Abstract
Background
Dopaminergic (DA) activity in the extended amygdala (EA) has been known to play a pivotal role in mediating drug and alcohol addiction. Alterations of DA activity within the EA after chronic exposure to alcohol or substances of abuse are considered a major mechanism for the development of alcoholism and addiction. To date, it is not clear how different patterns of chronic alcohol drinking affect DA receptor levels. Therefore, the current studies investigated the effects of chronic ethanol consumption, with or without deprivations, on D1 and D2 receptor densities within the EA.
Methods
Inbred alcohol-preferring (iP) rats were divided into 3 groups with the following treatments: (1) water for 14 weeks; (2) continuous alcohol (C-Alc) for 14 weeks [24-hour concurrent access to 15 and 30% (v/v) ethanol]; or (3) repeatedly deprived of alcohol (RD-Alc) (24-hour concurrent access to 15 and 30% ethanol for 6 weeks, followed by 2 cycles of 2 weeks of deprivation of and 2 weeks of reexposure to ethanol access). At the end of 14 weeks, the rats were killed for autoradiographic labeling of D1 and D2 receptors.
Results
Compared with the water control group, both the C-Alc and the RD-Alc groups displayed increases in D1 receptor binding density in the anterior region of the Acb core, whereas the RD-Alc group displayed additional increases in D1 receptor binding density in anterior regions of the lateral and intercalated nuclei of the amygdala. Additionally, both C-Alc and RD-Alc rats displayed increases in D2 receptor binding density in anterior regions of the Acb shell and core, whereas RDAlc rats displayed additional increases in D2 receptor binding density in the dorsal striatum.
Conclusion
The results of this study indicate that 14-week extended alcohol drinking with continuous chronic or repeated deprivations increase binding sites of D1 and D2 receptors in specific regions of the EA with greater sensitivity in the anterior regions. The repeated deprivation has greater effect on altering D1 and D2 receptor binding sites in the Acb, dorsal striatum, and subamygdala regions. The current result indicates that the two drinking paradigms may have common as well as differential mechanisms on alteration of dopamine receptor–binding sites in specific regions of the EA.
Keywords: Nucleus Accumbens, Receptor Autoradiography, SCH23390, Iodosulpiride, Neuroadaption
The dense dopaminergic (DA) projection from the ventral tegmental area (VTA) to the nucleus accumbens (Acb) is a key substrate mediating the natural rewarding properties of sex, food, and novelty (Hernandez and Hoebel, 1988; Pfaus et al., 1995). A consequence of obtaining these natural rewards is increased extracellular levels of DA in the Acb (Bozarth, 1991; Wise and Rompre, 1989). Similarly, administration of drugs of abuse (Di Chiara and Imperato, 1988; Imperato and Di Chiara, 1986) and ethanol (Melendez et al., 2002; Thielen et al., 2004) increase extracellular levels of DA in the Acb. While it is clear that the DA projection from the VTA to the Acb plays a key role in the rewarding effects of drugs of abuse, including ethanol, it is also apparent that multiple brain structures and neurotransmitter systems modulate this DA pathway (c.f., McBride, 2002; McBride and Li, 1998). In particular, the mesocorticolimbic circuit originates from the VTA with projections to the Acb, olfactory tubercle, frontal cortex, and in particular the amygdala complex (Asan, 1998). These DA innervations from the VTA are most intensely distributed to the central nucleus of amygdala (CeA), the bed nucleus of the stria terminalis (BNST), and Acb, collectively called the “extended amygdala” (EA) as has been suggested previously (Killcross et al., 1997; Palacios et al., 1990). The EA has extensive reciprocal connections with the VTA (Kalivas, 1993; Oades and Halliday, 1987) and is connected to the raphe nuclei, locus coeruleus, hippocampus, and ventral pallidum as well as other limbic structures implicated in reward (Azmitia and Segal, 1978; Fallon and Moore, 1978; Holstege et al., 1985; Krettek and Price, 1974; Nauta et al., 1978). Therefore, the EA and its interconnections with the mesocorticolimbic system form a complex reward circuit critical in mediating the rewarding properties of drugs of abuse, including ethanol, and the development of addiction and alcoholism (Koob and Le Moal, 2001; Koob et al., 1998a, 1998b).
It has been shown that innate differences in the mesocorticolimbic DA system are associated with high ethanol consumption (McBride and Li, 1998; Murphy et al., 2002). In particular, alcohol-preferring (P) rats have lower levels of DA and its metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) in the Acb and anterior striatum, compared with their non–alcohol-preferring (NP) counterparts (Murphy et al., 1982, 1987); our laboratory (Zhou et al., 1995) has reported a decreased number of DA neuronal projections from the VTA to the Acb in P rats, relative to NP rats. Moreover, operant oral self-administration of ethanol was found to increase DA overflow to a greater extent in the Acb of P rats than in unselected Wistar rats (Weiss et al., 1993), suggesting that the VTA DA system of the P line of rat may be more sensitive to the reinforcing actions of ethanol. Further support for this difference comes from the finding that P rats self-administer ethanol at lower concentrations directly into the VTA than Wistar rats (Rodd et al., 2004). Additionally, a role for DA activity in the Acb in mediating ethanol self-administration has been indicated by studies demonstrating changes in alcohol consumption following alterations in mesolimbic DA neurotransmission (Nowak et al., 2000; Rassnick et al., 1993; Samson et al., 1993).
In vitro and in vivo electrophysiological and microdialysis findings indicate that ethanol can activate VTA neurons (Brodie and Appel, 1998; Brodie et al., 1999). Systemic administration of ethanol increases activity of VTA neurons (for a review, see Gessa et al., 1985) and extracellular levels of DA within the Acb and CeA. Moreover, oral ethanol self-administration and anticipation of ethanol have both been reported to increase extracellular levels of DA in the Acb as well (Weiss et al., 1996; c.f., McBride and Li, 1998). Studies have also shown that chronic alcohol altered DA release in central reward circuitry (Nishiguchi et al., 2002; Murphy et al., 1983; Syvalahti et al., 1988; Rothblat et al., 2001). Most of these studies were carried out with relatively short periods of drinking and addressed direct responses of DA neuro-transmission due to alcohol exposure. Overall, these results suggest that oral alcohol self-administration activates DA inputs to major components of the EA, and anticipation of ethanol alone can have a similar effect on these DA projections (McBride and Li, 1998).
To better understand the effects of various patterns of chronic ethanol consumption on DA receptor levels, we investigated the effects of chronic alcohol including continuous access (C-Alc) and a repeated deprivation (RDAlc) protocol on dopamine D1 and D2 receptor binding site densities in brain structures associated with the EA. To date, there have been no published studies on changes in D1 and D2 receptor levels in the EA of iP, or P, rats using our extended 14-week chronic and repeated deprivation drinking protocols. The RD-Alc protocol was used to mimic the episodic drinking of alcohol abusers, where periods of high intake are interspersed with periods of abstinence, whether forced or voluntary (Burish et al., 1981; Hilbrom, 1990; McMillen, 1997).
