralateral side of the corpus callosum. J Neurosci. 2006;26:5840–8. doi: 10.1523/JNEUROSCI.1175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8:645–54. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- 59.Nemeth MJ, Topol L, Anderson SM, Yang Y, Bodine DM. Wnt5a inhibits canonical Wnt signaling in hematopoietic stem cells and enhances repopulation. Proc Natl Acad Sci U S A. 2007;104:15436–41. doi: 10.1073/pnas.0704747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamamoto S, Nishimura O, Misaki K, Nishita M, Minami Y, Yonemura S, et al. Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev Cell. 2008;15:23–36. doi: 10.1016/j.devcel.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 61.Nishita M, Yoo SK, Nomachi A, Kani S, Sougawa N, Ohta Y, et al. Filopodia formation mediated by receptor tyrosine kinase Ror2 is required for Wnt5a-induced cell migration. J Cell Biol. 2006;175:555–62. doi: 10.1083/jcb.200607127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nomachi A, Nishita M, Inaba D, Enomoto M, Hamasaki M, Minami Y. Receptor tyrosine kinase Ror2 mediates Wnt5a-induced polarized cell migration by activating c-Jun N-terminal kinase via actin-binding protein filamin A. J Biol Chem. 2008;283:27973–81. doi: 10.1074/jbc.M802325200. [DOI] [PubMed] [Google Scholar]
- 63.Boyan BD, Lossdorfer S, Wang L, Zhao G, Lohmann CH, Cochran DL, et al. Osteoblasts generate an osteogenic microenvironment when grown on surfaces with rough microtopographies. Eur Cell Mater. 2003;6:22–7. doi: 10.22203/ecm.v006a03. [DOI] [PubMed] [Google Scholar]
- 64.Cai Y, Mohseny AB, Karperien M, Hogendoorn PC, Zhou G, Cleton-Jansen AM. Inactive Wnt/beta-catenin pathway in conventional high-grade osteosarcoma. J Pathol. 2010;220:24–33. doi: 10.1002/path.2628. [DOI] [PubMed] [Google Scholar]
- 65.Dai J, Hall CL, Escara-Wilke J, Mizokami A, Keller JM, Keller ET. Prostate cancer induces bone metastasis through Wnt-induced bone morphogenetic protein-dependent and independent mechanisms. Cancer Res. 2008;68:5785–94. doi: 10.1158/0008-5472.CAN-07-6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buser D, Broggini N, Wieland M, Schenk RK, Denzer AJ, Cochran DL, et al. Enhanced bone apposition to a chemically modified SLA titanium surface. J Dent Res. 2004;83:529–33. doi: 10.1177/154405910408300704. [DOI] [PubMed] [Google Scholar]
- 67.Cochran DL, Buser D, ten Bruggenkate CM, Weingart D, Taylor TM, Bernard JP, et al. The use of reduced healing times on ITI implants with a sandblasted and acid-etched (SLA) surface: early results from clinical trials on ITI SLA implants. Clin Oral Implants Res. 2002;13:144–53. doi: 10.1034/j.1600-0501.2002.130204.x. [DOI] [PubMed] [Google Scholar]
- 68.DeChiara TM, Kimble RB, Poueymirou WT, Rojas J, Masiakowski P, Valenzuela DM, et al. Ror2, encoding a receptor-like tyrosine kinase, is required for cartilage and growth plate development. Nat Genet. 2000;24:271–4. doi: 10.1038/73488. [DOI] [PubMed] [Google Scholar]
- 69.Takeuchi S, Takeda K, Oishi I, Nomi M, Ikeya M, Itoh K, et al. Mouse Ror2 receptor tyrosine kinase is required for the heart development and limb formation. Genes Cells. 2000;5:71–8. doi: 10.1046/j.1365-2443.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- 70.Wall I, Donos N, Carlqvist K, Jones F, Brett P. Modified titanium surfaces promote accelerated osteogenic differentiation of mesenchymal stromal cells in vitro. Bone. 2009;45:17–26. doi: 10.1016/j.bone.2009.03.662. [DOI] [PubMed] [Google Scholar]
- 71.Schwartz Z, Olivares-Navarrete R, Wieland M, Cochran DL, Boyan BD. Mechanisms regulating increased production of osteoprotegerin by osteoblasts cultured on microstructured titanium surfaces. Biomaterials. 2009;30:3390–6. doi: 10.1016/j.biomaterials.2009.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


