Abstract
Wnt5a and 1α,25(OH)2D3 are important regulators of endochondral ossification. In osteoblasts and growth plate chondrocytes, 1α,25(OH)2D3 initiates rapid effects via its membrane-associated receptor protein disulfide isomerase A3 (Pdia3) in caveolae, activating phospholipase A2 (PLA2)-activating protein (PLAA), calcium/calmodulin-dependent protein kinase II (CaMKII), and PLA2, resulting in protein kinase C (PKC) activation. Wnt5a initiates its calcium-dependent effects via intracellular calcium release, activating PKC and CaMKII. We investigated the requirement for components of the Pdia3 receptor complex in Wnt5a calcium-dependent signaling. We determined that Wnt5a signals through a CaMKII/PLA2/PGE2/PKC cascade. Silencing or blocking Pdia3, PLAA, or vitamin D receptor (VDR), and inhibition of calmodulin (CaM), CaMKII, or PLA2 inhibited Wnt5a-induced PKC activity. Wnt5a activated PKC in Caveolin-1-silenced cells, but methyl-beta-cyclodextrin reduced its stimulatory effect. 1α,25(OH)2D3 reduced stimulatory effects of Wnt5a on PKC in a dose-dependent manner. In contrast, Wnt5a had a biphasic effect on 1α,25(OH)2D3-stimulated PKC activation; 50ng/ml Wnt5a caused a 2-fold increase in 1α,25(OH)2D3-stimulated PKC but higher Wnt5a concentrations reduced 1α,25(OH)2D3-stimulated PKC activation. Western blots showed that Wnt receptors Frizzled2 (FZD2) and Frizzled5 (FZD5), and receptor tyrosine kinase-like orphan receptor 2 (ROR2) were localized to caveolae. Blocking ROR2, but not FZD2 or FZD5, abolished the stimulatory effects of 1α,25(OH)2D3 on PKC and CaMKII. 1α,25(OH)2D3 membrane receptor complex components (Pdia3, PLAA, Caveolin-1, CaM) interacted with Wnt5a receptors/co-receptors (ROR2, FZD2, FZD5) in immunoprecipitation studies, interactions that changed with either 1α,25(OH)2D3 or Wnt5a treatment. This study demonstrates that 1α,25(OH)2D3 and Wnt5a mediate their effects via similar receptor components and suggests that these pathways may interact.
Keywords: 1,25-Dihydroxy vitamin D3; 1α,25(OH)2D3; Wnt5a; Pdia3; PLAA; PKC; MC3T3-E1 osteoblast-like cells; Costochondral cartilage growth zone chondrocytes
1. Introduction
Endochondral ossification encompasses multiple events during which the embryonic cartilaginous template of long bones is gradually calcified and replaced by bone (1). Previous reports by our lab and other groups have suggested that a complex network of interacting signaling pathways induced by hormones and growth factors also regulate post-natal growth plate chondrocytes and endochondral bone formation (2-5). However, limited information is available on the molecular basis of these interactions.
In the present study, we focus on inter-relation between two pathways involved in regulation of post-natal growth plate cartilage and osteoblast maturation: 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3] membrane-mediated signaling and non-canonical Wnt5a calcium-dependent signaling. 1α,25(OH)2D3 induces its effects via two mechanisms: classical vitamin D receptor (VDR) signaling and the more recently described calcium-dependent membrane-mediated pathway (6-8). In the membrane-mediated pathway, 1α,25(OH)2D3 initiates its effects via its specific membrane-associated receptor protein disulfide isomerase family A, member 3 (Pdia3, also known as ERp60, ERp57, Grp58, and 1,25-MARRS) (9,10) located in caveolae, which are 50-100 nm lipid rafts highly enriched with cholesterol and glycosphingolipids (11). Caveolae are characterized by caveolin coat proteins (Cav-1, Cav-2, Cav-3) that serve as signaling platforms for several steroid hormones (11). Upon binding to Pdia3, 1α,25(OH)2D3 induces interactions between Pdia3 and phospholipase-A2 (PLA2)-activating protein (PLAA) (12), stimulating increased calcium/calmodulin-dependent protein kinase II (CaMKII) activity in costochondral growth zone chondrocytes (GC) and MC3T3-E1 osteoblast-like cells (13). PLA2 is activated (14), producing lysophospholipid and releasing arachidonic acid (AA) (15) that is further processed into prostaglandin E2 (PGE2). AA can activate PKC directly (15). The PGE2 acts via its EP1 receptor to increase cyclic AMP (16), which results in downstream PKC and ERK1/2 activation (17). Together with Gαq, lysophospholipid activates phosphatidylinositol-specific phospholipase C beta (PLCβ), generating diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3) (14,15). DAG binds PKCα, recruiting it to the plasma membrane (18). IP3 activates the release of calcium ions required for PKCα activation from the endoplasmic reticulum.
1α,25(OH)2D3 stimulates differentiation of growth zone chondrocytes and osteoblasts, increasing alkaline phosphatase specific activity (16) and in the case of osteoblasts, by increasing production of osteopontin (9). While the VDR mediates many of the effects of 1α,25(OH)2D3 on these cells, Pdia3-dependent signaling has been shown to mediate the effects of the vitamin D metabolite on these two markers of osteoblast differentiation. Pdia3 gene knockout results in embryonic lethality (19,20) and as a result, a definitive demonstration of its role has not been possible. However, conditional knockout of Pdia3 in the intestinal epithelium results in severely blunted Ca2+ uptake in response to 1α,25(OH)2D3 (21), confirming its involvement in 1α,25(OH)2D3 dependent actions. Moreover, Pdia3+/− mice exhibit a long bone phenotype (19), further demonstrating the importance of this 1α,25(OH)2D3 receptor in skeletal development.
Wnts are signaling molecules that also regulate skeletal development and maintenance (22,23). In the growth plate, Wnt5a expression is low in the reserve zone, increased in proliferative and prehypertrophic zones, and decreased in the hypertrophic region (24). Wnt5a plays an important role in transition of chondrocytes between growth plate zones. Growth plates of Wnt5a−/− mice exhibit delayed chondrocyte hypertrophy, suggesting that Wn5a plays a critical role in promoting hypertrophy (25). Wnt5a is also expressed by osteoblasts at the interface between calcified cartilage and metaphyseal bone and it has been shown to promote osteoblast maturation in vitro (5,26). Osteoblasts isolated from Wnt5a−/− mice exhibit down-regulation of osteoblastic differentiation markers including runt related transcription factor 2, osterix and alkaline phosphatase (27) compared to wild type cells, suggesting Wnt5a regulates bone formation. This is supported by histological analysis of long bones of Wnt5a−/− mice, which exhibit significantly delayed chondrocyte hypertrophy and skeletal ossification compared to wild type mice (25). Wnt5a induces its effects via several known receptors and co-receptors including Frizzled2 (FZD2), Frizzled5 (FZD5), and receptor tyrosine kinase-like orphan receptor 2 (ROR2), activating intracellular release of calcium, thereby activating PLC, PKC, CaMKII and calcineurin (28-32).
Although 1α,25(OH)2D3 and Wnt5a both regulate osteoblast and chondrocyte maturation and signal via calcium-dependent mechanisms, little is known about the role of components of the 1α,25(OH)2D3 membrane-associated receptor complex in Wnt5a calcium-dependent signaling. The purpose of this study was to determine if the same receptor complex and pathway signaling proteins that are critical for the 1α,25(OH)2D3 membrane-mediated pathway via Pdia3 are also important for Wnt5a calcium-dependent signaling. To address this question, we first verified Wnt5a-dependent activation of CaMKII, PLA2, PKC, and PGE2 release in MC3T3-E1 osteoblast-like cells and GC cells. Next, we determined the role of the 1α,25(OH)2D3 activated Pdia3-dependent membrane receptor complex and its downstream signaling proteins in Wnt5a-stimulated PKC activation using the MC3T3-E1 cell model. Finally, we determined the interactions between the 1α,25(OH)2D3 membrane-receptor complex and Wnt5a receptors with or without 1α,25(OH)2D3 or Wnt5a treatment in MC3T3-E1 cells.
2. Materials and Methods
2.1. Reagent
Recombinant human/mouse Wnt5a was purchased from R&D Systems (Minneapolis, MN). 1α,25(OH)2D3 and PLAA were purchased from Enzo Life Sciences (Plymouth Meeting, PA). The anti-PLAA polyclonal antibody was designed and developed by Strategic Diagnostics Inc. (Newark, DE) (12). Rabbit antiserum against the N-terminal peptide of Pdia3 was purchased from Alpha Diagnostic International (San Antonio, TX) (33). A polyclonal antibody to Cav-1 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). ROR2 polyclonal antibody was from Cell Signaling Technology (Danvers, MA). Frizzled2 polyclonal antibody and Frizzled5 polyclonal antibody were from Abcam (San Francisco, CA). Monoclonal antibody to CaM was from Millipore (Billerica, MA). Myristoylated calmodulin kinase IINtide (mer-CaMKIINtide) peptide was from EMD Biosciences (Billerica, MA) and arachidonyl trifluoromethyl ketone (AACOCF3) was from Abcam. All other reagents were purchased from Sigma Aldrich (St. Louis, MO) unless specified.
2.2. Cell Culture
Wild type (WT) mouse MC3T3-E1 subclone 4 osteoblast-like cells (CRL-2593) were purchased from ATCC (Manassas, VA, USA). Stably silenced MC3T3-E1 cell lines for Pdia3 (shPdia3), PLAA (shPlaa), Cav-1 (shCav-1), VDR (shVdr), CaMKII-α (shCamk2a), and CaMKII-β (shCamk2b) were generated and characterized by our lab previously (9,12,34). Cells were plated at 10,000 cells/cm2 and cultured in Minimum Essential Medium Alpha (α-MEM) (Life Technologies, Carlsbad, CA) containing 10% fetal bovine serum (FBS) (Hyclone, Waltham, MA) and 1% penicillin-streptomycin (P/S) (Life Technologies). Osteoblastic differentiation of MC3T3-E1 cells was induced by culture in α-MEM supplemented with 10% FBS, 1% P/S and 50 μg/ml ascorbic acid 24 hours after plating, and then every 48 hours thereafter (35).