MATERIALS AND METHODS
Animals
The subjects were 18 ethanol-naïve adult (>postnatal day 90) male iP rats. These iP rats have propensity to develop a drinking habit to the level of intoxication without stress forcing paradigm. This drinking model is adopted to avoid the complication of sucrose induction or single bottle force drinking, which may effect the DA receptor expression. The iP rats were obtained from the Indiana University Medical Center/Veterans Affairs Medical Center (Indianapolis, IN) breeding colonies. At the beginning of the experiment, the animals weighed an average of 379 ± 7 (mean ± SEM) g, without group differences. Three experimental groups were examined: (a) a water control group (n = 6), (b) a C-Alc group (n = 6), and (c) a RD-Alc group (n = 6). After habituation to the vivarium, animals were single housed in hanging stainless-steel cages in a temperature (21 °C)- and humidity (50%)- controlled vivarium. The vivarium was maintained on a 12/12-hour reverse dark/light cycle (lights off at 0900 hours). All animals had ad lib access to water and food. Animals used in these procedures were maintained in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Indiana University School of Medicine (Indianapolis, IN), which are in accordance with the guidelines of the Institutional Animal Care and Use Committee of the National Institutes of Health and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, 1996).
Ethanol Drinking Procedures
For animals with access to ethanol, rats were given concurrent access to multiple concentrations of ethanol (15 and 30%, v/v). The CAlc group had free-choice access to ethanol for 14 weeks. The water control group was run in parallel for 14 weeks. The RD-Alc group had an initial 6 weeks of 24-hour free-choice access to ethanol followed by 2 cycles of 2 weeks of deprivation of and 2 weeks of re-exposure to ethanol access, for a total of 14 weeks. Although, the RD-Alc group received 10 weeks of alcohol access rather than 14 weeks, we believe that the prolonged drinking period or the length of the total time is one of the key factors once ethanol self-administration is initiated, as indicated by the development of tolerance or sensitization to ethanol-associated effects. It has been shown that P rats developed behavioral tolerance in less than = weeks of ethanol exposure (Stewart et al., 1991). With this in mind, the a priori hypothesis for the C-Alc group is that a longer ethanol treatment protocol (14 weeks) would result in greater neuroadaptations than a shorter protocol (10 weeks) that matches the total time of ethanol access for the RD-Alc group. Regarding the RD-Alc group, the a priori hypothesis is that neuroadaptations will take place not only because of ethanol exposure but also when the animals experience cycles of deprivation and reexposure. Reexposure for the RD-Alc group was initiated 1 hour after dark onset (1000 hours). Body weight, water bottle weight, and ethanol solution weights were obtained and recorded at least 3 days per week. Daily values for the intervening days, between the days when weights were recorded, were taken as the average of the prior and subsequent obtained measurements. At the end of the 14-week period, access to ethanol was terminated for both the C-Alc and RD-Alc groups.
Brain Tissue Harvesting
Four days before the end of the ethanol access period, animals were habituated to the decapitation room. Briefly, ethanol bottles were removed 3 hours before light offset (0600 hours). At 0900 hours, animals were transferred from their hanging wire mesh cages to plastic tubs with wood chip bedding and transported to the decapitation room. Animals were extensively handled in the decapitation room area and returned to the vivarium at 1300 hours with home cage access to ethanol restored. On the last day of treatment, ethanol was removed at 0900 hours and the animals were transported to the decapitation room, and at 1300 hours the animals were decapitated. For autoradiographic labeling, the brains were dissected, frozen, and stored at −80°C. Previous work from our laboratory indicates there is no blood alcohol concentration present, at decapitation, when these procedures are followed (unpublished observations).
Autoradiographic Labeling (D1 and D2 Receptor Levels)
Coronal sections, 20 mm thick, were cut using a cryostat-micro-tome (Leica, Northbrook, IL) at −20°C and thaw-mounted onto gelatin-coated slides. Four sets of alternative series sections were collected for the following binding studies. Each set consisted of sections for ligand-binding sites and control purposes in order to determine nonspecific binding. Radioactive binding ligands for D1 and D2 receptors were studied for quantified binding site analysis for each discrete region. Sections were thawed before being preincubated at room temperature for 10 minutes in 50 mM Tris-HCl buffer, pH 7.5. The sections were then incubated for 120 minutes at room temperature with Tris buffer containing 120 mM NaCl, 1 mM MgCl2, 5 mM KCl and 5 nM [3H] SCH23390 (Amersham Biosciences, Piscatway NJ; specific activity 2.63 TBq/mmol, 71.0 Ci/mmol), an antagonist for D1 receptors, or 1 nM [125I]iodosulpiride (Amersham Biosciences; specific activity 74 TBq/mmol, 2000 Ci/mmol), an antagonist for D2 receptors, and 40 nM ketanserin (Sigma Chemical Co., St. Louis, MO), which was included to prevent binding to serotonin-2A receptors. Nonspecific binding was determined in the presence of 5 mM butaclamol (Sigma Chemical Co.). After incubation in [3H]SCH23390 or [125I]iodosulpiride, the sections were washed twice with 50 mM Tris-HCl buffer at 4 °C for 5 minutes, dipped quickly three times in ice-cold distilled water and then dried in a stream of cold air. For the D1 receptor binding sites, the slides were apposed 2 weeks at 4 °C to tritium-sensitive film (Hyperfilm-3H, Amersham Biosciences), along with slide-mounted tritium micro-scale standards (3H-microscales, Amersham Biosciences). For the D2 receptor binding sites, the slides were apposed for 2 days to Hyperfilm-3H along with slide-mounted 125I micro scale standards. Films were developed in Kodak GBX in the dark for 4 minutes at 20 °C, rinsed in Kodak GBX Indicator stop (Eastman Kodak, Rochester, NY) fixed with Kodak X-ray fixer for 5 minutes, and then rinsed for 20 minutes in running water and dried. The autoradiograms in the films were captured with a Nikon (Melville, NY) camera and digitized using Scion (Frederick, MD) Image program. The density of D1 and D2 receptor binding sites were evaluated with an image analyzing system (NIH Image System, Bethesda, MD). Using the NIH Image System, the standard curve was measured and used to calibrate regional brain measurements. The optical density values of the digitized autoradiograms were converted into concentration values by referring to the coexposed calibrated standards of defined radioactive concentrations.
Statistical Analyses
To examine overall ethanol drinking behavior, a 2×10 (ethanol treatment by week) mixed ANOVA was conducted (the weeks of ethanol deprivation, for the RD-Alc group, were excluded to equalize the number of weeks with ethanol intake values). Week was the within-subjects variable and ethanol treatment was the between-subjects variable. To assess for an alcohol deprivation effect (ADE), a one-way repeated-measures ANOVA [ethanol access day: baseline (the average intake of the last 3 days before the deprivation interval) and each of the first 4 days of reexposure] was conducted for each deprivation cycle on the drinking data from the RD-Alc group. For the drinking data, significant interactions and main effects were followed by appropriate simple effect analyses. To evaluate changes in D1 or D2 receptor binding density, each subregion was examined separately using one-way ANOVAs, with ethanol treatment as the between-subjects factor. Post hoc multiple comparisons between ethanol treatment groups were conducted using the Student-Newman-Keuls test. The α level was set at 0.05 for all analyses.
RESULTS
Ethanol Drinking Behavior
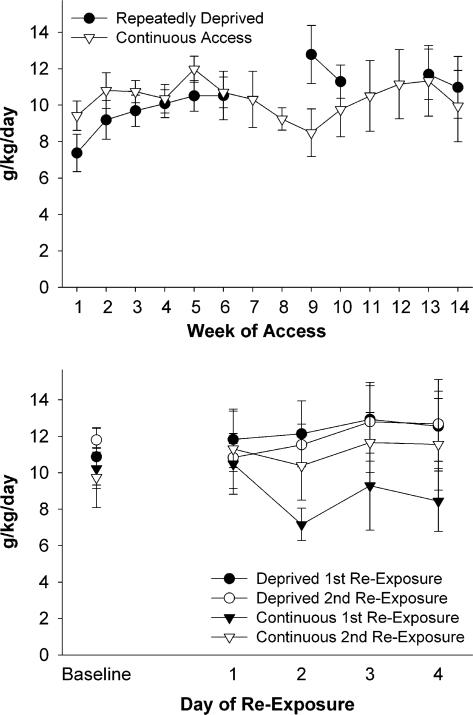
The omnibus 2×10 (ethanol treatment×week) ANOVA on ethanol drinking behavior across weeks did not reveal significant differences between the two ethanol treatment conditions. As seen in Fig. 1 (upper panel), even though the repeatedly deprived animals drank more ethanol during the first week of the first reexposure cycle, overall drinking behavior did not differ between the two groups across weeks. The 2×5 (ethanol treatment by day of reexposure) ANOVAs for the daily ethanol consumption across the first 4 days of each reexposure, compared with baseline intake, did not reveal overall significant increases in ethanol intake upon reexposure to access after either the first or second deprivation cycle (Fig. 1, lower panel). Nevertheless, previous work from our laboratory indicates that P and iP rats consuming the amounts of alcohol observed in this study would achieve blood alcohol concentrations (BACs) in excess of 50 mg% (with peak BACs approximating 90 mg%) during bouts at the beginning and end of the dark cycle and upon initial reexposure for the RD-Alc group (Murphy et al., 2002).
Fig. 1.
Histograms indicate overall ethanol (concurrent access to 15 and 30% ethanol) drinking behavior of male iP rats in top panel and initial (first 4 days) ethanol drinking behavior during reexposure to ethanol access of same animals in lower panel, with baseline referring to the average intake of the last 3 days of ethanol access before the 2-week deprivation interval. Values are expressed as mean ± SEM. Overall, no significant differences were seen in the drinking behavior between the continuous access (C-Alc) and the repeatedly deprived (RD-Alc) animals.
D1 Receptor Binding Sites by [3H]SCH23390
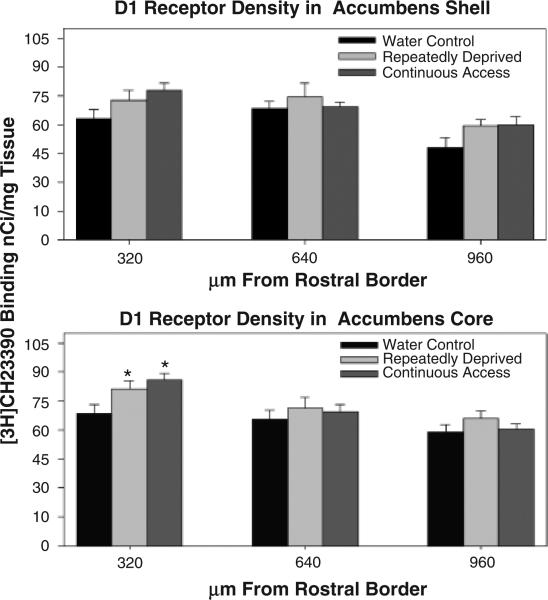
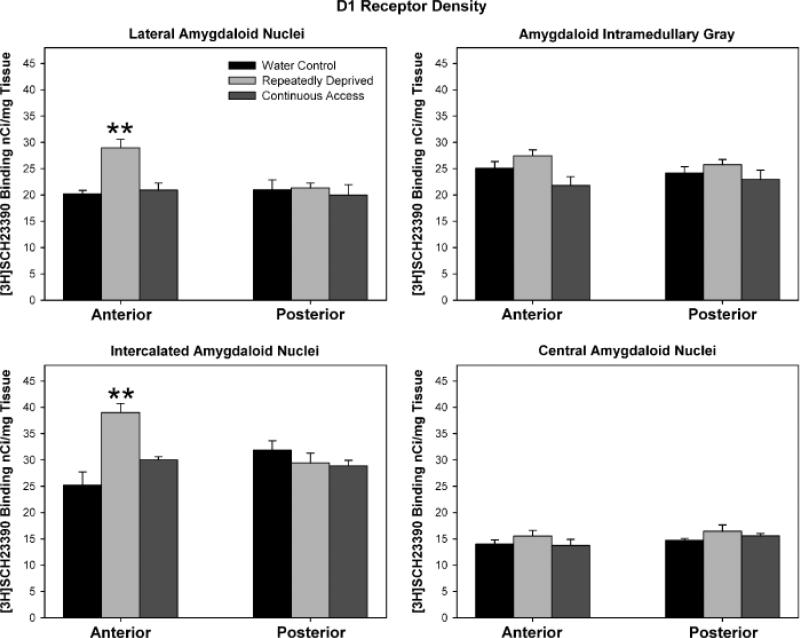
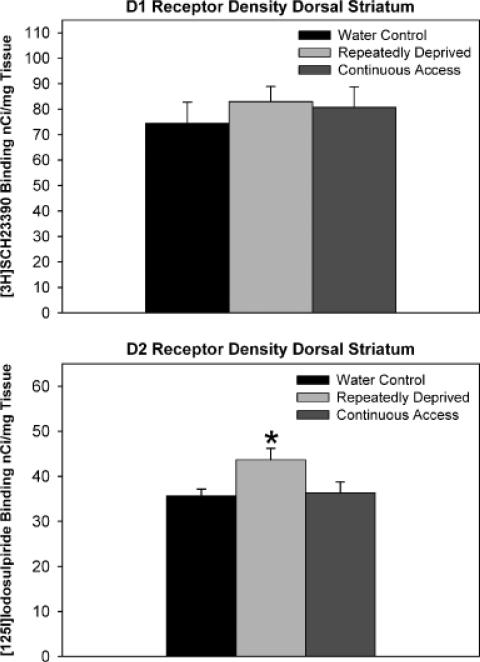
One-way ANOVAs did not reveal statistically signifi-cant differences in D1 receptor binding density levels between the ethanol treatment groups for the anterior, mid, and posterior regions of the Acb shell (p = 0.12), although there was an overall trend for increased levels of D1 receptor binding sites in the anterior region of the RD-Alc and C-Alc groups [Figs. 2 (autoradiographs) and 3 upper panel]. The one-way ANOVA examining ethanol treatment group differences in levels of D1 receptor binding sites was significant for the anterior region of the Acb core [F(2, 12) = 4.70, p = 0.031], with the Student-Newman-Keuls multiple comparisons test revealing that both the RD-Alc and the C-Alc groups had significantly (p<0.05) higher levels of D1 receptor binding sites than the water control group (Fig. 3, lower panel). There were no significant differences for the mid and posterior regions of the Acb core (Fig. 3, lower panel). Within the amygdala [Fig. 4 (autoradiographs)], statistical analyses for D1 receptor binding site density in (a) the lateral amygdaloid nuclei revealed significant differences in the anterior region [F(2, 9) = 9.40, p = 0.0063], with the RD-Alc group expressing significantly (p<0.05) greater D1 receptor binding density than both the C-Alc and the water control groups (Fig. 5, upper left panel); (b) differences within the amygdala intramedullary gray approached significance for the anterior region (p = 0.093: Fig. 5, upper right panel); (c) differences within the intercalated amygdaloid nuclei reached significance in the anterior region [F(2, 9) = 12.11, p = 0.0028], with the RD-Alc group expressing significantly (p<0.05) greater D1 receptor binding density than both the C-Alc and the water control groups (Fig. 5, lower left panel); and (d) differences within the central amygdaloid nuclei did not reach statistical significance (Fig. 5, lower right panel). The one-way ANOVA examining ethanol treatment group differences for D1 receptor binding site density within the pooled dorsomedial and dorsolateral striatum (dorsal striatum) did not reveal statistically significant differences.