GC cells were isolated from costochondral cartilage of 100-125g male Sprague-Dawley rats (Harlan, Indianapolis, IN). Rats were at the end of their adolescent growth spurt; therefore, their long bones were growing at a reduced rate. The culture system used in this study was described previously in detail (36,37). Briefly, fourth passage cultures of GC chondrocytes were plated at 10,000 cells/cm2 and cultured in Dulbecco's modified Eagle's medium (Mediatech) containing 10% FBS, 1% P/S, and 50 μg/ml ascorbic acid.
All cells were cultured at 37°C with 5% CO2 and 100% humidity. Confluent cultures were treated for experiments as described below. The approximate density of MC3T3-E1 osteoblast-like cells at the time of treatment was 80,000 cells/cm2.
2.3. Time Course and Dose Response of PKC Activity to Wnt5a
To assess the dose-dependent effects of Wnt5a on PKC, GC cells were treated for 9 minutes and MC3T3-E1 cells were treated for 15 minutes with 50, 125 and 200 ng/ml Wnt5a (26,38), time points previously demonstrated to be optimal for activation of PKC by 1α,25(OH)2D3 in these cell types (12,39). After treatment, cell layers were washed with phosphate buffered saline (PBS) and then were lysed in RIPA buffer (20 mM Tris-HCl, 150 mM NaCl, 5 mM disodium EDTA, 1% Nonidet P-40). PKC activity was measured in cell layer lysates using a commercially available kit following manufacturer's instructions (GE Healthcare, Piscataway, NJ) and data were normalized to total protein (Pierce BCA Protein Assay, Thermo Fisher Scientific, Waltham, MA).
Next, the time course of PKC activation by Wnt5a was examined. GC cells and MC3T3-E1 cells were treated with 125 ng/ml (26,38) Wnt5a for 6, 9, 15, or 30 minutes. PKC activity was measured as described above.
2.4. Time Course of Wnt5a Effect on CaMKII Activity
GC and MC3T3-E1 cells were treated for 6, 9, 15, and 30 minutes with 125 ng/ml Wnt5a and cell layer lysates were assayed for CaMKII activity. CaMKII activity was measured using a commercially available assay following manufacturer's instructions (SignaTECT® Calcium/Calmodulin-Dependent Protein Kinase Assay System, Promega, Madison, WI). CaMKII activity was normalized to total protein in the cell lysate.
2.5 Time Course of Wnt5a Effect on PLA2 Activity
GC and MC3T3-E1 cells were treated for 6, 9, 15, or 30 minutes with 125ng/ml Wnt5a. After washing the cell layers with PBS, the cell layers were lysed and assayed for PLA2 activity using a commercially available kit (cPLA2 Assay kit, 765021, Cayman Chemical, Ann Arbor, MI). PLA2 data were normalized to total protein.
2.6. Time Course of Wnt5a Effect on PGE2 Release
GC and MC3T3-E1 cells were treated for 6, 9, 15, and 30 minutes with 125ng/ml Wnt5a. At the end of incubation, conditioned media were acidified and PGE2 was measured using a commercially available kit (Prostaglandin E2 [125I]-RIA kit, Perkin Elmer, Waltham, MA). PGE2 levels were normalized to total DNA (Quant-iT™ PicoGreen® dsDNA Assay kit, Life Technologies).
2.7. Role of Vitamin D Signaling Components in Wnt5a-induced PKC Activity
Based on the results of time-course and dose-response studies, the treatment conditions resulting in the highest PKC activity (125ng/ml Wnt5a for 9 minutes in GC cells and 125ng/ml Wnt5a for 15 minutes in MC3T3-E1 osteoblast-like cells) were selected for subsequent experiments unless specified in the text.
To determine the effects of Pdia3, Vdr, and Cav1 silencing on Wnt5a-induced PKC activity, wild type (WT), shPdia3, shVdr, and shCav-1 cells were treated with Wnt5a and PKC measured. These results were confirmed using Pdia3 and VDR blocking antibodies in GC chondrocytes and MC3T3-E1 cells. Cells were pretreated with either anti-Pdia3 or anti-VDR antibodies for 30 minutes, and PKC activity measured after Wnt5a treatment.
Methyl-beta-cyclodextrin (β-CD) disrupts lipids rafts and caveolae by binding cholesterol and removing it from the plasma membranes. To determine the effects of caveolae destruction on PKC activation, MC3T3-E1 cells were treated with 10 mM of β-CD for 30 minutes in serum-free media, as described previously (40). At the end of incubation, cell layers were rinsed with serum free media. Cells were then treated with Wnt5a and cell layer lysates were assayed for PKC activity.
2.8. Role of Calmodulin in Wnt5a-induced PKC Activity
MC3T3-E1 cells were pre-treated with 0.1, 1, or 10 μM calmodulin inhibitor W-7 for 30 minutes (41), then cells were treated with Wnt5a for 15 minutes and cell layer lysates were assayed for PKC activity. To investigate the effect of CaMKII inhibition on 1α,25(OH)2D3- dependent PKC activation, MC3T3-E1 cells were treated for 30 minutes with 1.25, 2.5, and 5 μM CaMKII peptide-inhibitor mer-CaMKIINtide (42) followed by Wnt5a. Cell layer lysates were assayed for PKC activity. To investigate the effects of Camk2a and Camk2b silencing on Wnt5a-stimulated PKC activation, confluent WT, shCamk2a, and shCamk2a MC3T3-E1 cells were treated withWnt5a. Cell layer lysates were assayed for PKC.
2.9. Role of PLAA in Wnt5a-induced PKC Activity
Cells were pretreated with anti-PLAA antibody (PLAA Ab) for 30 minutes followed by Wnt5a for 15 minutes. Cell layer lysates we assayed for PKC activity. To confirm these findings, PKC activity was measured in WT and shPlaa MC3T3-E1 cells after Wnt5a treatment. To investigate the effects of PLA2 inhibition on PKC activity, MC3T3-E1 osteoblast-like cells were pretreated with 0.1, 1, and 10 μM AACOCF3 for 30 minutes followed byWnt5a. PKC activity was measured in cell layer lysates. AACOCF3 has been shown in other studies to inhibit PLA2 in these cells (43).
2.10. Effects of Wnt5a and 1α,25(OH)2 D3 Co-treatment on Regulation of PKC Activity
MC3T3-E1 cells were treated with 0, 10−10, 10−9, or 10−8 M 1α,25(OH)2D3. Wnt5a was added to one-half of the cultures at the concentration of 125 ng/ml. At the end of the 15 minute incubation, cell layer lysates were assayed for PKC. To assess the effects of Wnt5a in 1α,25(OH)2D3 regulation of PKC activity, MC3T3-E1 cells were treated with 10−8 M 1α,25(OH)2D3 and 0, 50, 87.5 or 125 ng/ml Wnt5a for 15 minutes. At the end of the incubation, the cell layer lysates were assayed for PKC.
2.11. Roles of ROR2, FZD2 and FZD5 in 1α,25(OH)2 D3-induced PKC Activity
MC3T3-E1 cells were pretreated with anti-Frizzled2 (FZD2 Ab), anti-Frizzled5 (FZD5 Ab), or anti-ROR2 (ROR2 Ab) antibodies for 30 minutes, then were treated with 10−8 M 1α,25(OH)2D3 for 15 minutes. PKC activity was measured in cell layer lysates.
2.12. Role of ROR2 in 1α,25(OH)2 D3-induced CaMKII Activity
MC3T3-E1 cells were pretreated with anti-ROR2 antibodies for 30 minutes, then were treated with 10−8 M 1α,25(OH)2D3 for 15 minutes. CaMKII activity was measured in cell layer lysates.
2.13. Role of ROR2 on PLAA-induced CaMKII Activity
MC3T3-E1 osteoblasts were pretreated with anti-ROR2 antibodies for 30 minutes, then were treated with 10−6 M PLAA peptide for 15 minutes. CaMKII activity was measured in cell layer lysates.
2.14. Caveolae Isolation
Pdia3 exists in plasma membrane caveolae of osteoblasts (9). To determine the plasma membrane localization of FZD2, FZD5 and ROR2, plasma membranes and caveolae were isolated using a detergent-free method as described previously (44). Briefly, confluent MC3T3-E1 cultures were harvested by scraping while in isolation buffer (0.25 M sucrose, 1 mM EDTA, 20 mM Tricine, pH 7.8) and were homogenized using a tissue grinder (20 strokes; 10 strokes clockwise and 10 strokes counter-clockwise). Homogenates were centrifuged at 20,000 g for 10 minutes. The supernatant was collected and layered on top of isolation buffer containing 30% Percoll® (GE Healthcare, Piscataway, NJ). The pellet, including nucleus, mitochondria, and endoplasmic reticulum, was discarded. Samples were centrifuged at 84,000 g for 30 minutes. Syringe needles (18G) were used to collect the plasma membrane fraction from the gradient column. The isolated fraction was layered over a 10-20% OptiPrep gradient (Sigma Aldrich, St. Louis, MO), then centrifuged at 52,000 g for another 4 hours. Plasma membrane sub-fractions were collected from the tube, resulting in isolation of thirteen fractions. Caveolae were observed as an opaque band that was collected in fraction 3.
2.15. Western Blots
Whole cell layer lysates and plasma membrane fractions (50μg protein) were loaded onto 4–20% Mini-PROTEAN® TGX™ precast polyacrylamide gels (Bio-Rad, Hercules, CA). Proteins were transferred to low-fluorescence PVDF membranes (Bio-Rad) using a Trans-Blot® Turbo™ Transfer System (Bio-Rad). Membranes were incubated with blocking buffer (LI-COR, Lincoln, NE) for 1 hour. Subsequently, the membranes were incubated with antibodies against Cav-1, FZD2, FZD5, and ROR2 overnight. The next day, the membranes were incubated for one hour with IRDye 800CW conjugated goat anti-rabbit IgG secondary antibodies (LI-COR) in blocking buffer containing 0.2% Tween-20, and 0.01% SDS. Following three washes with PBS containing 0.05% Tween-20 membranes were imaged using the LI-COR Odyssey® CLx Infrared Imaging System.