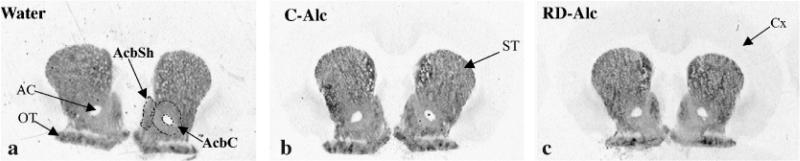
Fig. 2.
Autoradiographic images show the labeling for D1 receptors in na ïve (water, A), continuous access to alcohol (C-Alc, B), and repeatedly deprived of alcohol (RD-Alc, C) groups. AcbSh, nucleus accumbens shell; AcbC, nucleus accumbens core; ST, striatum, OT, olfactory tubercle.
Fig. 3.
Histograms indicate the density of D1 receptor binding sites in the nucleus accumbens (Acb) shell (upper panel) and Acb core (lower panel) among treatment groups. The X-axis refers to the distance from the rostral border. Values are expressed as mean ± SEM. *Significant (p<0.05) difference from water control values. Both animals with continuous access (C-Alc) and those repeatedly deprived (RD-Alc) displayed increased D1 receptor binding site density in the anterior region of the Acb core.
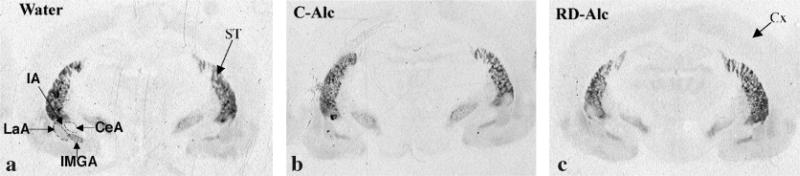
Fig. 4.
Autoradiographic images show the labeling for dopamine D1 receptors in the amygdala complex in naïve (water, A), repeatedly deprived of alcohol (RD-Alc, B), and continuous access to alcohol (C-Alc, C) groups. CeA, central amygdaloid nuclei; IA, intercalated nuclei amygdala; IMGA, amygdaloid intramedullarly gray; LaA, lateral amygdaloid nuclei; ST, striatum.
Fig. 5.
Histograms indicate the density of D1 receptor binding sites in subregions of the amygdala complex: lateral amygdaloid nuclei (upper left panel), amygdaloid intramedullary gray (upper right panel), intercalated amygdaloid nuclei (lower left panel), and central amygdaloid nuclei (lower right panel). Values are expressed as mean ± SEM. **Significantly (p<0.05) higher values than both the water control and the continuous access (C-Alc) groups. The D1 receptor binding site density in anterior regions of the lateral and intercalated amygdaloid nuclei was increased in repeatedly deprived (RD-Alc), but not C-Alc, animals.
D2 Receptor Binding Sites by [125I]Iodosulpiride
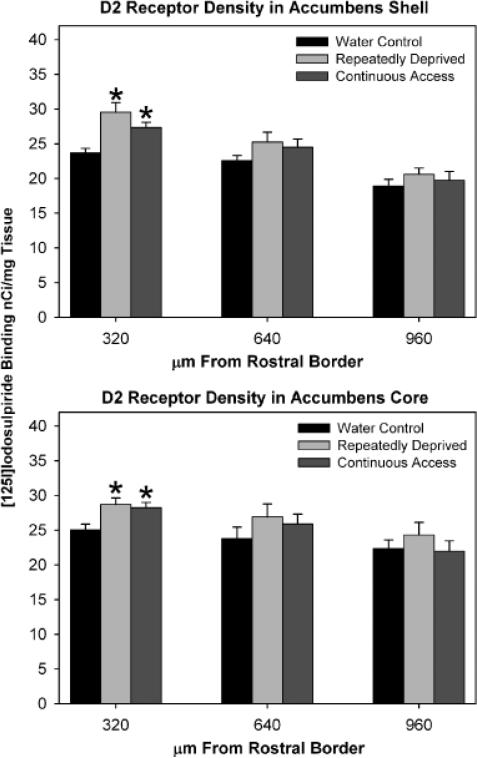
The one-way ANOVA and Student-Newman-Keuls multiples comparisons test examined ethanol treatment group differences in D2 receptor binding site density were significant for the anterior region of the Acb shell [F(2, 15) 5 8.99, p = 0.0027], such that both the RD-Alc and the C-Alc groups displayed higher levels of D2 receptor binding sites than the water control group [Figs. 6 (autoradio-graphs) and 7 upper panel]. There were no significant differences between groups in levels of D2 receptor binding density within the mid and posterior regions of the Acb shell (Fig. 7, upper panel). The statistical analyses revealed that ethanol treatment group differences in levels of D2 receptor binding site density were significant for the anterior region of the Acb core as well [F(2, 15) 5 5.65, p = 0.015]. Similar to the findings for the Acb shell, both the RD-Alc and the C-Alc groups expressed significantly (p<0.05) higher levels of D2 receptor binding sites than the water control group (Fig. 7, lower panel). Again, similar to the findings for the Acb shell, there were no significant differences between groups in D2 receptor binding site density of the mid and posterior regions of the Acb core (Fig. 7, lower panel). The one-way ANOVA examining ethanol treatment group differences in D2 receptor binding site density within the pooled dorsomedial and dorsolateral striatum (dorsal striatum) was significant [F(2, 15) = 4.02, p = 0.040], with the RD-Alc, but not C-Alc, group expressing significantly (p<0.05) greater levels of D2 receptor binding sites than the water control group (Fig. 8).
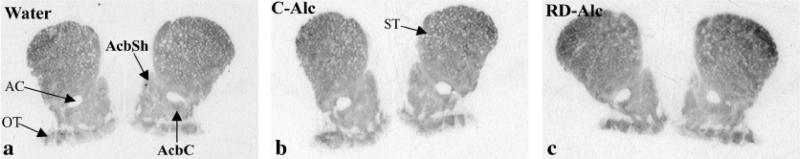
Fig. 6.
Autoradiographic images show the labeling for dopamine D2 receptors in the nucleus accumbens (Acb) of naïve (water, A), continuous access to alcohol (C-Alc, B), and repeatedly deprived of alcohol (RD-Alc, C) groups. AC, anterior commissure; AcbSh, nucleus accumbens shell; AcbC, nucleus accumbens core; ST: striatum, OT, Olfactory tubercle.
Fig. 7.
Histograms indicated the density of D2 receptor binding sites in the nucleus accumbens (Acb) shell (upper panel) and Acb core (lower panel). The X-axis refers to the distance from the rostral border. Values are expressed as mean ± SEM. *Significant (p<0.05) difference from water control values. Both animals with continuous access (C-Alc) and those repeatedly deprived (RD-Alc) displayed increased D2 receptor binding site density in the anterior regions of the Acb shell and core.
Fig. 8.
Histograms indicated the density of D1 receptor (upper level) and D2 receptor (lower level) binding sites in the dorsal striatum (data from the dorsolateral and dorsomedial striatum were pooled). Values are expressed as mean ± SEM. There was no significant difference in the density of D1 recep tor binding sites between the Water, C-Alc, and RD-Alc groups. *Significant (p<0.05) difference in the density of D2 receptor binding sites from water control values. Repeatedly deprived (RD-Alc), but not continuous access (C Alc), animals displayed increased D2 receptor binding site density in the dorsal striatum, compared with controls.
DISCUSSION
Findings from the present study indicate that (a) both RD-Alc and C-Alc rats had subregion-specific increases in D1 (Fig. 3) and D2 (Fig. 7) receptor binding site densities within the anterior Acb, compared with the water control group; (b) D1 receptor binding site density was also increased in the anterior lateral and intercalated nuclei of the amygdala in RD-Alc, compared with water control and CAlc, rats (Fig. 5); and (c) RD-Alc, but not C-Alc, rats displayed increased D2 receptor binding site density in the dorsal striatum, compared with water controls.
Drinking Score
Adult male iP rats in the present study drank approximately 10 g/kg/day [RD-Alc 10.4 ± 0.47 (overall mean-SEM); C-Alc 10.4 ± 0.32] of ethanol. Nevertheless, the absence of a robust ADE was unexpected (Fig. 1). The ADE is a temporary increase in ethanol intake and/or preference after resumption of drinking following a period of deprivation, compared with intake prior to the deprivation interval (Sinclair and Senter, 1968). In reviewing previous work investigating the development of the ADE in P rats, it was found that, for the most part, this has been limited to adult female P rats (Rodd-Henricks et al., 2000; Rodd-Henricks et al., 2001). When adult male P rats have been tested for the ADE, the expression of an ADE has been modest upon the first or subsequent re-exposures, but, at the same time, baseline drinking has also been substantially higher (Bell et al., 2002; Hoffman et al., 2004) than that displayed by adult female P rats (Rodd-Henricks et al., 2000; Rodd-Henricks et al., 2001). Despite the absence of an ADE, the amount of ethanol consumed by the adult male iP rats, in the present study, was very high (twice that of the criterion for selective breeding: 5.0 g/kg/ day), even for an alcohol-preferring line of rats. Together, these findings suggest that the length of deprivation, the number of deprivation cycles, and the sex of the animal can have an impact on ethanol drinking behavior and the expression of an ADE.
Time Course and Dose of Alcohol Drinking
The present study using a 14-week paradigm revealed that consequent to RD-Alc or C-Alc, there were significant increases in D1 and D2 receptor binding site densities within subregions of brain structures associated with the EA and within the dorsal striatum. Previous work has indicated that 4 weeks of alcohol drinking leads to a decrease in D1 receptor binding site density in the Acb of Fisher 344 rats (Pellegrino and Druse, 1992), and 5 weeks of alcohol drinking results in no change in D1 receptor binding site density in the Acb of Fawn-Hooded rats (Djouma and Lawrence, 2002). A major difference between these studies and the present study is the length of the alcohol drinking period and its effects on receptor binding site, such that shorter treatments (4–5 weeks) seem to reduce D1 receptor binding site density whereas longer treatments (10 or 14 weeks in the present study) appear to increase D1 receptor binding site density (see Table 1). One possible explanation for the present results is that an extended protocol of C-Alc self-administration may result in extracellular levels of DA in the Acb that have either returned to or decreased below levels of the naïve state after an initial increase. This would, conceivably, result in an upregulation of D1 receptor and/or binding site levels. Support for this hypothesis of decreased extracellular DA levels in the Acb, after chronic ethanol exposure, comes from the fact that adult female P rats with 12 weeks of C-Alc access to multiple concentrations of ethanol displayed reduced extracellular levels of DA in the Acb, compared with naïve controls (Melendez et al., 2002).
Table 1.
Summary of Previous Findings Investigating the Effect of Single and Chronic Alcohol Exposure on D1 and/or D2 Receptors
| Duration of Alcohol Exposure | Brain Regions |
||||
|---|---|---|---|---|---|
| ST | Acb | Amg | Strains | References | |
| Single injection (3g/kg, i.p.) | No effect on D1 or D2 receptors | — | — | Sprague–Dawley rats | Lucchi et al. (1988) |
| 21 d(6.2%v/v) | ↑ D1 and D2 receptors | — | — | Sprague–Dawley rats | Hruska (1988) |
| 25 d (6% v/v) | ↓ D1 and D2 receptors | — | — | Sprague–Dawley rats | Lucchi et al. (1988) |
| 4 wk (6.6% v/v) | No effect on D1 or D2 receptors | ↓ D1 receptors | — | Fisher 344 rats | Pellegrino and Druse (1992) |
| No effect on D2 receptors | |||||
| 5 wk (5% v/v) | No effect on D1 or D2 receptors | No effect on D1 or D2 receptors | — | Fawn-Hooded rats | Djouma and Lawrence (2002) |
| 5 wk (6% v/v) | No effect on D1 and D2 receptors | — | — | Wistar rats | Hietala et al. (1990) |
| 6 wk (6.6% v/v) | No effect on D2 receptors | ↑ D2 receptors | — | Fisher 344 rats | Tajuddin and Druse (1996) |
| 8 wk (3% v/v) | ↑ D1 receptors | — | — | Wistar rats | Lograno et al. (1993) |
| No effect on D2 receptors | |||||
| 14 wk (15% and 30% v/v) | No effect on D1 or D2 receptors | ↑ D1 receptors, Acb core | No effect on D1 receptors | iP rats | Sari et al., present report |
| ↑ D2 receptors, Acb shell and core | |||||
ST, striatum; Acb, nucleus accumbens; Amg, amygdala complex; iP, inbred alcohol-preferring rats; —, not determined.
Similarly for D2 receptor studies, it has been reported that 5 weeks of C-Alc (10%) resulted in increased D2 receptor mRNA in the caudate-putamen (Kim et al., 1997). However, it must be noted that an increase in D2 receptor mRNA may not be an indication of increased levels of functional D2 receptors. In other studies, it was found that 8 weeks of C-Alc (3%) increased D1 receptor, but not D2 receptor, binding site density in the caudate-putamen (Lograno et al., 1993), and 5 weeks of ethanol in a liquid diet did not alter D1 or D2 receptor levels of the caudateputamen (Hietala et al., 1990). Regarding the DA receptor functional state, the receptor alterations (e.g., increased binding) following chronic ethanol exposure, in the present study, could be due to conformational changes in the receptor complex, which may reflect cellular neuroadaptations to ethanol, as has been reported for other types of receptors (Kril et al., 1988; Ross et al., 1977). Therefore, the altered levels of D1 and D2 receptor binding sites may have been due to alterations in the binding capacity of the ligands such as affinity or conformation of receptor dime-rization (Rocheville et al., 2000) rather than changes in absolute receptor numbers.