2.16. Effects of 1α,25(OH)2 D3 and Wnt5a on Receptor Complex Interactions
MC3T3-E1 cells were treated with 10−8 M 1α,25(OH)2D3 or its vehicle (ethanol), or 125 ng/ml Wnt5a or its vehicle (cell culture medium) for 15 minutes. At the end of treatment, cell layers were washed with PBS and cell layers were lysed in RIPA buffer containing 100 mM sodium fluoride, protease inhibitor cocktail, and 1 mM phenylmethylsulfonyl fluoride. Protein samples (500 μg) were pre-cleared by incubation in 5 μg of rabbit IgG conjugated to Dynabeads® Protein A (Life Technologies) at 4°C for 1 hour. The beads were separated from solution using a magnet. To immunoprecipitate Pdia3, PLAA, Cav-1 and CaM protein complexes, anti-Pdia3, anti-PLAA, anti-Cav-1, and anti-CaM antibodies were covalently coupled to the Dynabeads Protein A according to the manufacturer's protocol. Pre-cleared protein samples were mixed with antibody coated Dynabeads and incubated at 4°C overnight with continuous agitation. Dynabeads were recovered using a magnet and were washed three times with 0.05% Tween-20 in PBS. Precipitated proteins were eluted in elution buffer, then diluted in Tris-glycine SDS sample loading buffer (Bio-Rad) and boiled for 5 minutes. Immunoprecipitated samples were examined by Western blot (ONE-HOUR Western™ Fluorescent Kit, Genscript, Piscataway, NJ).
2.17. Statistical Analysis
For each experiment, data points represent the mean ± standard error of the mean (SEM) of six individual cultures, per variable. Each experiment was repeated at least three times to ensure validity of the data. Statistical significance was assessed by analysis of variance and post hoc testing performed using Bonferroni's modification of Student's t-test for multiple comparisons (GraphPad Prism, GraphPad Software, Inc., San Diego, CA). P-values <0.05 were considered significant.
3. Results
3.1. Wnt5a rapidly activates PKC, CaMKII, and PLA2 and triggers PGE2 release
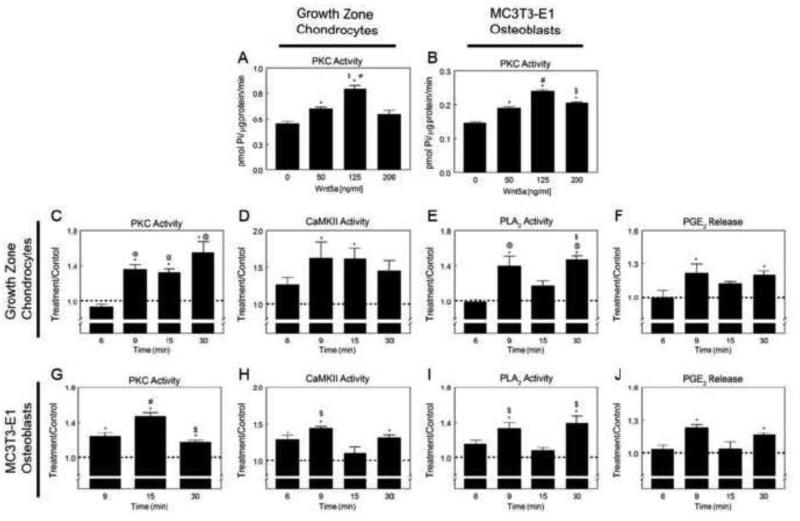
Wnt5a regulated the activity of PKC in GC and MC3T3-E1 cells in a dose- and time-dependent manner. Similarly, the effects of Wnt5a on CaMKII and PLA2 activities and PGE2 release were time-dependent and rapid. Wnt5a protein increased PKC-specific activity in a dose-dependent manner in GC cells with the highest stimulatory effects of the peptide observed at 125 ng/ml concentration (Fig. 1A). Wnt5a activated PKC in a dose-dependent manner in MC3T3-E1 cells, with the highest stimulatory effect observed at 125 ng/ml (Fig. 1B). Wnt5a activated PKC in GC cells within 9 minutes of treatment, and PKC remained significantly higher than control at 15 and 30 minutes after treatment (Fig. 1C).
Figure 1.
Effects of Wnt5a treatment on PKC, CaMKII, and PLA2 activation, and PGE2 release and growth zone chondrocytes (GC) (A) and MC3T3-E1 osteoblast-like cells (B). *p<0.05, versus 0 ng/ml Wnt5a; #p<0.05, versus 50 ng/ml Wnt5a; $p<0.05, versus 50 ng/ml Wnt5a. Time-course of Wnt5a-induced PKC (C,G), CaMKII (D,H) and PLA2 (E,I) activation in GC (C-F) and MC3T3-E1 (G-J) cells. Treatment over control ratios were calculated for each parameter. The dashed line represents the value for the control cultures, which was set to 1. *p<0.05, 1α,25(OH)2D3 treatment versus control; @p<0.05, versus 6 minutes; #p<0.05, versus 9 minutes; $p<0.05, versus 6 minutes.
We further assessed whether Wnt5a acts on cells by a mechanism similar to that used by 1α,25(OH)2D3. Wnt5a increased CaMKII activity at 9 and 15 minutes after treatment in GC chondrocytes (Fig. 1D). Wnt5a increased PLA2 activity (Fig. 1E) and PGE2 release (Fig. 1F) in GC cells at both 9 minutes and 30 minutes after treatment. Similarly, Wnt5a caused a rapid increase in PKC activity in MC3T3-E1 cells at 9 minutes that remained elevated until 30 minutes, with a peak at 15 minutes (Fig. 1G). Wnt5a increased CaMKII activity at 6, 9, and 30 minutes in MC3T3-E1 osteoblast-like cells (Fig. 1H). Similarly, the effect of Wnt5a on PLA2 activity (Fig. 1I) and PGE2 release (Fig. 1J) was time-dependent and significant increases were observed at 9 and 30 minutes after the treatment.
3.2. Pdia3, VDR, and lipid rafts, but not Cav-1, are required for the PKC activation in response to Wnt5a
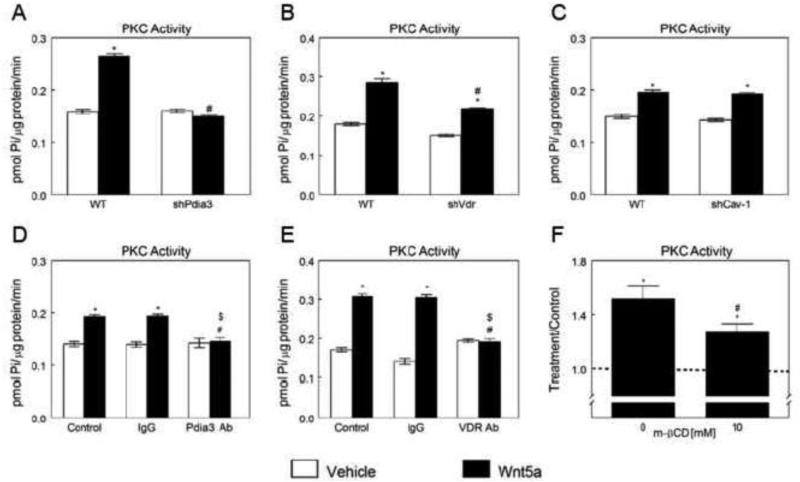
Pdia3, VDR, and plasma membrane lipid raft structures were involved in Wnt5a-stimulated rapid activation of PKC in MC3T3-E1 osteoblasts, while Cav-1 was not required. Unlike WT cells, shPdia3 osteoblasts did not increase in PKC activity in response to Wnt5a (Fig. 2A). Wnt5a stimulated PKC activity in shVdr cells was significantly lower than in WT cells (Fig. 2B). However, Wnt5a failed to increase PKC in shCav-1 osteoblasts (Fig. 2C). Likewise, Pdia3 antibody (Fig. 2D) and VDR antibody (Fig. 2E) significantly reduced the Wnt5a-induced increase in PKC activity in MC3T3-E1 cells. Although Wnt5a stimulated PKC activity in cells pretreated with lipid raft disruptor β-CD, the increase was significantly lower when compared to the β-CD untreated group (Fig. 2F).
Figure 2.
Effects of Pdia3, Vdr and Cav-1 silencing or blocking and β-CD on Wnt5a-stimulated activation of PKC in MC3T3-E1 cells. Wnt5a-induced PKC activation in wild-type (WT), shPdia3 (A), shVdr (B) and shCav-1 (C) MC3T3-E1 cells. *p<0.05, treatment versus control; #p<0.05, versus Wnt5a treated WT. Effect of Pdia3 blocking (D) or VDR blocking (E) on Wnt5a-induced PKC activation. *p<0.05, treatment versus control; #p<0.05, versus Wnt5a treated control group; $p<0.05, versus Wnt5a treated IgG group. (F) Effect of β-CD on Wnt5ainducedPKC activation. Treatment over control ratios were calculated for each parameter. The dashed line represents the value for the control cultures, which was set to 1. *p<0.05, treatment versus control.
3.3. CaM, CaMKII-α, PLAA, and PLA2 are required for the rapid activation of PKC in response to Wnt5a
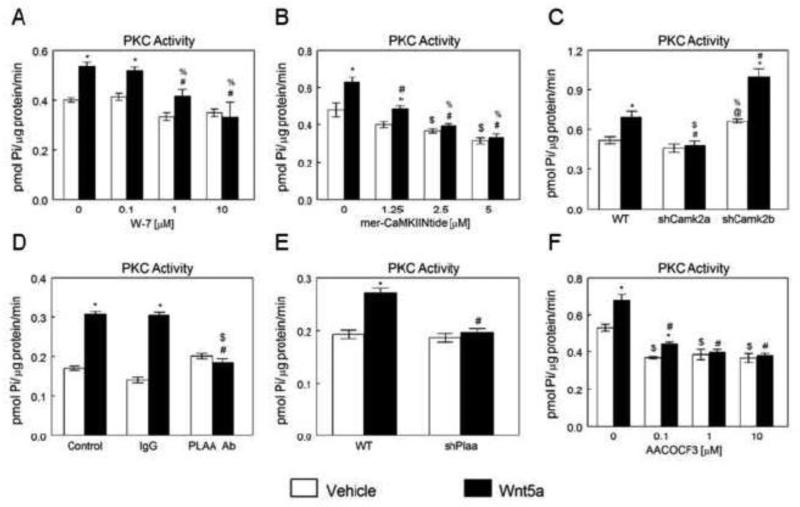
In MC3T3-E1 cells, Wnt5a-stimulated activation of PKC depends on upstream actions of CaM, CaMKII-α, PLAA, and PLA2. Calmodulin inhibitor W-7 blocked activation of PKC in response to Wnt5a at 1 and 10 μM, but not at 0.1 μM (Fig. 3A). mer-CaMKIINtide inhibited the Wnt5a induced increase in PKC activity in a comparable manner to W-7. mer-CaMKIINtide significantly reduced stimulatory effects of Wnt5a on PKC activation at 1.25μM, and completely blocked the effect at 2.5 and 5 μM (Fig. 3B). Wnt5a significantly increased PKC activity in WT and shCamk2b MC3T3-E1 cells, but this effect was prevented in shCamk2a cells (Fig. 3C). PLAA antibody significantly reduced the Wnt5a-induced increase in PKC activity in MC3T3-E1 cells, whereas IgG had no effect (Fig. 3D). Similarly, Wnt5a increased PKC activity in MC3T3-E1 cells, and effect was blocked in shPlaa cells (Fig. 3E). PLA2 inhibitor AACOCF3 significantly reduced stimulatory effects of Wnt5a on PKC activation at 0.1 μM, and completely blocked the effect at 1 and 10 μM (Fig. 3F).
Figure 3.
Effects of CaM, CaMKII, PLAA and PLA2 inhibition and Plaa, Camk2a and Camk2b silencing on Wnt5a-stimulated activation of PKC in MC3T3-E1 cells. (A) Effect of CaM inhibition on Wnt5a-induced PKC activation.. *p<0.05, treatment versus control; #p<0.05, versus 0 μM W-7 + Wnt5a; %p<0.05, versus 0.1 μM W-7 + Wnt5a. (B) Effect of CaMKII inhibition on Wnt5a-induced PKC activation. *p<0.05, treatment versus control; #p<0.05, versus 0 μM mer-CaMKIINtide + Wnt5a; $p<0.05, versus 0.1 μM mer-CaMKIINtide + vehicle; %p<0.05, versus 1.25 μM mer-CaMKIINtide + Wnt5a. (C) Effect of Wnt5a treatment on PKC activation in wild-type (WT), shCamk2a, and shCamk2b MC3T3-E1 cells. *p<0.05, treatment versus control; #p<0.05, versus Wnt5a-treated WT; $p<0.05, versus Wnt5a treated shCamk2b; @p<0.05, versus vehicle treated WT; %p<0.05, versus vehicle treated shCamk2a. (D) Effect on PLAA blocking on Wnt5a-induced PKC activation. *p<0.05, treatment versus control; #p<0.05, versus Wnt5a treated control group; $p<0.05, versus Wnt5a treated IgG group. (E) Effect of Plaa silencing on Wnt5-stimulated PKC activation. *p<0.05, treatment versus control; #p<0.05, versus Wnt5a-treated WT cells. (F) Effect of PLA2 inhibitor, AACOCF3, on Wnt5ainducedPKC activation. . *p<0.05, treatment versus control; #p<0.05, versus 0 μM AACOCF3 + Wnt5a; $p<0.05, 0 μM AACOCF3 + vehicle versus 0.1 μM AACOCF3 + Wnt5a.
3.4. Co-treatment with Wnt5a and 1α,25(OH)2 D3 reduces PKC activation in a dose-dependent manner
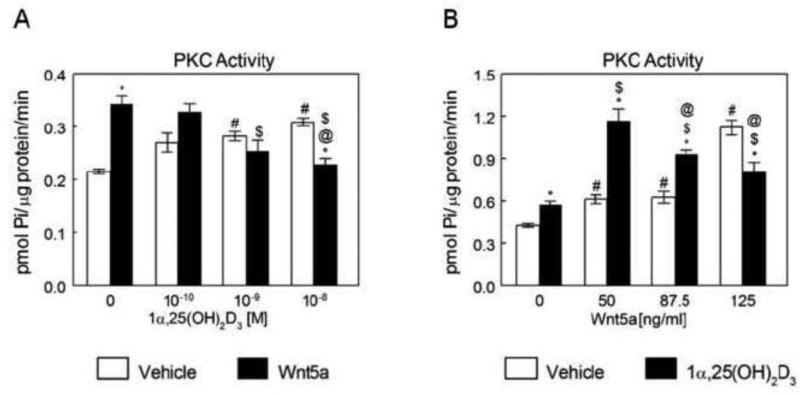
Given that both 1α,25(OH)2D3 and Wnt5a promote maturation of osteoblasts and use Pdia3 to induce their effects, we tested the effects of their co-treatment on the induction of their signaling pathways in MC3T3-E1 osteoblast-like cells, specifically examining PKC activation. In MC3T3-E1 cells, Wnt5a caused a 75% increase in PKC activity (Fig. 4A). Addition of 1α,25(OH)2D3 reduced the stimulatory effect of Wnt5a in a dose-dependent manner. At the highest concentration of the hormone, PKC was increased by 30% in control cultures but the effect of Wnt5a was abolished. The effect of Wnt5a on PKC in the presence of 1α,25(OH)2D3 also depended on Wnt5a concentration. Co-treatment with 50 ng/ml Wnt5a caused a 2-fold increase in 1α,25(OH)2D3 stimulated PKC activity compared to cultures treated with only 1α,25(OH)2D3 (Fig. 4B). However, as the concentration of Wnt5a increased, 1α,25(OH)2D3-stimulated PKC activation was suppressed.
Figure 4.
Effect of 1α,25(OH)2D3 on Wnt5a-dependent PKC activation and effect of Wnt5a in 1α,25(OH)2D3 regulation of PKC activity. (A) MC3T3-E1 cells were treated for 15 minutes with 10−10, 10−9 and 10−8 M 1α,25(OH)2D3. Wnt5a was added to one-half of the cultures at the concentration of 125 ng/ml, at the time of the treatment. At the end of incubation, cell layers were collected for PKC assay. *p<0.05, treatment versus control; #p<0.05, versus 0 M 1α,25(OH)2D3 + vehicle; $p<0.05, versus 0 M 1α,25(OH)2D3 + Wnt5a; @p<0.05 versus 10−10 M 1α,25(OH)2D3 + Wnt5a. (B) MC3T3-E1 cells were treated with 10−8 M 1α,25(OH)2D3 in the presence and absence of 50, 87.5 and 125 ng/ml Wnt5a. At the end of the incubation, the cell layers were collected for PKC assay. *p<0.05, treatment versus control; #p<0.05, versus 0 ng/ml Wnt5a + vehicle; $p<0.05, versus 1α,25(OH)2D3 + 0 ng/ml Wnt5a; @p<0.05 versus 1α,25(OH)2D3 + 5 ng/ml Wnt5a.
3.5. ROR2, FZD2, and FZD5 are localized in caveolae and ROR2 is crucial for 1α,25(OH)2 D3 membrane-mediated activation of PKC and CaMKII
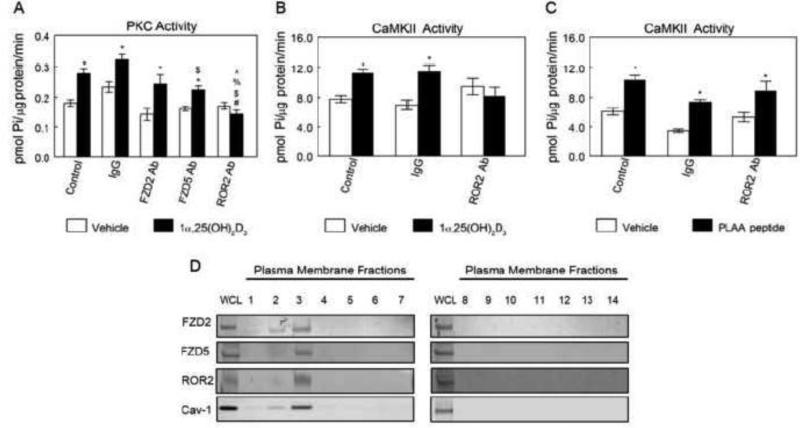
Given, the key roles ROR2, FZD2 and FZD5 play in Wnt5a signaling, we assessed their role on 1α,25(OH)2D3 stimulated rapid activation of PKC and CaMKII. ROR2, but not FZD2 and FZD5 mediated the 1α,25(OH)2D3 stimulated rapid activation of PKC and CaMKII in MC3T3-E1 osteoblasts-like cells. Anti-ROR2 antibody abolished the 1α,25(OH)2D3-dependent increase in PKC activity in MC3T3-E1 cells (Fig. 5A), whereas, IgG had no effect. Conversely, blocking FZD2 and FZD5 receptors had no effect on activation of PKC in response to 1α,25(OH)2D3 treatment. Similarly, anti-ROR2 antibody abolished the 1α,25(OH)2D3-dependent increase in CaMKII activity in MC3T3-E1 cells (Fig. 5B). Furthermore, anti-ROR2 antibody had no effect on PLAA-induced CaMKII activation (Fig. 5C). Western blots of the plasma membrane fractions of MC3T3-E1 cells indicated that ROR2, FZD2, and FZD5 were present in the plasma membranes with their greatest concentration in fraction 3, which represents caveolae microdomains (Fig. 5D).
Figure 5.
Roles of FZD2, FZD5 and ROR2 in 1α,25(OH)2D3 -dependent PKC activation and their subcellular localization. (A) Effects of FZD2, FZD5 or ROR2 blocking on 1α,25(OH)2D3-induced PKC activation. *p<0.05, treatment versus control; #p<0.05, versus vehicle treated Control group; $p<0.05, versus IgG + 1α,25(OH)2D3; %p<0.05 versus FZD2 Ab + 1α,25(OH)2D3; ^p<0.05 versus ROR2 Ab + 1α,25(OH)2D3. (B) Effect of ROR2 blocking on 1α,25(OH)2D3-inducedCaMKII activation. *p<0.05, treatment versus control. (C) Effect of ROR2 blocking on PLAA peptide-stimulated CaMKII activation. *p<0.05, treatment versus control. (D) Subcellular localization of FZD2, FZD5 and ROR2. Plasma membrane fractions were isolated as described. Presence of FZD2, FZD5, ROR2 and Cav-1 in fractions were examined by Western blot. Each figure is a representative experiment repeated three times with similar results.