In addition to the length of treatment differences, another major difference between the studies is the dose of ethanol self-administered. There is evidence for dose-dependent effects of ethanol on DA activity in the Acb and other mesolimbic structures. Our study with iP rats that drank multiple concentrations of ethanol (15 and 30%, v/v), with overall average intakes approximating 10 g/kg/ day, is likely to have resulted in greater effects on the central nervous system than studies examining the effects of self-administered ethanol using low to moderate concentrations (3–6% or 10%) by rats that do not show a propensity for ethanol preference over water (Hietala et al., 1990; Lograno et al., 1993; Lucchi et al., 1988; Pellegrino and Druse, 1992) (see Table 1). This is also in line with data collected from P rats, such that C-Alc access to multiple concentrations of ethanol concentrations for 12 weeks induced decreases in basal DA levels in the Acb (Melendez et al., 2002), whereas C-Alc access to a single concentration of ethanol (10%) for a total of 8 weeks increased extracellular levels of DA and reduced D2 autoreceptor function in the Acb (Thielen et al., 2004). Additionally, even though analyses of 24-hour ethanol intake did not reveal signifi-cant differences between the RD-Alc and C-Alc groups, the RD-Alc group may have displayed enhanced intake during the initial bouts following reexposure to ethanol access. The RD-Alc group has additional withdrawal, which may have a different mechanism mediating the reinforcing effect of alcohol drinking.
Continuous Alcohol versus Repeated Deprivation
The present findings indicate that RD-Alc, but not CAlc, animals displayed increases in D1 receptor binding density of the lateral and intercalated nuclei of the amygdala. The results also revealed an increase in D2 receptor binding density in the dorsal striatum of RD-Alc-treated, but not C-Alc-treated, animals. To the best of our knowledge, this is the first report of differential neuro-adaptive changes in levels of D1/D2 receptor binding sites following RD-Alc exposure.
The differences in D1 and D2 receptor bindings site levels between RD-Alc and C-Alc groups may be consequences of the length of ethanol access, 10-versus 14-week paradigm. The period of ethanol drinking may be a key factor in sensitivity modifications of D1 and/or D2 receptors. In the present study, it is likely that the effects of 10 versus 14 weeks of ethanol exposure differentially modified the densities of D1 and D2 receptors at specific brain regions: e.g., RD-Alc (10 week) induced increase in the densities of D1 receptor in the lateral and intercalated amygdaloid nuclei and D2 receptor in the dorsal striatum compared to C-Alc (14-week) treatment. However, although overall drinking levels did not differ between the RD-Alc and C-Alc groups, the patterns of ethanol exposure (i.e., presence of extended periods of deprivation in RD-Alc animals) may also have contributed to the alterations in DA receptor sensitivity. One possibility is that the initial 6 weeks of access may have induced an increase in extracellular levels of DA in the Acb with a concomitant decrease in D1 receptor levels. However, upon deprivation (withdrawal) there would have been a decrease, at least initially, in extracellular DA, as reported by others (Diana et al., 1992; Rossetti et al., 1992; Weiss et al., 1996) for outbred rats, with a subsequent increase in D1 receptor levels. It may be that after multiple cycles of deprivation and reexposure (2 in the present study) the homeostatic mechanism controlling D1/D2 receptor levels was sufficiently perturbed that the expected reduction in D1/D2 receptor levels upon reexposure to ethanol was not seen at the end of the second reexposure cycle (i.e., the end of the 14th week). Instead elevated levels of D1/D2 receptor binding sites were maintained across the last reexposure period. To evaluate these possibilities, our laboratory has ongoing work examining levels of DA receptor binding sites within the EA at the end of deprivation cycles and at other time points.
We suggest in the present report that the EA is more sensitive to changes in the density of D1/D2 receptor binding sites following cycles of repeated deprivations compared with a protocol of chronic ethanol. In particular, the findings suggest that repeated deprivations and the effects of associated withdrawal may induce greater sensitivity to neuroadaptive processes of subregion-specific DA receptor binding sites in RD-Alc animals.
Topographic Sensitivity
The present findings revealed changes in levels of D1/D2 receptor binding sites that occurred across functionally related brain regions (i.e., the EA). Additionally, these changes were subregion-specific, such that significant differences were detected in anterior, but not in mid or posterior, portions of the Acb shell and core and the lateral and intercalated nuclei of the amygdala. Thus, ethanol consumption alters DA receptor activity in anterior portions of interconnected regions within the EA, which suggests that these subregions are more sensitive than mid and posterior portions to the effects of high ethanol consumption. It is noteworthy that the anterior regions of the Acb have greater (a) DA receptor density levels and (b) DA innervation from the VTA, compared with posterior regions (Papa et al., 2002). Therefore, greater DA receptor density levels and DA innervation may allow for greater regulation, or dysregulation in the case of alcohol abuse, of ethanol self-administration.
It has been shown that intraperitoneal administration of ethanol elevates extracellular DA levels not only in the ventral (Acb) but also in the dorsal striatum (Melendez et al., 2003). Regarding subregion-specific sensitivity, Melendez et al. (2003) reported greater release of DA in the ventral (Acb) versus dorsal striatum following ethanol exposure as demonstrated previously by other researchers (Imperato and Di Chiara, 1986). Therefore, the dorsal striatum, similar to the ventral (Acb) striatum and the amygdaloid complex, displays altered DAergic activity after ethanol exposure (Melendez et al., 2003). The present findings indicate that ethanol exposure with repeated deprivations increased D2 receptor binding site density, which did not occur under conditions of chronic ethanol without deprivations. Given the above, the dorsal striatum may be involved in high ethanol consumption, either in concert with the EA or separate from this neuronal circuit.
In conclusion, it is known that the mesolimbic DA system, including the EA, mediates ethanol intake. The present findings indicate that the DAergic systems within anterior portions of these interrelated structures (Acb shell and core, lateral and intercalated nuclei of the amygdala, and the dorsal striatum) may play a preferential role, over that displayed by mid and posterior portions of these structures, in mediating this effect. This subregion specifi-city may be due to the fact that anterior portions of the Acb have greater DA receptor site density levels and DA-ergic projections from the VTA than those seen in the posterior Acb. Additionally, the present results indicate that the lateral and intercalated nuclei of the amygdala are especially sensitive to the effects of repeated deprivations, such that increases in D1 receptor binding density were limited to RD-Alc animals. It is well known that the amygdala is sensitive to the effects of ethanol withdrawal and mediates, at least in part, the anxiolytic effects of ethanol (c.f., Koob and Britton, 1996). Therefore, the DA system in the lateral and intercalated nuclei of the amygdala complex may be crucial in mediating continued excessive ethanol intake despite intervening periods of withdrawal and abstinence. To further delineate the neurophysiological significance of these upregulations in D1 and D2 receptor binding sites, our laboratory continues to investigate subregion differences in DAergic activity within the EA and associated brain structures, as well as examine the time course of these changes after animals are deprived of ethanol access.
Acknowledgments
Supported by NIAAA grants NIH U01 AA014829 (an INIA project) to FCZ and in part by P50 AA07611 to D. Crabb and AA13522 (an INIA project) to W. McBride.