3.6. 1α,25(OH)2 D3 alters the interactions between 1α,25(OH)2 D3 receptor complex and Wnt5a receptors
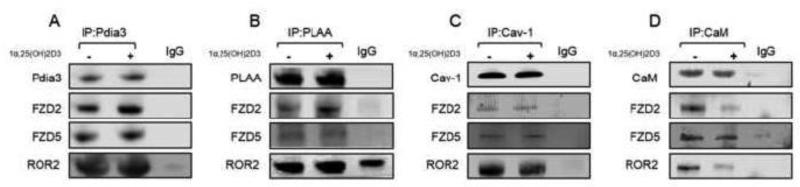
Immunoprecipitation studies confirmed the interaction between components of 1α,25(OH)2D3 receptor complex and Wnt5a receptors. 1α,25(OH)2D3 treatment altered some of these interactions. Samples of MC3T3-E1 whole cell lysates immunoprecipitated using antibodies to Pdia3 (IP:Pdia3) were positive for FZD2, FZD5, and ROR2 (Fig. 6A). Treatment with 1α,25(OH)2D3 for 15 minutes had no effect on Pdia3:FRZ5 but increased Pdia3:FZD2 and Pdia3:ROR2. Western blots of whole cell lysates immunoprecipitated with antibodies to PLAA (IP:PLAA) demonstrated an increase in FZD2-associated PLAA after addition of 1α,25(OH)2D3 (Fig. 6B). FZD5 and ROR2 interacted with PLAA, but 1α,25(OH)2D3 treatment had no effect on these interactions. Cav-1 (IP:Cav-1) interacted with FZD2, FZD5, and ROR2 (Fig. 6C). Treatment with 1α,25(OH)2D3 for 15 minutes had no effect on FZD2 and FZD5 but reduced ROR2. Immunoprecipitation of CaM (IP:CaM) demonstrated interaction with FZD5 with or without 1α,25(OH)2D3 treatment (Fig. 6D). FZD2 and ROR2 also interacted with CaM and 1α,25(OH)2D3 treatment reduced their interactions with CaM.
Figure 6.
Effect of 1α,25(OH)2D3 treatment on interactions between 1α,25(OH)2D3 receptor complex and Wnt5a receptors. MC3T3-E1 cells were treated with 1α,25(OH)2D3 for 15 minutes. Whole cell lysates were isolated as described. (A) Pdia3 was immunoprecipitated and subjected to Western blot. The membranes were incubated with Ab Pdia3, Ab FZD2, anti-FZD5, and anti-ROR2 antibodies. (B) PLAA was immunoprecipitated and subjected to Western blot. The membranes were incubated with the anti-PLAA, anti-FZD2, anti-FZD5, and anti-ROR2 antibodies. (C) Cav-1 was immunoprecipitated and subjected to Western blot. The membranes were incubated with the anti-Cav-1, anti-FZD2, anti-FZD5, and anti-ROR2 antibodies. (D) CaM was immunoprecipitated and subjected to Western blot. The membranes were incubated with the anti-CaM, anti-FZD2, anti-FZD5, and anti-ROR2 antibodies. Each figure is a representative experiment repeated three times with similar results.
3.7. Wnt5a alters the interactions between 1α,25(OH)2 D3 receptor complex and Wnt5a receptors
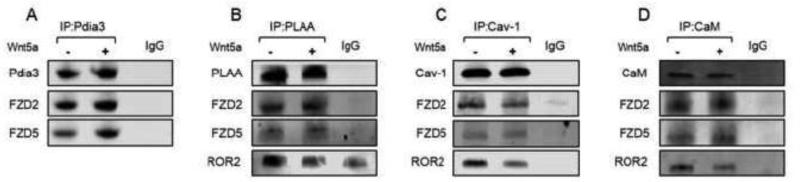
Immunoprecipitates of MC3T3-E1 whole cell lysates using antibodies to Pdia3 (IP:Pdia3) were positive for FZD2 and FZD5 (Fig. 7A). Treatment with Wnt5a for 15 minutes had no effect on FZD2's interaction with Pdia3 but increased FZD5. Western blots of whole cell lysates immunoprecipitated with antibodies to PLAA (IP:PLAA) demonstrated interaction with FZD2 and FZD5 with and without 1α,25(OH)2D3 treatment (Fig. 7B). ROR2 also interacted with PLAA, and Wnt5a treatment reduced its interaction with PLAA. Immunoprecipitation of Cav-1 (IP:Cav-1) demonstrated interactions with FZD2, FZD5 and ROR2 (Fig. 7C). While Wnt5a treatment reduced FZD2 and ROR2 interactions with Cav-1, it had no effect on FZD5 interactions with Cav-1. Immunoprecipitation of CaM (IP:CaM) demonstrated interaction with FRZD5 with or without Wnt5a treatment (Fig. 7D). FZD2 and ROR2 also interacted with CaM. 1α,25(OH)2D3 treatment increased FZD2 and CaM interaction and decreased ROR2 and CaM interaction.
Figure 7.
Effect of Wnt5a treatment on interactions between 1α,25(OH)2D3 receptor complex and Wnt5a receptors. MC3T3-E1 cells were treated with Wnt5a for 15 minutes. Whole cell lysates were isolated as described. (A) Pdia3 was immunoprecipitated and subjected to Western blot. The membranes were incubated with the anti-Pdia3, anti-FZD2 and anti-FZD5 antibodies. (B) PLAA was immunoprecipitated and subjected to Western blot. The membranes were incubated with the anti-PLAA, anti-FZD2, anti-FZD5 and anti-ROR2 antibodies. (C) Cav-1 was immunoprecipitated and subjected to Western blot. The membranes were incubated with the anti-Cav-1, anti-FZD2, anti-FZD5 and anti-ROR2 antibodies. (D) CaM was immunoprecipitated and subjected to Western blot. The membranes were incubated with the anti-CaM, anti-FZD2, anti-FZD5 and anti-ROR2 antibodies. Each figure is a representative experiment repeated three times with similar results.
4. Conclusions
This study demonstrates that signaling proteins critical for the 1α,25(OH)2D3 membrane-mediated pathway are also crucial for Wnt5a calcium-dependent signaling. Events at the signaling level indicated that Wnt5a calcium-dependent signaling works through a mechanism involving the CaMKII/PLA2/PGE2/PKC pathway. Moreover, silencing or blocking of Pdia3, PLAA and VDR and inhibition of CaM, CaMKII and PLA2 affected the activity of PKC in response to Wnt5a treatment. In contrast, PKC activity was unaffected in Cav-1 silenced cells, but the stimulatory effect of Wnt5a was decreased in cells treated with β-CD. Furthermore, Western blots of plasma membrane fractions indicated that ROR2, FZD2, and FZD5 are localized in caveolae fraction. Blocking of ROR2 abolished the stimulatory effects of 1α,25(OH)2D3 on PKC and CaMKII activations. This study also provides mechanistic information by showing that the 1α,25(OH)2D3 receptor complex and its downstream mediators form complexes with Wnt5a receptors, and these complexes respond to both 1α,25(OH)2D3 and Wnt5a treatment by altering some of their protein-protein interactions.
Similar to 1α,25(OH)2D3 signaling, Wnt5a time course studies indicate a rapid increase in CaMKII, PLA2 and PKC activities and PGE2 release in GC chondrocytes and MC3T3-E1 osteoblast-like cells. While the profiles of time points at which Wnt5a activated PLA2 and triggered PGE2 release were similar between GC and MC3T3-E1 cells, differences were observed in PKC and CaMKII activities. One reason that may contribute to such a difference is that while GC cells are primary cartilage cells isolated from the rat costochondral cartilage growth zone, MC3T3-E1 osteoblast-like cells are a cell line derived from mouse calvaria. The second potential reason for such an observation is that growth zone chondrocytes used in these experiments were isolated from 100-125g male Sprague-Dawley rats, and these rats were at the end of their adolescent growth spurt while the mouse osteoblast-like MC3T3-E1 cell line was derived from a newborn mouse.
When GC and MC3T3-E1 cells were treated with Wnt5a, the increase in PLA2 activity and PGE2 release occurred in a cyclic manner. A rapid elevation was observed at 9 minutes after the treatment, which was followed by a decline at 15 minutes and a second increase at 30 minutes post Wnt5 a treatment. The initial increase can be attributed to a rapid Wnt5a-induced use of available factors required for activation of PLA2 and downstream PGE2 release, as well as an increase in intracellular calcium and activation of CaMKII (13,45,46). The increase observed at 30 minutes might be the consequence of cross-talk with another pathway that involves in activation of PLA2or it could result from production of new factors triggered by Wnt5a action.Previously, we reported that PLAA, VDR, Cav-1 and caveolae are involved in 1α,25(OH)2D3 membrane-mediated signaling by Pdia3 (9,12,40,47). VDR has also been shown by others to mediate rapid effects of 1α,25(OH)2D3 on Ca2+ transport via voltage gated Ca2+ channels (48). Similar to 1α,25(OH)2D3 membrane-mediated signaling, our results indicated that Pdia3, PLAA, and VDR are critical for Wnt5a calcium-dependent pathway. However, to our surprise, silenced Cav-1 osteoblasts activated PKC in response to Wnt5a treatment, suggesting that Cav-1 is not necessary for the Wnt5a calcium-dependent pathway. To further investigate the role of lipid rafts in Wnt5a calcium-dependent pathway, we subjected the cells to β-CD. PKC activity increased in cells pretreated with β-CD but the increase was significantly lower than the β-CD-untreated group. Collectively, these studies indicate that Wnt5a induces its effects partially via lipid rafts and caveolae are not required to mediate Wnt5a effects. Previously, FZD5 and FZD2 proteins have been reported to be found in clathrin coated pits, suggesting Wnt5a regulates its pathway via this group of lipid rafts (49).
Our inhibitor study indicates that CaM, CaMKII, and PLA2 are critical for Wnt5a stimulated PKC activation. We previously reported that CaM plays a critical role in 1α,25(OH)2D3 membrane-mediated pathway and its inhibition blocks 1α,25(OH)2D3 stimulated rapid activation of PKC (13). In the present study, we found that CaM inhibition by W-7 abolished Wnt5a stimulated activation of PKC in a dose-dependent manner. Our hypothesis that CaM is required for Wnt5a stimulated activation of PKC is also supported by the observation that CaM inhibition suppresses PKC translocation in response to phorbol 12-myristate 13-acetate (PMA) treatment in the rat aorta (50). In our previous work, we reported that CaMKII, isoform α, is necessary for 1α,25(OH)2D3 membrane stimulated activation of PLA2, PKC and PGE2 release (13). Using mer-CaMKIINtide to inhibit the effect of CaMKII, we tested the role of CaMKII in Wnt5a induced PKC activation. In the current study, we found that CaMKII inhibition abolished Wnt5a stimulated activation of PKC in a dose-dependent manner. Our hypothesis that CaMKII is required for Wnt5a induced activation of PKC is also supported by the observation that CaMKII regulates PLA2 activity (45). PLA2 is known to act upstream of PKC in several signaling pathways, including 1α,25(OH)2D3 and dihydrotestosterone (14,51), hence CaMKII influences PKC activity in a PLA2-dependent mechanism. Additionally, in agreement with previous findings (14,51), we found that PLA2 inhibition abolished Wnt5a induced activation of PKC in a dose-dependent manner.
MC3T3-E1 cells respond to 125ng/ml Wnt5a with a rapid increase in PKC activity. Here we report that co-treatment with 10−10-10−8 M 1α,25(OH)2D3 abrogates the increase in PKC activation seen with exogenous 125 ng/ml Wnt5a treatment alone, in a dose dependent manner. Co-treatment of osteoblasts with 125 ng/ml Wnt5a and 10−8 M 1α,25(OH)2D3, returned PKC activity to the control level. Previously, we reported that MC3T3-E1osteoblast-like cells respond to 10−8 M 1α,25(OH)2D3 with a rapid increase in PKC activity (9). However, co-treatment with 50-125ng/ml Wnt5a leads to a strong increase in PKC activity. Surprisingly, we observed that co-treatment with Wnt5a induces enhanced effects on 1α,25(OH)2D3 stimulated PKC activation at low dose of Wnt5a and repressive effects at high dose of Wnt5a. These results may suggest that Wnt5a and 1α,25(OH)2D3 pathways compete at their receptor complex or downstream pathway mediators levels.
Previously, we reported that 1α,25(OH)2D3 membrane associated receptor, Pdia3, is present in plasma membrane compartments called caveolae (52). We also showed that PLAA and CaM are present in caveolae, where their interaction with Pdia3 receptor complex is critical for transducing the 1α,25(OH)2D3 signal (12,13). In the present study, our plasma membrane fractionation experiment indicates that ROR2, FZD2, and FZD5 are localized in fraction 3. Furthermore, our receptor antibody blocking experiments shows that blocking ROR2 abolishes 1α,25(OH)2D3 stimulated PKC activation while blocking FZD2 and FZD5 receptors has no effect on activation of PKC in response to 1α,25(OH)2D3 treatment. These results indicate that Wnt5a co-receptor, ROR2, is part of 1α,25(OH)2D3 membrane-associate receptor complex in caveolae. In agreement with our findings, another group has detected ROR2 in Cav-1-α positive cholesterol-rich, detergent-resistant microdomains (DRMs) of the plasma membrane in (53). Furthermore, they showed that ROR2 forms a complex with bone morphogenetic protein receptor type 1B (BMPR1B) in a ligand-independent manner and it inhibited the growth and differentiation factor 5 (GDF5)/BMPR1B induced Smad 1/5 signaling pathway in ATDC5 cells (54). Collectively, these findings suggest that ROR2 participates in multiple signaling pathways including Wnt5a, BMP and 1α,25(OH)2D3.
Wnt5a induces its non-canonical signaling via several receptors. ROR2 is a transmembrane receptor, previously identified to mediate Wnt5a actions (55). Wnt5a increases ROR2 expression, and knockdown of WNT5A dramatically decreases expression of ROR2 (30). ROR2 has regions of cysteine-rich domain that serve as its Wnt binding domain (56,57). Several conditions, including brachydactyly type B and autosomal recessive Robinow syndrome, which display severe skeletal dysplasia, are due to mutations in the ROR2 gene (58,59). Additionally, ROR2 is critically required for Wnt5a-induced migration of osteoblasts (60). Wnt5a can also act via the Frizzled family of receptors. In human cells, FZD2 and FZD5 are known to act as Wnt5a receptors and activate its non-canonical signaling cascades (61,62).
Our CaM immunoprecipitation studies suggest that CaM forms a complex with ROR2, FZD2, and FZD5. Wnt5a treatment does not alter the interactions between CaM-FZD2 and CaM-FZD5, while it reduces the interaction between CaM-ROR2. 1α,25(OH)2D3 treatment decreases the interactions between CaM-FZD2 and CaM-ROR2; however, it does not alter the interactions between CaM-FZD5. These results indicates that CaM serves as a mediator in both 1α,25(OH)2D3 and Wnt5a pathways; suggesting its potential role to mediate the cross-talk between these two pathways.
The results of this study suggest that Wnt5a and 1α,25(OH)2D3 mediate their effects via a network of interacting mediators rather than through a secluded linear pathway. In co-treatment studies, we speculate that 1α,25(OH)2D3 receptor competes with Wnt5a receptors for binding of PLAA, CaM and Cav-1, thus inhibiting downstream PKC signaling. Previously, similar 1α,25(OH)2D3-induced mechanisms in which one hormone can modulate the activity of a second, by competing for a shared mediator site, have been identified. Work from the Ross lab has shown that retinoid X receptor (RXR)-vitamin D receptor (VDR) and retinoid acid receptor (RAR)-RXR heterodimers compete for a novel steroid hormone response element containing elements responding to retinoid acid and 1α,25(OH)2D3 in the promoter region of the avian β3 integrin gene (63). Co-treatment with retinoid acid and 1α,25(OH)2D3 resulted in a response equal to that of retinoid acid alone (63). Furthermore, their results indicated RAR-RXR had a greater affinity for the shared promoter region than RXR-VDR, hence co-addition of retinoid acid and 1α,25(OH)2D3 resulted in preferred binding of RAR-RXR to the promoter and inhibition of 1α,25(OH)2D3 induced transcription (63). Similar to this finding, our results also indicated that Wnt5a and 1α,25(OH)2D3 compete for similar signaling mediators and co-treatment antagonizes the immunological effects of 1α,25(OH)2D3. 1α,25(OH)2D3 is known to stimulate alkaline phosphatase activity (16) and osteopontin production (9), markers of osteoblast maturation, via its membrane-mediated events. Wnt5a has also been shown to promote osteoblast differentiation and maturation. Wnt5a treatment increases osteocalcin and osteoprotegerin levels and alkaline phosphatase specific activity (26), while its knockout down regulates osteoblastic differentiation markers including runt related transcription factor 2, osterix and alkaline phosphatase (27). Future studies should focus on the in vivo consequences of the regulation of growth plate chondrocytes growth and differentiation by Wnt5a and 1α,25(OH)2D3.
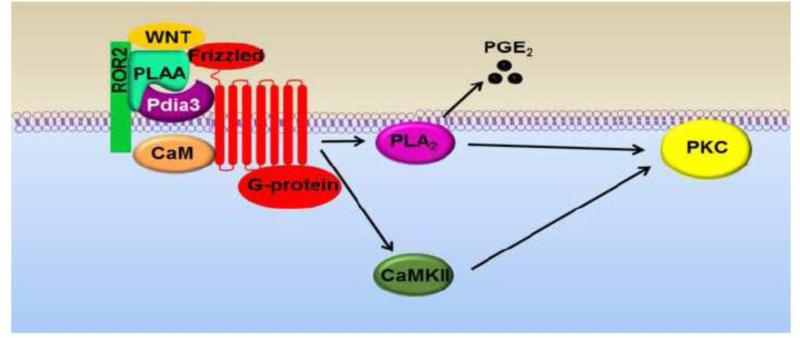
In conclusion, this study investigated the requirement for components of 1α,25(OH)2D3 membrane-associated receptor complex in Wnt5a calcium-dependent signaling. We found that Wnt5a stimulates its calcium-dependent actions via Pdia3 receptor complex (Fig. 8). The effects of Wnt5a are mediated through Pdia3, PLAA, and VDR. Inhibition of CaM, CaMKII, and PLA2 suppressed the activation of PKC in response to Wnt5a treatment. Silencing Cav-1 had no effect on Wnt5a-stimulated PKC activation, which reveals one of the differences between the mediators of 1α,25(OH)2D3 and Wnt5a calcium-dependent pathways. Our results also showed that ROR2, one of the receptors of Wnt5a, plays an important role in 1α,25(OH)2D3 membrane-mediated signaling. Blocking ROR2 abolished 1α,25(OH)2D3 induced PKC and CaMKII activations. Moreover, immunoprecipitation studies showed that 1α,25(OH)2D3 membrane receptor complex (Pdia3, PLAA, Cav-1 and CaM) interacts with Wnt5a receptors (ROR2, FZD2 and FZD5). While most of their protein-protein interactions were independent of either 1α,25(OH)2D3 or Wnt5a treatment, a few of the interactions changed with ligands treatments. In the co-treatment study, addition of 1α,25(OH)2D3 repressed Wnt5a stimulated PKC activation in a dose-dependent manner. We found that co-treatment with 1α,25(OH)2D3 repressed Wnt5a induced PKC activation in a dose-dependent manner, and was most inhibited at 10−8 M. Furthermore, co-treatment with 50 ng/ml Wnt5a caused a 2-fold increase in 1α,25(OH)2D3 stimulated PKC activity compared to cultures treated with only 1α,25(OH)2D3. However, as the concentration of Wnt5a increased, it had repressive effects on 1α,25(OH)2D3-induced PKC activation. The results of this study suggest that signaling components of Pdia3 receptor complex are required for mediating the calcium-dependent actions of Wnt5a and 1α,25(OH)2D3 may modulate the response of Wnt5a by competing for similar signaling mediators.
Figure 8.