REFERENCES
- Asan E. The catecholaminergic innervation of the rat amygdala. Adv Anat Embryol Cell Biol. 1998;142:1–118. doi: 10.1007/978-3-642-72085-7. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978;179:641–667. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd-Henricks ZA, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK. Binge drinking induces excessive alcohol in-take in the alcohol-preferring (P) rat. Alcohol Clin Exp Res. 2002;26(Suppl):61A. [Google Scholar]
- Bozarth MA. The mesolimbic dopamine system as a model reward system. In: Willner P, Scheel-Kruger J, editors. The Mesolimbic Dopamine System: From Motivation to Action. Wiley Press; Chichester: 1991. pp. 301–330. [Google Scholar]
- Brodie MS, Appel SB. The effects of ethanol on dopaminergic neurons of the ventral tegmental area studied with intracellular recording in brain slices. Alcohol Clin Exp Res. 1998;22:236–244. [PubMed] [Google Scholar]
- Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res. 1999;23:1848–1852. [PubMed] [Google Scholar]
- Burish TG, Maisto SA, Cooper AM, Sobell MB. Effects of voluntary short-term abstinence from alcohol on subsequent drinking patterns of college students. J Stud Alcohol. 1981;42:1013–1020. doi: 10.15288/jsa.1981.42.1013. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana M, Pistis M, Muntoni A, Rossetti ZL, Gessa G. Marked decrease of A10 dopamine neuronal firing during ethanol withdrawal syndrome in rats. Eur J Pharmacol. 1992;221:403–404. doi: 10.1016/0014-2999(92)90734-l. [DOI] [PubMed] [Google Scholar]
- Djouma E, Lawrence AJ. The effect of chronic ethanol consumption and withdrawal on mu-opioid and dopamine D(1) and D(2) receptor density in Fawn-Hooded rat brain. J Pharmacol Exp Ther. 2002;302:551–559. doi: 10.1124/jpet.102.035915. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1978;180:545–580. doi: 10.1002/cne.901800310. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG. Feeding and hypothalamic stimulation increase dopamine turnover in the accumbens. Physiol Behav. 1988;44:599–606. doi: 10.1016/0031-9384(88)90324-1. [DOI] [PubMed] [Google Scholar]
- Hietala J, Salonen I, Lappalainen J, Syvalahti E. Ethanol administration does not alter dopamine D1 and D2 receptor characteristics in rat brain. Neurosci Lett. 1990;108:289–294. doi: 10.1016/0304-3940(90)90656-t. [DOI] [PubMed] [Google Scholar]
- Hilbrom ME. Alcohol withdrawal seizures and binge versus chronic drinking. In: Port RJ, Mattson RH, Cramer JA, Diamond I, editors. Alcohol and Seizures: Basic Mechanisms and Clinical Concepts. FA Davis; Philadelphia: 1990. pp. 206–215. [Google Scholar]
- Hoffman PL, Hedman K, Bell RL, Strother WN, Tabakoff B. NMDA receptors in the amygdala of ethanol-treated P rats. Alcohol Clin Exp Res. 2004;24(Suppl):133A. [Google Scholar]
- Holstege G, Meiners L, Tan K. Projections of the bed nucleus of the stria terminalis to the mesencephalon, pons, and medulla oblong-ata in the cat. Exp Brain Res. 1985;58:379–391. doi: 10.1007/BF00235319. [DOI] [PubMed] [Google Scholar]
- Hruska RE. Effect of ethanol administration of striatal D1 and D2 dopamine receptors. J Neurochem. 1988;50:1929–1933. doi: 10.1111/j.1471-4159.1988.tb02499.x. [DOI] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239:219–228. [PubMed] [Google Scholar]
- Kalivas PW. Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Res Brain Res Rev. 1993;18:75–113. doi: 10.1016/0165-0173(93)90008-n. [DOI] [PubMed] [Google Scholar]
- Killcross S, Robbins TW, Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature. 1997;388:377–380. doi: 10.1038/41097. [DOI] [PubMed] [Google Scholar]
- Kim MO, Lee YK, Choi WS, Kim JH, Hwang SK, Lee BJ, Kang SG, Kim K, Baik SH. Prolonged ethanol intake increases D2 dopamine receptor expression in the rat brain. Mol Cells. 1997;7:682–687. [PubMed] [Google Scholar]
- Koob GF, Britton KT. Neurobiological substrates for the anti-anxiety effects of ethanol. In: Begleiter H, Kissin B, editors. The Pharmacology of Alcohol and Alcohol Dependence. Oxford University Press; New York: 1996. pp. 477–506. [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, Merlo-Pich E, Weiss F. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res. 1998b;22:3–9. [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998a;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Projections from the amygdala to the perirhinal and entorhinal cortices and the subiculum. Brain Res. 1974;71:150–154. doi: 10.1016/0006-8993(74)90199-1. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Dodd PR, Gundlach AL, Davies N, Watson WE, Johnston GA, Harper CG. Necropsy study of GABA/benzodiazepine receptor binding sites in brain tissue from chronic alcoholic patients. Clin Exp Neurol. 1988;25:135–141. [PubMed] [Google Scholar]
- Lograno DE, Matteo F, Trabucchi M, Govoni S, Cagiano R, Lacomba C, Cuomo V. Effects of chronic ethanol intake at a low dose on the rat brain dopaminergic system. Alcohol. 1993;10:45–49. doi: 10.1016/0741-8329(93)90052-p. [DOI] [PubMed] [Google Scholar]
- Lucchi L, Moresco RM, Govoni S, Trabucchi M. Effect of chronic ethanol treatment on dopamine receptor subtypes in rat striatum. Brain Res. 1988;449:347–351. doi: 10.1016/0006-8993(88)91051-7. [DOI] [PubMed] [Google Scholar]
- McBride WJ. Central nucleus of the amygdala and the effects of alcohol and alcohol-drinking behavior in rodents. Pharmacol Biochem Behav. 2002;71:509–515. doi: 10.1016/s0091-3057(01)00680-3. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Li TK. Animal models of alcoholism: Neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- McMillen BA. Toward a definition of a valid model of alcoholism: Multiple animal models for multiple diseases. Alcohol. 1997;14:409–419. doi: 10.1016/s0741-8329(97)90012-4. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Rodd-Henricks ZA, Engleman EA, Li TK, McBride WJ, Murphy JM. Microdialysis of dopamine in the nucleus accumbens of alcohol-preferring (P) rats during anticipation and operant self-administration of ethanol. Alcohol Clin Exp Res. 2002;26:318–325. [PubMed] [Google Scholar]
- Melendez RI, Rodd-Henricks ZA, McBride WJ, Murphy JM. Alcohol stimulates the release of dopamine in the ventral pallidum but not in the globus pallidus: A dual-probe microdialysis study. Neuropsychopharmacology. 2003;28:939–946. doi: 10.1038/sj.npp.1300081. [DOI] [PubMed] [Google Scholar]
- Murphy JM, McBride WJ, Lumeng L, Li TK. Regional brain levels of monoamines in alcohol-preferring and -nonpreferring lines of rats. Pharmacol Biochem Behav. 1982;16:145–149. doi: 10.1016/0091-3057(82)90026-0. [DOI] [PubMed] [Google Scholar]