Proposed role of components of 1α,25(OH)2D3 membrane-associated receptor complex in Wnt5a calcium-dependent signaling. The effects of Wnt5a are mediated through Pdia3, PLAA and VDR (not shown). Inhibition of CaM, CaMKII, and PLA2 suppressed the activation of PKC in response to Wnt5a treatment.
Highlights.
Wnt5a rapidly activates CaMKII, PLA2 and PKC in chondrocytes and osteoblasts.
Silencing or blocking Pdia3, PLAA or CaM inhibits Wnt5a signaling.
Blocking ROR2 inhibits 1α,25(OH)2D3-dependent PKC and CaMKII activation.
Pdia3, PLAA and CaM interact with Wnt5a receptors.
1α,25(OH)2D3 and Wnt5a competition may balance proliferation v. differentiation.
Acknowledgements
This research was supported by grants from the Price Gilbert, Jr. Foundation and Children's Healthcare of Atlanta. The authors thank Subhendu De and Lauren Cason for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Howell DS DD. Biology, chemistry and biochemistry of the mammalian growth plate. In: Coe FL, F. M., editors. in Disorders of Bone and Mineral Metabolism. New York. Raven; 1992. pp. 313–353. [Google Scholar]
- 2.Gurlek A, Kumar R. Regulation of osteoblast growth by interactions between transforming growth factor-beta and 1alpha,25-dihydroxyvitamin D3. Crit Rev Eukaryot Gene Expr. 2001;11:299–317. [PubMed] [Google Scholar]
- 3.Pedrozo HA, Schwartz Z, Mokeyev T, Ornoy A, Xin-Sheng W, Bonewald LF, Dean DD, Boyan BD. Vitamin D3 metabolites regulate LTBP1 and latent TGF-beta1 expression and latent TGF-beta1 incorporation in the extracellular matrix of chondrocytes. J Cell Biochem. 1999;72:151–165. [PubMed] [Google Scholar]
- 4.Alvarez J, Sohn P, Zeng X, Doetschman T, Robbins DJ, Serra R. TGFbeta2 mediates the effects of hedgehog on hypertrophic differentiation and PTHrP expression. Development. 2002;129:1913–1924. doi: 10.1242/dev.129.8.1913. [DOI] [PubMed] [Google Scholar]
- 5.Nemoto E, Ebe Y, Kanaya S, Tsuchiya M, Nakamura T, Tamura M, Shimauchi H. Wnt5a signaling is a substantial constituent in bone morphogenetic protein-2-mediated osteoblastogenesis. Biochem Biophys Res Commun. 2012;422:627–632. doi: 10.1016/j.bbrc.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 6.Nemere I, Norman AW. The rapid, hormonally stimulated transport of calcium (transcaltachia). J Bone Miner Res. 1987;2:167–169. doi: 10.1002/jbmr.5650020302. [DOI] [PubMed] [Google Scholar]
- 7.Boyan BD, Schwartz Z, Swain LD, Bonewald LF, Khare A. Regulation of matrix vesicle metabolism by vitamin D metabolites. Connect Tissue Res. 1989;22:3–16. doi: 10.3109/03008208909114115. discussion 53-61. [DOI] [PubMed] [Google Scholar]
- 8.Khanal RC, Nemere I. The ERp57/GRp58/1,25D3-MARRS receptor: multiple functional roles in diverse cell systems. Curr Med Chem. 2007;14:1087–1093. doi: 10.2174/092986707780362871. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Olivares-Navarrete R, Wang Y, Herman TR, Boyan BD, Schwartz Z. Protein-disulfide isomerase-associated 3 (Pdia3) mediates the membrane response to 1,25-dihydroxyvitamin D3 in osteoblasts. J Biol Chem. 2010;285:37041–37050. doi: 10.1074/jbc.M110.157115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nemere I, Farach-Carson MC, Rohe B, Sterling TM, Norman AW, Boyan BD, Safford SE. Ribozyme knockdown functionally links a 1,25(OH)2D3 membrane binding protein (1,25D3-MARRS) and phosphate uptake in intestinal cells. Proc Natl Acad Sci U S A. 2004;101:7392–7397. doi: 10.1073/pnas.0402207101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas CM, Smart EJ. Caveolae structure and function. Journal of cellular and molecular medicine. 2008;12:796–809. doi: 10.1111/j.1582-4934.2008.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doroudi M, Schwartz Z, Boyan BD. Phospholipase A(2) activating protein is required for 1alpha,25-dihydroxyvitamin D(3) dependent rapid activation of protein kinase C via Pdia3. J Steroid Biochem Mol Biol. 2012;132:48–56. doi: 10.1016/j.jsbmb.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Doroudi M, Plaisance MC, Boyan BD, Schwartz Z. Membrane Actions of 1α,25(OH)2D3 is Mediated by Ca2+/Calmodulin-dependent Protein Kinase II in Bone and Cartilage Cells. 2014 doi: 10.1016/j.jsbmb.2014.09.019. Under Review. [DOI] [PubMed] [Google Scholar]
- 14.Sylvia VL, Schwartz Z, Curry DB, Chang Z, Dean DD, Boyan BD. 1,25(OH)2D3 regulates protein kinase C activity through two phospholipid-dependent pathways involving phospholipase A2 and phospholipase C in growth zone chondrocytes. J Bone Miner Res. 1998;13:559–569. doi: 10.1359/jbmr.1998.13.4.559. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz Z, Shaked D, Hardin RR, Gruwell S, Dean DD, Sylvia VL, Boyan BD. 1alpha,25(OH)2D3 causes a rapid increase in phosphatidylinositol-specific PLC-beta activity via phospholipase A2-dependent production of lysophospholipid. Steroids. 2003;68:423–437. doi: 10.1016/s0039-128x(03)00044-8. [DOI] [PubMed] [Google Scholar]
- 16.Sylvia VL, Del Toro F, Dean DD, Hardin RR, Schwartz Z, Boyan BD. Effects of 1alpha,25-(OH)(2)D(3) on rat growth zone chondrocytes are mediated via cyclooxygenase-1 and phospholipase A(2). J Cell Biochem Suppl Suppl. 2001;36:32–45. doi: 10.1002/jcb.1072. [DOI] [PubMed] [Google Scholar]
- 17.Boyan BD, Sylvia VL, Dean DD, Schwartz Z. Membrane mediated signaling mechanisms are used differentially by metabolites of vitamin D(3) in musculoskeletal cells. Steroids. 2002;67:421–427. doi: 10.1016/s0039-128x(01)00178-7. [DOI] [PubMed] [Google Scholar]
- 18.Kishimoto A, Takai Y, Mori T, Kikkawa U, Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980;255:2273–2276. [PubMed] [Google Scholar]
- 19.Wang Y, Chen J, Lee CS, Nizkorodov A, Riemenschneider K, Martin D, Hyzy S, Schwartz Z, Boyan BD. Disruption of Pdia3 gene results in bone abnormality and affects 1alpha,25-dihydroxy-vitamin D3-induced rapid activation of PKC. J Steroid Biochem Mol Biol. 2010;121:257–260. doi: 10.1016/j.jsbmb.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Coe H, Jung J, Groenendyk J, Prins D, Michalak M. ERp57 modulates STAT3 signaling from the lumen of the endoplasmic reticulum. J Biol Chem. 2010;285:6725–6738. doi: 10.1074/jbc.M109.054015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nemere I, Garbi N, Hammerling GJ, Khanal RC. Intestinal cell calcium uptake and the targeted knockout of the 1,25D3-MARRS (membrane-associated, rapid response steroid-binding) receptor/PDIA3/Erp57. J Biol Chem. 2010;285:31859–31866. doi: 10.1074/jbc.M110.116954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yates KE, Shortkroff S, Reish RG. Wnt influence on chondrocyte differentiation and cartilage function. DNA and cell biology. 2005;24:446–457. doi: 10.1089/dna.2005.24.446. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Li YP, Paulson C, Shao JZ, Zhang X, Wu M, Chen W. Wnt and the Wnt signaling pathway in bone development and disease. Frontiers in bioscience. 2014;19:379–407. doi: 10.2741/4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrade AC, Nilsson O, Barnes KM, Baron J. Wnt gene expression in the post-natal growth plate: regulation with chondrocyte differentiation. Bone. 2007;40:1361–1369. doi: 10.1016/j.bone.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Y, Topol L, Lee H, Wu J. Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development. 2003;130:1003–1015. doi: 10.1242/dev.00324. [DOI] [PubMed] [Google Scholar]
- 26.Olivares-Navarrete R, Hyzy SL, Hutton DL, Dunn GR, Appert C, Boyan BD, Schwartz Z. Role of non-canonical Wnt signaling in osteoblast maturation on microstructured titanium surfaces. Acta Biomater. 2011;7:2740–2750. doi: 10.1016/j.actbio.2011.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo J, Jin J, Cooper LF. Dissection of sets of genes that control the character of wnt5a-deficient mouse calvarial cells. Bone. 2008;43:961–971. doi: 10.1016/j.bone.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 28.He X, Saint-Jeannet JP, Wang Y, Nathans J, Dawid I, Varmus H. A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science. 1997;275:1652–1654. doi: 10.1126/science.275.5306.1652. [DOI] [PubMed] [Google Scholar]
- 29.Bazhin AV, Tambor V, Dikov B, Philippov PP, Schadendorf D, Eichmuller SB. cGMP-phosphodiesterase 6, transducin and Wnt5a/Frizzled-2-signaling control cGMP and Ca(2+) homeostasis in melanoma cells. Cellular and molecular life sciences : CMLS. 2010;67:817–828. doi: 10.1007/s00018-009-0214-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.O'Connell MP, Fiori JL, Xu M, Carter AD, Frank BP, Camilli TC, French AD, Dissanayake SK, Indig FE, Bernier M, Taub DD, Hewitt SM, Weeraratna AT. The orphan tyrosine kinase receptor, ROR2, mediates Wnt5A signaling in metastatic melanoma. Oncogene. 2010;29:34–44. doi: 10.1038/onc.2009.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kikuchi A, Yamamoto H, Sato A, Matsumoto S. Wnt5a: its signalling, functions and implication in diseases. Acta physiologica. 2012;204:17–33. doi: 10.1111/j.1748-1716.2011.02294.x. [DOI] [PubMed] [Google Scholar]