- Murphy JM, McBride WJ, Lumeng L, Li TK. Monoamine and metabolite levels in CNS regions of the P line of alcohol-preferring rats after acute and chronic ethanol treatment. Pharmacol Biochem Behav. 1983;19:849–856. doi: 10.1016/0091-3057(83)90092-8. [DOI] [PubMed] [Google Scholar]
- Murphy JM, McBride WJ, Lumeng L, Li TK. Alcohol preference and regional brain monoamine contents of N/Nih heterogeneous stock rats. Alcohol Drug Res. 1987;7:33–39. [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li TK. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Nauta WJ, Smith GP, Faull RL, Domesick VB. Efferent connections and nigral afferents of the nucleus accumbens septi in the rat. Neuroscience. 1978;3:385–401. doi: 10.1016/0306-4522(78)90041-6. [DOI] [PubMed] [Google Scholar]
- Nishiguchi M, Kinoshita H, Taniguchi T, Utsumi T, Ouchi H, Minami T, Hishida S. Effects of chronic alcohol administration on changes of extracellular dopamine and serotonin concentration induced by methamphetamine—comparison of two different alcohol preference rat lines. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2002;37:555–576. [PubMed] [Google Scholar]
- Nowak KL, McBride WJ, Lumeng L, Li TK, Murphy JM. Involvement of dopamine D2 autoreceptors in the ventral tegmental area on alcohol and saccharin intake of the alcohol-preferring P rat. Alcohol Clin Exp Res. 2000;24:476–483. [PubMed] [Google Scholar]
- Oades RD, Halliday GM. Ventral tegmental (A10) system: Neurobiology. 1. Anatomy and connectivity. Brain Res. 1987;434:117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- Palacios JM, Waeber C, Hoyer D, Mengod G. Distribution of serotonin receptors. Ann N Y Acad Sci. 1990;600:36–52. doi: 10.1111/j.1749-6632.1990.tb16871.x. [DOI] [PubMed] [Google Scholar]
- Papa M, Diewald L, Carey MP, Esposito FJ, Gironi Carnevale UA, Sadile AG. A rostro-caudal dissociation in the dorsal and ventral striatum of the juvenile SHR suggests an anterior hypo- and a posterior hyperfunctioning mesocorticolimbic system. Behav Brain Res. 2002;130:171–179. doi: 10.1016/s0166-4328(01)00421-1. [DOI] [PubMed] [Google Scholar]
- Pellegrino SM, Druse MJ. The effects of chronic ethanol consumption on the mesolimbic and nigrostriatal dopamine systems. Alcohol Clin Exp Res. 1992;16:275–280. doi: 10.1111/j.1530-0277.1992.tb01376.x. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Damsma G, Wenkstern D, Fibiger HC. Sexual activity increases dopamine transmission in the nucleus accumbens and striatum of female rats. Brain Res. 1995;693:21–30. doi: 10.1016/0006-8993(95)00679-k. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Stinus L, Koob GF. The effects of 6-hydroxydopa-mine lesions of the nucleus accumbens and the mesolimbic dopamine system on oral self-administration of ethanol in the rat. Brain Res. 1993;623:16–24. doi: 10.1016/0006-8993(93)90004-7. [DOI] [PubMed] [Google Scholar]
- Rocheville M, Lange DC, Kumar U, Patel SC, Patel RC, Patel YC. Receptors for dopamine and somatostatin: Formation of hetero-oligomers with enhanced functional activity. Science. 2000;288:154–157. doi: 10.1126/science.288.5463.154. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Melendez RI, Kuc KA, Lumeng L, Li TK, Murphy JM, McBride WJ. Comparison of intracranial self-administration of ethanol within the posterior ventral tegmental area between alcohol-preferring and Wistar rats. Alcohol Clin Exp Res. 2004;28:1212–1219. doi: 10.1097/01.alc.0000134401.30394.7f. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of concurrent access to multiple ethanol concentrations and repeated deprivations on alcohol intake of alcohol-preferring rats. Alcohol Clin Exp Res. 2001;25:1140–1150. [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Shaikh SR, Murphy JM, McBride WJ, Lumeng L, Li TK. Alcohol deprivation effect is prolonged in the alcohol preferring (P) rat after repeated deprivations. Alcohol Clin Exp Res. 2000;24:8–16. [PubMed] [Google Scholar]
- Ross DH, Kibler BC, Cardenas HL. Modification of glycoprotein residues as Ca21 receptor sites after chronic ethanol exposure. Drug Alcohol Depend. 1977;2:305–315. doi: 10.1016/0376-8716(77)90033-3. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Hmaidan Y, Gessa GL. Marked inhibition of mesolimbic dopamine release: A common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. Eur J Pharmacol. 1992;221:227–234. doi: 10.1016/0014-2999(92)90706-a. [DOI] [PubMed] [Google Scholar]
- Rothblat DS, Rubin E, Schneider JS. Effects of chronic alcohol ingestion on the mesostriatal dopamine system in the rat. Neurosci Lett. 2001;300:63–66. doi: 10.1016/s0304-3940(01)01548-8. [DOI] [PubMed] [Google Scholar]
- Samson HH, Hodge CW, Tolliver GA, Haraguchi M. Effect of dopamine agonists and antagonists on ethanol-reinforced behavior: The involvement of the nucleus accumbens. Brain Res Bull. 1993;30:133–141. doi: 10.1016/0361-9230(93)90049-h. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Senter RJ. Development of an alcohol-deprivation effect in rats. Q J Stud Alcohol. 1968;29:863–867. [PubMed] [Google Scholar]
- Stewart RB, McBride WJ, Lumeng L, Li TK, Murphy JM. Chronic alcohol consumption in alcohol-preferring P rats attenuates subsequent conditioned taste aversion produced by ethanol injections. Psychopharmacology (Berl) 1991;105:530–534. doi: 10.1007/BF02244375. [DOI] [PubMed] [Google Scholar]
- Syvalahti EK, Hietala J, Roytta M, Gronroos J. Decrease in the number of rat brain dopamine and muscarinic receptors after chronic alcohol intake. Pharmacol Toxicol. 1988;62:210–212. doi: 10.1111/j.1600-0773.1988.tb01874.x. [DOI] [PubMed] [Google Scholar]
- Tajuddin NF, Druse MJ. Effects of chronic alcohol consumption and aging on dopamine D2 receptors in Fischer 344 rats. Alcohol Clin Exp Res. 1996;20:144–151. doi: 10.1111/j.1530-0277.1996.tb01057.x. [DOI] [PubMed] [Google Scholar]
- Thielen RJ, Engleman EA, Rodd ZA, Murphy JM, Lumeng L, Li TK, McBride WJ. Ethanol drinking and deprivation alter dopaminergic and serotonergic function in the nucleus accumbens of alcohol-preferring rats. J Pharmacol Exp Ther. 2004;309:216–225. doi: 10.1124/jpet.103.059790. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: Genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- Weiss F, Parsons LH, Schulteis G, Hyytia P, Lorang MT, Bloom FE, Koob GF. Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytrypta-mine release in dependent rats. J Neurosci. 1996;16:3474–3485. doi: 10.1523/JNEUROSCI.16-10-03474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Zhang JK, Lumeng L, Li TK. Mesolimbic dopamine system in alcohol-preferring rats. Alcohol. 1995;12:403–412. doi: 10.1016/0741-8329(95)00010-o. [DOI] [PubMed] [Google Scholar]