- 32.Kuhl M, Sheldahl LC, Malbon CC, Moon RT. Ca(2+)/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J Biol Chem. 2000;275:12701–12711. doi: 10.1074/jbc.275.17.12701. [DOI] [PubMed] [Google Scholar]
- 33.Pedrozo HA, Schwartz Z, Rimes S, Sylvia VL, Nemere I, Posner GH, Dean DD, Boyan BD. Physiological importance of the 1,25(OH)2D3 membrane receptor and evidence for a membrane receptor specific for 24,25(OH)2D3. J Bone Miner Res. 1999;14:856–867. doi: 10.1359/jbmr.1999.14.6.856. [DOI] [PubMed] [Google Scholar]
- 34.Chen J, Doroudi M, Cheung J, Grozier AL, Schwartz Z, Boyan BD. Plasma membrane Pdia3 and VDR interact to elicit rapid responses to 1alpha,25(OH)2D3. Cellular signalling. 2013;25:2362–2373. doi: 10.1016/j.cellsig.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 35.Wang D, Christensen K, Chawla K, Xiao G, Krebsbach PH, Franceschi RT. Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. J Bone Miner Res. 1999;14:893–903. doi: 10.1359/jbmr.1999.14.6.893. [DOI] [PubMed] [Google Scholar]
- 36.Boyan BD, Schwartz Z, Swain LD, Carnes DL, Jr., Zislis T. Differential expression of phenotype by resting zone and growth region costochondral chondrocytes in vitro. Bone. 1988;9:185–194. doi: 10.1016/8756-3282(88)90008-7. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz Z, Graham EJ, Wang L, Lossdorfer S, Gay I, Johnson-Pais TL, Carnes DL, Sylvia VL, Boyan BD. Phospholipase A2 activating protein (PLAA) is required for 1alpha,25(OH)2D3 signaling in growth plate chondrocytes. J Cell Physiol. 2005;203:54–70. doi: 10.1002/jcp.20212. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Rubin B, Bodine PV, Billiard J. Wnt5a induces homodimerization and activation of Ror2 receptor tyrosine kinase. J Cell Biochem. 2008;105:497–502. doi: 10.1002/jcb.21848. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz Z, Ehland H, Sylvia VL, Larsson D, Hardin RR, Bingham V, Lopez D, Dean DD, Boyan BD. 1alpha,25-dihydroxyvitamin D(3) and 24R,25-dihydroxyvitamin D(3) modulate growth plate chondrocyte physiology via protein kinase C-dependent phosphorylation of extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase. Endocrinology. 2002;143:2775–2786. doi: 10.1210/endo.143.7.8889. [DOI] [PubMed] [Google Scholar]
- 40.Boyan BD, Wong KL, Wang L, Yao H, Guldberg RE, Drab M, Jo H, Schwartz Z. Regulation of growth plate chondrocytes by 1,25-dihydroxyvitamin D3 requires caveolae and caveolin-1. J Bone Miner Res. 2006;21:1637–1647. doi: 10.1359/jbmr.060713. [DOI] [PubMed] [Google Scholar]
- 41.Nebigil C, Malik KU. Alpha adrenergic receptor subtypes involved in prostaglandin synthesis are coupled to Ca++ channels through a pertussis toxin-sensitive guanine nucleotide-binding protein. J Pharmacol Exp Ther. 1993;266:1113–1124. [PubMed] [Google Scholar]
- 42.Chang BH, Mukherji S, Soderling TR. Characterization of a calmodulin kinase II inhibitor protein in brain. Proc Natl Acad Sci U S A. 1998;95:10890–10895. doi: 10.1073/pnas.95.18.10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murakami M, Kuwata H, Amakasu Y, Shimbara S, Nakatani Y, Atsumi G, Kudo I. Prostaglandin E2 amplifies cytosolic phospholipase A2- and cyclooxygenase-2-dependent delayed prostaglandin E2 generation in mouse osteoblastic cells. Enhancement by secretory phospholipase A2. J Biol Chem. 1997;272:19891–19897. doi: 10.1074/jbc.272.32.19891. [DOI] [PubMed] [Google Scholar]
- 44.Huhtakangas JA, Olivera CJ, Bishop JE, Zanello LP, Norman AW. The vitamin D receptor is present in caveolae-enriched plasma membranes and binds 1 alpha,25(OH)2-vitamin D3 in vivo and in vitro. Mol Endocrinol. 2004;18:2660–2671. doi: 10.1210/me.2004-0116. [DOI] [PubMed] [Google Scholar]
- 45.Muthalif MM, Benter IF, Uddin MR, Malik KU. Calcium/calmodulin-dependent protein kinase IIalpha mediates activation of mitogen-activated protein kinase and cytosolic phospholipase A2 in norepinephrine-induced arachidonic acid release in rabbit aortic smooth muscle cells. J Biol Chem. 1996;271:30149–30157. doi: 10.1074/jbc.271.47.30149. [DOI] [PubMed] [Google Scholar]
- 46.Li L, Hutchins BI, Kalil K. Wnt5a induces simultaneous cortical axon outgrowth and repulsive axon guidance through distinct signaling mechanisms. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:5873–5883. doi: 10.1523/JNEUROSCI.0183-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen J, Doroudi M, Cheung J, Grozier AL, Schwartz Z, Boyan BD. Plasma membrane Pdia3 and VDR interact to elicit rapid responses to 1alpha,25(OH)D. Cell Signal. 2013 doi: 10.1016/j.cellsig.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 48.Norman AW. Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology. 2006;147:5542–5548. doi: 10.1210/en.2006-0946. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto H, Komekado H, Kikuchi A. Caveolin is necessary for Wnt-3a-dependent internalization of LRP6 and accumulation of beta-catenin. Dev Cell. 2006;11:213–223. doi: 10.1016/j.devcel.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 50.Chuprun JK, Bazan E, Chang KC, Campbell AK, Rapoport RM. Inhibition of phorbol ester-induced contraction by calmodulin antagonists in rat aorta. Am J Physiol. 1991;261:C675–684. doi: 10.1152/ajpcell.1991.261.4.C675. [DOI] [PubMed] [Google Scholar]
- 51.ElBaradie K, Wang Y, Boyan BD, Schwartz Z. Rapid membrane responses to dihydrotestosterone are sex dependent in growth plate chondrocytes. J Steroid Biochem Mol Biol. 2012;132:15–23. doi: 10.1016/j.jsbmb.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 52.Chen J, Lobachev KS, Grindel BJ, Farach-Carson MC, Hyzy SL, El-Baradie KB, Olivares-Navarrete R, Doroudi M, Boyan BD, Schwartz Z. Chaperone properties of pdia3 participate in rapid membrane actions of 1alpha,25-dihydroxyvitamin d3. Mol Endocrinol. 2013;27:1065–1077. doi: 10.1210/me.2012-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sammar M, Sieber C, Knaus P. Biochemical and functional characterization of the Ror2/BRIb receptor complex. Biochem Biophys Res Commun. 2009;381:1–6. doi: 10.1016/j.bbrc.2008.12.162. [DOI] [PubMed] [Google Scholar]
- 54.Sammar M, Stricker S, Schwabe GC, Sieber C, Hartung A, Hanke M, Oishi I, Pohl J, Minami Y, Sebald W, Mundlos S, Knaus P. Modulation of GDF5/BRI-b signalling through interaction with the tyrosine kinase receptor Ror2. Genes to cells : devoted to molecular & cellular mechanisms. 2004;9:1227–1238. doi: 10.1111/j.1365-2443.2004.00799.x. [DOI] [PubMed] [Google Scholar]
- 55.Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, Mundlos S, Shibuya H, Takada S, Minami Y. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes to cells : devoted to molecular & cellular mechanisms. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- 56.Cheung R, Kelly J, Macleod RJ. Regulation of villin by wnt5a/ror2 signaling in human intestinal cells. Frontiers in physiology. 2011;2:58. doi: 10.3389/fphys.2011.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saldanha J, Singh J, Mahadevan D. Identification of a Frizzled-like cysteine rich domain in the extracellular region of developmental receptor tyrosine kinases. Protein science : a publication of the Protein Society. 1998;7:1632–1635. doi: 10.1002/pro.5560070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Afzal AR, Rajab A, Fenske CD, Oldridge M, Elanko N, Ternes-Pereira E, Tuysuz B, Murday VA, Patton MA, Wilkie AO, Jeffery S. Recessive Robinow syndrome, allelic to dominant brachydactyly type B, is caused by mutation of ROR2. Nat Genet. 2000;25:419–422. doi: 10.1038/78107. [DOI] [PubMed] [Google Scholar]
- 59.van Bokhoven H, Celli J, Kayserili H, van Beusekom E, Balci S, Brussel W, Skovby F, Kerr B, Percin EF, Akarsu N, Brunner HG. Mutation of the gene encoding the ROR2 tyrosine kinase causes autosomal recessive Robinow syndrome. Nat Genet. 2000;25:423–426. doi: 10.1038/78113. [DOI] [PubMed] [Google Scholar]
- 60.Nishita M, Yoo SK, Nomachi A, Kani S, Sougawa N, Ohta Y, Takada S, Kikuchi A, Minami Y. Filopodia formation mediated by receptor tyrosine kinase Ror2 is required for Wnt5a-induced cell migration. J Cell Biol. 2006;175:555–562. doi: 10.1083/jcb.200607127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang H, Lee Y, Malbon CC. PDE6 is an effector for the Wnt/Ca2+/cGMP-signalling pathway in development. Biochemical Society transactions. 2004;32:792–796. doi: 10.1042/BST0320792. [DOI] [PubMed] [Google Scholar]
- 62.Blumenthal A, Ehlers S, Lauber J, Buer J, Lange C, Goldmann T, Heine H, Brandt E, Reiling N. The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood. 2006;108:965–973. doi: 10.1182/blood-2005-12-5046. [DOI] [PubMed] [Google Scholar]
- 63.Cao X, Teitelbaum SL, Zhu HJ, Zhang L, Feng X, Ross FP. Competition for a unique response element mediates retinoic acid inhibition of vitamin D3-stimulated transcription. J Biol Chem. 1996;271:20650–20654. doi: 10.1074/jbc.271.34.20650. [DOI] [PubMed] [Google Scholar]